Abstract
Fibroblast growth factor – 2 (FGF2) and interleukin – 1β IL-1β) stimulate the expression of matrix metalloproteinases (MMPs) in articular chondrocytes, which may contribute to cartilage degradation and development of osteoarthritis. Histone deacetylases (HDACs) have recently been implicated in the regulation of MMP gene expression. To investigate the functional involvement of HDACs in the signaling pathway of FGF2 and IL-1β, we examined the effects of HDAC inhibition on activities of FGF2 or IL-1β on gene expression of MMP-1, MMP-3, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs – 5 (ADAMTS5), collagen type II, and aggrecan. Human articular chondrocyte cultures were treated with FGF2 or IL-1β in the presence or absence of HDAC inhibitor (trichostatin A, TSA). Gene expression levels after treatments were assessed using quantitative real time PCR. Results showed that FGF2 and IL-1β both increased MMP-1 and -13 expression, while IL-1βalso increased MMP-3 mRNA levels. These effects were attenuated in the presence of TSA in a dose dependent manner. In contrast to the effects on MMPs, FGF2 decreased mRNA levels of ADAMTS–5, which was not affected by HDAC inhibition. FGF2, IL-1β, and TSA inhibited expression of aggrecan, while TSA also decreased mRNA levels of collagen type II. These findings showed that HDAC inhibition antagonized FGF2 and IL-1β induced MMP expression. Combination of FGF2 and the HDAC inhibitor decreases both anabolic and catabolic genes, which may slow the cartilage turnover and be beneficial for maintaining cartilage integrity.
Keywords: Fibroblast growth factor, interleukin –1β, histone deacetylase, matrix metalloproteinase, articular chondrocyte, trichostatin A
Introduction
Osteoarthritis (OA) is pathologically characterized by fibrillation and destruction of articular cartilage. Two families of enzymes, MMPs (matrix metalloproteinases) and ADAMTS (“A disintegrin and metalloproteinase with thrombospondin motifs”), act to degrade cartilage matrix proteins, such as collagens and proteoglycans (Arner 2002; Bramono et al. 2004). In vitro and in vivo studies have suggested that these enzymes contribute to the onset and progression of OA. First, many enzymes of the MMP and ADAMTS families are present in normal and OA cartilage, including MMP1 (collagenase 1), MMP3 (stromelysin 1), MMP13 (collagenase 3), and ADAMTS5 (aggrecanase 2) (Bau et al. 2002; Bramono et al. 2004). Expression of MMP-13, which degrades collagen type II with high efficiency (Knauper et al. 1996), is significantly increased in human chondrocytes of late stage OA(Aigner et al. 2001; Bau et al. 2002). Secondly, deletion of ADAMTS5 (aggrecanase 2) gene inhibits cartilage degradation in mouse models of OA and inflammatory arthritis(Glasson et al. 2005; Stanton et al. 2005), while over-expression of MMP-13 in articular chondrocytes results in joint degeneration in mice similar to human OA (Neuhold et al. 2001). Furthermore, MMP inhibitors have been shown to reduce cartilage degradation in explant cultures (Billinghurst et al. 1997) and in an animal OA model (Janusz et al. 2002). Concomitantly, OA also appears to reduce the expression of cartilage anabolic genes, including collagen type II A1 (COL2A1) and aggrecan. Therefore, inhibition of aggrecanase and MMP activities is a promising approach for the treatment of OA.
In addition to direct inhibition of enzyme activities, modulation of MMP gene expression has also been a strategy for the development of OA drugs (Vincenti and Brinckerhoff 2002). A recent study showed that histone deacetylase (HDAC) inhibitors, trichostatin A (TSA) and sodium butyrate, block MMP and aggrecanase expression, and inhibit cartilage resorption induced by pro-inflammatory factors, interleukin-1α (IL-1α and oncostatin M (Young et al. 2005). Studies have also demonstrated that treatment with HDAC inhibitors reduces joint swelling and inflammation, and inhibits cartilage and bone destruction in animal models of rheumatoid arthritis (Chung et al. 2003; Nishida et al. 2004). HDAC inhibitors are thus candidate agents for the treatment of arthritic diseases.
