Abstract
During the last decades a large number of cucurbitacins have been isolated from various plant species belonging to other plant families than Cucurbitaceae. Although the roots and the fruits of plant belong to these Cucurbitaceae species are very bitter, they have been used as folk medicines in some countries because of their wide spectrum of pharmacological activities such as anti-inflammation and anticancer effects. In the last ten years, cucurbitacins had been shown to inhibit proliferation and induced apoptosis utilizing a long array of in vitro and in vivo cancer cell models. Several molecular targets for cucurbitacins have been discovered, such as fibrous-actin, signal transducer and activator of transcription (STAT), cyclooxygenase-2, etc. This review aimed at elucidating the natural sources of some cucurbitacin compounds, their chemical structure and derivatives, physical properties, biological activities and mechanism by which they reduce the proliferation human cancer cells. This widens our armaments against a devastating disease that we are failing to face.
Keywords: Cucurbitacin, STAT, Janus kinase (JAK), anti-tumor, anti-inflammation, apoptosis
Introduction
Cucurbitacins are a class of highly oxidized tetracyclic triterpenoids. They are widely distributed in the plant kingdom, where they act as heterologous chemical pheromones that protect plants from external biological insults. The magnitude of their broad-spectrum pharmacological bioactivities first attracted attention in the 1960s. (1) Natural and semi-synthetic cucurbitacins show promising anticancer activities ranging from antiproliferation, cell cycle arrest to induction of apoptosis. (2–4) Cancer is responsible for 12% of the world’s mortality. Treatments include surgery, and radio- and/or chemo-therapy. However, chemotherapy suffers limitations of side-effects, toxicity and drug resistance. Also, most established chemotherapy drugs are lacking specificity toward tumor cells. (1, 5) Therefore, there has been a growing interest in the use of herbs as a promising source of more efficient new therapeutic anticancer drugs. In addition, recent trends in the management of cancer development include increasing awareness and chemoprevention that suggest using natural or synthetic chemicals to prevent initiation and promotional events associated with cancer development. Marine and terrestrial plants and animals are the main sources of natural products. They are considered as a fertile ground for finding novel antitumor drugs. Medicinal plants, used in folk medicine worldwide, are studied in ethno-botany and ethno-pharmacology. Up to the present date, more than 40 new cucurbitacins and cucurbitacin-derived compounds have been isolated from the cucurbitaceae family and from other species of the plant Kingdome. The most significant mechanisms with regard to the apoptotic effects of cucurbitacins are their ability to modify mitochondrial trans-membrane potential and transcriptional activities via nuclear factors or genes and their capability to activate or inhibit pro- or anti-apoptotic proteins. In general, cucurbitacins are considered to be selective inhibitors of the JAK/STAT pathways; however, other mechanisms may be implicated in their apoptotic effects, including the MAPK pathway (known to be important for cancer cell proliferation and survival), PARP cleavage, expression of active caspase-3, decreased pSTAT3 and JAK3 levels, as well as decreases in various downstream STAT3 targets such as Mcl-1, Bcl-2, Bcl-xL, and cyclin D3, all of which are implicated in apoptosis and the cell cycle control. (3, 5)
Chemicals structure of cucurbitacins
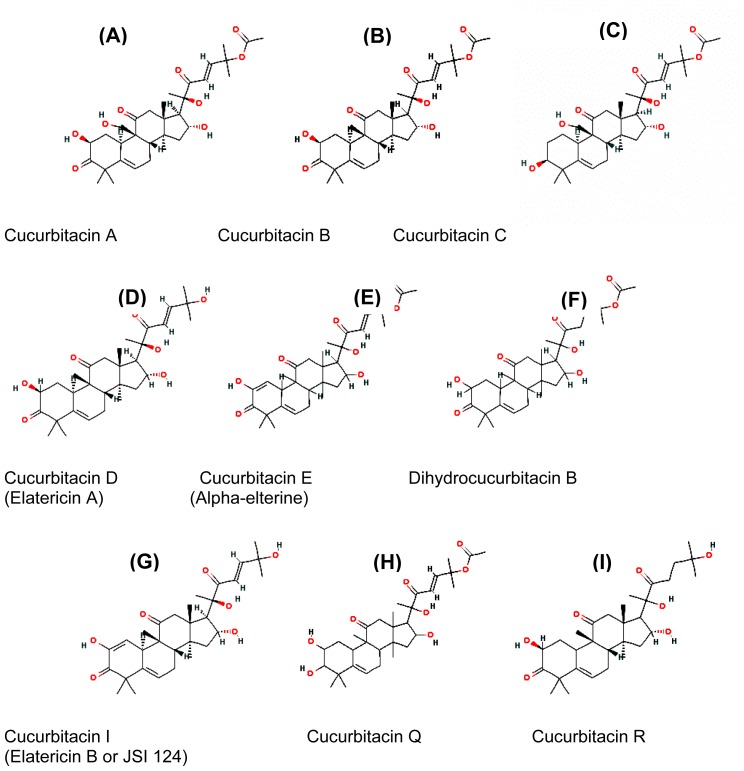
Structurally, cucurbitacins are characterized by the tetracyclic cucurbitane nucleus skeleton (triterpenes). The basic structure of triterpenes is built from six isoprene units. Triterpenes are accordingly C30-compounds. Cucurbitacins are derivatives of the hypothetical triterpene hydrocarbon cucurbitane named 19-(10→9-β)-abeo-5 alpha- lanostane (also known as 9-β-methyl-19-nor-lanosta-5-ene), with a variety of oxygen substitutions at different positions.(6) According to the characteristics of their structures, cucurbitacins are divided into twelve categories. (7) Of these, cucurbitacin E, (8, 9) cucurbitacin B, (10–12) cucurbitacin D and cucurbitacin I (13–16) are the most widely used for in vitro and in vivo tumor inhibition studies. Cucurbitacin glycosides usually have the saccharide linked to carbon atom 2 (2-O-β-glycosides). Cucurbitacins E and B result from the acetylation of cucurbitacins I and D, a feature that increased hydrophobicity and cytotoxicity. (17) Cucurbitacins E and I differ, respectively, from cucurbitacins B and D by the presence of a double bond between C1 and C2 which increases both the hydrophobicity and the cell toxicity; (17) see Figure 1.
