Abstract
Objectives
This study is intended to examine the effect of hypothyroidism and thyroxin replacement on axial and appendicular skeletal growth, growth plate thickness and to observe associated microscopic changes within the growth plates.
Methodology
Experimental albino rats were developed with carbimazole and carbimazole plus thyroxin. Animals were administered with these drugs throughout pregnancy (prenatally) and for a period of six weeks, postnatally. At the end of the experiment the animals were sacrificed, measured and processed to demonstrate the bony and cartilaginous parts. Ulna and tibia of both sides were removed and processed for light microscopy and growth plate measurement.
Results:
At the end of the experiment, reduction in the crown rump length was observed in the carbimazole treated animals. It was 8.77%, 13.26% and 7.25% in the prenatal, two weeks and six weeks age group animals respectively. In carbimazole plus thyroxin treated animals, this reduction was 3.22%, 2.94% and 3.42%, when compared to their age matched controls. Reduction in the thickness of the Epiphyseal growth plate (EGP) was 16.89% and 12.80% in the ulna of the two and six weeks age group and 18.06 % and 15.65% in the tibia of the these animals respectively. The carbimazole plus thyroxin treated animals showed an increase in the thickness of EGP as compared to their age-matched controls though the crown rump length of these animals was less than the controls. Prenatally treated hypothyroid rats showed disrupted growth plates without any well-formed microscopic zones.
Conclusion:
The results of this study showed that the pre and postnatally, carbimazole induced hypothyroidism and its replacement therapy affected the axial and appendicular skeletal growth. Proximal limb bones of the prenatally induced hypothyroid animals showed the greatest skeletal change in this study.
Keywords: Growth plate, Hypothyroidism, skeletal growth, Prenatal growth, Thyroid dysfunction
Introduction
Growth is a continuous biologic process influenced by genetic, nutritional, environmental, and hormonal factors. (1) Thyroid hormones exert an important effect on growth and differentiation of tissues in many mammalian species. (2) Euthyroid status is essential for normal skeletal development and adult bone maintenance. (3, 4) Hypothyroidism in children causes growth arrest, delayed bone maturation, and epiphyseal dysgenesis. T4 replacement, on the other hand results in rapid catch-up growth. (5) Thyroid hormones are important homeostatic regulators that act via nuclear thyroid hormone receptors (TRs) in virtually all tissues during development and throughout postnatal life. (6) Pregnancy influences thyroid function in multiple ways. Not only does the maternal hypothalamic-pituitary-thyroid (HPT) axis undergo a series of adjustments, the fetus develops its own HPT axis and the placenta plays an active role in iodide and T4 transport and metabolism. Thus, an integrated three-compartment thyroid model exists during gestation. (7) In normal pregnant women, the thyroid gland maintains a euthyroid state with only minor fluctuations in serum T4 and TSH. However, in women with limited thyroid reserve, such as in cases of thyroid autoimmunity or iodine deficiency, hypothyroidism can develop. (8)
Abnormal thyroid gland function may be restricted to the fetus, the expectant mother, or both. During prenatal life, thyroid hormone of the embryo and fetus are required for development, considering the maternal hormone crosses placenta very limitedly. (9) Contrary to this belief, some of the newer animal studies have reported that thyroid hormone crosses the placenta in a more liberal manner and that the maternal T4 does reach the fetus. (10)
New born with congenital deficiency of thyroid hormones develops short stature and mental retardation. Delay and retardation in skeletal growth have been described in human cretins at birth. (11) Thyroid hormones deficiency therefore is associated with adverse effect on skeletal growth. (12) The recognition and treatment of fetal hypothyroidism are believed to be important to optimize growth and intellectual development in affected fetuses.
The importance of monitoring pregnant women with known thyroid dysfunction, including those being treated with L-thyroxine, has been recognized for more than 10 years. A recent review of articles found that 10.0% (range, 2.8–19.6) of 12,592 women were positive for microsomal or TPO antibodies during or shortly after pregnancy. (13) Many of these women may have decreased thyroid reserve that would lead to maternal and fetal hypothyroidism in the setting of an increase in T4 catabolism during pregnancy. Furthermore, many women with known hypothyroidism that is being treated will have a substantially increased T4 dose requirement. (14)
Carbimazol is a thionamid compound and a good agent for inducing hypothyroidism. It has been used for treating hyper thyroidism and its utility as an antithyroid agent is beyond doubt. (15)
The purpose of this study is to investigate the extent of the effects of hypothyroidism on axial skeletal growth (Crown rump length which is indicative of the axial growth) and effect on the growth plates which are the ultimate final site of appendicular skeletal (limb bones) growth. This study also examines the effect of simultaneous thyroxin replacement on the recovery.
