Abstract
Objectives
To investigate the effects of exercise training and anabolic androgenic steroids (AAS) on hemodynamics, glycogen content, angiogenesis, apoptosis and histology of cardiac muscle.
Methods
Forty rats were divided into 4 groups; control, steroid, exercise-trained and exercise-trained plus steroid groups. The exercise-trained and trained plus steroid groups, after one week of water adaptation, were exercised by jumping into water for 5 weeks. The steroid and trained plus steroid groups received nandrolone decanoate, for 5 weeks. Systolic blood pressure and heart rate (HR) were monitored weekly. Heart weight/body weight ratio (HW/BW ratio) were determined. Serum testosterone, vascular endothelial growth factor (VEGF), cardiac caspase-3 activity and glycogen content were measured.
Results
Compared with control, the steroid group had significantly higher blood pressure, HR, sympathetic nerve activity, testosterone level, HW/BW and cardiac caspase-3 activity. Histological examination revealed apoptotic changes and hypertrophy of cardiomyocytes. In exercise-trained group, cardiac glycogen, VEGF and testosterone levels were significantly higher while HR was significantly lower than control. HW/BW was more than control confirmed by hypertrophy of cardiomyocytes with angiogenesis on histological examination. Trained plus steroid group, had no change in HR, with higher blood pressure and HW/BW than control, cardiac glycogen and serum VEGF were higher than control but lower than exercise-trained group. Histological examination showed hypertrophy of cardiomyoctes with mild angiogenesis rather than apoptosis.
Conclusion
When exercise is augmented with AAS, exercise-associated cardiac benefits may not be fully gained with potential cardiac risk from AAS if used alone or combined with exercise.
Introduction
Exercise training exerts its beneficial effects on cardiovascular system through reducing cardiovascular risk factors, and directly affecting the cellular and molecular remodeling of the heart. (1) The acute endocrine response to heavy resistance exercise includes increased secretion of testosterone, and this can explain muscle hypertrophy observed in athletes who routinely employ high power resistance exercise. (2) Testosterone is one of the most potent naturally secreted androgenic-anabolic hormones and is considered the major promoter of muscle growth. It has been shown to decrease pro-inflammatory cytokines and increase anti-inflammatory cytokines, cause coronary vasodilatation, improve insulin sensitivity, reduce body mass index, reduce abdominal fat with decreased risk of heart disease. (3,4) Men with low testosterone levels are at an increased risk of stroke as well as heart attack due to an increased accumulation of atherosclerotic plaque in their arteries. The physiological effects of testosterone are modulated through the interaction of testosterone and training. (5)
Anabolic androgenic steroids (AAS) are synthetic compounds, made up of testosterone and its derivatives. When these AAS work on androgen receptor, they produce nearly similar anabolic and androgenic effects of testosterone. (6) They are used in diseases, such as testosterone deficiency, malnutrition, aplastic anemia, hypogonadism and delayed male puberty. (7) AAS have attracted the attention of health researchers because some athletes have been using them without prescription and at supraphysiological doses, with the purpose of increasing muscle mass or to improve physical performance. (8)
Although testosterone has beneficial effects on the cardiovascular system, some studies linked the exogenous supraphysiologic doses of AAS with the development of cardiovascular abnormalities as hypertension, increased interventricular septum thickness, dilated cardiomyopathy, arrhythmia, heart failure and sudden cardiac death. (9, 10)
Muscle glycogen is an essential fuel for exercise training. Exercise produces a significant reduction in muscle glycogen. (11) However during recovery from prolonged exercise, muscle glycogen can be restored more than preexercise levels. Therefore, glycogen availability would be greater for subsequent exercise bouts which elevate exercise tolerance and increase resistance to fatigue. (12) Previous studies reported that capillarity in active skeletal muscle is significantly increased by exercise training. (13, 14) Angiogenesis refers to the formation of new capillaries from existing capillaries. Vascular endothelial growth factor (VEGF) is a potent mitogen of endothelial cells and plays a critical role in both physiological and pathological angiogenesis. (15, 16) However, little is known about the impact of training and AAS on cardiac glycogen and angiogenesis.
Apoptosis has been observed in a large spectrum of heart diseases, and constitutes a key event in the pathogenesis of cardiac failure. Previous observations emphasize the fact that cardiomyocyte apoptosis is a critical event in the transition between compensatory cardiac hypertrophy and heart failure.(17, 18) AAS exert primarily anabolic and growth-promoting effects in cardiac tissue; however, they also cause ultrastructural alterations of cardiomyocytes similar to those observed in the early stages of congestive heart failure. (19) Although the use of AAS is frequently associated with exercise, limited studies in animal models have attempted to find the relationship between AAS use with and without training and cardiovascular function. Therefore, the aim of the present work was to investigate the effects of training and AAS on the hemodynamic function, cardiac glycogen content as an energy store, sympathetic activity, angiogenesis, apoptosis, and histology of cardiac muscle in adult male rats. Also, as the cardiovascular functions are influenced by testosterone and because training and AAS have different effects on it, the serum testosterone levels will be also evaluated.
