Abstract
Purpose
Accurate detection of radiation-induced fibrosis (RIF) is crucial in management of breast cancer survivors. Tissue compliance meter (TCM) has been validated in musculature. We validate TCM in healthy breast tissue with respect to interobserver and intraobserver variability before applying it in RIF.
Methods and Materials
Three medical professionals obtained three consecutive TCM measurements in each of the four quadrants of the right and left breasts of 40 women with no breast disease or surgical intervention. The intraclass correlation coefficient (ICC) assessed interobserver variability. The paired t test and Pearson correlation coefficient (r) were used to assess intraobserver variability within each rater.
Results
The median age was 45 years (range, 24-68 years). The median bra size was 35C (range, 32A–40DD). Of the participants, 27 were white (67%), 4 black (10%), 5 Asian (13%), and 4 Hispanic (10%). ICCs indicated excellent interrater reliability (low interobserver variability) among the three raters, by breast and quadrant (all ICC ≥0.99). The paired t test and Pearson correlation coefficient both indicated low intraobserver variability within each rater (right vs. left breast), stratified by quadrant (all r ≥ 0.94, p < 0.0001).
Conclusions
The interobserver and intraobserver variability is small using TCM in healthy mammary tissue. We are now embarking on a prospective study using TCM in women with breast cancer at risk of developing RIF that may guide early detection, timely therapeutic intervention, and assessment of success of therapy for RIF.
Keywords: Tissue compliance meter, fibrosis, Radiation-induced fibrosis, Breast cancer, Hypofractionated accelerated radiotherapy, Partial breast irradiation, Postmastectomy irradiation, Boost, Radiation side effects
INTRODUCTION
With increased survival of breast cancer patients, one of the main challenges remains identification of radiation (RT)–induced late effects (1). Radiation-induced fibrosis (RIF) is a form of damage to normal tissues after RT, which can cause persistent symptoms and cosmetic disfigurement and can impair quality of life (QOL) (2-6). The reported incidence of moderate-to-severe RIF after breast conservation therapy (BCT) is 43% to 58% (7, 8).
Accurate detection and quantification of RIF are crucial for timely therapeutic intervention with various agents such as Cu/Zn superoxide dismutase (SOD) and Mn SOD (9-11). Delanian et al. demonstrated that superficial RIF is partially reversible with combination of pentoxifylline and tocopherol (12). However, these studies are difficult to interpret because of the variability in methodology of RIF detection and determination of effectiveness of intervention. The degree of severity of RIF depends on a variety of RT parameters (5, 13-15) and genetic components (1, 15). Consequently, accurate methodology for RIF identification of phenotypical changes due to genetic susceptibilities to fibrosis is critical (16).
RIF is characterized by skin retraction, atrophy, toughness to palpation, and decreased tissue compliance. Compliance is the relationship between the force acting upon tissue and the transformation that is caused by that force. There are two methodologies measuring compliance: Radiation Therapy Oncology Group (RTOG) and Late Effects of Normal Tissue–Subjective Objective Management Analytical (LENT-SOMA). However, clinical palpation used by these scales is qualitative and is flawed by subjectivity and high variability among observers (14, 17-24). Although histopathological assessment of RIF provides the most accurate identification of RIF, it is logistically difficult and invasive.
A noninvasive modality that can objectively and reliably quantify RIF renders an important alternative. A tissue compliance meter (TCM) (Fig. 1) is a user-friendly, noninvasive mechanical device that measures tissue compliance by external application (25-29). TCM has been used to quantify normal paraspinal tissue compliance, as well as soft tissue pathology in physical medicine and rehabilitation (PM&R) (28-32). Recently, TCM was applied to compare compliance of irradiated and nonirradiated breasts in patients treated with BCT. TCM was a more promising approach to qualitatively measure RIF as compared with palpation, and it had higher interobserver reliability (p < 0.001) (24).
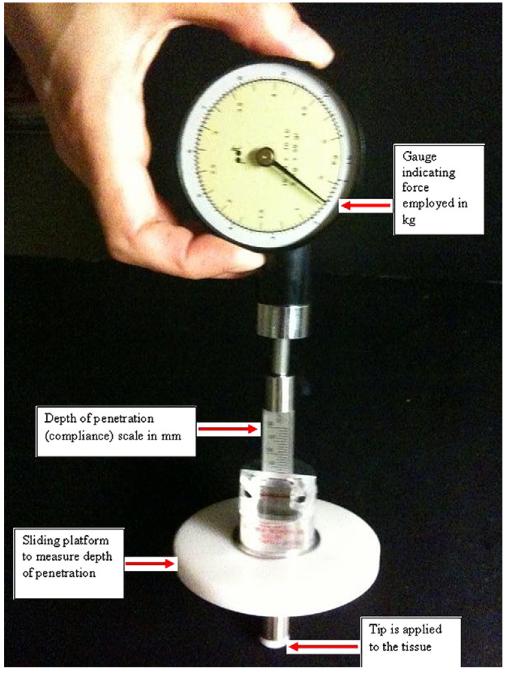
Fig. 1.
Tissue compliance meter (TCM): force gauge (kg), scale indicating depth of penetration or compliance (mm), sliding platform, and tip applied to tissues.
