Abstract
Objective
Oral submucous fibrosis (OSMF) is a premalignant condition of oral cavity characterized by inflammation and progressive mucosal fibrosis. It has questionable pathogenesis. Mast cells (MC) have been associated with variety of inflammatory and fibrotic conditions, but little is known about their role in OSMF. Mast cells have been studied in normal gingiva, chronic inflammatory gingivitis, desquamative gingivitis, lichen planus, OSMF and oral cancer. Mast cells exhibit phenotypic plasticity. There is variation in the mast cell mediators with the change in the microenvironment, which makes the study of this cell in various diseases interesting.
Study design
A retrospective study was conducted to find possible correlation between MC in 25 cases of OSF, 10 cases of oral squamous cell carcinoma (OSCC) and 10 cases of normal buccal mucosa by means of acidified toluidine blue staining method.
Results
The density of MC increased with disease progression. The densities of MC were significantly higher in OSMF than in normal buccal mucosa (p=0.001). The average numbers of MCs per square millimeter were 25, 49.50, 53.25 & 55.25 respectively.
Conclusion
The results suggest that MC have a definite role in initiation and progression of OSMF.
Keywords: mast cell, oral submucous fibrosis, premalignant, squamous cell carcinoma
Introduction
OSMF is a premalignant condition of the oral cavity characterized by inflammation and progressive mucosal fibrosis. It affects the mucosa of any part of oral cavity and occasionally extending into the pharynx and esophagus. (1, 2) The pathogenesis of the disease is not well established. Since its first description various etiopathological concepts have been put forward such as spices (chilies), nutritional deficiencies, genetic susceptibility, lysyl oxidase, autoimmunity and one of the most accepted is areca nut usage. (2)
MC are common constituent of connective tissue and have been associated with various inflammatory conditions such as lichen planus, gingivitis, pulpitis, and periapical inflammation, but their role in pathogenesis of OSMF is still not been evaluated. Extensive search in English literature for the role of MC in human OSMF showed few studies, which revealed high mast cell response in early stages of OSMF and subsequent decrease in MC density as the severity increased. (3, 4, 5, 6, 7)
It has been recently evaluated that MC and microvessels appear to increase in OSCC than in hyperkeratosis and normal buccal mucosa, (8) suggesting that MC density increases as it progresses from normal oral mucosa to premalignancy and malignancy. Since OSMF is also a premalignant condition, this study was aimed to determine the density of MC in OSMF.
Material and methods
Samples
OSMF cases which were diagnosed histologically using H&E staining were selected from the department of oral pathology P.M.N.M Dental College and Hospital, Bagalkot. Using Pindborg’s criteria, OSMF cases were histopathology graded into, very early, early, moderately advanced and advanced. (9) A total of 25 cases were selected in which 2 were of very early grade, 8 of early grade, 7 of moderately advanced and 8 of advanced grade. For the purpose of simplicity in analysis we grouped our lesions into (i) early (which included very early and early cases), and (ii) late (which included moderately advanced and advanced cases). For comparison, biopsies of 10 samples from normal buccal mucosa of patients without having any habits were also selected. Control tissues of squamous cell carcinoma (n=10) were used for MC demonstration since increase in MC density has been reported in OSCC. (8)
MC evaluation
MC density was evaluated using a modified toluidine blue stain that is the acidified toluidine blue technique described by Churukian and Schenk. (10) Mast cells are spindle to oval-shaped and have the same staining characteristics as the fibroblasts with hematoxylin and eosin staining. Therefore, they are difficult to differentiate from fibroblasts. Selective stain of 1% toluidine blue is used for mast cells. Mast cell granules are purplish red and the nuclei of mast cells appear sky blue in colour. The MCs were counted using an oculometer grid fields under a magnification of ×400 in a step ladder fashion. Mast cell count was expressed as the number of mast cells per square millimeter. (11)
Statistical analysis
All data were tabulated and statistical tests were performed. The nonparametric, Wilcoxon & Kruskal-Wallis tests were used when variables did not have a normal distribution. MC density between normal buccal mucosa, early OSMF, late OSF and OSCC were compared by using Mann-Whitney test. P values of < 0.05 were regarded as significant.