Material and methods
This study was carried out on 40 adult male albino rats aged 12–14 weeks old; weighing 200–250 g. They were obtained from Faculty of Medicine Animal House, Assuit University, Egypt. The rats were divided randomly into four groups, each with 10 rats: control, steroid, exercise-trained, and trained plus steroid groups. Food and water were provided ad libitum. Room temperature was kept at 23 ± 1°C. A 12:12-h light-dark cycle was maintained throughout the experiment. Over the course of 5 weeks, the steroid and trained plus steroid groups were treated with the anabolic androgenic steroid (AAS), nandrolone decanoate (Decadurabolin; Organon, Roseland, USA) administered intramuscular twice a week, in a dosage of 5 mg/kg body weight. The control and exercise-trained groups received the same volume of vehicle of the AAS, composed of peanut oil with benzyl alcohol (90: 10, V/V). (20) Initial and final body weights (BW) were recorded. Animal care and use were in accordance with procedures outlined in the National Institutes of Health Guidelines. The protocol was approved by the institutional animal experimentation ethics committee of Assuit University, Assuit, Egypt.
The training protocol was according to Cunha et al. (20) During the first week, the animals of the exercise-trained and trained plus steroid groups swam for 30 min/day in a tank (60 cm wide, 75 cm long, 80 cm deep) filled with water at 32 ± 1°C to adapt the environment. The exercise training was done once per day for 5 days, from the second to the sixth week of the experiment. The exercise regimen included 4 sets of 10 jumps from the bottom of the tank to the water surface with 30 seconds recovery time between sets. To augment the exercise intensity, an external load was added to the animal. The animals carried a load of 50% body weight strapped to the chest in the second week. In the third and fourth training weeks, the animals performed the same exercise carrying a load of 60% body weight, and in the last two weeks, this load was adjusted to 70% of body weight. All the procedure began at 8 a.m.
Systolic blood pressure was recorded in the first week as baseline then weekly twenty-four hours after the last training session for all groups throughout the experiment. It was recorded with pneumatic tail – cuff device (NARCO, Biosystems, Inc., Houston, Texas) after the animals had been pre warmed for 30 min. in a metabolic chamber maintained at approximately 30 °C, mean values of three consecutive measurements (10-sec interval) were calculated.
Recoding of electrocardiogram (ECG) was done in the first week as baseline then weekly after twenty-four hours of the last training session for all groups. It was recorded by inserting the needle electrodes in the skin of four limbs of all groups under light ether anesthesia. The needle was connected to an ECG recorder (ECG Cardiofax Nih Onkohn Kohden, Kogyo Co. Ltd, Kogyo, Japan). The animals were left for 15 min before recording LI, LII and LIII of ECG from which the heart rate (HR) (beats/min) of each rat was determined.
Recording of sympathetic nerve activity was done by measuring sciatic nerve activity (SNA) as described by McLeod (21). After the animals were sacrificed, the right leg was dissected to expose the sciatic nerve. A strong thread was passed around the main sciatic nerve and tied. Sciatic nerve traffic was recorded after stimulation with a microelectrode connected to the stimulator cable (electronic stimulator SEN-3201). The stimulator frequency was set at 25/sec, for the duration of 2 sec and strength of 3–5 V. The sciatic signals were magnified with a Washington coupler (814-80950-0). Responses of the nerve were recorded on the physiograph. SNA was determined as burst/min.
Blood and Tissue Preparation
At the end of the experimental period, blood samples were collected, and serum was separated for assays. Then the rats were sacrificed. The intraabdominal fat was dissected, weighed and normalized by total BW of the animal. To measure cardiac mass, the heart was removed from the thoracic cavity and dissected to separate the left ventricle (LV). The interventricular septum remained as part of the LV. To evaluate cardiac hypertrophy, the heart LV weight (HW) was normalized by total BW of the animal (HW/BW in mg/g). The HW/BW ratio was used as an index of cardiac hypertrophy as reported by Rocha et al. (22) For analytical assay, the apex half of the LV was cut into pieces that were snap-frozen in liquid nitrogen then homogenized and stored at −20°C until used for biochemical analysis, while the other half was used for histological analysis.
For light microscopy, cardiac samples were fixed in 10% neutral buffered formaldehyde then processed for paraffin sections, cut into 5 mm thickness and stained with hematoxylin and eosin (H&E) for the visualization of cellular structures. (23)
Serum testosterone level was measured by ELISA method (ALPCO Diagnostics. NH, USA, Cat No 55-TESMS-E01). Serum vascular endothelial growth factor (VEGF) as a marker of angiogenesis (15) was evaluated by ELISA method (R&D Systems, Minneapolis, Minnesota, USA). Cardiac glycogen content was determined by chemical method according to the Siu et al. (24) Cardiac caspase-3 activity as a marker of apoptosis (19) was measured by ELISA method using Apotarget Apoptosis kit supplied by Biosource International, Inc (Camarillo, California 93012USA). Data were normalized to the amount of protein measured by the Lowery method, (25) using the bovine serum albumen as a slandered.
Statistical Analysis
Data were presented as means ± standard error (SEM). Comparison between groups was done by using Unpaired Student’s “t” test. Comparison between means of different parameters in the same group before and after the intervention was done by Paired Student’s “t” test. The level of significance was accepted with P < 0.05. Prism computer program (graph pad version 3.0) was used for statistical analysis according to that described by Knapp and Miller. (26)
Results
A significant rise of systolic blood pressure (mmHg) was observed in steroid group at the 4th, 5th, and 6th weeks (P<0.05, P<0.01 and P<0.01 respectively) in comparison to 1st week and to control group during 4th, 5th, and 6th weeks (P<0.01, P<0.001 and P<0.001 respectively). In trained plus steroid group, significant rise of systolic blood pressure was observed starting from 5th week (P<0.05) and 6th week (P<0.05) as compared with 1st week and with control group. In exercise-trained group, systolic blood pressure was non significantly varied from control levels (Table 1).
