Abstract
Objective
Mesenchymal stem cells (MSCs) are promising candidate cells for cartilage tissue engineering. Expression of cartilage hypertrophy markers, e.g., collagen type X, by MSCs undergoing chondrogenesis raises concern for tissue engineering application for MSCs, because hypertrophy would result in apoptosis and ossification. To analyze the biological basis of MSC hypertrophy, we examine the response of chondrifying MSCs to culture conditions known to influence chondrocyte hypertrophy, using an array of hypertrophy-associated markers.
Methods
Human MSC pellet cultures are pre-differentiated for two weeks in a chondrogenic medium, and hypertrophy induced with TGF-β withdrawal, dexamethasone reduction, and addition of thyroid hormone (T3). Cultures are characterized by histological, immunohistochemical, and biochemical methods, and for gene expression using quantitative RT-PCR.
Results
A combination of TGF-β withdrawal, dexamethasone reduction, and T3 addition is essential for hypertrophy induction. Cyto-morphological changes are accompanied by increased alkaline phosphatase activity, matrix mineralization, and changes in various hypertrophy markers, including collagen type X, receptors (FGFR1-3, PTHrPR, RARγ), MMP-13, Indian hedgehog, osteocalcin, and the pro-apoptotic gene, p53. However, hypertrophy is not induced uniformly throughout the pellet culture, with distinct regions of dedifferentiation seen.
Conclusions
Chondrogenically differentiating MSCs behave functionally similar to growth plate chondrocytes, expressing a very similar hypertrophic phenotype. Under the in vitro culture conditions used here, MSC-derived chondrocytes undergo a differentiation program analogous to that observed during endochondral embryonic skeletal development, with the potential for terminal differentiation. This culture system is applicable for the screening of hypertrophy-inhibitory conditions and agents that may be useful to enhance MSC performance in cartilage tissue engineering.
Keywords: mesenchymal stem cells, chondrogenesis, hypertrophy, differentiation, cartilage tissue engineering, apoptosis
Introduction
Adult mesenchymal stem cells (MSCs) are considered a promising candidate cell source for cartilage tissue engineering and regeneration. The chondrogenic differentiation potential of MSCs has been shown in many matrix-free and matrix-based cell culture systems (1–10). Specifically, in the widely used pellet culture system of Johnstone and Yoo (10, 11), MSCs are packed to achieve a high cell density to mimic mesenchymal chondrogenesis during developmental chondrogenesis. The cultures are maintained in a defined, serum free chondrogenic medium, containing transforming growth factor-β (TGF-β) and dexamethasone, for induction of chondrogenesis. A requirement for chondrogenesis in this model is high cell density and cell-cell-contact (9). Interestingly, in cultures of MSCs undergoing chondrogenesis, the expression of chondrocyte hypertrophy associated genes, including collagen type X, alkaline phosphatase, matrix metalloproteinase (MMP)-13, vascular endothelial growth factor (VEGF) and parathyroid hormone-related protein receptor (PTHrP-R), has been reported (7, 10–14). These gene expression activities suggest that MSC chondrogenic differentiation may have proceeded towards the chondrocyte hypertrophy stage, typical of endochondral ossification during skeletal development. Moreover, after in vivo ectopic implantation of MSC-derived cartilage constructs, chondrocyte dedifferentiation or vascular invasion and mineralization takes place, depending on the pre-differentiation state of the constructs, whereas differentiated implanted articular chondrocytes are able to maintain their phenotype (14–16). It should be noted that these reported hypertrophy related changes are not specific for terminal chondrocyte differentiation, but can also be detected under pathological conditions, such as osteoarthritis (17–27). This phenomenon is thus a concern for the clinical application of MSCs in articular cartilage repair, because chondrocyte hypertrophy in the neo-cartilage could ultimately lead to apoptosis, vascular invasion and ossification, as observed in the cartilage growth plate. To ascertain whether and to what extent chondrifying MSCs undergo maturation and terminal differentiation, including apoptosis, a wider array of hypertrophy markers is needed for more complete characterization, as well as investigation of the response of the differentiating MSCs to culture conditions known to affect terminal differentiation in growth plate chondrocytes.
A number of conditions have been shown to influence chondrocyte hypertrophy in vitro. Chondrocyte hypertrophy is induced by thyroid hormones in embryonic mesenchymal chondroprogenitor cells and growth plate chondrocytes (28–30). TGF-β (31) and dexamethasone (32) at high concentrations inhibit hypertrophy. Withdrawal of TGF-β, reduction of dexamethasone, and addition of L-thyroxine and β-glycerophosphate induces cellular hypertrophy and increases expression of collagen type X in chondrogenically differentiating pellet cultures of human MSCs (33). In this study, various culture conditions were screened in terms of their ability to induce a hypertrophic phenotype in chondrogenically differentiating MSCs. The hypertrophic phenotype was then further characterized on the basis of gene expression of an array of hypertrophy associated genes analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR), and by histological and immunohistochemical techniques.
Materials and Methods
Human Bone Marrow Derived Mesenchymal Stem Cells
Human MSCs were isolated from proximal femora of eight male patients (age 46 to 65) undergoing total hip replacement with Institutional Review Board approval (George Washington University) as previously described (34). In brief, bone marrow was curetted and irrigated from the cutting planes of the femoral neck, washed in Dulbecco’s Modified Eagle Medium with high glucose content, L-glutamine and sodium pyruvate (DMEM) (Invitrogen, Carlsbad, CA) and separated from debris by passing the eluate through a 20-gauge needle and 40 μm cell strainers (BD Biosciences, San Jose, CA). Cell suspensions were plated in 150 cm2 tissue culture flasks in DMEM containing 10% fetal bovine serum of selected lots (Invitrogen) and antibiotic-antimycotic (Invitrogen). Medium was changed after 24 hours and adherent cells were cultivated to 80% confluence and either split 1:3 or frozen for later use. For the experiments described below, cells were used at passages 2 to 4.