HDACs are a family of enzymes responsible for the deacetylation of histone and non-histone proteins, while histone acetyltransferases (HATs) catalyze the reverse reaction – acetylation (de Ruijter et al. 2003; Docmanovic and Marks 2005). In human, at least 18 HDACs have been identified and grouped into three classes. Class 1 HDACs (HDAC 1, 2, 3, and 8) are related to yeast RPD3 deacetylase and are expressed in most cell types, whereas class 2 HDACs (HDAC 4, 5, 7, 9, and 10) share homology with yeast HDA1 deacetylase, and expression of these HDACs is more limited to certain cell types (de Ruijter et al. 2003). A third class of HDACs, called Sir2 family of deacetylase, share low homology with class 1 and 2 HDACs. HDAC inhibitors, such as TSA, inhibit class 1 and 2 HDACs, but not class 3 HDACs (Docmanovic and Marks 2005).
Generally, acetylation of histones results in relaxed chromatin and transcriptional activation, whereas deacetylation of histones is associated with compacted chromatin and transcriptional repression (de Ruijter et al. 2003). However, hyperacetylation of proteins as a result of HDAC inhibition, increases expression of some genes, and may also repress others (Mitsiades et al. 2004; Peart et al. 2005). Up or down regulation of gene expression depends on the acetylation status of both histones and non-histone proteins, such as transcriptional factors and components of transcriptional machinery (Docmanovic and Marks 2005; Drummond et al. 2005). In addition to modulating gene expression, HDAC inhibitors also cause growth arrest, cell differentiation, or cell death (Docmanovic and Marks 2005; Drummond et al. 2005).
In addition to pro-inflammatory factors, such as IL-1 and tumor necrosis factor-α (TNFα, fibroblast growth factor –2 (FGF2) has also been demonstrated by us and other investigators to increase MMP1 and MMP-13 expression in articular chondrocytes (Mitchell et al. 1996; Shlopov et al. 1997; Tardif et al. 1999; Bau et al. 2002; Vincent et al. 2002; Wang et al. 2004; Young et al. 2005). Studies have shown that FGF2 is sequestered in cartilage matrix, and tissue damage results in the release of FGF2, which in turn stimulates MMP expression (Vincent et al. 2002). Increased levels of FGF2 are detected in the synovial fluid of OA joints (Orito et al. 2003; Im et al. 2007). Taken together, these studies suggest that FGF2 could initiate tissue remodeling upon cartilage damage in vivo, and may also contribute to the progression of OA through up-regulation of MMPs.
Although HDAC inhibitors block MMP expression induced by pro-inflammatory factors(Young et al. 2005), it is not clear whether HDAC inhibitors would also inhibit FGF2 effects on MMPs. In this study, we investigated the interaction of FGF2 and IL-1βwith the HDAC inhibitor, trichostatin A (TSA), on the expression of MMPs, ADAMTS5, COL2A1, and aggrecan. Here, we report for the first time that the HDAC inhibitor, TSA, blocked MMP expression in chondrocytes induced by FGF2. We also showed that FGF2 inhibited ADAMTS5 expression. In addition to antagonizing IL-1β and FGF2-induced MMP expression, HDAC inhibition also reduced COL2A1 and aggrecan basal expression. Therefore, HDAC activities not only mediate FGF2 and IL-1β induced catabolic gene expression, but also are essential for maintaining anabolic gene expression in human articular chondrocytes.
Materials and methods
Reagents
Recombinant human FGF2 and IL-1β were purchased from R&D systems (Minneapolis, MN) and reconstituted as recommended by the manufacturer. TSA from Upstate USA, Inc. (Lake Placid, NY) was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) as a 20 μM stock solution, and then diluted with cell culture medium.
Chondrocyte isolation and culture
Human joint specimens were obtained with Institutional Review Board approval and informed consent from patients undergoing knee or hip replacement (George Washington University, Washington, DC). Patients had grade III or IV cartilage defects (Outerbridge classification) with ages ranging from 42 to 70 years. Specimens were stored at 4° C and cartilage tissue was dissected from the underlying bone within 24 hours. Chondrocytes were isolated from the intact region of the condylar cartilage. The cartilage was first incubated in 0.1% trypsin in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA) with antibiotics and antimycotics (100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 250 ng/ml amphotericin B) (Invitrogen) at 37°C for one hour. After three washes with DMEM, the cartilage was minced in DMEM with 200 U/ml bacterial collagenase type 2 (Worthington, Lakewood, NJ) at a ratio of 10 ml of collagenase solution per gram wet weight of cartilage, and incubated with shaking at 37°C for 18 hours. The suspension was then filtered through 40 μm mesh filter and the cells were washed three times in DMEM with 10% fetal bovine serum (FBS). Cells were plated in 10 cm tissue culture dishes at a concentration of 3 × 106 cells/dish. Cells were passaged at approximately 90% confluence in DMEM with 10% FBS, and used between passages one and four.