Table (1).
Systolic blood pressure (mmHg) in control, steroid, exercise-trained and trained plus steroid groups.
| Groups (n=10) | 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week |
|---|---|---|---|---|---|---|
| Control | 100±4.7 | 101±5.5 | 103±5.8 | 102±5.5 | 105±4.6 | 104±4.7 |
| Steroid | 107±4.2 | 112±6.4 | 120±8.6 | 129±7.1 **, # | 142±8.2 ***, ## | 147±9.3 ***, ## |
| Exercise-trained | 104±5.3 | 110±7.2 | 112±7.5 | 116±6.2 | 115±5.5 | 112 ± 9.2 |
| Trained plus steroid | 105±4.9 | 114±7.4 | 115±6.6 | 119±6.5 | 122±6.3 *, # | 124±7.4 *, # |
Data are presented as mean ± SEM.
P<0.05,
P<0.01,
P<0.001 versus control group.
P<0.05,
P<0.01 versus first week.
In steroid group, the HR (beats/min) was increased significantly at the 3rd, 4th, 5th, and 6th weeks ( P<0.01, P<0.001, P<0.001 and P<0.001 respectively) in comparison to 1st week and in comparison to control group during 3rd, 4th, 5th, and 6th weeks (P<0.001 for each). In exercise-trained group, HR was significantly lower at 2nd, 3rd 4th, 5th and 6th weeks as compared to the 1st week (P<0.05, P<0.01, P<0.001, P<0.01 and P<0.001 respectively) and as compared to control during 2nd, 3rd 4th, 5th, and 6th weeks (P<0.01, P<0.01, P<0.001, P<0.001 and P<0.001 respectively). In trained plus steroid group HR was non significantly varied as compared to 1st week or to control group (Table 2).
Table (2).
Heart rate (beats/min) in control, steroid, exercise-trained and trained plus steroid groups.
| Groups (n=10) | 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week |
|---|---|---|---|---|---|---|
| Control | 410±27 | 433±28 | 421±33 | 461±28 | 452±29 | 466±34 |
| Steroid | 422±29 | 505±35 | 631±36***, ## | 677±38***, ### | 793±43***, ### | 851±50***, ### |
| Exercise-trained | 405±25 | 326±19**, # | 311±18**, ## | 279±31***, ### | 301±21***, ## | 260±23***, ### |
| Trained plus steroid | 401±24 | 386±26 | 391±22 | 485±34 | 474±39 | 492±42 |
Data are presented as mean ± SEM.
P<0.01,
P<0.001 versus control group.
P<0.05,
P<0.01,
P<0.001 versus first week.
The SNA (burst/min) was significantly higher in steroid group as compared to control, exercise-trained and trained plus steroid groups (P<0.01 for each) (Figure 1).
Fig. (1).
Sciatic nerve activity (burst/min) in control, steroid, exercise-trained and trained plus steroid groups. Data are means ±SEM. ** P< 0.01versus control, ##P< 0.01 versus steroid group.
The body weight (BW) increased significantly at the sixth week compared to the first week in all groups with P value <0.001 for each. Compared to control the BW measured at week 6 were significantly lower in steroid, exercise-trained, trained plus steroid groups (P <0.001 for each). Trained plus steroid group has less BW as compared to steroid group (P<0.05). Compatible with BW, the intraabdominal fat was significantly lower in steroid and trained plus steroid (P<0.05 for each) and non significantly lower in exercise-trained group than control group (Table 3).
Table (3).
Body weight (BW), heart weight (HW), HW/BW ratio and intraabdominal fat in control, steroid, exercise-trained and trained plus steroid groups.
| Groups | Body weight (g) | Heart weight (g) | HW/BW Ratio (mg/g) | Intraabdominal fat (g/100g BW) | |
|---|---|---|---|---|---|
| Weak 1 | Weak 6 | ||||
| Control (n=10) | 219±4.2 | 395±8.2§§§ | 1.13±0.05 | 2.86 | 5.18±0.63 |
| Steroid (n=10) | 223±5.1 | 351±6.6 ***, §§§ | 1.45±0.07** | 4.13** | 3.16±0.42* |
| Exercise-trained (n=10) | 227±4.5 | 335±7.7 ***, §§§ | 1.24±0.04# | 3.7* | 4.29±0. 53 |
| Trained plus steroid (n=10) | 222±3.9 | 324±6.9 ***, #,§§§ | 1.30±0.04* | 4.01* | 3.65±0.23* |
Data are presented as mean ± SEM.
P<0.05,
P<0.01,
P<0.001 versus control group.
P<0.05 versus steroid group.
P<0.001 versus weak 1.
Steroid and trained plus steroid had significantly increased HW in comparison to control group (P<0.01, P<0.05 respectively). In exercise-trained group, HW was non significantly varied from control or trained plus steroid groups while significantly lower than steroid group (P<0.05) (Table 3).