Results
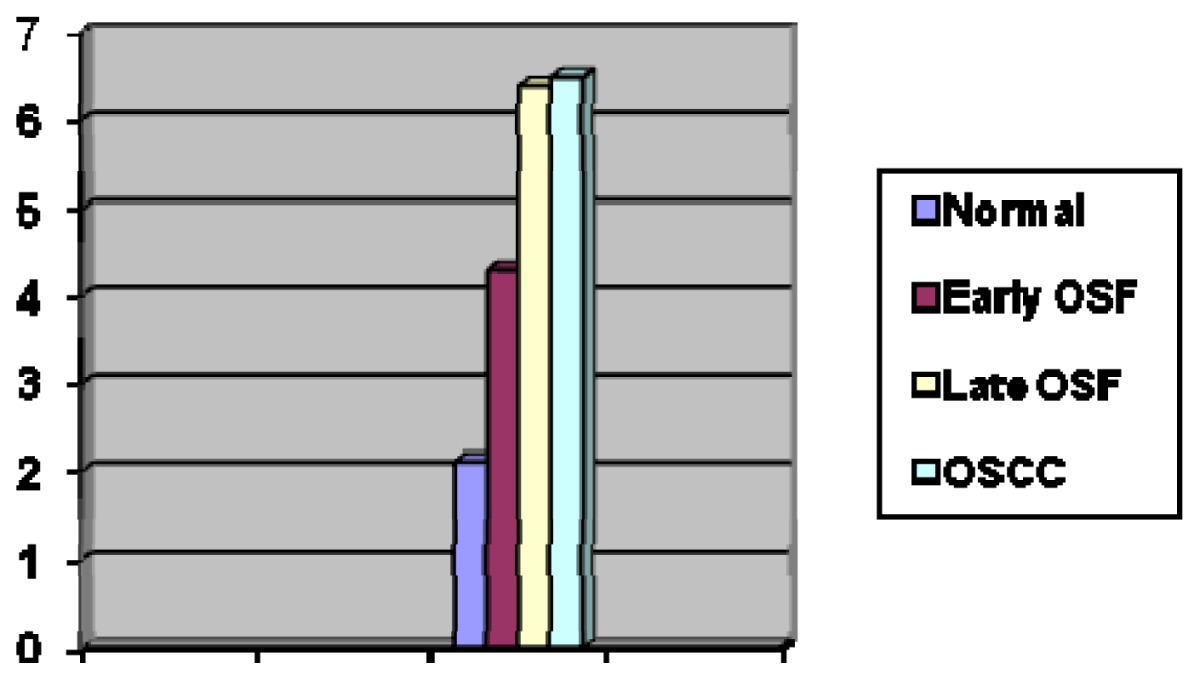
MCs were characterized by blue nuclei and purple cytoplasm, when stained with acidified toluidine blue technique. The mean densities of MC were found to be 2.1 in normal buccal mucosa (fig 1), 4.3 in early OSMF (fig 2), 6.4 in late OSMF (fig 3) and 6.5 in OSCC (fig 4). It was observed that MC density in normal buccal mucosa, when compared with early OSF cases did not show statistical significance with p value of 0.076, but when compared with late OSMF cases and OSCC showed statistical significance with p value of 0.001 and 0.003 respectively.
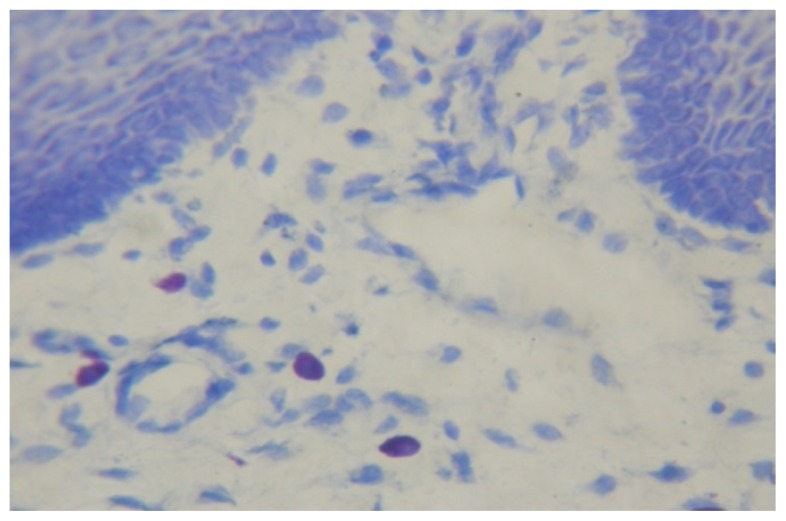
Fig. (1).