To our knowledge, there are no reports in the literature that validate application of TCM in healthy breast tissue. The purpose of studying a group of healthy volunteers is to examine interobserver and intraobserver variability and to determine the “normal” variation in compliance between both breasts. Such knowledge is fundamental to characterize changes in compliance caused by RT (i.e., RIF). Before embarking on measuring RIF after RT, we explore and validate this technology in healthy volunteers.
MATERIALS AND METHODS
Tissue compliance meter
The TCM is a hand-held mechanical device used for quantification of soft tissue consistency (firmness or softness) that can be used during physical examination (25). It consists of a force gauge (kg) similar to an analog watch face with a dial indicating the applied force (Fig. 1). A disc with 1-cm2 surface attached to a force gauge is pressed into the examined tissue. The tissue compliance, or amount of penetration of the disc in relation to the force used, is read on a millimeter (mm) scale attached to the force gauge. The deformation of the tissue (i.e., its depth of displacement) is indicated by a ring sliding on the shaft of the force gauge. Depth of penetration of the tip as related to the force used expresses tissue compliance. This quality is the reciprocal value of the tissue’s resistance to the deforming force. TCM determines that the tissue is less compliant when there is a smaller transformation as a result of a certain force, e.g., a fibrotic region.
Subjects and positioning for the study
After obtaining institutional review board approval for the study, 40 women who volunteered to participate in this protocol were enrolled. The women were required to have no history of or active breast disease, to have no surgical intervention, and to not be lactating or pregnant. Five subjects participated in the pilot study. In the pilot study, the participating physicians first trained in TCM application and then established maximally tolerated force (MTF) in the breast tissue.
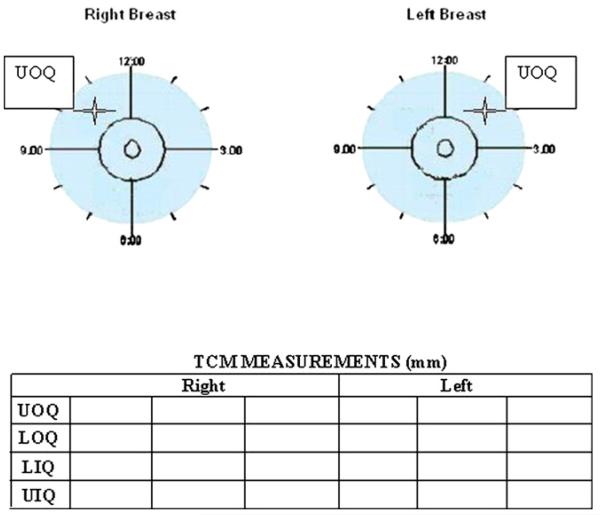
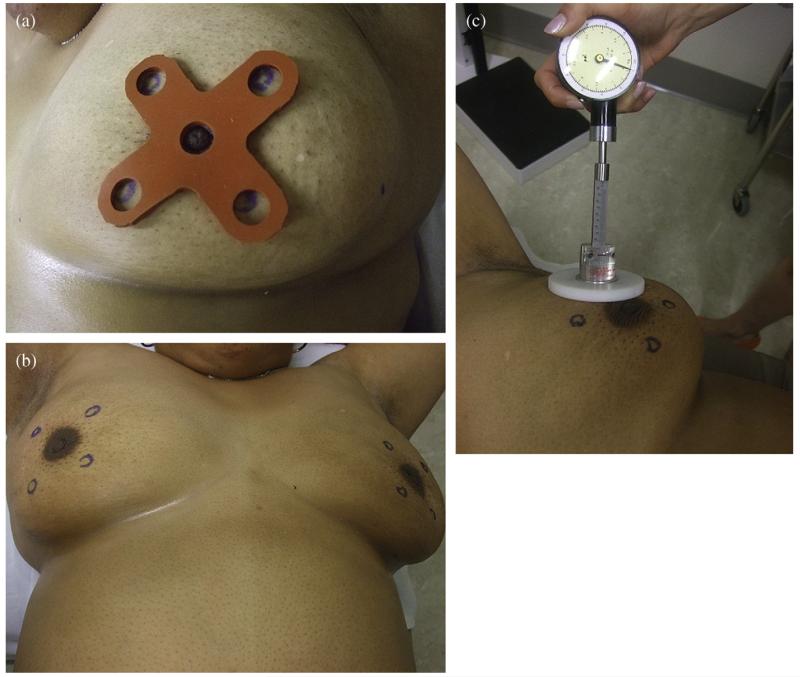
All 40 participants were placed in supine position with the arms above the head. The chest had to be parallel to the table. An example of marking of the point in a quadrant in the right and left breasts, respectively, and documenting the results are provided in Fig. 2. A measurement point for TCM was denoted with a removable ink marker in each of the four quadrants in both breasts, as determined by the predesigned templates (a small template for a bra cup ≤C and large for ≥D) (Fig. 3a). Using these templates, the point in the quadrants was determined as if using the face of a clock for the right (R) and left (L) breasts, respectively, in the following manner: UOQ, medially to the upper side of the axillary line and above the areola at 10:30 o’clock (R) and 1:30 o’clock (L); LOQ, medially to the lower side of the axillary line and below the areola at 7:30 (R) and 4:30 (L); UIQ, medially to the sternum and above the areola at 1:30 (R) and 10:30 (L); and ULQ, medially to the sternum and below the areola at 4:30 (R) and 7:30 (L) (Fig. 3b). Each subject was tested three times in four premarked quadrants of each breast with TCM perpendicular (at 90°) to the chest (Fig. 3c).