Cell number counting and cell death assay
Human articular chondrocytes were plated at a concentration of 4 ×104 cells/well in 12 well plates in a medium of DMEM/F12 (a 1:1 mixture of DMEM and F12 Nutrients Mixture, Invitrogen) containing 10% FBS. After cells reached approximately 50% confluency, the medium was removed and replaced with DMEM/F12 supplemented with 1% FBS. After 24 hours, cells were treated in triplicates with FGF2 (25 ng/ml) or IL-1β (5 ng/ml) in the absence or presence of HDAC inhibitor (TSA at 200 nM). After 1, 3, and 6 days of culture, cells were harvested and cell numbers were counted with a Coulter Z-1 particle counter (Beckman Coulter Corp. Miami, FL). For live and dead cell assay, a LIVE/DEAD Viability/Cytotoxicity Assay Kit (Molecular Probes, Eugene, OR) was used according to the manufacturer’s instruction. Briefly, after removal of the medium, cells were gently washed with Dulbecco’s phosphate-buffered saline (PBS) and incubated for 45 minutes at room temperature in 2 μM calcein AM and 4μM ethidium homodimer, probes for live cells and dead cell nuclei, respectively. Cells were counted by epifluorescence imaging using an Axiovert 135 microscope (Carl Zeiss Microimaging, Inc. Thornwood, NY) equipped with Open Lab software 3.1.4 (Improvision Inc. Lexington, MA).
RNA isolation and gene expression analysis
Cells in culture plates were washed in PBS and lysed in 350 μl RLT lysis buffer with 1% β-mercaptoethanol (vol/vol) for RNA isolation using the RNeasy mini kit (Qiagen Inc. Valencia, CA) according to the manufacturer’s instructions. RNA was quantified spectrophotometrically based on A260 using ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE).
First strand cDNA was synthesized from total RNA using a SuperScript First-Strand Synthesis System kit (Invitrogen). Levels of mRNA were analyzed by real time PCR with SYBR green detection using the iCycler (Bio-Rad, Hercules, CA) and values of each gene were calculated using individual standard curves and normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Gene specific primer sequences are shown in Table 1, and include those for GAPDH, MMP-1, -3, and -13, ADAMTS5, COL2A1, and aggrecan.
Table 1.
Primer sequences for RT-PCR analysis
| Gene | Primer | Accession # |
|---|---|---|
| MMP1 | Forward: 5′- GGGAGCAAACACATCTGACCTA-3′ | NM_002421 |
| Reverse: 5′- GCTGGACAGGATTTTGGGAACG-3′ | ||
| MMP3 | Forward: 5′- GTCTCTTTCACTCAGCCAACAC-3′ | NM_002422 |
| Reverse: 5′- CAGCTCGTACCTCATTTCCTCT-3′ | ||
| MMP13 | Forward: 5′- ACTTTATGCTTCCTGATGACGATG-3′ | NM_002427 |
| Reverse: 5′- TGCTGTATTCAAACTGTATGGGTC-3′ | ||
| COL2A1 | Forward: 5′- GAGCAGCAAGAGCAAGGAGAAGA-3′ | NM_001844, NM_033150 |
| Reverse: 5′- CAATGATGGGGAGGCGTGAGGTC-3′ | ||
| Aggrecan | Forward: 5′- TCGTGGTGAAAGGCATCGTGTT-3′ | NM_001135, NM_013227 |
| Reverse: 5′- TCATAGGTCTCGTTGGTGTCTC-3′ | ||
| ADAMTS5 | Forward: 5′- GATGTCTGTGCTCGCCTGTGGT-3′ | NM_007038 |
| Reverse: 5′- CTGTGCAGTAGCGTCCGTTGTT-3′ | ||
| GAPDH | Forward: 5′- ACCACAGTCCATGCCATCAC-3′ | NM_002046 |
| Reverse: 5′- TCCACCACCCTGTTGCTGTA-3′ |
Statistical analysis
The interaction of HDAC inhibitors with FGF2 or IL-1β was investigated in factorial experiments, in which the first factor was treatment with/without FGF2 or IL-1βand the second factor was treatment with TSA. These effects were examined using chondrocytes from different patients in a randomized complete block design. ANOVA was conducted using a general linear model. When standard deviations among groups differ significantly, data were analyzed after transformation into logarithmic values. When the main effect of treatments was significant, Tukey’s test was used to separate treatment means. Differences were considered to be significant at a level of p < 0.05.