Mast cells in normal buccal mucosa (×400).
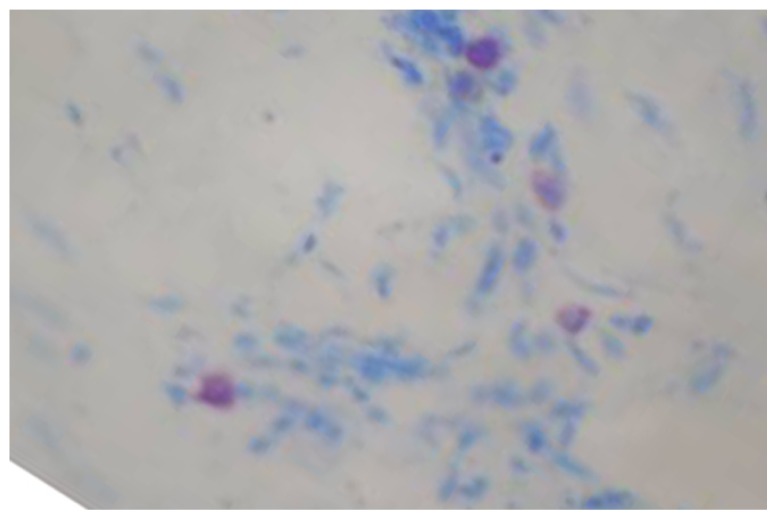
Fig. (2).
Mast cells in early OSMF (×400).
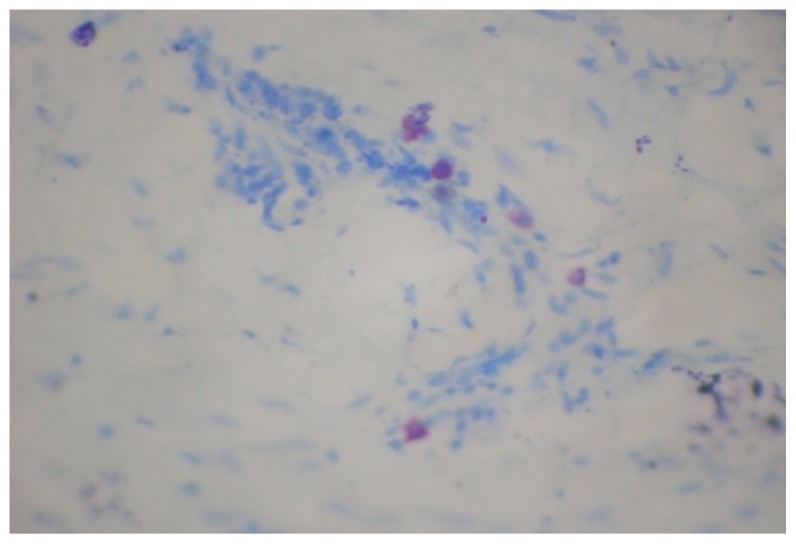
Fig. (3).
Mast cells in late of OSMF (×400).