HW/BW ratio was significantly higher in steroid (P<0.01), exercise-trained (P<0.05) and trained plus steroid (P<0.05) groups in comparison to control group. This ratio was non significantly higher for steroid and trained plus steroid groups than exercise-trained group (Table 3).
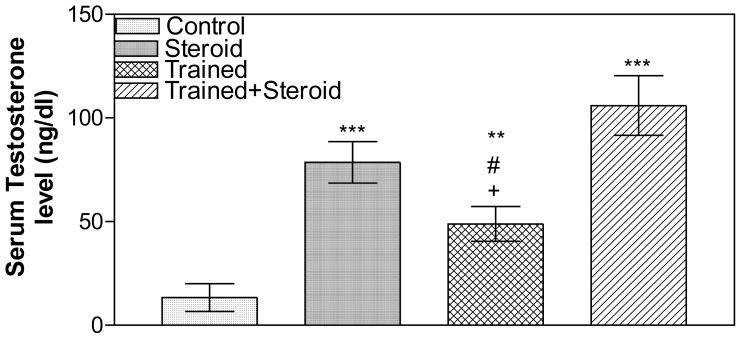
The levels of serum testosterone (ng/dl) in steroid, exercise-trained, and trained plus steroid groups were significantly higher than control (P <0.001, P <0.01 and P <0.001 respectively). In between the 3 groups exercise-trained group had lower testosterone levels than steroid and trained plus steroid groups (P value < 0.05 for each) (Figure 2).
Fig. (2).
Serum testosterone level (ng/dl) in control, steroid, exercise-trained and trained plus steroid groups. Data are means ±SEM. ** P< 0.01, *** P< 0.001 versus control, #: P< 0.05 versus steroid group and + P<0.05 versus trained plus steroid group.
It was found that serum levels of VEGF (pg/ml) were significantly higher in both exercise-trained and trained plus steroid groups when compared to control and steroid groups (P<0.001 for all) with higher level observed in exercise-trained than in trained plus steroid group (p<0.01) (Figure 3).
Fig. (3).
Serum level of VEGF (pg/ml) in control, steroid, exercise-trained and trained plus steroid groups. Data are means ±SEM. *** P<0.001versus control, ###P<0.001 versus steroid and ++P<0.01 versus trained plus steroid group.
Cardiac glycogen content (mg/100mg protein) was significantly higher in exercise-trained and trained plus steroid groups than control and steroid groups (P<0.001 for all) with higher level (P<0.05) observed in exercise-trained than in trained plus steroid group (Figure 4). Steroid group presented with the highest cardiac caspase-3 activity (nmol/mg protein) than control, tra exercise-trained ined and trained plus steroid (P<0.001 for each) whereas the levels in exercise-trained and in trained plus steroid groups non significantly varied from control (Figure 5).
Fig. (4).
Cardiac glycogen content (mg/100mg protein) in control, steroid, exercise-trained and trained plus steroid groups. Data are means ±SEM. *** P<0.001 versus control, ###P<0.001 versus steroid and +P<0.05 versus trained plus steroid group.
Fig. (5).
Cardiac caspase-3 (nmol/mg protein) in control, steroid, exercise-trained and trained plus steroid groups. Data are means ±SEM. ***: P<0.001 versus control, and ###P<0.001 versus steroid group.
Histological Changes
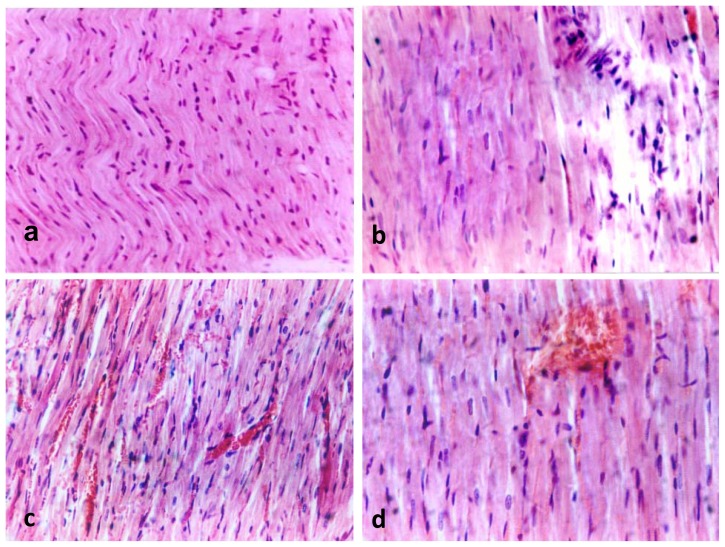
Figure 6a–d showed histological sections of the studied groups of animals, stained with H&E. The histological structure of cardiac muscle fibers of the LV in control group is shown in Figure 6a. Normal blood capillaries were seen among the muscle fibers. The muscle fibers appeared elongated and branched with oval central nuclei and pale acidophilic cytoplasm. Figure 6b showed histological features of the steroid group with the nuclei of the cardiac muscle fibers appeared enlarged with less frequency (hypertrophy of cardiomyocytes) in comparison to that of the control group. Focal areas of degeneration and vaculation as well as damage of sarcolemma and myofibril (apoptotic changes) were observed. The most characteristic features of exercise-trained group were the increased capillaries density around the cardiomyocytes (angiogenesis) and hypertrophy of the cardiomyocytes (Figure 6c). In trained plus steroid group hypertrophy of the cardiomyocytes was found. No apoptotic changes were noticed but mild angiogenesis was seen among the muscle fibers (Figure 6d).