Materials and Methods
The present study included Fifty four albino rats of different age groups. The animals were kept in an experimental room for one week prior to the commencement of the study, for acclimatization to the experimental conditions with 12 hours light and dark cycles. The animals were fed laboratory chow and water ad libitum. Of 54 rats, at the commencement of study, 18 were two weeks old, another 18 were six weeks old and the remaining 18 animals were of age 10th postnatal day. The 36 animals of group B&C underwent experimentation for a period of six weeks.
Groups
Prenatally treated
Two weeks old
Six weeks old
Groups A and B and C were further divided into three sub groups: A1, A2, & A3, Bl, B2, & B3 and group C1, C2 and C3. Each of the group contained six animals. Sub group B1 and C1 received anti-thyroid agent carbimazole dissolved in 0.9% NaCl subcutaneously at a dose of 6 μg/gm body weight daily for six weeks. Sub groups B2 and C2 received carbimazole subcutaneously at a dose of 6μg/gm body weight and Thyroxin 5 μg/animal in 0.9%saline and 0.01 M NaOH solution intraperitoneally (16) daily for six weeks. Sub group B3 and C3, received 0.5 cc (volume equal to the volume of Thyroxin) of normal saline (0.9% NaCI) intraperitoneally and acted as control groups for group B and C. The animals were weighed weekly and dosage reviewed accordingly. At the end of experimental period (six weeks), these animals were sacrificed by deep ether anesthesia. To obtain group A rats (prenatally treated), nine adult virgin female albino rats, weighing 175–200 gms, were mated with adult male albino rats of same age and strain in a ratio of three females with one male rat in one cage. (17)
Next morning each female rat was examined for any sign of mating in form of blood stained vagina or a vaginal plug. (18,19) Absence of any apparent sign of mating led to vaginal wash which was obtained on glass slide and observed under microscope for presence of spermatozoa. Their presence was again considered as day zero of pregnancy. The pregnant albino rats were then separated from male rats and divided into three groups, P1, P2 and P3 each comprising three rats. Animals of group P1 were given carbimazole at a dose of 6μg/gm body weight subcutaneously from 10th day of pregnancy to parturition. (9) Group P2 animals were given carbimazole subcutaneously at a dose of 6μg/gm body weight as well as thyroxin 5μg intraperitoneally (16) daily from 10th day of gestation until parturition. Group P3 animals received 0.5 cc [volume equal to thyroxin volume] of normal saline (0.9% Nacl) intraperitoneally for same period and acted as control group. All the animals were injected between 10:00–12:00 hours daily.
On birth six pups were obtained from each of the groups P1, P2 and P3 (18 pups in all) for study. The pups obtained were then cleaned, weighed, measured and marked. They were then left with their mothers up to 10th postnatal day. On 10th day the pups were again weighed and measured and sacrificed by deep ether anesthesia. (20) All the sacrificed animals of group A, B and C, were then eviscerated and fixed in formal saline, for one week. (21) After one week they were processed through 95% ethanol and acetone and placed in 4% KOH for clearing. (6) Group A, animals were processed for a period of 1–2 weeks and group B and C animals for a 4–6 week period.
After clearing, they were bulk stained using 0.001% alizarin red in 1% KOH. (22)
This technique clearly demonstrated the ossified bone while unossified bone remained unstained. Appendicular skeleton was then separated from the axial skeleton and long bones of fore and hind limbs were disarticulated.
Ulna (fore limb) and tibia (hind limb) from each animal were taken, decalcified in 5% nitric acid, blocked in paraffin wax, cut longitudinally at 5micron thickness and stained with hematoxylin and eosin. Stained sections were observed under light microscope for measuring the thickness of epiphyseal growth plate (EGP) and for observing any changes. MICROMETRY
For measurement of thickness of EGP, micrometry was done by using stage and ocular scales. A stage micrometer of 1mm (1000 micron meters) with 100 small divisions (each of 10 micron meters) placed on stage and an eye piece micrometer was placed in the left eye piece of light microscope. Calibration of eye piece micrometer was done with stage micrometer under 10 1nd 40 objectives.
Statistical Analyses
The data was subjected to student’s t-test. Difference between the means of the parameter between different groups was evaluated and regarded as significant if the “p-value” was less than 0.05 and nonsignificant if the “p-value” was greater than 0.05.