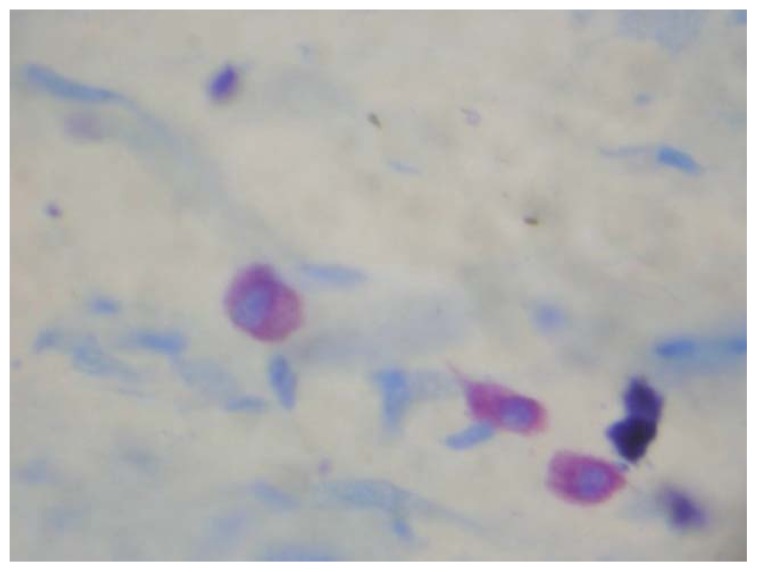
Fig. (4).
Degranulating Mast cells in Squamous Cell Carcinoma (×1000).
The average number of MCs per grid field for normal buccal mucosa, early OSMF, late OSMF & OSCC were 1.0, 1.98, 2.13 & 2.21 respectively (Table 1). All the results were statistically significant.
Table (1).
Mast Cells Count in Normal Buccal Mucosa, Early & Late OSMF & OSCC.
| Sample | Avg no of MCs/grid field | Avg no of MCs/sq mm |
|---|---|---|
| Normal buccal mucosa | 1.00 | 25.00 |
| Early OSMF | 1.98 | 49.50 |
| Late OSMF | 2.13 | 53.25 |
| OSCC | 2.21 | 55.25 |
Discussion
OSMF has been described as an insidious chronic disease affecting any part of the oral cavity and sometimes pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with juxtaepithelial inflammatory reaction followed by fibroelastic change of the lamina propria, with epithelial atrophy leading to stiffness of the oral mucosa and causing trismus and inability to eat. (9) Various theories of etiology are proposed, but areca nut is considered to be most important for OSMF. The major products of areca nut alkoloids such as the arecadine, guvacain, isoguvacain, arecolidine, arecoline and guvacoline, of these arecoline and arecadine have been proposed to cause fibrosis. (2) Very little focus has been put on the role of MC in OSMF.
MC are common constituent of connective tissue and has been associated with various chronic inflammatory disorders like ulcerative colitis, Crohn’s disease, some soft tissue tumors, neurofibromas, benign and malignant breast lesions, (10) in certain oral conditions like lichen planus, gigivits, pulpitis and periapical inflammation.
In our study, we found that there was increased MC density in early and late OSMF when compared to normal buccal mucosa. Continuous chronic irritants in the form of areca nut and their products may cause continuous MC activation and degranulation. Following degranulation MC mediators are deposited in large quantities in extracellular environment, where they exert effects on various cell types. MC may subsequently synthesize and secrete additional mediators like proteases (tryptase, chymase and cathepsin G), histamine, serotonin acid hydrolases and cytokines like TNF-α and IL-16 that are preformed in their granules. (10) Thus release of the mediators from degranulated MC may lead to the initial inflammatory signs of burning sensation, stomatitis and glossitis in OSF with occasional vesicle formation (Table 1). Mast cell mediators like prostaglandins and leukotrienes are potent secretogouges for the serous and mucous cells. This could attribute to the increased salivation seen in OSMF.
Earlier published studies of MC density in OSMF, revealed decreased MC density as the severity increased. They suggested that release of MC granules may initiate a change in the connective tissue ground substance by changing the intracellular fluid or free tissue water into mucinous fluid. (12) The MC histamine chain may be terminated by fibroblastic production of hyaluronic acid or by deposition, fibrosis or other processes. In contrast, our results showed increased MC density with increased severity of the disease, thus suggesting that MC have a definite role not only in the initiation, but also in the progression of OSMF. With the persistence of the habit, there is a continuous chronic inflammation, which leads to MC activation and degranulation. This in turn produces fibrosis resulting in trismus along with burning sensation, stomatitis, and glossitis (Table 2).
Table (2).