Chondrogenic Differentiation
Cells were trypsinized and seeded into V-bottom 96 well plates (Nunc, Rochester, NY), pelleted for 5 minutes at 250 × g, and chondrogenically pre-differentiated for 2 weeks in pellet culture (200,000 cells per pellet) in chondrogenic medium, consisting of DMEM with 1% ITS+ (BD Biosciences, Franklin Lakes, NJ), antibiotic-antimycotic, 50 μg/ml ascorbate-2-phosphate (Sigma, St. Louis, MO), 40 μg/ml L-proline (Sigma), 100 nM dexamethasone (Sigma), 10 ng/ml recombinant human TGF-β3 (R&D, Minneapolis, MN). In initial experiments with cells of two patients, the cultures were exposed to different medium conditions on day 15, by changing the following parameters either alone or in combination: TGF-β withdrawal, addition of 1 nM triiodothyronine (T3) (Sigma) or 10 mM β-glycerophosphate (β-GP; Sigma), and reduction of dexamethasone to 1 nM. Hypertrophy was induced upon addition of T3, withdrawal of TGF-β, and reduction of dexamethasone (with or without β-GP; see Results). These two conditions were used for further experiments using cells of six patients, referred to as hypertrophic medium and hypertrophic medium with β-GP, and the characteristics of the cultures compared to pellet cultures cultivated in chondrogenic medium as control. Medium was changed every three days.
Histology and Immunohistochemistry
For histology, pellets were fixed in 4% paraformaldehyde and embedded in paraffin. Six μm thick sections were stained with hematoxylin and eosin (Sigma), alcian blue and alizarin red (both from Rowley Biochemical, Danvers, MA). Alkaline phosphatase activity was determined histochemically on paraffin embedded sections using a commercial reagent kit (Sigma). Immunohistochemical staining for cell surface receptors and collagen type II was carried out after antigen retrieval with 0.2% hyaluronidase (Sigma) digestion for 1 hour at 37°C. For immunohistochemical detection of collagen type X, additional treatment with 0.1% Subtilisin A (Sigma) for 15 minutes at 37°C was performed. Primary antibodies against fibroblast growth factor (FGF)-receptor 1, 2 and 3 (FGFR1-3) were purchased from Novus Biologicals (Littleton, CO), and against PTHrP-receptor (PTHrPR) from Upstate (Charlottesville, VA). The anti-collagen type II antibody (II-II6B3) was from Developmental Studies Hybridoma Bank (Iowa City, IA), and the anti-collagen type X antibody (clone X53) was kindly provided by Dr. Klaus von der Mark (University of Erlangen, Germany). Incubation with primary antibodies was carried out at 4°C overnight, and immunolabeling detected using a Zymed SuperPicTure™ Polymer Detection kit (Invitrogen) with horseradish peroxidase conjugate and diaminobenzidine as substrate.
Biochemical Analyses
Samples were digested with Sigma papain digestion solution (1:50 in 0.1 M sodium acetate, 0.01 M cysteine HCl, 0.05 M EDTA, pH 6.0). DNA concentration was determined with Hoechst 33258 dye (Sigma) using calf thymus DNA as standard. Sulfated glycosaminoglycan (sGAG) concentration was measured with the dimethylmethylene blue (DMMB) method. Total collagen content was estimated by means of hydroxyproline assay after acid hydrolysis, using a hydroxylproline to total collagen ratio of 1:10.
Immunoblot
The antibody X53 was used for collagen type X western blot. Cell pellets were homogenized in 6 M urea with 2 % SDS, separated by reducing SDS-PAGE and blotted on PVDF membranes. Equal amounts of total collagen were loaded in the sample lanes (based on hydroxyproline content). After primary antibody incubation, collagen type X collagen was detected using Super Signal West Dura kit (Pierce Biotechnology, Rockford, IL).
Collagen Biosynthesis
Cell pellets were metabolically radiolabeled for 24 h with 1 μCi/ml [14C]-proline (GE Healthcare, Piscataway, NJ) in fully supplemented medium containing 1 mM β-aminopropionitrile to inhibit collagen crosslinking. Pellets were washed in PBS, lysed and extracted in 6M urea/2% SDS, and proteins separated by reducing SDS-PAGE. Gels were dried and autoradiography was performed using storage phosphor screens and a Typhoon variable mode imager (GE Healthcare). The ratio of collagen type II to type I expression was calculated from the α1:α2 ratio (based on [α1]2α2 for collagen type I and [α1]3 for collagen type II), after image quantitation with NIH ImageJ software (35).
Apoptosis Analysis
TUNEL histochemistry was performed using an In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions.
Gene Expression Analysis
Cell pellets were homogenized in Trizol reagent (Invitrogen). Chloroform (1:5 volume) was added and vigorously mixed. After phase separation by centrifugation, the aqueous phase was mixed with 1 volume 70% ethanol, transferred to RNeasy Micro Columns (Qiagen, Valencia, CA, USA) and RNA was extracted according to the manufacturer’s instructions, including an on-column DNA digestion step. Reverse transcription was performed with Omniscript RT kits (Qiagen), and gene expression analysis with semi-quantitative real-time-PCR using Brilliant Sybr Green QPCR mix (Stratagene, La Jolla, CA) and the MX3000P QPCR system (Stratagene). Gene expression levels were normalized to that of the housekeeping gene, HPRT. Primer sequences are listed in Table 1.
Table 1.
Primer sequences for RT-PCR gene expression analysis
| Gene | Sense Primer | Anti-sense Primer |
|---|---|---|
| COL10A1 | GCTTCAGGGAGTGCCATCATC | CTCACATTGGAGCCACTAGGAATC |
| COL1A1 | ATGGGAGGAGAGCGTGTG | GAGGTCGGAGAGCAGAGG |
| COL2A1 | CCACACTCAAGTCCCTCAAC | GCTGCTCCACCAGTTCTTC |
| FGFR1 | CCCAGCCACAACCCAGAGG | TGCCAGGTCTCGGTGTATGC |
| FGFR2 | CATCCTGTGCCGAATGAAGAAC | TGAAGAGAGGCGTGTTGTTATCC |
| FGFR3 | GCTAACACCACCGACAAGGAG | CACCACCAGGATGAACAGGAAG |
| HPRT | CGAGATGTGATGAAGGAGATGG | GCAGGTCAGCAAAGAATTTATAGC |
| IHH | CCTCAGTTGATGCTGCTAAATTC | AACAGTCTCTGGATGTGTCTTG |
| MMP-13 | GGACCCTGGAGCACTCATGTTTC | TCGGAGACTGGTAATGGCATCAAG |
| Osteocalcin | AGGAGGGCAGCGAGGTAG | GAAAGCCGATGTGGTCAGC |
| p21 | TCCTCATCCCGTGTTCTC | GAATTTCATAACCGCCTGTG |
| p53 | GGAGCACTAAGCGAGCACTG | ATGGCGGGAGGTAGACTGAC |
| PTHrP | CGACGACACACGCACTTGAAAC | CGACGCTCCACTGCTGAACC |
| PTHrPR | CCCGCCTACTGCCCACTG | GCCGTTGAGGAACCCATCG |
| RAR-α | GGCTTCACCACCCTCACCATC | CGTCTCCGCATCATCCATCTCC |
| RAR-γ | ACCCAGTATGTAGAAGCCAGTC | GCTGCGGTGTGAGAGTCC |
| RUNX2 | ATACCGAGTGACTTTAGGGATGC | AGTGAGGGTGGAGGGAAGAAG |
| SOX9 | GCAGGCGGAGGCAGAGGAG | GGAGGAGGAGTGTGGCGAGTC |
| VEGF | GGCGAAGAGAAGAGACACATTG | AGGAAGGTCAACCACTCACAC |
Abbreviations: COL10A1, collagen type X α1; COL1A1, collagen type I α1; COL2A1, collagen type II α1; FGFR1-3, fibroblast growth factor receptor types 1-3; HPRT, hypoxanthine guanine phosphoribosyl transferase; IHH, Indian hedgehog; MMP-13, matrix metalloproteinase 13; PTHrP, parathyroid hormone related peptide; PTHrPR, PTHrP-receptor; RAR-α and -γ, retinoic acid receptor-α and -γ; RUNX2, runt related transcription factor 2; SOX9, SRY (sex determining region Y)-box 9; VEGF, vascular endothelial growth factor
Statistical analysis
Statistical analysis was carried out using Microsoft Excel. For real-time RT-PCR data, results of six independent experiments were combined, and a paired two-tailed t-test was carried out. Data for biochemical composition were determined in four replicates per condition, and analyzed using an unpaired two-tailed t-test with unequal variance.