Figure (1).
The chemical structure of major cucurbitacins
Physical properties and solubility of cucurbitacins
At room temperature, cucurbitacins are generally crystalline substances. The chemical structure of cucurbitacins reveals their possession of hydrophobic properties, and thus poor water solubility. (18) To date, only a limited number of polymeric micellar systems have shown positive results in tumor targeted delivery of poorly soluble drugs after systemic administration. (19,20) Polyethylene oxide block micelles are nanoscopic carriers (20 – 100 nm in size) with a hydrophilic shell/hydrophobic core structure that have shown great promise in the solubility and controlled delivery of hydrophobic drugs. (19) Poly (ethylene oxide)-block-poly (ε-caprolactone) (PEO-b-PCL) and poly (ethylene oxide)-block-poly (α-benzyl carboxylate ε-caprolactone) (PEO-b-PBCL) micelles (<90 nm) were engineered by a co-solvent evaporation technique as nanocarriers for the delivery of cucurbitacins I and B (figure 1. G, B), which are inhibitors of the signal transducer and activator of transcription 3 (STAT3). It was determined that the anti-cancer and STAT3 inhibitory activity of the polymeric micellar cucurbitacins were comparable to the free drugs on a B16-F10 melanoma cell line in vitro. Intra-tumoral injection of 1 mg/kg/day cucurbitacin I (figure 1.G), resulted in the regression of established B16-F10 mouse melanoma tumors in vivo. In comparison to free cucurbitacin I (figure 1.G), PEO-b-PBCL micellar cucurbitacin I was found to provide comparable anti-cancer effects against B16-F10 tumors and limit drug levels in animal serum while maintaining high drug levels in tumor following intra-tumoral administration. The results indicate the potential of polymeric micelles as suitable vehicles for the delivery of cucurbitacins I and B (figure 1. G, B). Interestingly, the toxicity associated with cucurbitacins I and B (figure 1. G, B) was significantly reduced when these drugs were loaded into the nanoparticles. (21)
Bioactivity of cucurbitacins
Most of cucurbitacins have a potent biological activities depending on the target cells such as cytotoxic, anti-tumor properties, hepatoprotective, anti-inflammation, antimicrobial, anthelmintic, cardiovascular and anti-diabetic effects. Indeed, these activities were investigated for the most widely used cucurbitacins in vivo and in vitro studies. (22–24) For instance; the antioxidant capacities and free-radical scavenging activities of cucurbitacin B/E glucosides have been demonstrated (figure 1.B). These results show the promising potential of cucurbitacin glucosides in preventing human diseases involving free radical and oxidative damage.(25)
Also, some of the cucurbitacins possess anti-inflammation or analgesic effects. Considering their anti-inflammation activities, it has been demonstrated that they involve the inhibition of the expression of tumor necrosis factor alpha (TNFα) in lymphocytes and in macrophages, (13) and interference with the activity of nuclear factor-kappa-B (NF-κB). (26–28) Also, cucurbitacins are able to inhibit the activity of cyclooxygenases 2 (COX2)(2) and inhibit the production of pro-inflammation mediators through inducible nitric oxide synthase (iNOS). (26, 28) Cucurbitacin R (figure 1.I) anti-inflammation activity was proven on several experimental models of pain and inflammation. (29) In addition, clinical trials with two compounds hemslecins A (25-acetoxy-23, 24-dihydrocucurbitacin F) and B (23, 24-dihydrocucurbitacin F), isolated initially from the genus Hemsleya, showed their efficacy against infectious diseases, such as enteritis, bronchitis, acute tonsillitis, and bacillary dysentery.
Triterpenes have been reported to induce cell death. Several different cucurbitacin compounds have been found to exhibit antiproliferative on numerous human cancer cell lines and tumor xenografts, including breast, prostate, lung, uterine cervix, liver, skin, and brain cancers (see Table 1). (3,17,25,30,31) Moreover, the effectiveness of cucurbitacins B, D, E, and I, (figure 1 .B, D, E. G) has so far been shown in colon (HCT-116), breast (MCF-7), lung (NCI-H460) and brain (SF-268) cancer cell lines, where cucurbitacin B demonstrated more than 80% proliferation inhibitory effect. (30) Likewise, cucurbitacins A, B, E, I and Q (figure 1, A, B, E, G) were antiproliferative on lung cancer cells (A549). Cucurbitacin I (figure 1G) caused reduction of growth in breast and prostate carcinoma cell lines (MDA-MB-231, MDA-MB-468, Panc-1), in vitro, as well as in nude mice xenograft models. (31, 32) Growth inhibition was accompanied by cell cycle arrest and apoptosis in breast cancer cell lines (MCF-7 and MDA-MB-231) treated with cucurbitacins B and E (figure 1. B, E). (1, 33) Also, cucurbitacin glucosides (B and E) (figure 1 .B, E) isolated from Citrullus colocynthis have antiproliferative effect on human breast cancer cells, through accumulation of cells in the growth phase II/mitotic phase (G2/M phases) of the cell cycle accompanied with induction of apoptosis. They also modulated the expression of proteins involved in cell-cycle regulation in both of the estrogen-dependent (MCF-7) and estrogen-independent (MDA-MB-231) human breast cancer cell lines.