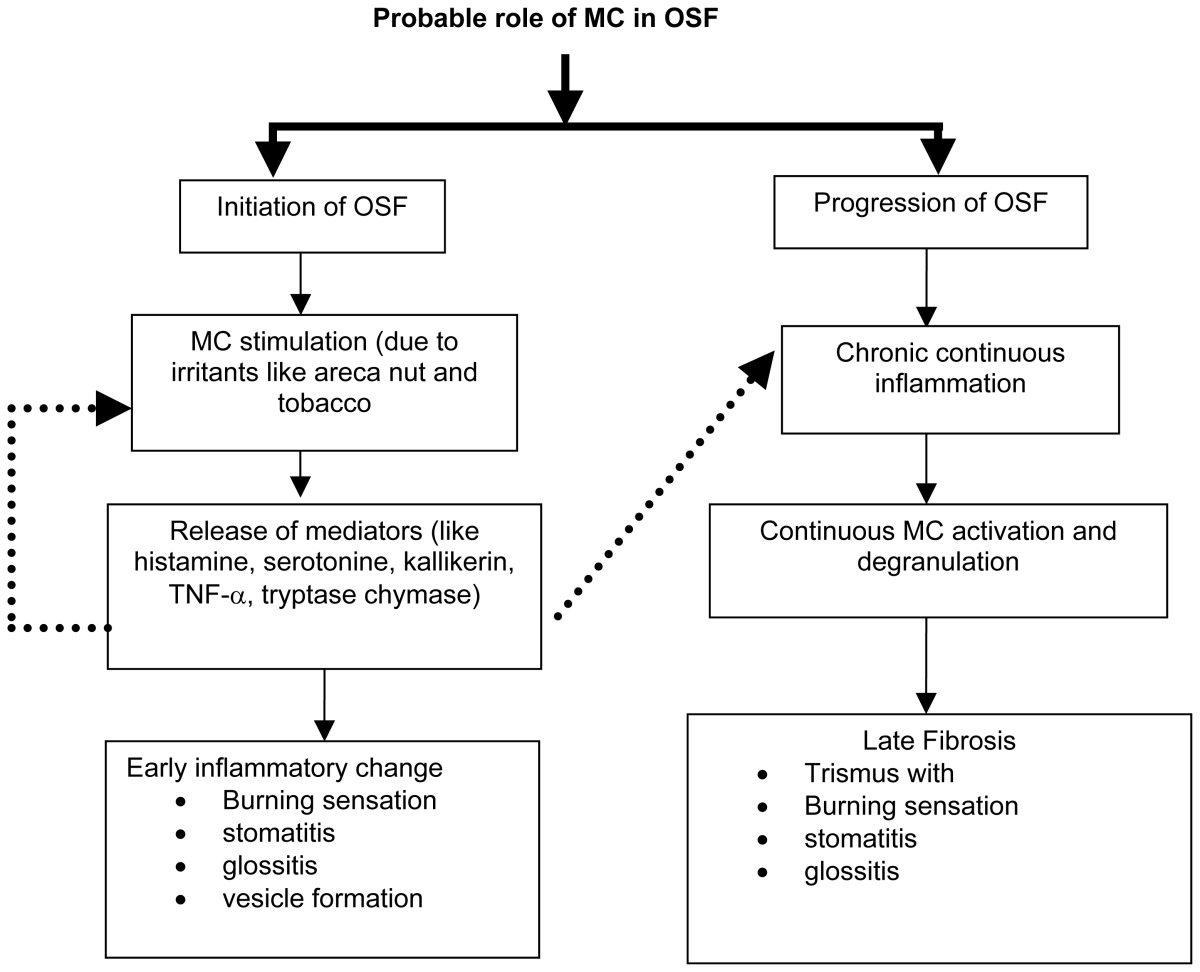
In a recent study by Huang s, et al, on affection of MC on collagen metabolism of OSMF induced by aqueous areca nut extract in rats was studied by transmission electron microscopy, histochemical and biochemical analyses. The results revealed that there was a close relationship between MC and fibroblasts in the pathogenesis of OSMF; MC which appeared in the buccal mucosa in the model groups increased significantly and became more active in the function. A great deal of collagen compacted in the buccal mucosa in the model groups. The contents of tissue hydroxyproline in the model groups were higher than those in the control groups. Their results are in correlation with our findings suggesting a close association between MC and fibroblast in pathogenesis of OSMF.