Results
In initial experiments, pellet cultures of MSCs were first induced to undergo chondrogenesis in chondrogenic medium (CM), and were then tested for their hypertrophic response when placed in different medium conditions previously reported to influence hypertrophy in growth plate (29, 31) or sternal (30, 32) chondrocytes. A distinctly hypertrophic phenotype was seen only after withdrawal of TGF-β, reduction of dexamethasone, and addition of thyroid hormone, in combination. Any of these measures alone did not elicit this cyto-morphological alteration. After addition of β-GP, mineralization was evident in the hypertrophic cultures, whereas continuous application of TGF-β either reduced or inhibited calcification. Addition of β-GP to cultures maintained under hypertrophy conditions also increased the abundance of apoptotic cells.
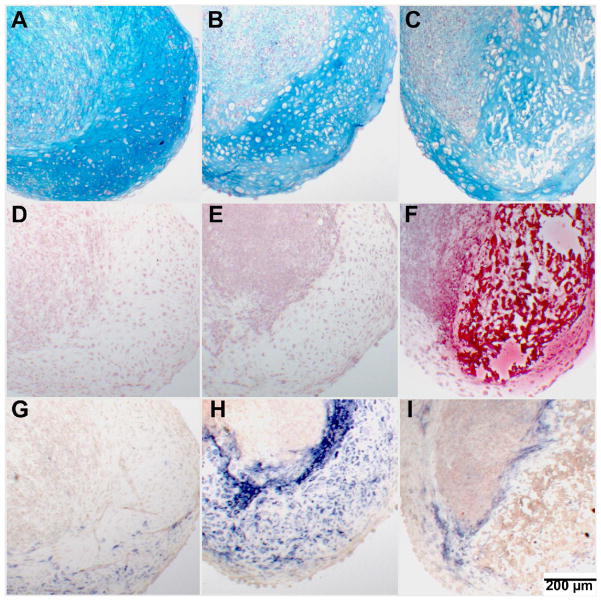
We next focused our analysis on cultures that underwent hypertrophy under the condition stated above, with cultures maintained in CM as controls. Histological observations (Figure 1) of day 28 pellet cultures clearly showed hypertrophic zones as well as areas containing dedifferentiated cells in cultures maintained in hypertrophy medium (Figure 1B & C). The CM control group exhibited a more homogeneous hyaline cartilage-like morphology, with little sign of cellular hypertrophy (Figure 1A). Mineralization of the extracellular matrix surrounding the hypertrophic chondrocytes was only seen when the cultures were maintained in hypertrophy medium supplemented with β-GP (Figure 1F); cells also appeared to be mostly not viable in these mineralized areas of the culture (Figure 1C, F, & I). Cells adjacent to these mineralized areas were strongly positive for alkaline phosphatase (Figure 1I). In cultures maintained in hypertrophy medium without β-GP, cells were also strongly positive for alkaline phosphatase in the hypertrophic areas (Figure 1H), compared to the control group (Figure 1G).
Figure 1.
Histology of pellets on culture day 28. (A–C) Alcian blue stain with nuclear fast red counterstain. (D–H) Alizarin red stain. (G–I) Alkaline phosphatase stain with nuclear fast red counterstain. Control group (A,D,G): Homogeneous alcian blue staining, no mineralization and low alkaline phosphatase activity. Hypertrophy group without β-GP (B,E,H): Hypertrophic next to dedifferentiated areas, with no mineralization and strong alkaline phosphatase activity in hypertrophic areas. Hypertrophy group with β-GP (C,F,I): Heterogeneous differentiation with mineralization of hypertrophic areas, few viable cells seen in mineralized areas, alkaline phosphatase positive cells present adjacent to mineralized areas.
Biochemical analyses revealed that while DNA content was unchanged in hypertrophy cultures, sGAG and total collagen levels were both reduced compared to control cultures (Figure 2). Total collagen normalized to DNA was, however, not significantly reduced under the hypertrophic condition with β-GP, probably related to the reduced cell number in this group due to apoptosis, particularly on day 28.
Figure 2.