Table (1).
Cucurbitacin compounds from different plant species and their bioactivity on cancer cells.
| Cucurbitacin | Plant Source | Effectiveness on cancer cell lines | Reference |
|---|---|---|---|
| Cucurbitacin A | Trichosanthes cucumerina. (Snake gourd) | Lung: A549 cell lines | 30 |
| Cucurbitacin B |
Trichosanthes cucumerina. (Snake gourd) Cucurbita andreana (Buttercup squash). Wilbrandia ebracteata. (no common name) Luffa operculata. (Sponge Cucumber) |
Leukemia and lymphoma: HL60, U937, THP1, NB4, K562, BALL1, Reh, RCH, LY4, Daudi, MD901, SP49, Jeko1 and NCEB1. Hepatocellular: Hep-2. Breast: SKBR2, MCF-7, T47D and MDA-MB435. Lung: A549, SK LU1 and NCI-H460. Colon: COCA-2 and HCT-116. Brian: SF-268. Pancreatic cancer cell lines. |
2, 17, 30, 38 |
| Cucurbitacinglucosides | Citrullus colocynthis (Bitter cucumber) | Breast: ER+MCF-7 and ER−MDA-MB231. | 25 |
| Cucurbitacin E &itsglucoside (Elaterin) |
Bacopa monnieri (Water hyssop) Cucurbita andreana (Winter Squash) Citrullus colocynthis. (Bitter cucumber) |
Ovarian sarcoma: M5076. Colon: HCT-116. Breast: MCF-7 and ZR-75-1. Lung: NCI-H460. Brian: SF-268. Prostate: PC-3. Hepatocelluar: HepG2 |
30, 32, 37, 42 |
| Cucurbitacin D (Elatericin A) |
Trichosanthes kirilowii (Chinese Cucumber) Cucurbita andreana (Winter Squash) |
Hepatocellular: Hep-2. Leukemia and lymphoma: HL60, U937, THP, BALL1, Reh, RCH, LY4, Daudi, MD901, SP49, Jeko1 and NCEB1. Breast: MCF-7. Colon: HCT-116. Lung: NCI-H460. Brain: SF-268. |
30, 32 |
| Dihydrocucurbitacin B |
Wilbraandia ebracteata (no common name) Trichosanthes kirilowii (Chinese Cucumber) Cayaponia tayuya (Tayuya) |
Leukemia. Hepatocellular Hep-2. Breast: Bcap37 Hela, SW620, SMMC-7721, K562 and MCF-7. Colon: HCT116 and Hke3. |
13 |
| Cucurbitacin I &itsglucoside (Elatericin B) (JSI 124) |
Momordica balsamina L (Balsam pear). Cayaponia tayuya. (Tayuya) Cucurbita andreana. (Winter Squash) Citrullus colocynthis. (Bitter cucumber) |
Colon: HCT-116. Breast: MCF-7, MDA-MB-231, MDA-MB-468, and Panc-1. Lung: NCI-H460. Brain: SF-268. Prostate. Gliboblastomamultiforme: U251 and A172. Hepatocellular: Hep-G2 |
30, 31, 32, 37 |
| Cucurbitacin Q | Cayaponia tayuya. (Tayuya) | Lung: A549. Human and murine cancers: A549, MDA-MB-435, and v-Src/NIH 3T3. |
32 |
| Cucurbitacin R | Cayaponia tayuya. (Tayuya) | Colon: HCT116 and Hke-3. | 13 |
Cucurbitacin Q (figure 1.H) induces apoptosis more potently in human and murine tumors. Furthermore, in HeLa cells, cucurbitacins inhibited DNA, RNA, and protein synthesis. (34) Another two cucurbitacins compounds, isolated from the roots of Cayaponia tayuya and identified as 23, 24-dihydrocucurbitacin B and cucurbitacin R, inhibit proliferation and/or induce apoptosis in colon cancer cell lines. (13, 23) Although cucurbitacin E (Figure 1.E) was capable of inducing and maintaining high proliferation rates in lymphocytes, (35) it inhibited the proliferation of prostate cancer cells and caused disruption of the cytoskeleton structure of actin and vimentin.(8,9) Moreover, cucurbitacins also inhibited proliferation of normal mitogen-induced T-lymphocytes (36) and endothelial cells accompanied by a disruption of the F-actin and tubulin microfilaments cytoskeleton and reduced cell motility. (9) The latter effects suggest an anti-angiogenesis and anti-metastasis role for cucurbitacins. Both of cucurbitacin E glucoside and cucurbitacin I glucoside – isolated from Citrullus colocynthis growing in Saudi Arabia - had potent in vitro cytotoxic activity against Hepatoma HepG2 cell line and prolonged the survival time, life span and normalizes the biochemical parameters of mice-bearing tumor of Ehrlich’s ascites carcinoma. (37) Growth inhibition and cytotoxic effect of cucurbitacin B (figure 1.B) on breast cancer cell lines SKBR-3 and MCF-7 were attributed to G2/M phase arrest and apoptosis. Cyclin D1, c-Myc, and β-catenin expression levels were reduced. Western blot analysis showed increased PARP cleavage suggesting induced caspase activity and decreased mitogenic Wnt-associated signaling molecules β-catenin, galectin-3, cyclin D1 and c-Myc, and corresponding changes in phosphorylated GSK-3β levels. Cucurbitacin B treatment inhibited translocation to the nucleus of β-catenin and galectin-3. T-cell factor (TCF)/lymphoid enhancer factor (LEF)-dependent transcriptional activity was disrupted in cucurbitacin B treated cells as tested by a TCF reporter luciferase activity assay. (38)