Results
CROWN RUMP LENGTH (CRL)
Prenatally treated group
The mean initial CRL (on the day of birth) was 4.51±0.01 cm, 3.89±0.48 cm, and 4.20±0.06 cm in the pups born to control, Carbimazole treated, and Carbimazole plus Thyroxin treated mothers respectively (table-1) whereas the final CRL (on 10th postnatal day) was 7.75 ± 0.03 cm, 7.07±0.08cm, and 7.50±0.02 cm. Increment in the CRL in these pups was 3.24±0.03 cm, 3.17±0.04 cm, and 3.30±0.09 cm respectively (table-2).
Table (1).
Comparison of Crown Rump Length (cm) between the Control and Treated Animals at different age groups
| Age Group | Control | carbimazole | Carbimazole plus thyroxin | |||
|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | Initial | Final | |
| Prenatally treated | 4.5±0.01 | 7.75±0.03 | 3.89±0.48 | 7.07±0.08 | 4.20±0.06 | 7.50±0.02 |
| Two weeks | 8.30±0.07 | 13.26±0.11 | 8.25±0.08 | 11.5±0.06 | 8.27±0.07 | 12.87±0.13 |
| Six weeks | 11.93±0.04 | 14.88±0.08 | 11.83±0.10 | 13.80±0.10 | 11.83±0.10 | 14.37±0.10 |
| Statistical Comparision | ||||||
|---|---|---|---|---|---|---|
| Prenatally treated | Two weeks age group | Six weeks age group | ||||
| Groups | P value | S/HS/NS | P value | S/HS/NS | P value | S/HS/NS |
| C vs Ca | <.001 | HS | <.001 | HS | <.001 | HS |
| C vs Ca + T4 | <.001 | HS | <.001 | HS | <.01 | S |
| Ca vs Ca+T4 | <.001 | HS | <.001 | HS | <.001 | HS |
Key: C = control, Ca = carbimazole, T4= Thyroxin, S= significant, HS =highly significant, NS= non significant
Table (2).
Increment in Crown Rump Length (cm)
| Age Group | Control | carbimazole | Carbimazole plus thyroxin | |||
|---|---|---|---|---|---|---|
| Total increment | Rate of increment | Total increment | Rate of increment | Total increment | Rate of increment | |
| Prenatally treated | 3.24±0.03 | 0.36/day | 3.17±0.04 | 0.34/day | 3.30±0.09 | 0.36/day |
| Two weeks | 4.97±0.05 | 0.82/day | 3.25±0.06 | 0.54/day | 4.60 ±0.07 | 0.76/day |
| Six weeks | 2.95±0.07 | 0.49/day | 1.96±0.03 | 0.32/day | 2.53 ±0.11 | 0.42/day |
Decrease in the CRL in the pups born to Carbimazole treated mothers was approximately 8.77%, statistically highly significant (P<0.001) and in the pups born to Carbimazole plus Thyroxin treated mothers was approximately 3.22%, statistically highly significant (P<0.001) also, when compared to their age matched controls. Comparing the CR length between the carbimazole and carbimazole plus thyroxin treated group, the difference found was approximately, 6.08%, which was also highly significant (P<0.001).
Two weeks age group
The mean initial CRL was 8.30±0.07 cm in the control, 8.25±0.08 cm in the Carbimazole treated, and 8.27±0.07 cm in the Carbimazole plus Thyroxin treated animals (table-1) while the final CRL in these animals was 13.26±0.11 cm in the control, 11.50±0.06 cm in the Carbimazole treated, and 12.87±0.13 cm in the Carbimazole plus Thyroxin treated animals (table-1). Increment in the CRL observed was 4.97±0.05 cm, 3.25±0.06 cm, and 4.60±0.76 cm in these animals (table-2). Decrease in the CRL noted in the Carbimazole treated animals was approximately 13.27%, statistically highly significant (P<0.001) and in the Carbimazole plus Thyroxin treated animals was approximately 2.94%, statistically highly significant (P<0.001) also, when compared to their age matched controls. Comparing the CR length between the carbimazole and carbimazole plus thyroxin treated group, the difference found was approximately, 11.33%, which was also highly significant (P<0.001).
Six weeks age group
The mean initial CRL was 11.93±0.04 cm, 11.83±0.10 cm, and 11.83±0.10 cm in the control, Carbimazole treated, and Carbimazole plus Thyroxin treated animals respectively (table-1) while the final CRL in these animals was 14.88±0.08 cm, 13.80±0.10 cm, and 14.37±0.09. Increment in the CRL observed was 2.95±0.07 cm, 1.96±0.03 cm, and 2.53±0.11 cm respectively (table-2). Decrease in the CRL noted in the Carbimazole treated animals was approximately 7.25%, statistically highly significant (P<0.001) and in the Carbimazole plus Thyroxin treated animals was approximately 3.42%, statistically significant (P<0.01) when compared to their age matched controls. Comparing the CR length between the carbimazole and carbimazole plus thyroxine treated group, the difference found was approximately, 4.13%, which was also highly significant (P<0.001).