Fig. 2.
Diagram illustrating division of the breasts into four quadrants, with template-designated points (e.g., UOQs are marked by a star) to be measured with TCM and recorded by each investigator.
Fig. 3.
Application of a pre-designed template establishes four locations for Tissue compliance meter (TCM) measurements in each breast. (a). A template is secured at the nipple and ink marks are placed at the apertures in each quadrant. (b). Bilateral marks reflect mirror-image points for each respective quadrant. (c). Application of TCM to quantify compliance.
Measuring technique
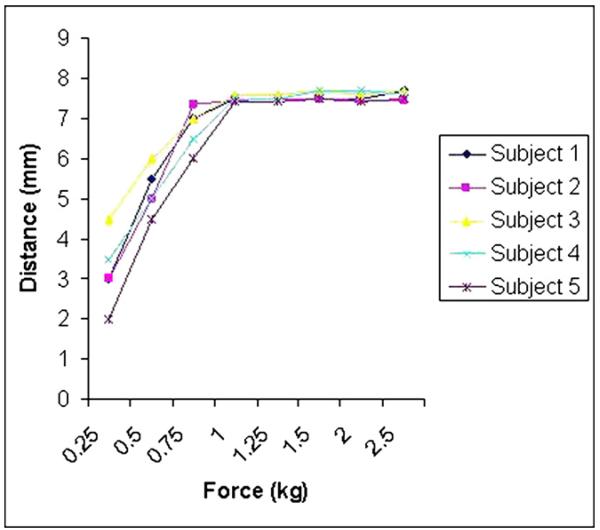
Three medical professionals participated in the data collection process. From the pilot study of force escalation, a pain-free MTF was established at 1.0 kg (Fig. 4). Each physician was blind to the measurements of the others. As the force of 1 kg was reached at the ink-marked areas of each quadrant, TCM was withdrawn and the reading of the depth of penetration scale (mm) was recorded on the data collection form for every investigator (Fig. 2). Each radiation oncologist performed eight measurements by TCM (one per every quadrant in each breast) and, after a 5-min interval, obtained the second and subsequently the third sets of readings, the same time intervals.
Fig. 4.
Pilot study established the maximally tolerated force (MTF) in five normal volunteers. The x-axis represents force (kg), and the y-axis represents depth of penetration (mm).
Statistical analysis
Descriptive statistics (means, frequencies, proportions) were calculated for demographic and clinical characteristics. The intraclass correlation coefficient (ICC), based on a two-way random effects model (i.e., assumes volunteers and raters were selected at random from a population of volunteers and raters, respectively), was used to assess interobserver variability (i.e., interrater reliability), stratified by breast and quadrant. The paired t test and Pearson correlation coefficient (r) were used to assess intraobserver variability within each rater (by comparing the right vs. left breast), stratified by quadrant. All p values were two-sided, with statistical significance evaluated at the 0.05 alpha level. To assess the precision of the obtained estimates, 95% confidence intervals (CIs) were calculated. All analyses were performed in SPSS version 17 (SPSS Inc., Chicago, IL).
RESULTS
The median age of women was 45 years (range, 24—68 years). The median bra size was 35 C (range, 32A–40DD). Of the participants, 27 were whites (67%), 4 blacks (10%), 5 Asian (13%), and 4 Hispanic (10%). All subjects were evaluated for oral contraceptive pill (OCP) use and hormonal replacement therapy (HRT), menopausal and ovulatory status, parity, and nursing. Baseline characteristics of the healthy participants in this study are presented in Table 1.
Table 1.
Baseline characteristics of healthy volunteers (n = 40)
| Characteristic | No. of women (%) |
|---|---|
| Race/Ethnicity | |
| Asian | 5 (13) |
| Black | 4 (10) |
| Hispanic | 4 (10) |
| White | 27 (67) |
| Breast cup size | |
| A | 7 (18) |
| B | 9 (22) |
| C | 12 (30) |
| D | 8 (20) |
| DD | 4 (10) |
| Menopausal status | |
| Premenopausal | 20 (50) |
| Postmenopausal | 20 (50) |
| Place in the menstrual cycle in premenopausal women (n = 20) |
|
| Preovulatory | 11 (55) |
| Postovulatory | 9 (45) |
| History of oral contraceptive pill use | |
| Yes | 11 (28) |
| No | 29 (72) |
| History of hormone replacement therapy use | |
| Yes | 8 (40) |
| No | 12 (60) |
| History of parity | |
| 0 or 1 child | 14 (35) |
| 2 or 3 children | 22 (55) |
| 4 or more children | 4 (10) |
| History of nursing | |
| Yes | 22 (55) |
| No | 18 (45) |
| History of breast disease and/or history of surgical intervention in a breast |
|
| Yes | 0 (0) |
| No | 40 (100) |
Interobserver variability
The ICCs for each quadrant, stratified by breast, indicated low interobserver variability (i.e., excellent interrater reliability) among the three raters. The ICCs ranged from 0.999 to 1.000 with precise 95% CIs (Table 2).