On the other hand, combination of cucurbitacin with standard anticancer drugs produced synergistic effects. The combination of cucurbitacin E with doxorubicin resulted in effective cytotoxicity for tumor cells in culture and in vivo, and in decreased tumor size and tumor weight. (39) Moreover, in comparison with single agent treatment, the combination of cucurbitacin B with docetaxel on Hep-2, a human laryngeal cancer cell line produced a greater efficacy in growth inhibition, cell cycle arrest at G2/M phases, and apoptosis induction in vitro, and synergistically inhibition of tumor growth in vivo. (10) A total of six cucurbitacins promoted TRAIL-induced apoptosis (B, I, E, C, D, and K), whereas P was inactive. They sensitized renal adenocarcinoma cells to anticancer effects of TRAIL. The synergistic effect was apparent after short exposure and did not require continued presence of cucurbitacin. Active cucurbitacins induced the proapoptotic caspase-8 activation only after subsequent TRAIL addition. Cucurbitacin-sensitized TRAIL-induced cytotoxicity was inhibited by N-acetyl cysteine suggesting a prooxidant mechanism. However, their TRAIL-sensitizing activity is STAT3-independent. (40) Cucurbitacin D inhibited proliferation and induce apoptosis of T-cell leukemia cells correlating NF-κB inhibition and down-regulation of the expression of antiapoptotic proteins Bcl-xL and Bcl-2. Furthermore, cucurbitacin D induced the accumulation of inhibitor of NF-κB (IκB) by inhibition of proteasome activity. Low doses of cucurbitacin D synergistically potentiated the antiproliferative effects of the histone deacetylase inhibitor VPA. Finally, the proapoptotic and proteasome inhibitory activities of cucurbitacin D also were demonstrated using SCID mice in an in vivo study. (41)
Cucurbitacins, STATs and tumorigenesis
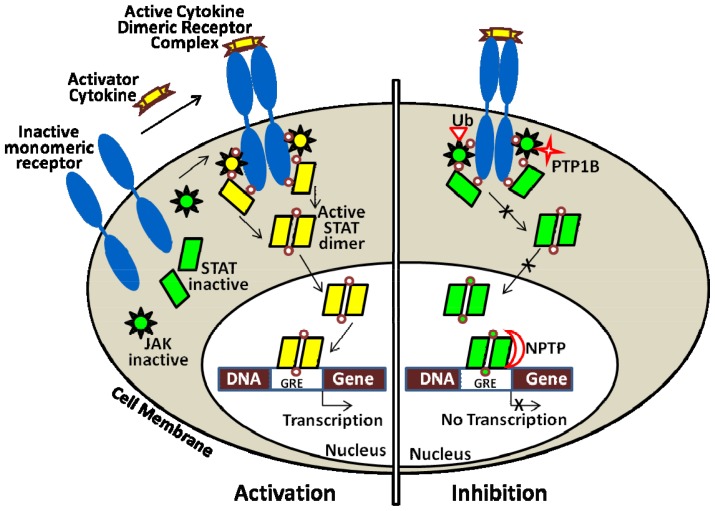
Signal transducers and activators of transcription (STATs) are a family of seven proteins including STAT 1, 2, 3, 4, 5a, 5b, and 6. STAT3 protein is ubiquitously expressed in most tissues. (43) Each has its unique function to transduce extracellular signals and directly modulate transcription. STAT proteins play important role in regulation of immune response, inflammation, proliferation, differentiation, development, cell survival, and apoptosis. (44–47) STATs are initially present in inactive forms in the cytoplasm. Upon stimulation by a wide variety of receptor-mediated growth factors such as platelet-derived growth factor (PDGF) or epidermal growth factor (EGF) (45, 48) and cytokines such as interleukin-6 (IL-6) or interferon (49) signaling, STATs associate the cell membrane receptors to be activated via phosphorylation at conserved tyrosine 705 residues either directly by receptor tyrosine kinases, or indirectly by non-receptor tyrosine kinases, e.g., Janus kinases (JAKs) and Src oncogenic kinase. The JAKs then phosphorylation of tyrosine 705 induces STAT dimerization, nuclear translocation, and DNA binding at STAT-specific sequence in the promoter regions of their target genes to stimulate their transcription. (50, 51) Additionally, serine at the 727th residue in the same domain must also be phosphorylated for complete transcriptional activity. (51) In normal physiological conditions, the activation duration of STAT protein especially STAT3 is temporary and strictly controlled. Figure (2) illustrates the JAK/STAT pathway for activation and inhibition.
Figure (2).
Mechanism of activation and inhibition of Janus kinases and signal transducer and activator transcription (JAK/STAT) pathway. Upon activator cytokine binding to its receptor on cell surface (e.g., IL-6 receptor), JAK/STATpathway is activated (left) leading to sequential cell response. Inhibition of signaling process (right) is induced by a particularly inhibitory cytokine, JAK degradation through ubiquitin-proteasome system (Ub), dephosphorylation by cytoplasmic PTP1B or nuclear phosphatase (NPTP), or by inhibition the dimerization of STAT (adapted with modification from Escandell et al.,2008).