EPIPHYSEAL GROWTH PLATE THICKNESS
Two weeks age group
Ulna
The mean EGP thickness of the ulna in the control group was 134.71±0.77 um, in Carbimazole treated was 112.08±1.02 um, and in Carbimazole plus Thyroxin treated group was 153.22±1.72um (Table-3). Decrease in the thickness, in the Carbimazole treated groups was 16.79% and observed to be highly significant (p<0.001) statistically, whereas in the Carbimazole plus Thyroxin treated group the increase in the thickness of EGP of ulna was 13.74% and found to be highly significant (p<0.001), when compared with their age matched controls. Comparing the mean EGP thickness between the carbimazole and carbimazole plus thyroxin treated group, the difference found was approximately, 36.70%, which was also highly significant (P<0.001).
Table (3).
Comparison of Epiphyseal Growth Plate thickness (μm) between the Control and Treated Animals at different age groups
| Bone | Two weeks age group | Six weeks age group | ||||
|---|---|---|---|---|---|---|
| control | Carbimazol treated | Carbimazol plus thyroxin treated | control | Carbimazol treated | Carbimazol plus thyroxin treated | |
| Ulna | 134.71±0.77 | 112.08±1.02 | 153.22±1.72 | 119.28±2.02 | 104.00±1.73 | 140.95±2.50 |
| Tibia | 159.39±2.47 | 130.58±1.90 | 181.71±2.19 | 137.79±1. | 116.22±1.03 | 159.03±0.87 |
| Statistical Comparison | ||||||
|---|---|---|---|---|---|---|
| Two weeks age group | Six weeks age group | |||||
| P value | P value | |||||
| Ulna | Tibia | S/HS/NS | Ulna | Tibia | S/HS/NS | |
| Groups | ||||||
| C vs Ca | <.001 | <.001 | HS | <.001 | <.001 | HS |
| C vs Ca + T4 | <.001 | <.001 | HS | <.001 | <.001 | HS |
| Ca vs Ca+T4 | <.001 | <.001 | HS | <.001 | <.001 | HS |
Key: C = control, Ca = carbimazole, T4= Thyroxin, S= significant, HS =highly significant, NS= non significant
Tibia
The mean EGP thickness of the tibia in the control group was 159.39±2.47 um, in Carbimazole treated was 130.58±1.90 um, and in Carbimazole plus Thyroxin treated group was 181.71±2.19um (Table-3). Decrease the in thickness in the Carbimazole treated groups was 18.06% and observed to be highly significant (p<0.001) statistically, whereas in the Carbimazole plus Thyroxin treated group the increase in the thickness of EGP of tibia was 14% and found to be highly significant (p<0.001), when compared with their age matched controls. Comparing the mean EGP thickness between the carbimazole and carbimazole plus thyroxin treated group, the difference found was approximately, 39.15%, which was also highly significant (P<0.001).
Six weeks age group
Ulna
The mean EGP thickness of the ulna in the control group was 119.28±2.02 um, in Carbimazole treated was 104.00±1.73 um, and in Carbimazole plus Thyroxin treated group was 140.95±2.50 um (Table-3). Decrease in the thickness, in the Carbimazole treated groups was 12.80% and observed to be highly significant (p<0.001) statistically, whereas in the Carbimazole plus Thyroxin treated group the increase in the thickness of EGP of ulna was 18.16% and found to be significant (p<0.05), when compared with their age matched controls. Comparing the mean EGP thickness between the carbimazole and carbimazole plus thyroxin treated group, the difference found was approximately, 35.52%, which was also highly significant (P<0.001)
Tibia
The mean EGP thickness of the tibia in the control group was 137.79±1.30 um, in Carbimazole treated was 116.22±1.03 um, and in Carbimazole plus Thyroxin treated group was 159.03±0.87um (Table-3 and photomicrograph 6&7). Decrease in the thickness, in the Carbimazole treated groups was 15.65% and observed to be highly significant (p<0.001) statistically, whereas in the Carbimazole plus Thyroxin treated group the increase in the thickness of EGP of tibia was 13.3% and found to be highly significant (p<0.001), when compared with their age matched controls. Comparing the mean EGP thickness between the carbimazole and carbimazole plus thyroxin treated group, the difference found was approximately, 36.83%, which was also highly significant (P<0.001).