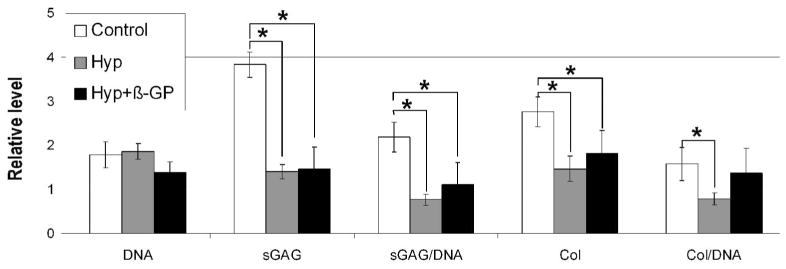
Biochemical composition of pellets on culture day 28. Data shown are from one experiment with four replicates. Similar trends were seen in independent experiments. Data are normalized to those of day 14 (mean ± SD). DNA = total DNA content; sGAG = total sulfated glycosaminoglycan content; sGAG/DNA = sGAG normalized to DNA; Col = total collagen content; Col/DNA = Col normalized to DNA. * p<0.05 (unpaired two-tailed t-test with unequal variance)
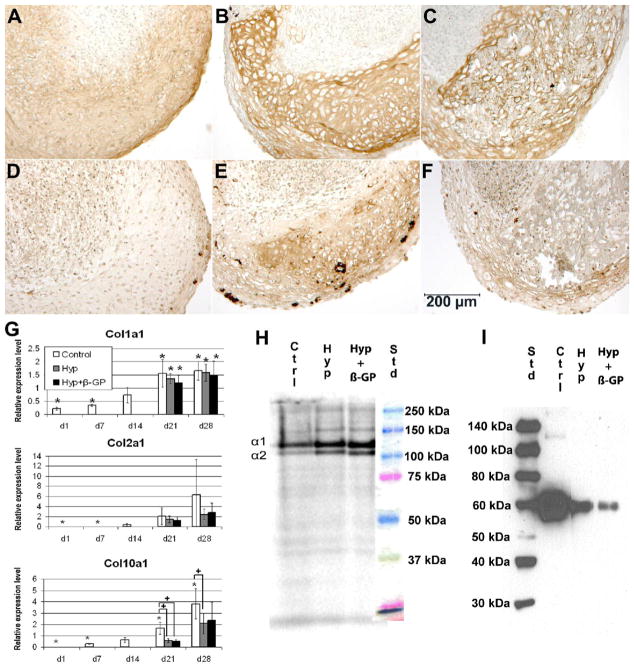
Expression of collagen types I, II, and X was seen under all three culture conditions (Figure 3). Immunohistochemistry showed low level of homogeneous staining of collagen type II in the control group (Figure 3A), and intense staining in the hypertrophic areas of cultures maintained in hypertrophy medium with or without β-GP (Figure 3 B & C); no collagen type II staining was seen in the dedifferentiated areas. Collagen type X showed similar distribution as collagen type II, i.e., low homogeneous staining in both the control and hypertrophy group without β-GP (Figure 3D–E). In the hypertrophy group with β-GP, collagen type X staining was positive in the periphery of hypertrophic regions (Figure 3F). Within the center of hypertrophic regions in this group, matrix mineralization most likely interfered with or obstructed collagen type X immunostaining. Real-time RT-PCR analysis (Figure 3G) showed upregulated gene expression of collagen types I, II, and X over time. Differences in expression of these genes between the control and hypertrophy cultures were not significant for collagen types I and II. The lack of significant difference in collagen type II expression, even at day 28, was due in part to the large variation within the control group, likely resulting from the different chondrogenic potential of MSCs of different donors. On the other hand, mRNA levels of collagen type X in the hypertrophy groups were significantly lower in the hypertrophy cultures compared to control cultures. As shown in Figure 3H, collagen synthesis analysis by metabolic [14C]-proline radiolabeling and autoradiography on day 28 showed distinct collagen α chain production; however, no clear ~65 kDa band corresponding to collagen type X was detected. Densitometric analysis showed an α1:α2 ratio of 3.5 for control cultures, and approximately 2 for the hypertrophy cultures, suggesting that a collagen type II to type I ratio of 0.5:1 for the control cultures, but primarily collagen type I with little collagen type II synthesis in the hypertrophy cultures, as measured on culture day 28. Interestingly, immunoblot (Figure 3I) confirmed the presence of collagen type X, with the highest level detected in the control cultures. In the control group, there was apparently a homogeneous, low level of accumulation of collagen type X throughout the pellets, whereas collagen type X deposition in the hypertrophy groups was restricted to the hypertrophic areas but absent in the dedifferentiated regions. The small volume and low celluarity of hypertrophic tissue in the hypertrophy groups, relative to the control group with homogeneous collagen type X distribution throughout the pellet, probably accounted for the lower level of total collagen type X accumulation and gene expression per pellet observed in the hypertrophy groups compared to the control groups.
Figure 3.
Collagen expression in pellets. (A–F) Immunohistochemistry on culture day 28: collagen type II (A–C), collagen type X (D–F). Control group (A,D), hypertrophy groups without (B,E) and with (C,F) β-GP. (G) Time course of gene expression of COL1A1, COL2A1 and COL10A1 normalized to the housekeeping gene, HPRT. (mean ± SD; * = significantly different from Day 14, p<0.05; + = significant different from control, p<0.05 (paired two-tailed t-test)). (H) Metabolic radiolabeling of collagen synthesis on culture day 28 analyzed by electrophoretic autoradiography (collagen α1 and α2 chains as indicated). (I) Western blot for collagen type X on day 28 (Std = molecular weight standards in kDa, Ctrl = Control, Hyp = hypertrophy medium without β-GP; Hyp + β-GP = hypertrophy medium with β-GP)
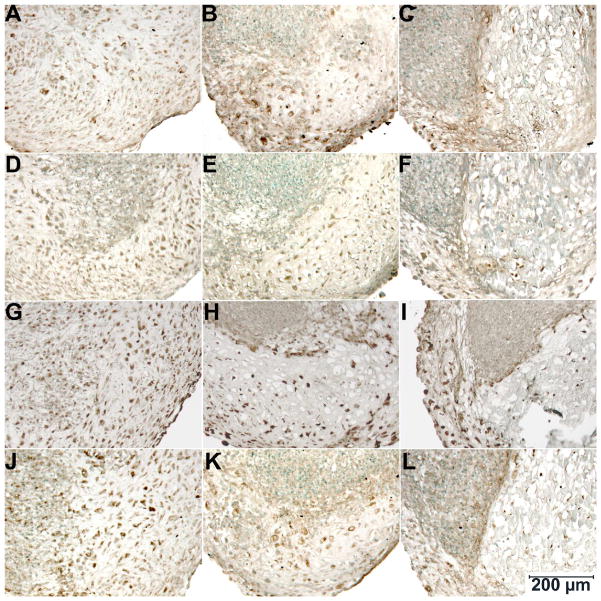
We next analyzed the expression of various growth factor receptors by means of immunohistochemistry (Figure 4). On day 28, FGFR1-3 and PTHrPR were detected in both control and hypertrophy cultures; specifically, only hypertrophic regions but not the dedifferentiated areas of the latter showed immunostaining for these receptors. While the core of the mineralized areas generally showed little immunostaining because of the absence of viable cells, positive staining cells were detected adjacent to these mineralized areas. Also, staining for all receptors was lower on day 21 both in terms of staining intensity and number of positive cells. In the hypertrophy cultures, similar to day 28, immunopositive cells were seen in hypertrophic areas but not in dedifferentiated areas. Immunostaining was undetectable on day 14.
Figure 4.