STAT3 regulates the expression of genes that mediate proliferation (e.g., c-myc and cyclin D1), (52, 53) suppress proapoptotic genes (e.g., Bcl-xL, Bcl -2 and survivin), (54) and/or promote angiogenesis through vascular endothelial growth factor (VEGF). (55, 56) Conversely, cytokines can inhibit STAT3 signaling. Cytokine inducible genes constituting the suppressors of cytokine signaling (SOCS) protein family can bind to and inhibit JAKs, thus repressing STAT3 activation. (57) Recently, it is commonly accepted that STAT3 can also be activated by many other cytokines, such as IL-7, IL-10, IL-20, leptin, granulocyte colony-stimulating factor, and epidermal growth factor. Of the seven human STAT genes, STAT3, a common oncogenic signaling pathway, is constitutively activated in many types of cancers, (58, 59) including 82% of prostate cancers, (60) 70% of breast cancers, (61) more than 82% of the carcinomas of the head and neck, (62) 71% of nasopharyngeal carcinoma, (63) over 50% lung cancers (64) and 50% of HCC, leukemias, lymphomas, and multiple myelomas. Unregulated activation of STAT3 was demonstrated in a variety of tumor types, including breast carcinoma, prostate cancer, melanoma, multiple myeloma, and leukemia among others. (54, 60, 65–68) Various genetic mutations can lead to constitutive activation of STAT3, e.g., over-expression and constitutive activation of epidermal growth factor receptor (EGFR). (69, 70) STAT3 can contribute to tumor growth by initiating the cell cycle, preventing apoptosis, and up-regulating oncogenes such as c-Myc and Bcl-X. (71) Furthermore, STAT3 has recently been demonstrated to augment prostate cancer metastasis by promoting prostate cancer cell migration. (72)
Accumulating evidence shows that blocking aberrant activation of STAT3 in tumor results in the inhibition of cancer cell growth, induction of apoptosis and enhancement of anti-cancer immune responses. (59, 73–76) Cucurbitacins are recognized as anti-tumour agents involving - among other mechanisms - interference with STAT3 signaling, and they also affect the integrity of the actin cytoskeleton. For example, cucurbitacin E inhibits the proliferation of prostate cancer cells and causes disruption of the cytoskeleton structure of actin and vimentin. (9) However, the more potent cucurbitacins A, B, E, I and Q inhibit the phosphorylation of STAT3 and/or JAK2 and thereby preventing STAT3 DNA binding and STAT3-mediated gene transcription in lung cancer A549 cell line. (31,32) Likewise, cucurbitacin I caused reduction of phospho-STAT3 in breast, prostate and pancreatic carcinoma cell lines (MDA-MB-231, MDA-MB-468 and Panc-1). (31,32) Surprisingly, cucurbitacins B and E have been shown to induce phosphorylation of STAT3 in breast cancer cell lines (MDA-MB-231 and MCF-7) while still exhibiting growth inhibition. (25) Cucurbitacins I, Q and B inhibited phosphorylation of STAT3 and induce apoptosis in v-Src-transformed NIH3T3 cells, but had limited biological activity in cells with no activated STAT3. (31) This study led to the conclusion that such cucurbitacins exert anti-tumorigenic activity selectively in cells with activated STAT3.
In structure-activity relationship studies with five cucurbitacins; A, B, E, I, and Q, showed that Q inhibited the activation of STAT3 but not JAK2; A inhibited JAK2 but not STAT3 activation; and B, E, and I, inhibited the activation of both. Furthermore, these studies demonstrated that conversion of the C3 carbonyl of the cucurbitacins to a hydroxyl results in loss of anti-JAK2 activity, whereas addition of a hydroxyl group to C11 of the cucurbitacins results in loss of anti-STAT3 activity. Cucurbitacin Q selectively inhibited the activation of STAT3 and induced apoptosis without inhibition of JAK2, Src, Akt, Erk, or JNK activation. Furthermore, it induced apoptosis more potently in human and murine tumors that contain constitutively activated STAT3 (e.g., A549, MDA-MB-435 and v-Src/NIH 3T3) as compared to those that do not (e.g., H-Ras/NIH 3T3, MDA-MB-453 and NIH 3T3 cells). Finally, in a nude mouse tumor xenograft model, cucurbitacin Q but not A, suppressed tumor growth. This indicated that JAK2 inhibition is not sufficient to inhibit tumor growth and suggesting that the ability of cucurbitacin Q to inhibit tumor growth is related to its anti-STAT3 activity. These studies further validate STAT3 as a drug discovery target and provide evidence that pharmacological agents - such as cucurbitacin Q - that can selectively reduce the P-STAT3 levels in human cancer cells result in tumor apoptosis and growth inhibition. (31) Although activation of STAT3 has been demonstrated in primary colon tumors, the majority of established colon cancer cell lines lack constitutively activated STAT3. (77, 78) In contrast, K-Ras mutations are found in 30 - 50% of primary colorectal cancers as well as in established colon cancer cell lines. (79) Thus, the presence of oncogenic K-Ras significantly decreased the sensitivity of cells to dihydrocucurbitacin B, cucurbitacin R and cucurbitacin I (13) possibly through K-Ras antagonism with STAT3 activation. Moreover, p53 and p21 protect cells from apoptosis induced by cucurbitacins. Other study confirmed that sensitivity of human colon cancer cell lines to these three cucurbitacins depends on the extent of oncogenic K-Ras and p53/p21 status, and established that cucurbitacins can exert antitumorigenic activity in the absence of activated STAT3.
Conclusion
Cucurbitacin structures are characterized by the tetracyclic cucurbitane nucleus (triterpenes) with a variety of oxygen substitutions at different positions. Because of the possession of hydrophobic properties and poorly soluble water, polymeric micellar systems exhibited improved antitumor efficacy because of a better solubilization and targeting after local and/or systemic administration. Different cucurbitacin compounds exhibit antitumor proliferation inhibition and induce apoptosis alone or synergistically with other proven anticancer chemicals and cytokines - using numerous human cancer cell lines and tumor xenografts of leukemia, lymphoma, breast, prostate, lung, uterine cervix, liver, skin, colon, laryngeal, brain and pancreatic cancers. In a structure-function related manner, cucurbitacins’ inhibition of phosphorylation of STAT3 and/or JAK2 and their subsequent activation seamed as the major mechanism of their action. Cucurbitacins deserve future investigations targeting their discovery in uninvestigated sources and their derivatives for improving their anticancer abilities. Moreover, preclinical and clinical studies using combined treatment composed of cucurbitacins and standard chemo-, immuno- and/or radio-therapies should be planned for.