Fig. (6a–d).
showed histological sections of cardiac muscle fibers (LV) of the studied groups of animals, stained with H&E a: Control group shows normal blood capillaries among the muscle fibers. The muscle fibers elongated and branched with oval central nuclei and pale acidophilic cytoplasm. b: Steroid group shows that the nuclei of the cardiac muscle fibers are enlarged with less frequency in comparison to that of the control group with apoptotic changes. c: exercise-trained group shows increased capillaries density around the cardiomyocytes (angiogenesis) with hypertrophy of the cardiomyocytes. d: Trained plus steroid shows hypertrophy of the cardiomyocytes with no apoptotic changes and mild angiogenesis among the muscle fibers. × 250 (a, c) & × 400 (b, d).
Discussion
Exercise training fosters the health and performance of the cardiovascular system, and represents nowadays a powerful tool for cardiovascular therapy. (1) The abuse of AAS which are commonly used to enhance sport performance in athletes has been associated to cardiovascular disorders. The heart is the most frequently affected organ by administration of these steroids. (27)
In the present study, hemodynamic measurements in steroid group showed a significant increase of systolic blood pressure and HR after receiving AAS compared to the initial values and to control group. These results could be attributed to increased sympathetic activity (SNA) observed in this group. These results are in accordance other investigators’ showed that AAS treatment leads to dysfunction in tonic cardiac autonomic regulation with marked impairment of parasympathetic cardiac modulation and sympathetic hyperactivity. (28, 29) It was also found that in presence of supraphysiological dose of AAS there may be changes in the sensitivity of the heart by increasing in adrenergic β receptors, causing super-sensitiveness in the cardiac sinoatrial node. (30) The decrease in HR observed in exercise-trained group at 2nd, 3rd 4th, 5th, and 6th weeks as compared to the initial value in 1st week and to control group confirms the effectiveness of exercise training in this study. These results are in line with previous studies which reported that the exercise-induced resting bradycardia is associated with exercise-induced adaptation in cardiac autonomic nervous activities. (31–33) However nandrolone treatment in trained plus steroid group block this cardiac autonomic adaptation as we didn’t detect any exercise-induced resting bradycardia in this group but a significant rise of systolic blood pressure was found at 5th and 6th week.
All groups had increased BW after 6 weeks however this BW was less in steroid, exercise-trained, trained plus steroid groups in comparison to control group indicating less weight gain in the 3 groups with decrease of intraabdominal fat in comparison to control group. This may reflect a higher body fat metabolism with decreased weight gain achieved by training and ASS administration which caused an increase in fat metabolism in adipose tissue or blunted the appetite, resulting in less weight gain in rats. (22)
The HW per se was not significantly different in exercise-trained group as compared to control; however, the training protocol was efficient in increasing the HW/BW ratio compared with that in control. This response is typical of physical training and represents physiological cardiac hypertrophy. (34, 35) Rats received AAS in steroid and trained plus steroid groups presented with significantly higher HW. Recent studies reported that recurring high dose AAS administration and physical training in mice produce significant increase in HW. (8, 36) Both groups had significantly higher HW/BW ratio than control and non significantly higher than exercise-trained group. Riezzo et al (27) observed that exogenous AAS administration induced cardiac hypertrophy in vitro and in vivo, and when combined with exercise, these steroids change exercise-induced physiological cardiac hypertrophy to pathophysiological cardiac hypertrophy. Histological examination revealed also that in steroid, exercise-trained and trained plus steroid groups, hypertrophy of the cardiomyocytes was evident compared to control group. These results are in line with previous studies. (27, 37)
Administration of AAS in steroid and trained plus steroid groups increased serum testosterone levels than control group. These results are consistent with the previously described that chronic AAS administration causes a significant increase in serum testosterone level in trained rats treated with steroids. They speculated that both endogenous and exogenous testosterone or testosterone like substances were measured. (22, 38) On the other hand, other study reported that low testosterone level was observed throughout AAS treatment due to negative feedback mechanism with reduced endogenous testosterone secretion. (39) Increased serum testosterone levels than control was also detected in exercise-trained group but less than those levels found in steroid and trained plus steroid groups. This agrees with data of Fry and Lohnes (2) who reported that physical exercise can elicit a high testosterone response and can contribute to the muscle hypertrophy observed in athletes.
Marsh et al (40) reported that presence of androgen receptors in cardiac myocytes can directly mediate a significant hypertrophic response to androgens in heart. In the present study, higher testosterone levels in response to training and with administration of AAS are likely to share such influences on the cardiac hypertrophic response through actions on the androgen receptors. The use of supraphysiological dose of AAS and not the physiological amounts of natural testosterone could induce pathophysiological cardiac hypertrophy since natural testosterone is beneficial with regard to cardiovascular health. AAS resemble the testosterone molecule and work like it in some ways but not in others. One of the ways they don’t work like testosterone is in terms of toxicity. (5)
The pattern of energy use by the heart is combined competition between fatty acids, lactate and glucose and varies according to workload and hormonal status. (41) In the present work, training resulted in significantly higher cardiac glycogen content in exercise-trained and in trained plus steroid groups compared with other groups. AAS administration alone did not increase cardiac glycogen content in steroid group as compared to control group. Lapier and Rodnick (42) demonstrated that physical training, could induce increase of cardiac glycogen storage and glucose uptake via enhancing the activity of glycogen synthase and considered these effects as metabolic adaptations in cardiac function and energy metabolism. Foss and Keteyian (43) reported that testosterone can increase glycogen synthase activity with subsequent increase in glycogen synthesis in male animal. Accordingly, in this study the increased cardiac glycogen in both trained groups could be due to endogenous testosterone secretion in response to exercise training and not AAS administration. On the contrary; Silva et al (30) reported an increase in glycogen content of the cardiac muscle in presence of AAS and suggested that this increase may reflect the effect of these steroids on changing the tissue responsiveness to other hormones, such as insulin-like growth factor on the glycogenic pathway.