Prenatally treated group
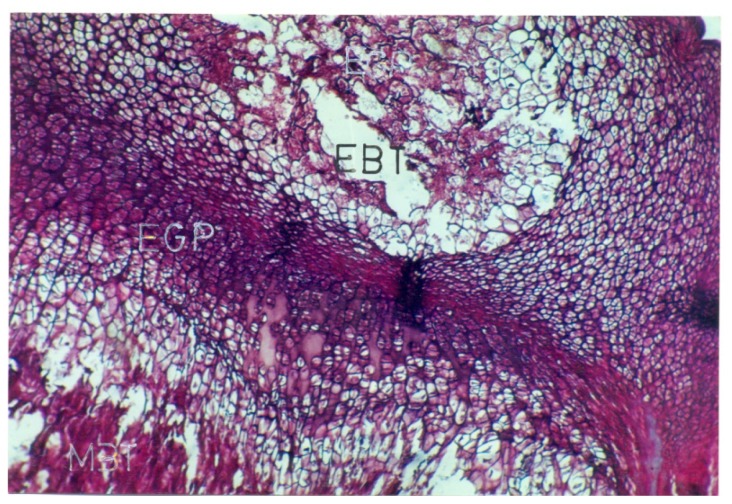
In the (P1) group, typical growth plate (EGP) formation was not seen in the pups born to carbimazole receiving hypothyroid mothers (photomicrograph-5) therefore thickness of EGP was not recordable and hence comparison was not possible
Discussion
Short stature may be a disability and a cause of distress in itself. (23) Carbimazole administered in pregnant rats crosses the placenta and concentrates in the thyroid of developing pups. (24) Thus, it blocks the synthesis of fetal thyroid hormones which are ultimately required for growth and differentiation in developing pups since maternal thyroxin crosses the placenta in a very limited amount. (9, 25, 26) The placenta acts as a very effective barrier, preventing maternal iodothyronins from reaching the fetus. This would be a consequence of both, the poor permeability and active the deiodinating mechanism of fetal membranes. (27, 28) However some maternal T4 and T3 do get transferred to the fetus in both rats and humans. (29) But administered carbimazole in pregnant rats also results in marked reduction in maternal levels of circulating T3 and T4 (24) thus reducing the chances of maternal hormones for crossing the placenta to nil. The effectiveness of carbimazole as a chemical method of inducing hypothyroidism in rats is shown by studies conducted by Redmond et al (16) and Berthier & Lamarchand. (30)
Crown rump length (CRL) amongst the all age group animals has shown a similar pattern, in that, carbimazole treated hypothyroid animals were observed to have shorter CRL when compared to their age matched controls and carbimazole plus thyroxin treated animals and this difference was more marked in younger than older age group animals. This Less amount of decrease in older age group may be attributed to the aging effect where animal has already achieved the major norms of development. Bassett et al, 2008, in their study have reported that it is an intact hypothalamic-pituitary-thyroid axis which regulates the skeletal development via the actions of T3(31). Thyroid hormone (T3) actions are mediated by thyroid hormone receptor (TR)α1 and TRβ nuclear receptors, which are expressed in chondrocytes and osteoblasts. (32)
Though thyroxin replacement treatment has mitigated the effect of carbimazole but full recovery was not possible in this study. Similar findings have been reported by the other researchers (3, 5, 33) who have reported that thyroid hormone replacement induces catch-up growth, though maximum predicted height may not be reached and any height deficit correlates with the duration of untreated hypothyroidism. Increase in bone length is the result of endochondral ossification and the epiphyseal growth plate plays a vital role in this increase. Ahmed et al 2007, in their study have reported that carbimazole induced hypothyroidism affects the endochondral as well as periosteal bone growth. (34) The growth plate (figures-1&4) is the final target organ for longitudinal growth and results from chondrocyte proliferation and differentiation (35) In the developing bone growth plate, thyroid hormones act via two thyroid hormone receptors (TRs) which act as hormone inducible transcription factor. (33) TRs are expressed by chondrocytes of reserve, proliferative and pre-hypertrophic zones as well as by the osteoblast of the primary spongiosum. Hypertrophic chondrocytes do not express T3 receptors suggesting that reserve and proliferative zone chondrocytes are the primary target cells for T33. This study has demonstrated a higher reduction in the epiphyseal growth plate thickness of the hind limb bone (tibia) as compared to the fore limb (ulna) bone amongst the hypothyroid rats in both age groups (figures 2&6). Ours this finding is consistent with the findings of Ahmed et al 2007 (34) who have reported that the skeletal growth of the hind limb bones is affected more as compared to the forelimb bones amongst carbimazole induced hypothyroid rats in the all age groups. Thickness of the EGP, in carbimazole plus thyroxin treated animals (figures 3&7) is more than the age matched controls. It appears to be overcorrected in this study. This shows that thyroxin treatment has resulted in recovery and has surpassed the controls. A possible explanation of this could be that thyroxin treatment in this study began from day one of the experiment when the animals were still having their own thyroxin in circulation and were not hypothyroid since carbimazole requires some time to show its anti-thyroid effect. This overtreatment with thyroxin may have stimulated too much skeletal maturation (33) of the growth plate.