Immunohistochemical staining of day 28 cultures for FGFR1 (A–C), FGFR2 (D–F), FGFR3 (G–I) and PTHrPR (J–L) (methyl green counterstain). (A, D, G, J) Control group; (B, E, H, K) hypertrophy cultures without β-GP, and (C, F, I, L) hypertrophy cultures with β-GP.
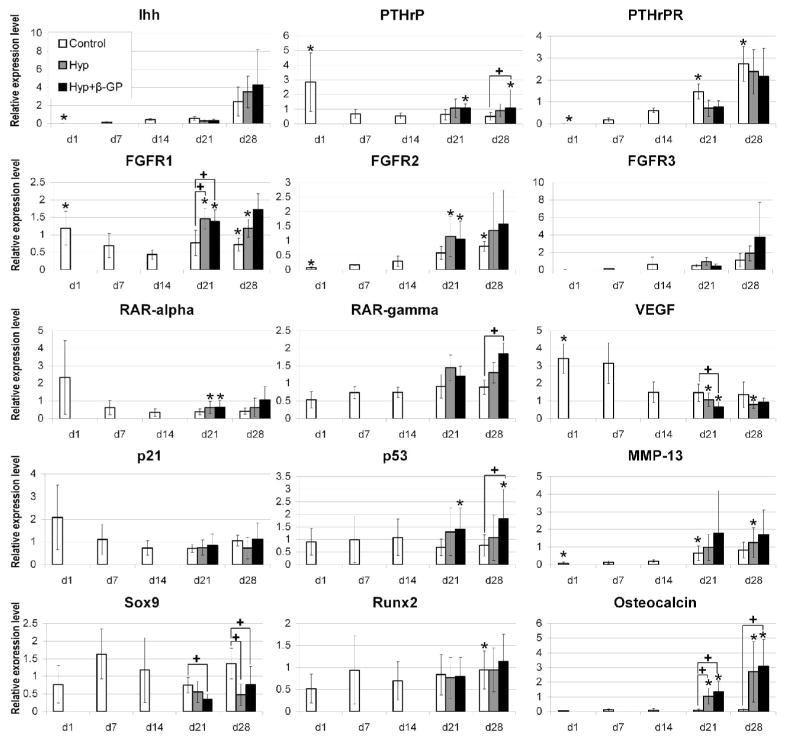
Quantitative analysis of a number of genes relevant to chondrocyte maturation and hypertrophy was carried out using real-time RT-PCR. These genes included Indian hedgehog (IHH), parathyroid hormone related peptide (PTHrP), PTHrP receptor (PTHrPR), FGF receptors 1, 2 and 3 (FGFR1, FGFR2 and FGRF3), retinoic acid receptor-α and -γ (RAR-α and -γ), vascular endothelial growth factor (VEGF), p21, p53, matrix metalloproteinase-13 (MMP-13), SOX9, RUNX2, and Osteocalcin. IHH and PTHrPR were up-regulated over time with significantly higher expression of PTHrPR under control condition on day 21 and 28 compared to day 14. FGFR1 expression profile was biphasic with high expression in undifferentiated MSCs on day 1, transient significant down-regulation on day 14 and significantly higher expression on day 21 under hypertrophy conditions compared to the control. FGFR2 was up-regulated over time and preceded up-regulation of FGFR3. RAR-α did not show a distinct expression pattern and RAR-γ was significantly more highly expressed under hypertrophy conditions with β-GP than in the control group on day 28. MMP-13 was up-regulated over time while VEGF appeared to be down-regulated. p53 was significantly more highly expressed under hypertrophy conditions with β-GP on day 28 compared to the control, and osteocalcin was significantly induced under hypertrophy conditions both on day 21 and day 28. RUNX2 expression was not regulated as a function of hypertrophy, while SOX9 was significantly suppressed under hypertrophy conditions.
Discussion
In this study, we have shown that chondrocyte hypertrophy could be experimentally induced in chondrogenic pellet cultures of human MSCs. Our results are consistent with those reported by Mackay et al. (33), and in addition demonstrated that the addition β-GP is not necessary to obtain hypertrophy like chondrocyte morphology. Our initial screen showed that only the combination of reduction of dexamethasone, TGF-β withdrawal and T3 addition was able to elicit phenotypic alterations consistent with hypertrophy, whereas any of these measures alone was not sufficient. The response of chondrogenically differentiated MSCs is thus similar to those of growth plate and sternal chondrocytes, where it has been shown that TGF-β inhibits hypertrophy, thyroid hormones induce hypertrophy, and high doses of steroid hormones inhibit the induction of hypertrophy by thyroid hormones (28–32).
It is noteworthy that the induction of hypertrophy in MSC pellet cultures on day 14 leads to a highly heterogeneous response within the pellets, i.e., regions of cellular hypertrophy are seen, as well as areas containing dedifferentiated cells. The unchanged DNA contents suggest that the lack of distinct morphology in the “dedifferentiated” areas is not a result of extensive cell death, such as necrosis. Extensive cell death in these areas would have instead resulted in a higher normalized collagen type X gene expression levels, since expression of the housekeeping gene, HPRT, would have decreased while the hypertrophic areas express high levels of collagen type X – this was not observed in the hypertrophy groups. In fact, the sGAG and total collagen contents of cultures maintained under hypertrophy conditions are significantly lower. We postulate the following scenario to explain this observation. MSCs consist of a non-homogeneous cell population, with varying proliferative and differentiation potentials. The withdrawal of TGF-β from the medium after 14 days of pre-differentiation removes the major chondro-inductive influence from the cultures. It is possible that only cells that have reached a sufficient state of chondrogenic differentiation by day 14 can proceed towards terminal differentiation, i.e., hypertrophy, whereas others are likely to de-differentiate. It should be noted that collagen type II expression at day 14 is only just beginning, rising rapidly to a high level by day 28. Day 14 cultures are thus at a relatively early stage of chondrogenesis. In the non-homogeneous day 14 cell pellet, perhaps the less chondrogenically differentiated cells require a more prolonged TGF-β exposure to remain differentiated, thus contributing to the de-differentiated regions seen in the hypertrophy cultures that lack TGF-β. In future studies, the timing and duration of TGF-β treatment will be varied, and the hypertrophic response of cells fractionated from day 14 cultures will be analyzed to examine this possibility.