The exercise training-induced increase of capillary density in this study is a beneficial adaptation for the heart because capillary network participates in maintaining the supply of oxygen and energy substances in the heart. (44) The molecular mechanisms underlying the exercise-induced improvement of angiogenesis in the heart might be related to VEGF. (14. 45) We observed that the highest serum level of VEGF was found in exercise-trained followed by trained plus steroid groups in comparison either to control or steroid groups in which the levels were very minimal. In harmony with these data the histological examination of the cardiac muscle showed angiogenesis in exercise-trained group which was more prominent than trained plus steroid group. Our findings are in line with Ellison et al (1) who showed that exercise training induces vascular remodeling of the cardiac muscle by increasing angiogenesis. Our results also agree with previous studies showed increased VEGF expression in rat heart after exercise training. (46, 47) It was found that reduced oxygen tension in active muscles during exercise may increase a hypoxia-inducible factor that stimulates transcription of the VEGF gene in cardiac muscle. (48) VEGF appears to enable and/or facilitates the mobilization of endothelial progenitor cells from the bone marrow (16, 49) which are known to have a great capacity for neovascularization. (50) Based on these observations, in this study the angiogenesis induced within the cardiac muscle of the exercise-trained and trained plus steroid groups was dependent on the availability of VEGF in response to the increased myocardial oxygen demand imposed by cardiac hypertrophy.
It’s described that the AAS abuse is clinically associated with sudden cardiac death, myocardial infarct; ventricular remodelling and cardiomyopathy, the molecular mechanisms of these events were closely related to apoptosis. (9) Results of the current study revealed that caspase-3 activity of the cardiac muscle as a marker of apoptosis was significantly increased in steroid group than other groups. Concomitant with these results, histological finding showed apoptotic changes in the cardiac muscle fibers of this group. These results are in accordance with other animal studies found that AAS resulted in cardiac apoptotic lesions. (19, 51, 52) In a recent study, it has been shown that AAS indirectly mediates the processes that precede mitochondrial damage, apoptosis and sarcomere disruption. (53)
In trained plus steroid group, cardiac caspase-3 activity was minimal and no apoptotic changes were observed on histological examination. These data support the hypothesis that exercise training is able to reduce the extent of apoptosis in rat cardiac muscle, where Siu et al (54) reported that 8 weeks endurance training led to decrease proapoptotic and increase antiapoptotic gene levels in rat cardiac muscle.
In Conclusion; impaired angiogenesis, pathophysiological hypertrophy, adverse hemodynamics and apoptotic changes of cardiac muscle on AAS administration may affect viability of the heart. Physiological cardiac hypertrophy with exercise training was associated with increase glycogen content, angiogenesis and improvement of hemodynamics of the heart; effects that can improve cardiac function and energy metabolism. Co-administration of AAS with exercise partially reduced those benefits with adverse hemodynamic effects and pathophysiological hypertrophy of the cardiac muscle suggesting increased cardiovascular risks in training exercise associated with AAS abuse.
Acknowledgements
The authors are indebted to Dr. Heba K Mohamed lecturer in Anatomy Department, Assuit University, Faculty of Medicine for describing the histological sections of the heart.