Fig. (1).
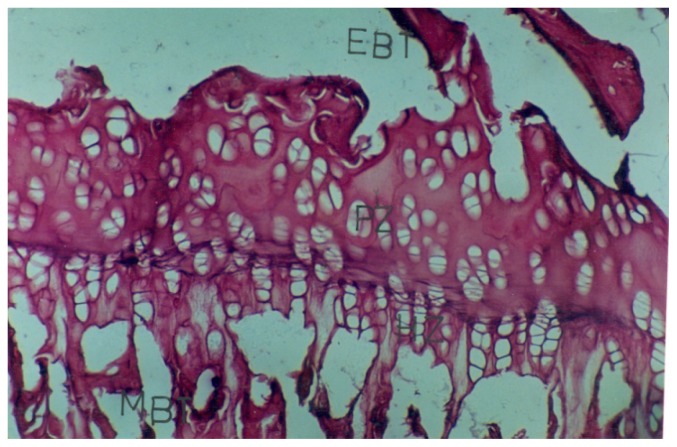
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a two weeks old control rat showing metaphyseal bone trabeculae (MBT), calcified cartilage zone (CCZ), hypertrophy zone(HZ), proliferative zone(PZ), reserve zone(RZ) and epiphyseal bone trabeculae
Figure- 4.
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a rat born to control mother( prenatally treated group) showing EGP wih well-formed zones and metaphyseal bone trabeculae (MBT) and epiphyseal bone trabeculae (EBT).
Fig. (2).
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a two weeks old carbimazole treated hypothyroid rat showing narrower hypertrophy zone(HZ) and proliferative zone(PZ)
Fig. (6).
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a six weeks old carbimazole treated hypothyroid rat showing severe narrowing of EGP with fewer metaphyseal (MBT) and epiphyseal bone trabeculae (EBT).
Fig. (3).
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a two weeks old rat treated with carbimazole plus thyroxin, showing wider hypertrophy zone (HZ), proliferative zone (PZ) and well-formed linear chondrocyte columns.
Fig. (7).
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a six weeks old rat treated with carbimazole plus thyroxin, showing well formed EGP with wider, hypertrophy (HZ), proliferative zone (PZ), linear chondrocyte columns and metaphyseal and epiphyseal bone trabeculae.
This study has demonstrated structural changes in various zones, and has shown overall narrowing of the growth plate (EGP) in carbimazole treated hypothyroid animals. Widening of the reserve and narrowing of proliferative and hypertrophic zones with a few metaphyseal bone trabeculae have been shown in the sections from the fore limb bones; while the sections from hind limb bones have shown narrowing with severe loss in the proliferative and hypertrophic zones and only a few metaphyseal bone trabeculae (figures-2,5&6). Proliferating chondrocytes have not shown the formation of the discrete columns in any of the limb bones amongst all age group animals in this study.
Fig. (5).
Photomicrograph of a 5 u thick section of the EGP of the proximal end of tibia, from a rat born to mother received carbimazole( prenatally treated group )showing metaphyseal bone trabeculae (MBT) and epiphyseal cartilage. No EGP is seen in this photomicrograph.