In terms of collagen type expression, both control and hypertrophy cultures express collagen types I, II, and X. The continued increase in collagen type I expression as a function of culture time in all cultures suggests that a fibrocartilage like phenotype is present in the chondrifying MSC cultures, most likely associated with the peripheral fibroblast-like cells surrounding the pellets, as observed previously (10). In comparison, the 28 day culture period is accompanied by substantial up-regulation of collagen type II gene expression, which is absent at the beginning of culture. Both RT-PCR and metabolic labeling analyses show that control cultures display higher level of collagen type II expression on day 28, compared to the hypertrophy cultures, most likely due to the higher cellularity and more homogeneous nature of the former. For collagen type X, at a protein level, there is high intensity immunostaining in the hypertrophy cultures localized to regions containing hypertrophic cells, but negative in the dedifferentiated areas (Figure 3). Interestingly, at an mRNA level, there is higher level of collagen type X expression in the control cultures, which is also seen in the immunoblot. These findings suggest that in MSC cultures maintained under optimal hypertrophy conditions, a high degree of hypertrophy is formed in focal areas that demonstrate advanced chondrocyte maturation, including mineralization, which is not seen in the control chondrogenic cultures that demonstrate a more homogeneous collagen type X distribution without high focal accumulation.
Hypertrophic chondrocytes found in the hypertrophy MSC cultures show high alkaline phosphatase activity, and are capable of matrix mineralization when supplemented with β-GP. In this respect, hypertrophic chondifying MSCs are functionally similar to hypertrophic growth plate chondrocytes. However, within the mineralized matrix of the MSC pellet cultures, few viable cells are detected. A likely cause of the loss of viable cells could be a localized increase in inorganic phosphate concentration for cells with high alkaline phosphatase activity when β-GP is added to the cultures. Inorganic phosphate and other components of the mineralized growth plate matrix have been shown to induce apoptosis in chondrocytes (36). This scenario is supported by the observed significant increase in p53 gene expression under hypertrophic conditions with β-GP, without increased p21 gene expression. p53 is a pro-apoptotic tumor suppressor gene, and p21 is an anti-apoptotic cyclin-dependent kinase inhibitor that induces cell-cycle arrest and is involved in DNA-repair (25, 37). p53 has been shown to be involved in growth plate chondrocyte maturation and apoptosis (38, 39). In addition, in initial experiments, we detected TUNEL positive apoptotic cells in the hypertrophic areas in the hypertrophy group with β-GP (data not shown). However, it should be noted that there is no significant change in the total DNA contents, perhaps because of the low percentage of cells in the mineralized areas (Figure 2).
The hypertrophy program of MSC-derived chondrocytes also parallels that of growth plate chondrocytes, e.g., in the developing limb, in terms of the expression profiles of a number of relevant genes. In the developing limb, FGFR1 is expressed in the loose, pre-condensation mesenchyme. At condensation, FGFR2 expression is induced in the condensing mesenchyme, while FGFR1 expression is maintained in the periphery of the condensation. After overt chondrogenesis, FGFR3 is expressed in the proliferating chondrocytes in a pattern overlapping that of FRGR2. FGFR1 is then up-regulated again in the pre-hypertrophic and hypertrophic chondrocytes (41). Qualitatively similar gene expression profiles are seen in the chondrogenesis-hypertrophy program of the MSC pellet cultures studied here. Thus, gene expression of the FGFR’s increases with time during the 28-day culture period. Under hypertrophy conditions, FGFR2 expression precedes that of FGFR3. Specifically, the level of FGFR1 expression is significantly higher under hypertrophy conditions on day 21, which is likely a sign of accelerated maturation induced by the hypertrophy medium, or an effect of dedifferentiation. However, the latter is unlikely because immunohistochemistry using antibodies to FGFR1-3 did not reveal any staining for FGFR in the apparently dedifferentiated areas of the hypertrophic cultures.
Other hypertrophy-associated genes are also up-regulated during MSC hypertrophy, including PTHrP/PTHrPR, IHH, and MMP13. The PTHrP-IHH feedback loop is a well studied regulatory mechanism in growth plate physiology. PTHrP is secreted by periarticular chondrocytes. Its receptor PTHrPR is up-regulated in pre-hypertrophic and hypertrophic chondrocytes, and paracrine PTHrP-signaling via this receptor delays terminal differentiation. IHH is up-regulated in hypertrophic chondrocytes and induces expression of PTHrP in periarticular chondrocytes (40, 41). MMP-13 up-regulation has been reported in chondrocyte hypertrophy. Preparation of the cartilage matrix for subsequent mineralization is discussed as a function of MMP-13 (42). However, expression of VEGF, a proangiogenic factor expressed in hypertrophic chondrocytes (43), is not up-regulated. A reason for this may be uneven expression within the heterogeneous pellets under hypertrophy conditions. Finally, there is significant up-regulation of osteocalcin gene expression in both hypertrophy groups on days 21 and 28, while no up-regulation is seen in the control group. Osteocalcin is a calcium binding extracellular matrix protein expressed by osteoblasts as well as hypertrophic chondrocytes (44). RUNX2 is a transcription factor involved in chondrocyte maturation and osteogenesis (41). Interestingly, osteocalcin up-regulation is not accompanied by up-regulated RUNX2, but by a significant suppression of SOX9 gene expression, perhaps acting to mediate the down-regulation of cartilage marker genes. It is thus of relevance that, in rib chondrocytes, down-regulation of SOX9 gene expression is considered a potential mechanism for the induction of hypertrophy (29). Whether SOX9 plays a similar regulatory role in MSC hypertrophy remains to be tested.
In the developing growth plate, expression of RAR subtypes is highly differentially regulated. RARα is expressed diffusely throughout limb development, whereas RARγ; expression increases during hypertrophy (19, 45). In the MSC pellet cultures, RARα is only highly expressed in undifferentiated cells, whereas RARγ is up-regulated over time with hypertrophy medium containing T3. A significant difference in RARγ expression is seen on day 28 between the control cultures and β-GP treated hypertrophy cultures.
Gene expression data shown in this experiment represent the average gene expression per pellet culture. The obvious heterogeneity within the cultures, especially within the hypertrophy groups, necessitates in situ techniques in order to map gene expression to specific cells. By immunohistochemistry, we could show that many of the chondrocyte hypertrophy related genes are indeed expressed in areas with hypertrophy-like cell morphology.
In summary, our observations have clearly shown that MSC pellet cultures that have been chondrogenically pre-differentiated may be induced to undergo hypertrophic maturation. The hypertrophic phenotype is demonstrated on the basis of histology, histochemistry, immunostaining, and biochemical and gene expression analyses. The hypertrophy conditions used, the combination of reduction of dexamethasone, TGF-β withdrawal and T3 addition, are similar to those previously shown to induce hypertrophy in growth plate and sternal chondrocytes (29–32). MSC-derived chondrocytes are thus functionally similar to growth plate chondrocytes in terms of their endochondral-like maturation properties.