Therapeutic implications of these findings include strategies directed toward mast cell-endothelial cell axis. Based on the concept that MC plays an important role in chronicity of inflammation, it may be possible to use drugs therapeutically in order to influence MC secretion and there by thwart inflammation (13) and fibrosis. In conclusion, we suggest that, MC considered, as “gatekeepers” may become “destructors” playing a definite role in initiation and progression of OSMF. The use of MC stabilizers may give new hope for the treatment of this condition. Further studies are needed to investigate possible role of MC in pathogenesis of OSMF. (12) The authors concluded that the biologically and pharmacologically active agents in the mast cells might contribute to inflammatory reaction seen in OSMF. They postulated that the MC may subsequently synthesize and secrete additional mediators like proteases (tryptase, chymase and cathepsin G), histamine, serotonin acid hydrolases and cytokines like TNF-α and IL-16 that are preformed in their granules. This will make the conclusions and findings are non specific or descriptive to the pathogenesis of OSMF, hence further studies are recommended for studying a panel of these biochemical and immunological markers.
Table (3).
showing mean values of MC
Acknowledgements
Principal of PMNM Dental College, HOD & staff of Department of Oral Pathology, PMNM Dental College & Hospital, Bagalkot, India.
References
- 1.Tsai CC, Sheih TY. Deficiency in collagen and fibronectine phagocytosis by human mucosa fibroblasts in vitro as possible mechanisms for oral submucous fibrosis. J Oral Pathol Med. 1999;28:59–63. doi: 10.1111/j.1600-0714.1999.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 2.Murti RR, Bhosale BB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS. Etiology of oral submucous fibrosis with special refrence to role of areca nut chewing. J Oral Pathol Med. 1995;24:145–52. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt AP, Dholakia HM. Mast cell density in oral submucous fibrosis. Journal Indian Dent Association. 1977;49:187–91. [Google Scholar]
- 4.Desai JV. submucous fibrosis of palate and cheek. Annals of Otology, Rhinology and Laryngology. 1957;66:1143–59. doi: 10.1177/000348945706600420. [DOI] [PubMed] [Google Scholar]
- 5.Rao ABN. Idiopathic palatal fibrosis. British journal of surgery. 1962;50:23–5. doi: 10.1002/bjs.18005021907. [DOI] [PubMed] [Google Scholar]
- 6.Pindborg, Singh Formation of vesicles in oral submucous fibrosis. Acta Pathologica Et Microbiologica Scandinavic. 1964;62:562–6. doi: 10.1111/apm.1964.62.4.562. [DOI] [PubMed] [Google Scholar]
- 7.Sirsat SM, Pindborg Mast cell response in early and advanced oral submucous fibrosis, 1967. Acta Pathologica Et Microbiologica Scandinavic. 70:174–8. doi: 10.1111/j.1699-0463.1967.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 8.Lamaroon Pongs Iriwel, Jittidecharaks Dattanaporn, Prapayasatok Wanachantarak. Increase of mast cell and tumor angiogenesis in oral squamous cell carcinoma. J oral pathol med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 9.Walsh LJ, Davis MF, Xu LJ, Savegen W. Relationship between mast cell degranulation and inflammation in oral cavity. J oral pathol med. 1995;24:266–272. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh LJ. Mast cell and oral inflammation. Crit rev Oral Biol Med. 2003;14(3):188–98. doi: 10.1177/154411130301400304. [DOI] [PubMed] [Google Scholar]
- 11.Madhuri AR, Alka KD, Ramakanth N. Mast cells are increased in leukoplakia, oral submucous fibrosis, oral lichen planus and oral squamous cell carcinoma. JOMFP. 2007;11(1):18–22. [Google Scholar]
- 12.Asboe-Hansen Gustav, Dyrbye MO, Moltke E, Wegelius O. Tissue edema – a stimulus of connective tissue regeneration. J of Investigative Dermatology. 1959;32:505–570. [PubMed] [Google Scholar]
- 13.Walsh LJ, Davis MF, Xu LJ, Savegen W. Relationship between mast cell degranulation and inflammation in oral cavity. J oral pathol med. 1995;24:266–272. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]