Sections from the carbimazole plus thyroxin treated animals in this study have shown wider proliferative and hypertrophic zones as compared to their age matched controls. Hypertrophic chondrocytes show well-formed linear columns and calcified cartilage zones (figures 3&7). Sections from the pups born to carbimazole receiving mothers have not shown the presence of any well formed EGP (figure-5). Wide epiphyseal cartilage and a few metaphyseal bone trabeculae have been demonstrated in the sections. T3 is necessary for stimulating the resting zone cells to differentiate into proliferating chondrocytes, for chondrocyte hypertrophy, differentiation and as well as for vascular invasion of growth plate. (3) Recent studies have revealed an essential role for the local control of T3 availability in osteoblasts and chondrocytes during maintenance and repair of bone and cartilage. (35) Enzyme type 2 iodothyronine deiodinase (DIO2), which catalyzes the conversion of T4 to T3 plays a major role in the control of the intracellular T3 concentration and also in its availability to the nucleus, and the saturation of the nuclear T3 receptor in the target tissues. (36)
More over many growth factors ie; IGF-I, Indian hedgehog, PTHrHP, fibroblast growth factors, bone morphogenetic proteins, and vascular endothelial growth factor, are now considered as crucial regulators of chondrocyte proliferation and differentiation. (32)
References
- 1.Frietas FR, Capelo LP, Oshea PJ, Jorgetti V, Moriscot AS, Scanlan TS, et al. The thyroid hormone receptor beta specific agonist GC-1 Selectively affects the bone development of hypothyroid rats. J Bone Miner Res. 2005;20(2):294–304. doi: 10.1359/JBMR.041116. [DOI] [PubMed] [Google Scholar]
- 2.Ganong WF Endocrine system, editor. Review of medical physiology. Stanford: Appleton and Lange; 1999. p. 303. [Google Scholar]
- 3.Murphy E, Williams GR. The thyroid and the skeleton. Clin Endocrinol (Oxf) 2004;61:285–298. doi: 10.1111/j.1365-2265.2004.02053.x. [DOI] [PubMed] [Google Scholar]
- 4.Harvey CB, O’Shea PJ, Scott AJ, Robson H, Siebler T, Shalet SM, et al. Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol Genet Metab. 2002;75:17–30. doi: 10.1006/mgme.2001.3268. [DOI] [PubMed] [Google Scholar]
- 5.Rivkees SA, Bode HH, Crawford JD. Long-term growth in juvenile acquired hypothyroidism: the failure to achieve normal adult stature. N Engl J Med. 1988;318:599–602. doi: 10.1056/NEJM198803103181003. [DOI] [PubMed] [Google Scholar]
- 6.Bassett JH, Harvey CB, Williams GR. Mechanisms of thyroid hormone receptor-specific nuclear and extra nuclear actions. Mol Cell Endocrinol. 2003;213:1–11. doi: 10.1016/j.mce.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331:1072– 1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- 8.Smallridge RC, Ladenson PW. Hypothyroidism in Pregnancy: Consequences to Neonatal Health. The Journal of Clinical Endocrinology & Metabolism. 2001;86(6):2349–2353. doi: 10.1210/jcem.86.6.7577. [DOI] [PubMed] [Google Scholar]
- 9.Farwell AP, Braverman LE. Thyroid and Antithyroid Drugs. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Tenth edition. McGraw-Hill; New York: 2001. pp. 1563–96. [Google Scholar]
- 10.Morreale de Escobar G, Jesús Obregón M, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 11.Scow RO, Simpson ME, Asling CW, et al. Response by the rat thyroparathyroidectomized at birth to growth hormone and to thyroxin given separately or in combination. Anat Rec. 1949;104:445–463. doi: 10.1002/ar.1091040405. [DOI] [PubMed] [Google Scholar]
- 12.Lewinson D, Harel Z, Shenzer p. Effect of thyroid hormone and growth hormone on recovery from hypothyroidism of epiphyseal growth plate cartilage and its adjacent bone. Endocrinology. 1989;124:937–45. doi: 10.1210/endo-124-2-937. [DOI] [PubMed] [Google Scholar]
- 13.Smallridge RC. Postpartum thyroid disease: a model of immunologic dysfunction. Clin Appl Immunol Rev. 2000;1:89–103. [Google Scholar]
- 14.Brent GA. Maternal hypothyroidism: recognition and management. Thyroid. 1999:661–665. doi: 10.1089/thy.1999.9.661. [DOI] [PubMed] [Google Scholar]
- 15.Redmond O, Tuffery AR. Thyroid proliferation, body weight and thyroid hormones in chronic carbimazole treatment in rats. J Anat. 1981;137:37–47. [PMC free article] [PubMed] [Google Scholar]