Results
Cell growth and cell death
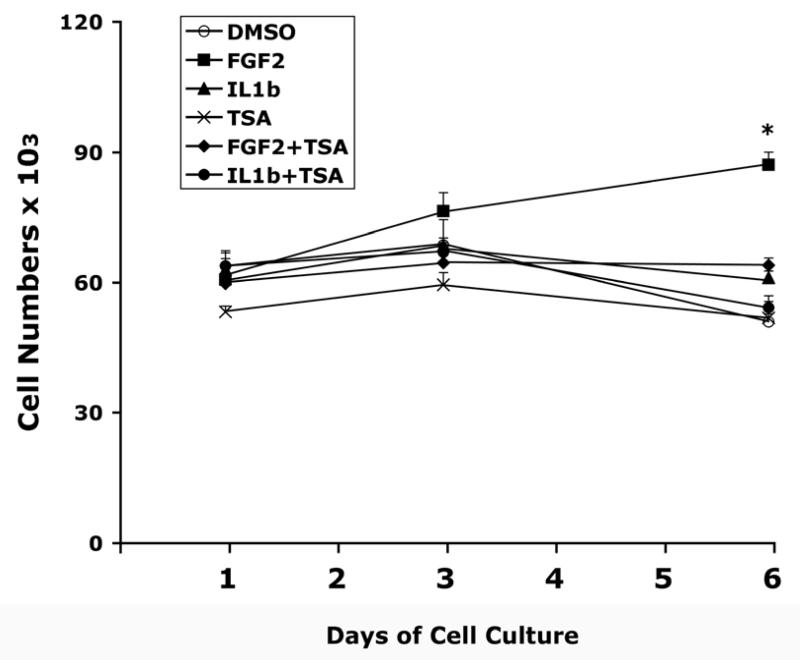
Since HDAC inhibitors are known to cause cell growth arrest and cell death (Docmanovic and Marks 2005; Drummond et al. 2005), we first investigated the in vitro effects of TSA on chondrocyte growth and survival. In addition, we also examined the interaction between the effects of TSA and those of FGF2 and IL-1β. In chondrocytes receiving DMSO (control), TSA, or IL1β with/without TSA, cell number remained constant or slightly decreased over the 6-day course of treatments (Fig. 1). FGF2 treatment increased cell numbers after 6 days (p<0.05). TSA blocked the increase in cell numbers induced by FGF2 (Fig. 1).
Figure 1.
Time course of cell growth in human articular chondrocyte cultures. Cells were cultured in 12-well culture plates with 1% FBS DMEM/F12 medium. Triplicate wells received treatment with FGF2 (25 ng/ml) and IL-1β (5 ng/ml) in the presence or absence of TSA (200 nM) for 1, 3, or 6 days. Cells were harvested by trypsinization and cell numbers were counted with a Coulter Z-1 particle counter. Data represent mean ± SEM from two separate experiments. Control: DMSO vehicle. *, p<0.05, when compared to the group at day 1 receiving the same treatment.
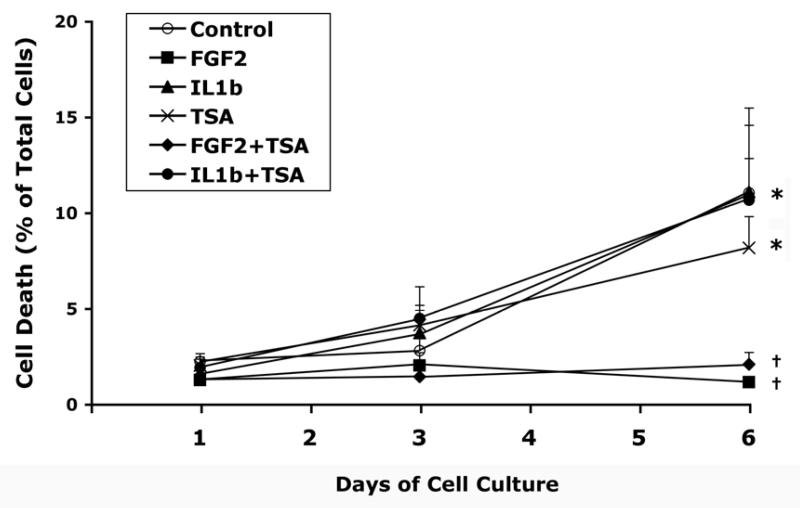
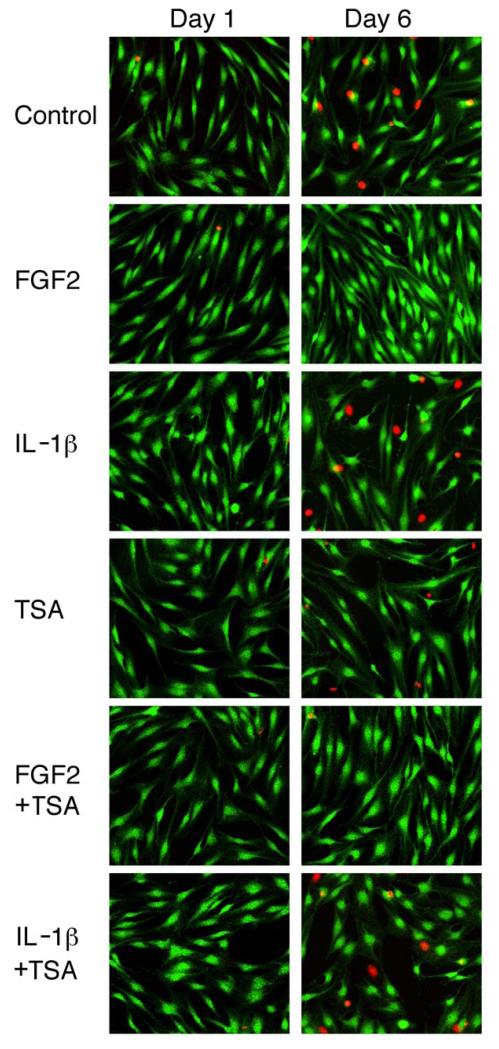
During the first 3 days of culture under the condition used in this study, cell death was observed in less than 5% of the cell population with or without any of the treatments (Fig. 2). The number of dead cells was significantly increased after 6 days in the control cultures and those receiving TSA, IL-1β, or TSA and IL-1β (p<0.05) (Fig. 2). FGF2, with or without TSA, blocked the increase in the percentage of cell death. The number of dead cells in groups receiving FGF2, with or without TSA, was significantly lower than the control after 6 days of treatment, while that in groups receiving TSA, IL-1β, or both did not differ from the control (Fig. 2). Examples of microscopy of live and dead cell assay were shown in Figure 3.