References
- 1.Chen X, Bao J, Guo J, Ding Q, Lu J, Huang M, Wang Y. Biological activities and potential molecular targets of cucurbitacins: a focus on cancer. Anticancer Drugs. 2012;23:777–87. doi: 10.1097/CAD.0b013e3283541384. [DOI] [PubMed] [Google Scholar]
- 2.Lang KL, Silva IT, Zimmermann LA, Machado VR, Teixeira MR, Lapuh MI, Galetti MA, Palermo JA, Cabrera GM, Bernardes LS, Simões CM, Schenkel EP, Caro MS, Durán FJ. Synthesis and cytotoxic activity evaluation of dihydrocucurbitacin B and cucurbitacin B derivatives. Bioorg Med Chem. 2012;20:3016–30. doi: 10.1016/j.bmc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Ríos JL, Andújar I, Escandell JM, Giner RM, Recio MC. Cucurbitacins as inducers of cell death and a rich source of potential anticancer compounds. Curr Pharm Des. 2012;18:1663–76. doi: 10.2174/138161212799958549. [DOI] [PubMed] [Google Scholar]
- 4.Pan L, Yong Y, Deng Y, Lantvit DD, Ninh TN, Chai H, Carcache de Blanco EJ, Soejarto DD, Swanson SM, Kinghorn AD. Isolation, structure elucidation, and biological evaluation of 16, 23-epoxycucurbitacin constituents from Eleaocarpus chinensis. J Nat Prod. 2012;75:444–52. doi: 10.1021/np200879p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeVita VT, Jr, Chu E, DeVita VT, Jr, Lawrence TS, Rosenberg SA, DePinho RA, Weinberg RA. DeVita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology. 9th edition. Lippincott Williams & Wilkins; NY: 2011. Part III: Principles of cancer treatment: Medical Oncology. [Google Scholar]
- 6.Teuscher E, Lindequist U. BiogeneGifte – Bio-logie, Chemie, Pharmakologie. Vol. 2. Auflage Gustav Fischer Verlag; Stuttgart, Jena, New York: 1994. Triterpene; pp. 159–75. [Google Scholar]
- 7.Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep. 2005;22:386–99. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 8.Duncan KL, Duncan MD, Alley MC, Sausville EA. Cucurbitacin E-induced disruption of the actin and vimentin cytoskeleton in prostate carcinoma cells. Biochem Pharmacol. 1996;52:1553–60. doi: 10.1016/s0006-2952(96)00557-6. [DOI] [PubMed] [Google Scholar]
- 9.Duncan MD, Duncan KL. Cucurbitacin E targets proliferating endothelia. J Surg Res. 1997;69:55–60. doi: 10.1006/jsre.1997.5028. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Zhang M, Zhang H, Sun C, Yang X, Deng JW. Combined antitumor activity of cucurbitacin B and docetaxel in laryngeal cancer. Eur J Pharm. 2008;587:78–84. doi: 10.1016/j.ejphar.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Haritunians T, Gueller S, Zhang, Badr R, Yin D, Xing H, Fung MC, Koeffler HP. Cucurbitacin B induces differentiation cell cycle arrest and actin cytoskeletal alterations in myeloid leukemia cells. Leukemia Res. 2008;32:1366–73. doi: 10.1016/j.leukres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Wakimoto N, Yin D, O’Kelly J, Haritunians T, Karlan B, Said J, Xing H, Koeffler HP. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008;99:1793–7. doi: 10.1111/j.1349-7006.2008.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Escandell JM, Kaler P, Recio MC, Sasazuki T, Shirasawa S, Augenlicht L, Ríos J-L, Klampfer L. Activated K-Ras protects colon cancer cells from cucurbitacin induced apoptosis; the role of p53 and p21. Biochem Pharmacol. 2008;76:198–207. doi: 10.1016/j.bcp.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Kester MS, Luiting JJ, von dem Borne PA, Willemze R, Tensen CP, Vermeer MH. Cucurbitacin I inhibits STAT3 and induces apoptosis in Sézary cells. J Invest Dermatol. 2008;128:1691–5. doi: 10.1038/sj.jid.5701246. [DOI] [PubMed] [Google Scholar]
- 15.Shi X, Franko B, Frantz C, Amin HM, Lai R. JSI-124 (cucurbitacin I) inhibits Janus kinase-3/signal transducer and activator of transcription-3 signaling, downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and induces apoptosis in ALK-positive anaplastic large cell lymphoma cells. Brit J Haematol. 2006;135:26–32. doi: 10.1111/j.1365-2141.2006.06259.x. [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Li G, Zhang X, Gu J, Zhang C, Tian Z, Zhang J. JSI-124 inhibits Glioblastoma multiforme cell proliferation through G(2)/M cell cycle arrest and apoptosis augment. Cancer Biol Ther. 2008;7:1243–1249. doi: 10.4161/cbt.7.8.6263. [DOI] [PubMed] [Google Scholar]
- 17.Bartalis J, Halaweish FT. Relationship between cucurbitacins reversed-phase high performance liquid chromatography hydrophobicity index and basal cytotoxicity on HepG2 cells. J. Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:159–66. doi: 10.1016/j.jchromb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Gry J, Søborg I, Andersson HC. Cucurbitacins in plant food. Vol. 556. Tema Nord Nordic Council of Ministers; Ekspressen Tryk & Kopicenter; Copenhagen: 2006. Identity physical and chemical properties and analytical methods; pp. 17–22. [Google Scholar]
- 19.Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv. 2006;3:139–62. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 20.Kwon GK, Forrest ML. Amphiphilic block copolymer micelles for nanoscale drug delivery. Drug Dev Res. 2006;67:15–22. [Google Scholar]
- 21.Molavi O, Ma Z, Mahmud A, Alshamsan A, Samuel J, Lai R, Kwon GS, Lavasanifar A. Polymeric micelles for the solubilization and delivery of STAT3 inhibitor cucurbitacins in solid tumors. Int J Pharmaceutics. 2008;347:118–27. doi: 10.1016/j.ijpharm.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clericuzio M, Mella M, Vita-Finzi P, Zema M, Vidari G. Cucurbitane triterpenoids from Leucopaxillus gentianeus. J Nat Prod. 2004;67:1823–8. doi: 10.1021/np049883o. [DOI] [PubMed] [Google Scholar]