Table 2.
Interobserver variability as reported with ICC indicates excellent reliability among the three raters, by breast and quadrant
| Right breast |
| UOQ: ICC = 0.999; 95% CI = (0.999, 1.000) |
| LOQ: ICC = 0.994; 95% CI = (0.989, 0.996) |
| LIQ: ICC = 1.000; 95% CI = (1.000, 1.000) |
| UIQ: ICC = 1.000; 95% CI = (0.999, 1.000) |
| Left breast |
| UOQ: ICC = 1.000; 95% CI = (1.000, 1.000) |
| LOQ: ICC = 0.999; 95% CI = (0.999, 1.000) |
| LIQ: ICC = 1.000; 95% CI = (1.000, 1.000) |
| UIQ: ICC = 1.000; 95% CI = (1.000, 1.000) |
Abbreviation: UOQ = upper outer quadrant; LOQ = lower outer quadrant; LIQ = lower inner quadrant; UIQ = upper inner quadrant; CI = confidence interval.
Intraobserver variability
The median of the original three TCM measurements taken by each rater on a patient for each quadrant was used as the unit of analysis. The mean differences in TCM compliance between the right and left breasts, stratified by quadrant, for each rater, ranged from 0.24 to 0.38 (Table 3). Although most of the 95% confidence intervals exclude zero (p < 0.05), the narrow confidence limits for the mean differences demonstrated that the TCM measurements taken on the right and left breasts, by quadrant, were in close agreement, indicating that the raters had low intraobserver variability. This is further demonstrated by using the Pearson correlation coefficient (r), comparing the right and left breasts within each rater and quadrant (Table 4). The correlation coefficients ranged from 0.94 to 0.98, again indicating low intraobserver variability for each rater.
Table 3.
Intraobserver variability for each rater by breast and quadrant, reported with the paired t test.
| Rater 1 (right breast vs. left breast) | ||
| UOQ: | 8.73 vs. 8.41; | diff =0.31; 95% CI for diff = (0.08, 0.54) |
| LOQ: | 11.04 vs. 10.68; | diff = 0.36; 95% CI for diff = (0.10, 0.63) |
| LIQ: | 9.86 vs. 9.63; | diff = 0.24; 95% CI for diff = (−0.01, 0.49) |
| UIQ: | 8.41 vs. 8.14; | diff = 0.28; 95% CI for diff = (0.03, 0.52) |
| Rater 2 (right breast vs. left breast) | ||
| UOQ: | 8.74 vs. 8.41; | diff =0.33; 95% CI for diff = (0.09, 0.56) |
| LOQ: | 11.08 vs. 10.73; | diff = 0.35; 95% CI for diff = (0.03, 0.67) |
| LIQ: | 9.88 vs. 9.63; | diff = 0.25; 95% CI for diff = (0.004, 0.50) |
| UIQ: | 8.40 vs. 8.14; | diff = 0.26; 95% CI for diff = (0.02, 0.50) |
| Rater 3 (right breast vs. left breast) | ||
| UOQ: | 8.73 vs. 8.41; | diff = 0.31; 95% CI for diff = (0.07, 0.55) |
| LOQ: | 11.08 vs. 10.70; | diff = 0.38; 95% CI for diff = (0.09, 0.66) |
| LIQ: | 9.86 vs. 9.61; | diff = 0.25; 95% CI for diff = (−0.01, 0.51) |
| UIQ: | 8.40 vs. 8.14; | diff = 0.26; 95% CI for diff = (0.02, 0.50) |
Abbreviations: CI = confidence interval; diff = difference.
Table 4.
Intraobserver variability for each rater by breast and quadrant, reported with the Pearson correlation coefficient (r)
| Rater 1 (right breast vs. left breast) | |
| UOQ: | r = 0.96; p < 0.0001 |
| LOQ: | r = 0.98, p <0.0001 |
| LIQ: | r = 0.97; p < 0.0001 |
| UIQ: | r = 0.94; p < 0.0001 |
| Rater 2 (right breast vs. left breast) | |
| UOQ: | r = 0.95; p < 0.0001 |
| LOQ: | r = 0.97, p < 0.0001 |
| LIQ: | r = 0.97; p < 0.0001 |
| UIQ: | r = 0.95; p < 0.0001 |
| Rater 3 (right breast vs. left breast) | |
| UOQ: | r = 0.95; p < 0.0001 |
| LOQ: | r = 0.98, p <0.0001 |
| LIQ: | r = 0.97; p < 0.0001 |
| UIQ: | r = 0.95; p < 0.0001 |
There were no significant differences in mean TCM compliance (for all four quadrants) between pre-ovulation vs. postovulation status (p ≥ 0.55) or between OCP-use vs. non-OCP-use among premenopausal women (p ≥ 0.15). No significant difference in mean TCM compliance was observed for HRT-use vs. non-HRT-use among post-menopausal women (p ≥ 0.05). Significant differences in mean TCM compliance in all 4 quadrants were observed between the following: women with one or none vs. two or more children (p ≤ 0.006); women with bra size 32 to 34 vs. 36 to 40 (p < 0.001); and women with cup size A to C vs. D or DD (p < 0.0001). Specifically, women in the latter categories had elevated and more variable TCM measurements. These findings established a “normal” variance in the healthy breast tissue.