Our study represents a detailed characterization of hypertrophy of MSC-derived chondrocytes that includes both morphological characterization and analysis of a wide array of hypertrophy markers. Although the expression profiles of the known regulatory genes of chondrocyte differentiation have been examined, a number of questions clearly remain. In particular, the mechanisms responsible for inducing hypertrophy in MSC-derived chondrocytes are not known. By analogy with growth plate chondrocytes, possible hypertrophy effectors for the MSC-derived chondocytes include bone morphogenetic proteins (BMPs), thyroid hormone, and retinoic acid (32, 44, 45). Other possible mechanisms include the involvement of other cytokine and growth factor systems, such as growth hormone and insulin like growth factor-I, FGFs, the IHH-PTHrP feedback loop, and Wnt signalling (46–50).
Finally, the expression of a hypertrophic phenotype in MSC-derived chondrocytes is clearly a concern for MSC application in cartilage tissue engineering, which largely targets the joint articular cartilage, a permanent hyaline cartilage that normally does not undergo hypertrophy. The in vitro MSC model studied here, in addition to its use in studying the mechanism of chondrocyte hypertrophy, is thus a potentially useful model to screen culture conditions or bioactive factors with the aim to inhibit the terminal hypertrophic differentiation of tissue-engineered cartilage, based on the experimental parameters reported here. Naturally, the biological relevance of this system will need to be tested in more three-dimensional tissue engineering systems and by in vivo experiments, specifically testing candidate “anti-hypertrophy” agents and/or conditions.
Figure 5.
Temporal profiles of gene expression analyzed by real-time RT-PCR. Genes analyzed include IHH, PTHrP, PTHrPR, FGFR1, FGFR2, FGFR3, RAR-alpha, RAR-gamma, VEGF, p21, p53, MMP-13, SOX9, RUNX2, and Osteocalcin. Data shown are raw data normalized to the housekeeping gene, HPRT. Values are mean ± S.D. (n = 3 to 6 patients, depending on group and time point). * = significantly different from day 14, + = significantly different from control group (two-tailed paired t-test).
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Arthritis, and Musculsokeletal and Skin Diseases, National Institutes of Health (Z01 AR41131), and Deutsche Forschungsgemeinschaft (Fellowship MU2318/1 to MBM).
Supported by Deutsche Forschungsgemeinschaft (MBM) Fellowship and NIAMS Intramural Research Program, NIAMS, NIH (Z01 AR41131)
References
- 1.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 2.Ichinose S, Tagami M, Muneta T, Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res. 2005;322:217–26. doi: 10.1007/s00441-005-1140-6. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone B, Yoo JU. Autologous mesenchymal progenitor cells in articular cartilage repair. Clin Orthop Relat Res. 1999:S156–62. doi: 10.1097/00003086-199910001-00017. [DOI] [PubMed] [Google Scholar]
- 4.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Lisignoli G, Cristino S, Piacentini A, Toneguzzi S, Grassi F, Cavallo C, et al. Cellular and molecular events during chondrogenesis of human mesenchymal stromal cells grown in a three-dimensional hyaluronan based scaffold. Biomaterials. 2005;26:5677–86. doi: 10.1016/j.biomaterials.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 6.Noth U, Tuli R, Osyczka AM, Danielson KG, Tuan RS. In vitro engineered cartilage constructs produced by press-coating biodegradable polymer with human mesenchymal stem cells. Tissue Eng. 2002;8:131–44. doi: 10.1089/107632702753503126. [DOI] [PubMed] [Google Scholar]
- 7.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A. 2002;99:4397–402. doi: 10.1073/pnas.052716199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004;6:596–601. doi: 10.1080/14653240410005276-1. [DOI] [PubMed] [Google Scholar]
- 9.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–36. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 10.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–57. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 12.Mwale F, Girard-Lauriault PL, Wang HT, Lerouge S, Antoniou J, Wertheimer MR. Suppression of genes related to hypertrophy and osteogenesis in committed human mesenchymal stem cells cultured on novel nitrogen-rich plasma polymer coatings. Tissue Eng. 2006;12:2639–47. doi: 10.1089/ten.2006.12.2639. [DOI] [PubMed] [Google Scholar]
- 13.Mwale F, Stachura D, Roughley P, Antoniou J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J Orthop Res. 2006;24:1791–8. doi: 10.1002/jor.20200. [DOI] [PubMed] [Google Scholar]
- 14.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–66. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 15.De Bari C, Dell’Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50:142–50. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- 16.Dell’Accio F, De Bari C, Luyten FP. Microenvironment and phenotypic stability specify tissue formation by human articular cartilage-derived cells in vivo. Exp Cell Res. 2003;287:16–27. doi: 10.1016/s0014-4827(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 17.Nakase T, Ariga K, Meng W, Iwasaki M, Tomita T, Myoui A, et al. Distribution of genes for parathyroid hormone (PTH)-related peptide, Indian hedgehog, PTH receptor and patched in the process of experimental spondylosis in mice. J Neurosurg. 2002;97:82–7. doi: 10.3171/spi.2002.97.1.0082. [DOI] [PubMed] [Google Scholar]
- 18.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J Rheumatol. 2005;32:876–86. [PubMed] [Google Scholar]
- 19.Terkeltaub R, Lotz M, Johnson K, Deng D, Hashimoto S, Goldring MB, et al. Parathyroid hormone-related proteins is abundant in osteoarthritic cartilage, and the parathyroid hormone-related protein 1-173 isoform is selectively induced by transforming growth factor beta in articular chondrocytes and suppresses generation of extracellular inorganic pyrophosphate. Arthritis Rheum. 1998;41:2152–64. doi: 10.1002/1529-0131(199812)41:12<2152::AID-ART10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Derfus B, Kranendonk S, Camacho N, Mandel N, Kushnaryov V, Lynch K, et al. Human osteoarthritic cartilage matrix vesicles generate both calcium pyrophosphate dihydrate and apatite in vitro. Calcif Tissue Int. 1998;63:258–62. doi: 10.1007/s002239900523. [DOI] [PubMed] [Google Scholar]