- 23.Recio MC, Prieto M, Bonucelli, Orsi C, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Anti-inflammation activity of two cucurbitacins isolated from Cayaponia tayuya roots. Planta Med. 2004;70:414–20. doi: 10.1055/s-2004-818968. [DOI] [PubMed] [Google Scholar]
- 24.Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas JM, Gil-Benso R, Ríos JL. Dihydrocucurbitacin B inhibits delayed-type hypersensitivity reactions by suppressing lymphocyte proliferation. J Pharmacol Exp Ther. 2007;322:1261–8. doi: 10.1124/jpet.107.122671. [DOI] [PubMed] [Google Scholar]
- 25.Tannin-Spitz T, Bergman M, Grossman S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Bioch Biophys Res Commun. 2007;364:181–6. doi: 10.1016/j.bbrc.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 26.Park CS, Lim H, Han KJ, Baek SH, Sohn HO, Lee DW. Inhibition of nitric oxide generation by 23, 24-dihydrocucurbitacin D in mouse peritoneal macrophages. J Pharmacol Exp Ther. 2004;309:705–10. doi: 10.1124/jpet.103.063693. [DOI] [PubMed] [Google Scholar]
- 27.Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Dihydrocucurbitacin B, isolated from Cayaponia tayuya, reduces damage in adjuvant-induced arthritis. Eur J Pharmacol. 2006;532:145–154. doi: 10.1016/j.ejphar.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 28.Escandell JM, Recio MC, Manez S, Giner RM, Cerda-Nicolas M, Rios JL. Cucurbitacin R reduces the inflammation and bone damage associated with adjuvant arthritis in Lewis rats by suppression of tumor necrosis factor-alpha in T lymphocytes and macrophages. J Pharmacol Exp Ther. 2007;320:581–90. doi: 10.1124/jpet.106.107003. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira JM, Jr, Peters RR, Gazola AC, Krepsky PB, Farias MR, Rae GA, et al. Anti-inflammation effects of a triterpenoid isolated from Wilbrandia ebracteata Cogn. Life Sci. 2007;80:1382–7. doi: 10.1016/j.lfs.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Jayaprakasam B, Seeram NP, Nair MG. Anticancer and antiinflammation activities of cucurbitacins from Cucurbita andreana. Cancer Letters. 2003;189:11–6. doi: 10.1016/s0304-3835(02)00497-4. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–45. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 32.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- 33.Tehila TS, Shlomo G, Sara D, Hugo EG, Margalit B. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73:56–67. doi: 10.1016/j.bcp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Witkowski A, Woynarowska B, Konopa J. Inhibition of the biosynthesis of deoxyribonucleic acid, ribonucleic acid and protein in HeLa S3 cells by cucurbitacins, glucocorticoid-like cytotoxic triterpenes. Biochem Pharmacol. 1984;33:995–1004. doi: 10.1016/0006-2952(84)90506-9. [DOI] [PubMed] [Google Scholar]
- 35.Attard E, Cuschieri A, Scicluna-Spiteri A, Brincat MP. The effects of cucurbitacin E on two lymphocyte models. Pharm Biol. 2004;42:170–5. [Google Scholar]
- 36.Smit HF, van den Berg AJ, Kroes BH, Beukelman CJ, Quarles van Ufford HC, van DH, Labadie RP. Inhibition of T-lymphocyte proliferation by cucurbitacins from Picrorhizas crophulariae flora. J Nat Prod. 2000;63:1300–2. doi: 10.1021/np990215q. [DOI] [PubMed] [Google Scholar]
- 37.Ayyad SE, Abdel-Lateff A, Alarif WM, Patacchioli FR, Badria FA, Ezmirly ST. In vitro and in vivo study of cucurbitacins-type triterpene glucoside from Citrullus colocynthis growing in Saudi Arabia against hepatocellular carcinoma. Environ Toxicol Pharmacol. 2012;33:245–51. doi: 10.1016/j.etap.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 38.Dakeng S, Duangmano S, Jiratchariyakul W, U-Pratya Y, Bögler O, Patmasiriwat P. Inhibition of Wnt signaling by cucurbitacin B in breast cancer cells: reduction of Wnt-associated proteins and reduced translocation of galectin-3-mediated β-catenin to the nucleus. J Cell Biochem. 2012;113:49–60. doi: 10.1002/jcb.23326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadzuka Y, Hatakeyama H, Daimon T, Sonobe T. Screening of biochemical modulator by tumor cell permeability of doxorubicin. Int J Pharm. 2008;354:63–9. doi: 10.1016/j.ijpharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Henrich CJ, Thomas CL, Brooks AD, Booth NL, Lowery EM, Pompei RJ, McMahon JB, Sayers TJ. Effects of cucurbitacins on cell morphology are associated with sensitization of renal carcinoma cells to TRAIL-induced apoptosis. Apoptosis. 2012;17:79–89. doi: 10.1007/s10495-011-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding N, Yamashita U, Matsuoka H, Sugiura T, Tsukada J, Noguchi J, Yoshida Y. Apoptosis induction through proteasome inhibitory activity of cucurbitacin D in human T-cell leukemia. Cancer. 2011;117:2735–46. doi: 10.1002/cncr.25711. [DOI] [PubMed] [Google Scholar]
- 42.Attard E, Brincat MP, Cuschieri A. Immunomodulatory activity of cucurbitacin E isolated from Ecballium elaterium. Fitoterapia. 2005;76:439– 41. doi: 10.1016/j.fitote.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Deng J, Grande F, Neamati N. Small Molecule Inhibitors of STAT3 Signaling Pathway. Current Cancer Drug Targets. 2007;7:91–107. doi: 10.2174/156800907780006922. [DOI] [PubMed] [Google Scholar]
- 44.Ihle JN, Kerr IM. JAKs and STATs in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 45.Schindler C, Darnell JE., Jr Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 46.Horvath CM, Darnell JE. The state of the STATs: recent developments in the study of signal transduction to the nucleus. Curr Opin Cell Biol. 1997;9:233–9. doi: 10.1016/s0955-0674(97)80067-1. [DOI] [PubMed] [Google Scholar]