DISCUSSION
As the number of long-term cancer survivors increases, late complications of RT manifest as a spectrum of degrees of complexity, from the mild to the severe. It is incumbent upon the medical professionals to be able to detect, characterize, and ultimately treat long-term sequelae of RT. The challenge lies in a considerable range of variability in the incidence and presentation of these effects while the patients are exposed to the same treatment fields, doses, and fractionation.
RIF has been receiving much-deserved attention with regard to prevention, causation, quantification, and treatment (1, 5, 10-18). This common form of late damage to normal tissues after high-dose RT (33-35) is mainly characterized by changes in the vascular connective tissues involving extracellular matrix deposition, fibroblast proliferation, and presence of inflammatory infiltrate (36-43). In organs such as liver, lung, skin, subcutis, and muscle, it can produce severe limitations on function (24). Furthermore, fibrosis is thought to progress and may last a lifetime, and has been called “a wound that does not heal” (37, 38). This may cause untoward persistent symptoms and diminish QOL (2-6). Recognizing and treating early functional impairment induced by RIF is critical not only when it is anatomically established but even more importantly while it is in evolution and reversible. A number of therapies have been explored, including Cu/Zn and Mn SOD, as well as a combination of pentoxifylline and tocopherol for superficial RIF (9-12). Other treatment options for RIF are being inferred from radiation-induced pulmonary fibrosis and are currently being investigated in the animal models (44-46).
Although prevention of complications of RT by primarily limiting the radiation dose and volume is warranted, the relationship between RT dose, volume of issue irradiated, development of complications, and tumor control is complex and not precisely defined for most normal tissues and malignancies (5, 13, 14, 47, 48). Furthermore, the complexity of RIF is not only defined by the RT parameters but also compounded by certain genetic factors that may predispose to developing RIF. Quarmby et al. reported an association of the tumor growth factor–β1 (TGF-β1) (C-509T) gene promoter polymorphism and increased risk of fibrosis as a late radiotherapy injury phenotype (49). Giotopolous et al. demonstrated that there is a highly significant threefold increased risk of RIF after RT with at least one variant tumor TGF-β1 (−509T) allele (p = 0.00006), and this applies to more than 50% of breast cancer patients (1). Of interest, approximately 8% of breast cancer patients are homozygous for the variant allele with a 15-fold increased risk of RIF (1). These findings make an even stronger argument for finding a more precise methodology of assessing fibrosis, as RIF may be a manifestation of phenotypic expression associated with patient’s genetic predisposition. In fact, a more reliable method of measuring RIF may be one of the crucial steps leading to correlating the degree of RIF to future optimization of RT dose, volume, and fractionation schedules.
The validated scales use palpation to assess RIF (17, 18). On palpation, RIF is an area of tissue with diminished or lost compliance. Keeping the force of exertion constant, the area of RIF or diminished compliance will sustain less deformation than the area free of changes associated with fibrosis (25-29). RTOG and LENT-SOMA are the scales that use a palpation method of assessment of clinical perception of RIF (14, 17-21). However, palpation is flawed by subjectivity and interobserver variability, insensitivity to detect change, and a ceiling effect, and it is limited to only four scores of severity from 0 to 3, i.e., from no fibrosis to severe fibrosis (19, 22-24). The interobserver variability for assessment of RIF by LENT-SOMA is substantial with a Cohen’s k = 0.4 (95% CI, 0.18–0.8) and for retraction/atrophy k = 0.3 (95% CI, 0.07–0.5) and with the RTOG scale 0.6 for both (14).
The pursuit of a reproducible method to measure compliance rendered several pressure gauge tools to objectively assess soft tissue consistency and compliance in the area of PM&R (50-52). One such method uses TCM as a noninvasive and hand-held mechanical device to test compliance in musculoskeletal system (25-29). Jansen et al. successfully tested the reliability of TCM in 20 normal subjects at four paraspinal locations of musculature with repeated measurements (30). In addition to detecting existing pathology, TCM proved to be a convenient tool for evaluation of the worsening/improvement and a natural course of soft tissue pathology, as well as for objectively and quantitatively quantifying an immediate and long-term effect of therapeutic interventions (25-30). The principles of TCM application were applied by the investigators in the Netherlands to assess interobserver variability of the TCM and palpation method of RTOG and LENT-SOMA in determining RIF of the breasts in 68 women (38 patients and 30 controls). Two radiation oncologists performed measurements by the palpation methods, and two physiotherapists used TCM. The interobserver reliability of the TCM method and compliance of tissue that had undergone operation or irradiation were determined more reliably with the TCM than with the palpation method (24).