Figure 2.
Time course of cell death in human articular chondrocyte cultures treated with FGF2 or IL-1β in the presence or absence of TSA. Cells were cultured and treated as described in Figure 1. Live cells and dead cells were detected as described in Materials and Methods. Cell death is determined as the percentage of dead cells in representative fields (also see Fig. 3). Data represent mean ± SEM from two separate experiments. Control: DMSO vehicle. *, p<0.05, when compared to the group mean at day 1 receiving the same treatment. ✝, p<0.05, when compared to the control group mean at the same time point.
Figure 3.
Detection of live cells and dead cell nuclei in human articular chondrocyte cultures treated with FGF2, IL-1β, and TSA. Cells were cultured and treated as described in Figure 1. Green fluorescence indicates live cells and red fluorescence shows nuclei of dead cells.
HDAC inhibition attenuates induction of MMP expression by FGF2 AND IL-1β
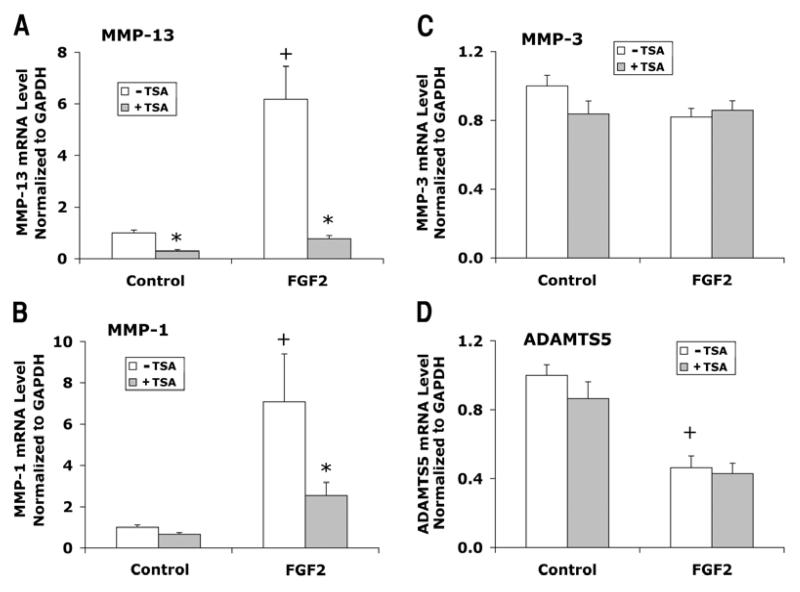
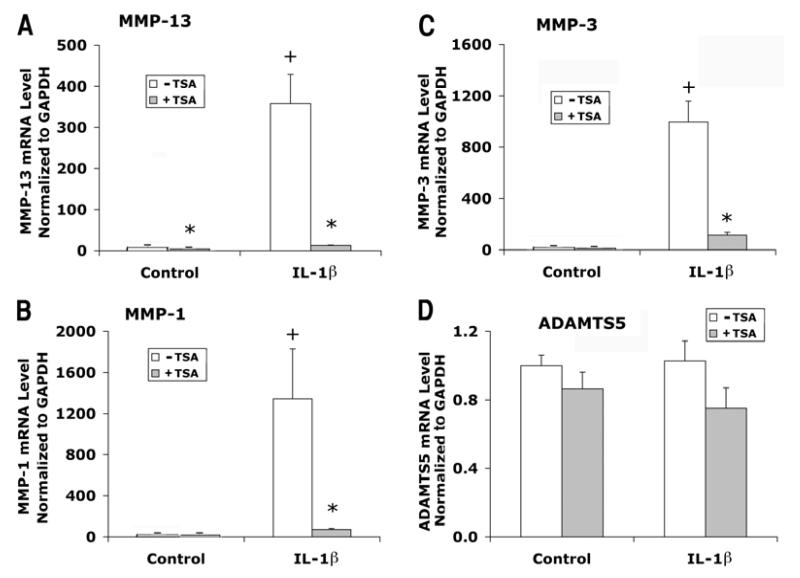
Articular chondrocytes cultured in 1% FBS DMEM/F12 medium were treated with FGF2 and IL-1β in the presence or absence of TSA for 24 hours. Chondrocytes from three patients were examined in separate experiments. FGF2 treatment increased MMP-13 mRNA levels by an average of 6-fold (Fig. 4A) and MMP-1 by 7-fold (Fig. 4B), but did not change MMP-3 mRNA levels significantly (Fig. 4C). In contrast to the effects on MMPs, FGF2 decreased ADMATS5 expression (Fig. 4D). Treatment with TSA totally blocked the inductive effects of FGF2 on MMP-13 expression (Fig. 4A), and also reduced FGF2 effects on MMP-1 expression by more than 50% (Fig. 4B).
Figure 4.