References
- 1.Ellison GM, Waring CD, Vicinanza C, Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012 Jan;98(1):5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- 2.Fry AC, Lohnes CA. Acute testosterone and cortisol responses to high power resistance exercise. Fiziol chelovka. 2010;36(4):102–106. [PubMed] [Google Scholar]
- 3.Bhasin S. Effects of testosterone administration on fat distribution, insulin sensitivity and atherosclerosis progression. Clin Infect Dis. 2003;37:2142–2149. doi: 10.1086/375878. [DOI] [PubMed] [Google Scholar]
- 4.Jones RD, Nettleship JE, Kapoor D, Jones HT. Testosterone and atherosclerosis in aging men: purported association and clinical implications. Am J Cardiovasc Drugs. 2005;5(3):141–154. doi: 10.2165/00129784-200505030-00001. [DOI] [PubMed] [Google Scholar]
- 5.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40(12):1037–1053. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Creutzberg EC, Schols AM, Beduschi G. Anabolic steroids. Curr Opin Clin Nutr Metab Care. 1999;2:243–253. doi: 10.1097/00075197-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Snyder PJ. Androgens. In: Hardman Limbird JGLE, Goodman, Gilman A, editors. The pharmacological basis of therapeutics. 10th ed. New York: McGraw Hill; 2001. pp. 1635–1648. [Google Scholar]
- 8.Fineschi V, Di Paolo M, Neri M, Bello S, D’Errico S, Dinucci DC. Anabolic steroid- and exercise-induced cardio-depressant cytokines and myocardial β1 receptor expression in CD1 mice. Pharm Biotechnol. 2011;12(2):275–284. doi: 10.2174/138920111794295792. [DOI] [PubMed] [Google Scholar]
- 9.Payne JR, Kotwinski PJ, Montgomery HE. Cardiac effects of anabolic steroids. Heart. 2004;90:473–475. doi: 10.1136/hrt.2003.025783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fineschi V, Riezzo I, Centini F, Silingardi E, Licata M, Karch SB. Sudden cardiac death during anabolic steroid abuse: morphologic and toxicologic finding in two fatal cases of bodybuilders. Int J Legal Med. 2005;15:1–6. doi: 10.1007/s00414-005-0055-9. [DOI] [PubMed] [Google Scholar]
- 11.Green HJ, Ball-Burnett M, Symon S, Grant S, Jamieson G. Short-term training, muscle glycogen, and cycle endurance. Can J Appl Physiol. 1995;20:315–324. doi: 10.1139/h95-024. [DOI] [PubMed] [Google Scholar]
- 12.Cadefau J, Green HJ, Cusso R, Ball-Burnett M, Jamieson G. Metabolic adaptations to short-term training are expressed early in submaximal exercise. Can J Physiol Pharmacol. 1995;73:474–482. doi: 10.1139/y95-060. [DOI] [PubMed] [Google Scholar]
- 13.Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Handbook of Physiology. Exercise. Regulation and Integration of Multiple Systems. chapt. 16. sect. 12. Bethesda, MD: Am. Physiol. Soc; 1996. Control of blood flow to cardiac and skeletal muscle during exercise; pp. 705–769. [Google Scholar]
- 14.Prior BM, Yang HT, Terjung RL. What makes vessels grow with exercise training? J Appl Physiol. 2004;97:1119–1128. doi: 10.1152/japplphysiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 15.Cucina A, Borrelli V, Randone B, Coluccia P, Sapienza P, Cavallaro A. Vascular endothelial growth factor increases the migration and proliferation of smooth muscle cells through the mediation of growth factors released by endothelial cells. J Surg Res. 2003;109:16–23. doi: 10.1016/s0022-4804(02)00042-2. [DOI] [PubMed] [Google Scholar]
- 16.Laufs U, Werner N, Link A, Endres M, Wassmann S, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 17.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 18.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, Muller W. Loss of a gp 130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 19.Fanton L, Belhani D, Vaillant F, Tabib A. Heart lesions associated with Anabolic steroid abuse: Comparison of post –mortem findings in athletes and norethandrolone –induced lesions in rabbits. Exp Toxicol Pathol. 2009;61(4):317–323. doi: 10.1016/j.etp.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Cunha TS, Tanno AP, Moura MJCS, Marcondes FK. Influence of high intensity exercise training and anabolic androgenic steroid treatment on rat tissue glycogen content. Life Sci. 2005;73:1030–1043. doi: 10.1016/j.lfs.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.McLeod LJ. Pharmacological experiments on intact preparation. 2nd ed. 1970. pp. 40–45. [Google Scholar]
- 22.Rocha FL, Carmo EC, Roque FR, Hashimoto NY, Rossoni LV. Anabolic steroids induce cardiac renin-angiotensin system and impair the beneficial effects of aerobic training in rats. Am J Physiol Heart Circ Physiol. 2007;293:H3575–H3583. doi: 10.1152/ajpheart.01251.2006. [DOI] [PubMed] [Google Scholar]
- 23.Drury RA, Wallington EA. Carleton’s Histological Techniques. 5th ed. Oxford: Oxford University Press; 1980. [Google Scholar]
- 24.Siu LO, Russeau JC, Taylor AW. Determination of glycogen in small tissue samples. Journal of Applied Physiology. 1970;28(2):234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 25.Lowery OH, Rosebrough NJ, Randoll RJ. Protein measurement with the folin phenol reagent. J boils Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Knapp GR, Miller MC. Clinical Epidemiology and Biostatistics. 1st Eddition. Williams and Wilkins; Baltimore Maryland: 1992. Tests of statistical significance: Regression and correlation; pp. 255–274. [Google Scholar]
- 27.Riezzo I, De Carlo D, Neri M, Nieddu A, Turillazzi E, Fineschi V. Heart disease induced by AAS abuse, using experimental mice/rats models and the role of physical exercise. Mini Rev Med Chem. 2011;11(5):409–424. doi: 10.2174/138955711795445862. [DOI] [PubMed] [Google Scholar]