To our knowledge, TCM has not been validated in the mammary tissue. Before using TCM in the settings of RIF in BCT, we designed a study to validate this device in 40 female volunteers with no history or active breast disease. The goal of this study was to assess interobserver and intraobserver variability. There was virtually a full agreement on all locations of the measurements obtained by all the physicians. This corroborates the Marinus et al. report of nearly full agreement in the measurements obtained in the control group in his study of comparing compliance in patients with fibrosis versus controls (24).
In our study, the locations for measurements were very specifically determined and are easily reproducible with an easy application of the predesigned templates (Fig. 3). The data assessing intraobserver variability using the paired t test indicated that the TCM measurements taken on the right and left breasts were in close agreement. Our study established the “normal” variation in compliance between the breasts of a healthy individual, thus potentially allowing us to draw inferences about what is “abnormal” compliance or RIF if the setting of a surgical intervention or irradiation to the breasts for future studies.
TCM objectively and quantitatively documents breast tissue consistency and thus may become a methodology of great clinical importance. Application of TCM to the breast is reliable (low intraobserver variability) and reproducible with minimal training (low interobserver variability). TCM can detect differences at smaller increments in contrast to the categorical and broad range of data set produced by clinical palpation of RTOG or LENT-SOMA scales. TCM is potentially more accurate than palpation and can therefore be applied to breast tissue with the goal of assessment of RIF while in evolution. Further, TCM is quantitative and objective method for diagnosis of soft tissue dysfunction, such as RIF, which escapes the conventional diagnostic methods such as imaging (53-56). Its clinical application may be relevant clinically since RIF causes pain and loss of function (57). Finally, the success of therapeutic intervention for RIF can be objectively measured with TCM.
CONCLUSION
To our knowledge, this is the first report in the literature validating the application of TCM in the mammary tissue. TCM is a noninvasive and user-friendly modality to obtain quick and simple measurements with no pain. In our study of healthy volunteers, variability (interobserver variability) among the raters measuring breast tissue compliance with TCM was low. Further, there was no clinically meaningful intraobserver variability. This study established a “normal” variance in tissue compliance in women with healthy breast tissue, thus allowing us to use the “normal” variation to draw inferences about what is “abnormal” compliance, such as in RIF. We have achieved our objectives of validating this instrument in healthy breast tissue. Therefore, we conclude that TCM is a reliable method that can be used to measure RIF in women undergoing BCT for breast cancer.
Acknowledgments
Supported in part by Clinical Translational Science Center Grant UL1-RR024996 (to P.J.C.).
Footnotes
Presented in part at the American Society of Therapeutic Radiation Oncology conference, November 1-5, 2009, Chicago, IL.
Conflict of interest: none.
REFERENCES
- 1.Giotopoulos G, Symonds RP, Foweraker K, et al. The late radiotherapy normal tissue injury phenotypes of telangiectasia, fibrosis and atrophy in breast cancer patients have distinct genotype-dependent causes. Br J Cancer. 2007;96:1001–1007. doi: 10.1038/sj.bjc.6603637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore GJ, Mendenhall NP, Kamath SS, et al. Persistent symptomatology after breast conservation therapy: Prevalence and impact on quality of life. Int J Radiat Oncol Biol Phys. 1998;42:2058. [Google Scholar]
- 3.Delanian S, Lefaix J-L. The radiation-induced fibro-atrophic process: Therapeutic perspective via antioxidant pathway. Radiother Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Hill C, Rodemann H, Hendry J, et al. Normal tissue radiobiology: From the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2001;49:353–365. doi: 10.1016/s0360-3016(00)01484-x. [DOI] [PubMed] [Google Scholar]
- 5.Bentzen SM, Thames HD, Overgaard M. Latent-time estimation for late cutaneous and subcutaneous radiation reactions in a single-follow-up clinical study. Radiother Oncol. 1989;15:267–274. doi: 10.1016/0167-8140(89)90095-9. [DOI] [PubMed] [Google Scholar]
- 6.Bentzen SM, Dorr W, Anscher MS, et al. Normal tissue effects: Reporting and analysis. Sem Radiat Oncol. 2003;13:189–202. doi: 10.1016/S1053-4296(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 7.Fehlauer F, Tribius S, Höller U, et al. Long-term radiation sequelae after breast conserving therapy in women with early-stage breast cancer: An observational study using the LENT-SOMA scoring system. Int J Radiat Oncol Biol Phys. 2003;55:651–658. doi: 10.1016/s0360-3016(02)04120-2. [DOI] [PubMed] [Google Scholar]