- 47.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 48.Leaman DW, Pisharody S, Flickinger TW, Commane MA, Schlessinger J, Kerr IM. Roles of JAKs in activation of STATs and stimulation of c-fos gene expression by epidermal growth factor. Mol Cell Biol. 1996;16:369–75. doi: 10.1128/mcb.16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–52. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 50.Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–53. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 51.Heim MH, Kerr IM, Stark GR, Darnell JE., Jr Contribution of STAT SH2 groups to specific interferon signaling by the JAK-STAT pathway. Science. 1995;267:1347–9. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- 52.Sinibaldi D, Wharton W, Turkson J, Bowman T, Pledger WJ, Jove R. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene. 2000;19:5419–27. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- 53.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–42. doi: 10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 54.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernandez-Luna JL, Nunez G, Dalton WS, Jove R. Constitutive activation of STAT3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 55.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–08. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 56.Aggarwa BL, Sethi G, Ahn KS, Sandur S, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting Signal-Transducer-and-Activator-of-Transcription-3 for Prevention and Therapy of Cancer. Ann NY Acad Sci. 2006;1091:151–69. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 57.Starr R, Hilton DJ. Negative regulation of the JAK/STAT pathway. Bioassays. 1999;21:47–52. doi: 10.1002/(SICI)1521-1878(199901)21:1<47::AID-BIES6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Bromberg J. STAT proteins and oncogenesis. J Clin Invest. 2002;109:39–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu H, Jove R. The STATs of cancer: new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 60.Mora LB, Buettner R, Seigne J, Diaz J, Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, Muro-Cacho C, Livingston S, Karras J, Pow-Sang J, Jove R. Constitutive activation of STAT3 in human prostate tumors and cell lines: direct inhibition of STAT3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–66. [PubMed] [Google Scholar]
- 61.Dolled-Filhart M, Camp RL, Kowalski DP, Smith BL, Rimm DL. Tissue microarray analysis of signal transducers and activators of transcription 3 (STAT3) and phospho-STAT3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin Cancer Res. 2003;9:594–600. [PubMed] [Google Scholar]
- 62.Nagpal JK, Mishra R, Das BR. Activation of STAT-3 as one of the early events in tobacco chewing-mediated oral carcinogenesis. Cancer. 2002;94:2393–400. doi: 10.1002/cncr.10499. [DOI] [PubMed] [Google Scholar]
- 63.Hsiao JR, Jin YT, Tsai ST, Shiau AL, Wu CL, Su WC. Constitutive activation of STAT3 and STAT5 is present in the majority of nasopharyngeal carcinoma and correlates with better prognosis. Br J Cancer. 2003;89:344–9. doi: 10.1038/sj.bjc.6601003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of STAT3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2004;22:4150–65. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 65.Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–54. [PubMed] [Google Scholar]
- 66.Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R. Constitutive activation of STAT3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267–76. [PubMed] [Google Scholar]
- 67.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of STAT3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 68.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, Daud A, Chang A, Kraker A, Jove R, Yu H. Roles of activated Src and STAT3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–10. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 69.Fernandes A, Hamburger AW, Gerwin BI. ErbB-2 kinase is required for constitutive stat 3 activation in malignant human lung epithelial cells. Int. J. Cancer. 1999;83:564–70. doi: 10.1002/(sici)1097-0215(19991112)83:4<564::aid-ijc20>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 70.Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol. 2001;19:1155–60. doi: 10.3892/ijo.19.6.1155. [DOI] [PubMed] [Google Scholar]
- 71.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, Russo A. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–61. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 72.Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Nevalainen T, Zellweger K, Alanen T, Mirtti T, Visakorpi L, Bubendorf MT. STAT3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–28. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burdelya L, Kujawski M, Niu G, Zhong B, Wang T, Zhang S, Kortylewski M, Shain K, Kay H, Djeu J, Dalton W, Pardoll D, Wei S, Yu H. STAT3 activity in melanoma cells affects migration of immune effector cells and nitric oxide-mediated antitumor effects. J Immunol. 2005;174:3925–31. doi: 10.4049/jimmunol.174.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Darnell JE. Validating STAT 3 in cancer therapy. Nat Med. 2005;11:595–6. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 75.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–27. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 76.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 77.Klampfer L. The role of signal transducers and activators of transcription in colon cancer. Front Biosci. 2008;13:2888–99. doi: 10.2741/2893. [DOI] [PubMed] [Google Scholar]
- 78.Bowman Garcia R, Turkson J, Jove R. STATs in oncogenesis, Oncogene. 2000;19:2474–88. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 79.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]