- 28.Beutel A, Bergamaschi CT, Campos RR. Effects of chronic anabolic steroid treatment on tonic and reflex cardiovascular control in male rats. J Steroid Biochem Mol Biol. 2005;93:43–48. doi: 10.1016/j.jsbmb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Pereira-Junior PP, Chaves EA, Costa RH, Masuda MO. Cardiac autonomic dysfunction in rats chronically treated with anabolic steroid. Eur J Appl Physiol. 2006;96:487–494. doi: 10.1007/s00421-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 30.Silva CA, Pardi AC, Gonçalves TM, Borin SH. Electrocardiographic Profile and Muscle Glycogen Content of Rats Treated with Nandrolone. Arq Bras Cardiol. 2010;95(6):720–725. doi: 10.1590/s0066-782x2010005000144. [DOI] [PubMed] [Google Scholar]
- 31.Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- 32.Alom MM, Bhuiyan NI, Hossain MM, Hoque MF, Rozario RJ, Nessa W. Physical training induced resting bradycardia and its association with cardiac autonomic nervous activities. Mymensingh Med J. 2011;20(4):665–670. [PubMed] [Google Scholar]
- 33.Matsukawa K. Central command: control of cardiac sympathetic and vagal efferent nerve activity and the arterial baroreflex during spontaneous motor behavior in animals. Experimental Physiology. 2012;97:20–28. doi: 10.1113/expphysiol.2011.057661. [DOI] [PubMed] [Google Scholar]
- 34.Iemitsu M, Miyauchi T, Maeda S, Sakai S, Kobayashi T, Fujii N. Physiological and pathological cardiac hypertrophy induce different molecular phenotypes in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2029–R2036. doi: 10.1152/ajpregu.2001.281.6.R2029. [DOI] [PubMed] [Google Scholar]
- 35.Medeiros A, Oliveira EM, Gianolla R, Casarini DE. Swimming training increases cardiac vagal activity and induces cardiac hypertrophy in rats. Braz J Med Biol Res. 2004;37:1909–1917. doi: 10.1590/s0100-879x2004001200018. [DOI] [PubMed] [Google Scholar]
- 36.Ahmadiasl N, Najafipour H, Soufi FG, Jafari A. Effect of short- and long-term strength exercise on cardiac oxidative stress and performance in rat. J Physiol Biochem. 2012;68(1):121–8. doi: 10.1007/s13105-011-0125-z. [DOI] [PubMed] [Google Scholar]
- 37.Medei E, Marocolo M, Rodrigues D, Arantes P. Chronic treatment with anabolic steroids induces ventricular repolarization disturbances: Cellular, ionic and molecular mechanism. J Mol Cel Cardiol. 2010;49:165–175. doi: 10.1016/j.yjmcc.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M, Tatsugi Y, Kohno P. Endocrinological and pathological effects of anabolic androgenic steroid in male rats. Endocrine Journal. 2004;51(4):425–434. doi: 10.1507/endocrj.51.425. [DOI] [PubMed] [Google Scholar]
- 39.Bahrke MS, Yesalis CE, Kopstein AN. Risk factors associated with anabolic androgen steroid use among adolescents. Sport Med. 2000;29:397–405. doi: 10.2165/00007256-200029060-00003. [DOI] [PubMed] [Google Scholar]
- 40.Marsh JD, Lehmann MH, Ritchie RH, Gwathmey JK. Androgen receptors mediate cardiac hypertrophy in cardiac myocytes. Circulation. 1998;98:256–261. doi: 10.1161/01.cir.98.3.256. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin GW, Yaegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. American Journal of Physiology Heart and Circulatory Physiology. 2000;279(4):H1490–H1501. doi: 10.1152/ajpheart.2000.279.4.H1490. [DOI] [PubMed] [Google Scholar]
- 42.Lapier KL, Rodnick K. Effects of aerobic exercise on energy metabolism in the hypertensive heart. Physical Therapy. 2001;81(4):1006–1017. [PubMed] [Google Scholar]
- 43.Foss ML, Keteyian SJ. Sources of energy. In: Foss ML, editor. Physiological Basis of Exercise and Sport Guanabara Koogan, Rio de Janeiro. 2000. pp. 17–45. [Google Scholar]
- 44.White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85:1160–1168. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- 45.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy. Cardiovasc Res. 2012;67(2):187–197. doi: 10.1016/j.cardiores.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 46.Husain K. Physical conditioning modulates rat cardiac VEGF gene expression in nitric oxide deficient hypertension. Biochem Biophys Res Commun. 2004;320:1169–1174. doi: 10.1016/j.bbrc.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 47.Wagatsuma A, Tamaki H, Ogita F. Expression of VEGF and its receptor in the heart tissue following short- term swimming training. Int J of Sport and Health Sci. 2005;(3):91–99. [Google Scholar]
- 48.Shohet R, Maslov K, Lihong V, Jeffrey M, Sohn R. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. PNAS. 2011;108(32):13264–13269. doi: 10.1073/pnas.1101321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45(3):321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 50.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 51.Zaugg M, Jamali NZ, Lucchineti E, Weimin XU, Alam M. Anabolic-Androgenic Steroids Induce Apoptotic Cell Death in Adult Rat Ventricular Myocytes. J Cellular Physiology. 2001;187:90–95. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1057>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 52.Belhani D, Fanton L, Vaillant F, Descotes J, Manati W. Cardiac lesions induced by testosterone: protective effects of dexrazoxane and trimetazidine. Cardiovasc Toxicol. 2009;9:64–69. doi: 10.1007/s12012-009-9041-7. [DOI] [PubMed] [Google Scholar]
- 53.Golestani R, Slart RH, Dullaart RP, Glaudemans AW, Zeebregts CJ, Boersma HH. Adverse cardiovascular effects of anabolic steroids: pathophysiology imaging. Eur J Clin Invest. 2012:1–9. doi: 10.1111/j.1365-2362.2011.02642.x. [DOI] [PubMed] [Google Scholar]
- 54.Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004;(18):1152–1159. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]