- 8.Hoeller U, Tribius S, Kuhlmey A, et al. Increasing the rate of late toxicity by changingthe score? A comparison of RTOG/EORTC and LENT/SOMA scores. Int J Radiat Oncol Biol Phys. 2003;55:1013–1018. doi: 10.1016/s0360-3016(02)04202-5. [DOI] [PubMed] [Google Scholar]
- 9.Delanian S, Lefaix JL. Current management for late normal tissue injury: Radiation induced fibrosis and necrosis. Semin Radiat Oncol. 2007;17:99–107. doi: 10.1016/j.semradonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Delanian S, Baillet F, Huart J, et al. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: Clinical trial. Radiother Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 11.Lefaix JL, Delanian S, Leplat JJ, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: An experimental study. Int J Radiat Oncol Biol Phys. 1996;35:305–312. doi: 10.1016/0360-3016(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 12.Delanian S, Porcher R, Rudant J, et al. Kinetics of response to long-term treatment combining pentoxyfullline and tocophelrol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 13.Overgaard M, Bentzen SM, Christensen JJ, et al. The value of the NSD formula in equation of acute and late radiation complications in normal tissue following 2 and 5 fractions per week in breast cancer patients treated with post-mastectomy irradiation. Radiother Oncol. 1987;9:1–11. doi: 10.1016/s0167-8140(87)80213-x. [DOI] [PubMed] [Google Scholar]
- 14.Borger JN, Kemperman H, Smitt HS, et al. Dose and volume effects on fibrosis after breast conservation therapy. Int J Radiat Oncol Biol Phys. 1994;30:1073–11081. doi: 10.1016/0360-3016(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 15.Rosenstein BS, Lymberis SC, Formenti SC. Biologic comparison of partial breast irradiation protocols. Int J Radiat Oncol Biol Phys. 2004;60:1393–1404. doi: 10.1016/j.ijrobp.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 16.Ho AY, Atencio DP, Peters S, et al. Genetic predictors of adverse radiotherapy effects: The Gene-PARE project. Int J Radiat Oncol Biol Phys. 2006;65:646–655. doi: 10.1016/j.ijrobp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncologic Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 18.Pavy JJ, Denekamp J, Letschert J, et al. Late effects toxicity scoring: The SOMA scale. Radiother Oncol. 1995;35:11–15. doi: 10.1016/0167-8140(95)97448-m. [DOI] [PubMed] [Google Scholar]
- 19.Bentzen SM, Overgaard M. Early and late normal tissue injury after postmastectomy radiotherapy. Recent Results Cancer Rec. 1993;130:59–78. doi: 10.1007/978-3-642-84892-6_6. [DOI] [PubMed] [Google Scholar]
- 20.Johansen J, Bentzen SM, Overgaard M, et al. Evidence for a positive correlation between in vitro radiosensitivity of normal human skin fibroblasts and the occurrence of subcutaneous fibrosis after radiotherapy. Int J Radiat Oncol Biol Phys. 1994;66:407–412. doi: 10.1080/09553009414551361. [DOI] [PubMed] [Google Scholar]
- 21.Bentzen SM, Overgaard M. Realtionship between early and late normal-tissue injury after postmastectomy radiotherapy. Radiother Oncol. 1991;20:159–165. doi: 10.1016/0167-8140(91)90092-u. [DOI] [PubMed] [Google Scholar]
- 22.Johansen J, Bentzen SM, Overgaard J, et al. Relationship between in vitro radiosensitivity of skin fibroblasts and the expression of subcutaneous fibrosis, telangiectasia, and skin erythema after radiotherapy. Radiother Oncol. 1996;40:101–109. doi: 10.1016/0167-8140(96)01777-x. [DOI] [PubMed] [Google Scholar]
- 23.Cooper JS, Fu K, Marks J, et al. Late effects of radiation therapy in the head and neck region. Int J Radiat Oncol Biol Phys. 1995;31:1141–1164. doi: 10.1016/0360-3016(94)00421-G. [DOI] [PubMed] [Google Scholar]
- 24.Marinus J, Niel CGJH, de Bie RA, et al. Measuring radiation fibrosis: The interobserver reliability of two methods of determining the degree of radiation fibrosis. Int J Radiat Oncol Biol Phys. 2000;47:1209–1217. doi: 10.1016/s0360-3016(00)00528-9. [DOI] [PubMed] [Google Scholar]
- 25.Fischer AA. Tissue compliance recording: Method for objective documentation of soft tissue pathology. Arch Phys Med Rehabil. 1981;62:122–125. [PubMed] [Google Scholar]
- 26.Fischer AA. Current therapy in physiatry. WB Saunders; Philadelphia: 1984. Diagnosis and management of chronic pain in physical medicine and rehabilitation; pp. 123–145. [Google Scholar]
- 27.Fischer AA. Tissue compliance meter for objective, quantitative documentation of soft tissue consistency and pathology. Arch Phys Med Rehabil. 1987;68:122–125. [PubMed] [Google Scholar]
- 28.Fischer AA. Clinical use of tissue compliance meter for documentation of soft tissue pathology. Clin J Pain. 1987;3:23–30. [PubMed] [Google Scholar]
- 29.Fischer AA. Muscle tone in normal persons measured by tissue compliance. J Neuro Orthop Med Surg. 1987;8:227–234. [Google Scholar]
- 30.Jansen RD, Nansel DD, Slosberg M. Normal paraspinal tissue compliance: The reliability of a new clinical and experimental instrument. J Manip Physiol Ther. 1990;13:243–246. [PubMed] [Google Scholar]