- 21.Pfander D, Swoboda B, Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol. 2001;159:1777–83. doi: 10.1016/S0002-9440(10)63024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8:294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 23.Walker GD, Fischer M, Gannon J, Thompson RC, Jr, Oegema TR., Jr Expression of type-X collagen in osteoarthritis. J Orthop Res. 1995;13:4–12. doi: 10.1002/jor.1100130104. [DOI] [PubMed] [Google Scholar]
- 24.Hayami T, Funaki H, Yaoeda K, Mitui K, Yamagiwa H, Tokunaga K, et al. Expression of the cartilage derived anti-angiogenic factor chondromodulin-I decreases in the early stage of experimental osteoarthritis. J Rheumatol. 2003;30:2207–17. [PubMed] [Google Scholar]
- 25.Shibakawa A, Yudoh K, Masuko-Hongo K, Kato T, Nishioka K, Nakamura H. The role of subchondral bone resorption pits in osteoarthritis: MMP production by cells derived from bone marrow. Osteoarthritis Cartilage. 2005;13:679–87. doi: 10.1016/j.joca.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Smith JO, Oreffo RO, Clarke NM, Roach HI. Changes in the antiangiogenic properties of articular cartilage in osteoarthritis. J Orthop Sci. 2003;8:849–57. doi: 10.1007/s00776-003-0717-8. [DOI] [PubMed] [Google Scholar]
- 27.Walsh DA. Angiogenesis in osteoarthritis and spondylosis: successful repair with undesirable outcomes. Curr Opin Rheumatol. 2004;16:609–15. doi: 10.1097/01.bor.0000133662.60223.ee. [DOI] [PubMed] [Google Scholar]
- 28.Mello MA, Tuan RS. Effects of TGF-beta1 and triiodothyronine on cartilage maturation: in vitro analysis using long-term high-density micromass cultures of chick embryonic limb mesenchymal cells. J Orthop Res. 2006;24:2095–2105. doi: 10.1002/jor.20233. [DOI] [PubMed] [Google Scholar]
- 29.Okubo Y, Reddi AH. Thyroxine downregulates Sox9 and promotes chondrocyte hypertrophy. Biochem Biophys Res Commun. 2003;306:186–90. doi: 10.1016/s0006-291x(03)00912-4. [DOI] [PubMed] [Google Scholar]
- 30.Quarto R, Campanile G, Cancedda R, Dozin B. Modulation of commitment, proliferation, and differentiation of chondrogenic cells in defined culture medium. Endocrinology. 1997;138:4966–76. doi: 10.1210/endo.138.11.5522. [DOI] [PubMed] [Google Scholar]
- 31.Ballock RT, Heydemann A, Wakefield LM, Flanders KC, Roberts AB, Sporn MB. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev Biol. 1993;158:414–29. doi: 10.1006/dbio.1993.1200. [DOI] [PubMed] [Google Scholar]
- 32.Leboy PS, Sullivan TA, Nooreyazdan M, Venezian RA. Rapid chondrocyte maturation by serum-free culture with BMP-2 and ascorbic acid. J Cell Biochem. 1997;66:394–403. doi: 10.1002/(sici)1097-4644(19970901)66:3<394::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 34.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–66. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Groessner-Schreiber B, Tuan RS. Enhanced extracellular matrix production and mineralization by osteoblasts cultured on titanium surfaces in vitro. J Cell Sci. 1992;101(Pt 1):209–17. doi: 10.1242/jcs.101.1.209. [DOI] [PubMed] [Google Scholar]
- 36.Pucci B, Adams CS, Fertala J, Snyder BC, Mansfield KD, Tafani M, et al. Development of the terminally differentiated state sensitizes epiphyseal chondrocytes to apoptosis through caspase-3 activation. J Cell Physiol. 2007;210:609–15. doi: 10.1002/jcp.20857. [DOI] [PubMed] [Google Scholar]
- 37.O’Reilly MA. Redox activation of p21Cip1/WAF1/Sdi1: a multifunctional regulator of cell survival and death. Antioxid Redox Signal. 2005;7:108–18. doi: 10.1089/ars.2005.7.108. [DOI] [PubMed] [Google Scholar]
- 38.Chrysis D, Nilsson O, Ritzen EM, Savendahl L. Apoptosis is developmentally regulated in rat growth plate. Endocrine. 2002;18:271–8. doi: 10.1385/ENDO:18:3:271. [DOI] [PubMed] [Google Scholar]
- 39.Ohyama K, Chung CH, Chen E, Gibson CW, Misof K, Fratzl P, et al. p53 influences mice skeletal development. J Craniofac Genet Dev Biol. 1997;17:161–71. [PubMed] [Google Scholar]
- 40.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107:295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J, Chung UI, Yang D, Karsenty G, Bringhurst FR, Kronenberg HM. PTH/PTHrP receptor delays chondrocyte hypertrophy via both Runx2-dependent and -independent pathways. Dev Biol. 2006;292:116–28. doi: 10.1016/j.ydbio.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 42.D’Angelo M, Yan Z, Nooreyazdan M, Pacifici M, Sarment DS, Billings PC, et al. MMP-13 is induced during chondrocyte hypertrophy. J Cell Biochem. 2000;77:678–93. [PubMed] [Google Scholar]
- 43.Horner A, Bishop NJ, Bord S, Beeton C, Kelsall AW, Coleman N, et al. Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J Anat. 1999;194(Pt 4):519–24. doi: 10.1046/j.1469-7580.1999.19440519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gerstenfeld LC, Shapiro FD. Expression of bone-specific genes by hypertrophic chondrocytes: implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J Cell Biochem. 1996;62:1–9. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C1::AID-JCB1%3E3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.Koyama E, Golden EB, Kirsch T, Adams SL, Chandraratna RA, Michaille JJ, et al. Retinoid signaling is required for chondrocyte maturation and endochondral bone formation during limb skeletogenesis. Dev Biol. 1999;208:375–91. doi: 10.1006/dbio.1999.9207. [DOI] [PubMed] [Google Scholar]
- 46.Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab. 2003;14:356–64. doi: 10.1016/s1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 47.Dong YF, Soung do Y, Schwarz EM, O’Keefe RJ, Drissi H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol. 2006;208:77–86. doi: 10.1002/jcp.20656. [DOI] [PubMed] [Google Scholar]
- 48.Harvey CB, O’Shea PJ, Scott AJ, Robson H, Siebler T, Shalet SM, et al. Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol Genet Metab. 2002;75:17–30. doi: 10.1006/mgme.2001.3268. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson O, Marino R, De Luca F, Phillip M, Baron J. Endocrine regulation of the growth plate. Horm Res. 2005;64:157–65. doi: 10.1159/000088791. [DOI] [PubMed] [Google Scholar]
- 50.Shao YY, Wang L, Ballock RT. Thyroid hormone and the growth plate. Rev Endocr Metab Disord. 2007 doi: 10.1007/s11154-006-9012-2. [DOI] [PubMed] [Google Scholar]