- 16.Inauwa I, Williams MA. Morphometric study on the uterine horn and thyroid gland in hypothyroid and thyroxin treated hypothyroid rats. J Anat. 1995;188:383–393. [PMC free article] [PubMed] [Google Scholar]
- 17.Rough R. The mouse Reproductive system. Minneapolis: Burgess Publishing Company; 1968. pp. 269–99. [Google Scholar]
- 18.Patton J, Kaufman H. Timing of ossification of limb bones and growth rates of various long bones of the fore and hind limbs of the prenatal and early postnatal laboratory mouse. J Anat. 1995;186:175–85. [PMC free article] [PubMed] [Google Scholar]
- 19.Smerdely P, Pitsiavas V, Boyages SC. Methimazole inhibits FTRL-5 thyroid cell proliferation by inducing-S-phase arrest of cell cycle. Endocrinology. 1993;133:2403–2406. doi: 10.1210/endo.133.5.8404692. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed M, Janjua Z. Effect of hypothyroidism and thyroxin replacement on growth of long bones in prenatally treated albino rats. J Pak Med Assoc. 2003;53:18–21. [PubMed] [Google Scholar]
- 21.Ronning O, Kantoma T. The growth pattern of the clavicle in the rat. J Anat. 1988;159:173–179. [PMC free article] [PubMed] [Google Scholar]
- 22.Culling CFA. Handbook of histolopathological and histochemical techniques. London: Butterworth; 1974. [Google Scholar]
- 23.Frindik JP, Kemp SF, Hunold JJ. Near adult heights after growth hormone treatment in patients with idiopathic short stature or idiopathic growth hormone deficiency. J Pediatr Endocrinol Metab. 2003;16:607–612. [PubMed] [Google Scholar]
- 24.Greenspan FS, Dong BJ. Thyroid and anti-thyroid drugs. In: Katzung BG, editor. Basic and clinical pharmacology. Appleton and Lange; London: 1989. pp. 466–478. [Google Scholar]
- 25.Calvo R, Obregon MJ, Del Rey FE. The rat placenta and the transfer of hormones from the mother to the foetus: effects on maternal thyroid status. Endocrinology. 1992;131:357–365. doi: 10.1210/endo.131.1.1612015. [DOI] [PubMed] [Google Scholar]
- 26.De escobar GM, Obregon MJ, Del Rey FE. Comparison of maternal to foetal transfer of 3,5, 3 triiodothyronine versus thyroxin in rats as assessed from the 3,5, 3 triiodothyronine levels in foetal tissues. Acta Endocrinology. 1989;120:489–490. doi: 10.1530/acta.0.1200020. [DOI] [PubMed] [Google Scholar]
- 27.Vulsma T, Gons MH, De Vijlder J. Maternal foetal transfer of thyroxin in congenital hypothyroidism due to total organification defect or thyroid agenesis. N Engl J Med. 1989;321:3–16. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]
- 28.Roti E, Fang SI, Green K. Human placenta is an active site of thyroxine and 3, 3, 5 triiodothyronine tyrosol ring deiodination. J Clin Endocrinol Metab. 1981;53:498–501. doi: 10.1210/jcem-53-3-498. [DOI] [PubMed] [Google Scholar]
- 29.De escobar GM, Calvo R, Del Rey FE, Obregon MJ. Differential effect of thyroid hormones on growth and thyrotropic hormones in rat foetuses near term. Endocrinology. 1993;132:2056–2064. doi: 10.1210/endo.132.5.8477656. [DOI] [PubMed] [Google Scholar]
- 30.Berthier C, Lamarchand BT. Importance of thyroid iodine and cyclic AMP and TSH concentration on goitre formation in rats. Acta Endocrinol. 1978;89:567–580. doi: 10.1530/acta.0.0890567. [DOI] [PubMed] [Google Scholar]
- 31.Duncan Bassett JH, Williams Allan J, Murphy Elaine, Boyde Alan. A Lack of Thyroid Hormones Rather than Excess Thyrotropin Causes Abnormal Skeletal Development in Hypothyroidism. Molecular Endocrinology. 2008;22(2):501–512. doi: 10.1210/me.2007-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003;14:356–364. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 33.Boersma B, Otten BJ, Stoelinga GB, Wit JM. Catch-up growth after prolonged hypothyroidism. Eur J Pediatr. 1996;155(5):362–367. doi: 10.1007/BF01955262. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed M, Sarwar M, Ahmed I, Qureshi GA, Makhdoom A, Parvez SH. Effect of carbimazole induced hypothyroidism and thyroxine replacement on the growth of the long bones in albino rats of different age groups. Neuro Endocrinol Lett. 2007;28:484–488. [PubMed] [Google Scholar]
- 35.waung JA, Bassett JH, Williams GR. Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends Endocrinol Metab. 2011 doi: 10.1016/j.tem.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Williams Graham R, Duncan Bassett JH. Local control of thyroid hormone action: role of type 2 deiodinase Deiodinases: the balance of thyroid hormone. J Endocrinol. 2011;209(3):259–260. doi: 10.1530/JOE-10-0448. [DOI] [PubMed] [Google Scholar]