- 31.Waldorf T, Devlin L, Nansel DD. The comparative assessment of paraspinal tissue compliance in asymptomatic female and male subjects in both prone and standing positions. J Manip Physiol Ther. 1991;14:457–461. [PubMed] [Google Scholar]
- 32.Sanders G, Lawson D. Stability of paraspinal tissue compliance in normal subjects. J Manipulative Physio Ther. 1992;15:361–364. [PubMed] [Google Scholar]
- 33.Rodemann HP, Binder A, Burger A, et al. The underlying cellular mechanism of fibrosis. Kidney Int. 1996;49:32–36. [PubMed] [Google Scholar]
- 34.Delanian S, Lefaix J-L. The radiation-induced fibro-atrophic process: Therapeutic perspective via antioxidant pathway. Radiother Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Hill C, Rodemann H, Hendry J, et al. Normal tissue radiobiology: From the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2001;49:353–365. doi: 10.1016/s0360-3016(00)01484-x. [DOI] [PubMed] [Google Scholar]
- 36.Burger A, Loffler H, Hendry J, et al. Molecular and cellular basis of radiation fibrosis. Int J Radiat Oncol Biol Phys. 1998;73:401–408. doi: 10.1080/095530098142239. [DOI] [PubMed] [Google Scholar]
- 37.Denham J, Hauer-Jensen M. The radiotherapeutic injury: A complex wound. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin M, Lefaix J-L, Delanian S. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 39.Hom DB, Adams GL, Monyak D. Irradiated bone and its management. Otolaryngol Clin North Am. 1995;28:1003–1018. [PubMed] [Google Scholar]
- 40.Rezvani M, Hopewell JW, Robbins ME. Initiation of non-neoplastic late effects: The role of endothelium and connective tissue. Stem Cells. 1995;13:248–256. doi: 10.1002/stem.5530130730. [DOI] [PubMed] [Google Scholar]
- 41.Rubin P. The Franz Buschke Lecture: Late effects of chemotherapy and radiation therapy: A new hypothesis. Int J Radiat Oncol Biol Phys. 1984;10:5–34. doi: 10.1016/0360-3016(84)90408-5. [DOI] [PubMed] [Google Scholar]
- 42.Michalowski A. The pathogenesis and conservative management of radiation injuries. Neoplasma. 1995;42:289–292. [PubMed] [Google Scholar]
- 43.Gabka CJ, Benhaim P, Mathes SJ, et al. An experimental model to determine the effect of irradiated tissue on neutrophil function. Plastic Reconstruct Surg. 1995;96:1676–1688. doi: 10.1097/00006534-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 44.Haydont V, Bourgier C, Pocard M, et al. Pravastatin inhibits the Rho/CCN2/extracellular matrix cascade in human fibrosis explants and improves radiation-induced intestinal fibrosis in rats. Clin Cancer Res. 2007;13:5331–5340. doi: 10.1158/1078-0432.CCR-07-0625. [DOI] [PubMed] [Google Scholar]
- 45.Anscher MS, Garst J, Marks LB, et al. Assessing the ability of the antiangiogenic and anticytokine agent thalidomide to modulate radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2006;66:477–482. doi: 10.1016/j.ijrobp.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Machtay M, Scherpereel A, Santiago J, et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81:196–205. doi: 10.1016/j.radonc.2006.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vujaskovic Z, Marks L, Anscher M. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000;10:296–307. doi: 10.1053/srao.2000.9424. [DOI] [PubMed] [Google Scholar]
- 48.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 49.Quarmby S, Fakhoury H, Levine E, et al. Association of transforming growth factor beta-1 single nucleotide polymorphisms with radiation-induced damage to normal tissues in breast cancer patients. Int J Radiat Oncol Biol Phys. 2003;79:137–143. [PubMed] [Google Scholar]
- 50.Jaeger G, Reeves L. Quantification of changes in myofascial trigger point sensitivity with pressure algometer following passive stretch. Pain. 1986;27:203–210. doi: 10.1016/0304-3959(86)90211-3. [DOI] [PubMed] [Google Scholar]
- 51.Reeves JL, Jaeger B, Graff-Radford SB. Reliability of the pressure algometer as a measure of myofascial trigger point sensitivity. Pain. 1986;24:313–321. doi: 10.1016/0304-3959(86)90117-X. [DOI] [PubMed] [Google Scholar]
- 52.Steinbroker O. Simple pressure gauge for the measured palpation in physical diagnosis and therapy. Arch Phys Med. 1949;30:389–390. [PubMed] [Google Scholar]
- 53.Johansen J, Taagejoi F, Christensen T, et al. Quantitative magnetic resonance for assessment of radiation fibrosis after postmastectomy radiotherapy. Br J Radiol. 1994;67:1238–1242. doi: 10.1259/0007-1285-67-804-1238. [DOI] [PubMed] [Google Scholar]
- 54.Kimming B, Engenhart R, Muller M, et al. Quantification of subcutaneous fibrosis after combined photon neutron therapy. Strahlenther Onkol. 1990;166:76–77. [PubMed] [Google Scholar]
- 55.Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: Impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 1989;17:531–537. doi: 10.1016/0360-3016(89)90103-x. [DOI] [PubMed] [Google Scholar]
- 56.Overgaard M. The clinical implication of non-standard fractionation. Int J Radiat Oncol Biol Phys. 1985;11:1225–1226. doi: 10.1016/0360-3016(85)90074-4. [DOI] [PubMed] [Google Scholar]
- 57.O’Young B. Spinal segmental sensitization. PM&R Secrets. 3rd edition Mosby/Elsevier; Philadelphia: 2007. [Google Scholar]