Effects of FGF2 with or without TSA on gene expression of MMP-13, MMP-1, MMP-3, and ADAMTS5 in human articular chondrocytes. Cells were cultured and treated with FGF2 and TSA for 24 hours as described in Figure 1. mRNA levels were quantified by real time RT-PCR and normalized to GAPDH. Chondrocytes from three patients were examined in separate experiments. Data were combined from these experiments after the group mean of the control without TSA is adjusted to 1. Data are presented as mean ± SEM. + (p<0.05) indicates significant FGF2 effects after comparison of the two group means with and without FGF2 in the absence of TSA; * (p<0.05) indicates significant TSA effects by comparing data with and without TSA at the same level of FGF2.
IL-1β was substantially more potent in stimulating MMP expression than FGF2, and drastically elevated mRNA levels of MMP-13, MMP-1, and MMP-3 hundred to thousand-fold (Fig. 5A, B, C). TSA attenuated the stimulatory effects of IL-1β on MMP-13, MMP-1 and MMP-3 expression by an average of 90% or more (Fig. 5A, B, C). IL-1β did not significantly affect ADAMTS5 expression (Fig. 5D).
Figure 5.
Effects of IL-1β with or without TSA on gene expression of MMP-13, MMP-1, MMP-3, and ADAMTS5 in human articular chondrocytes. Cells were cultured and treated with IL-1β and TSA for 24 hours as described in Figure 1. mRNA levels were quantified by real time RT-PCR and normalized to GAPDH. Chondrocytes from three patients were examined in separate experiments. Data were combined from these experiments after the group mean of the control without TSA is adjusted to 1. Data are presented as mean ± SEM. + (p<0.05) indicates significant IL-1β effects after comparison of the two group means with and without IL-1β in the absence of TSA; * (p<0.05) indicates significant TSA effects by comparing data with and without TSA at the same level of IL-1β.
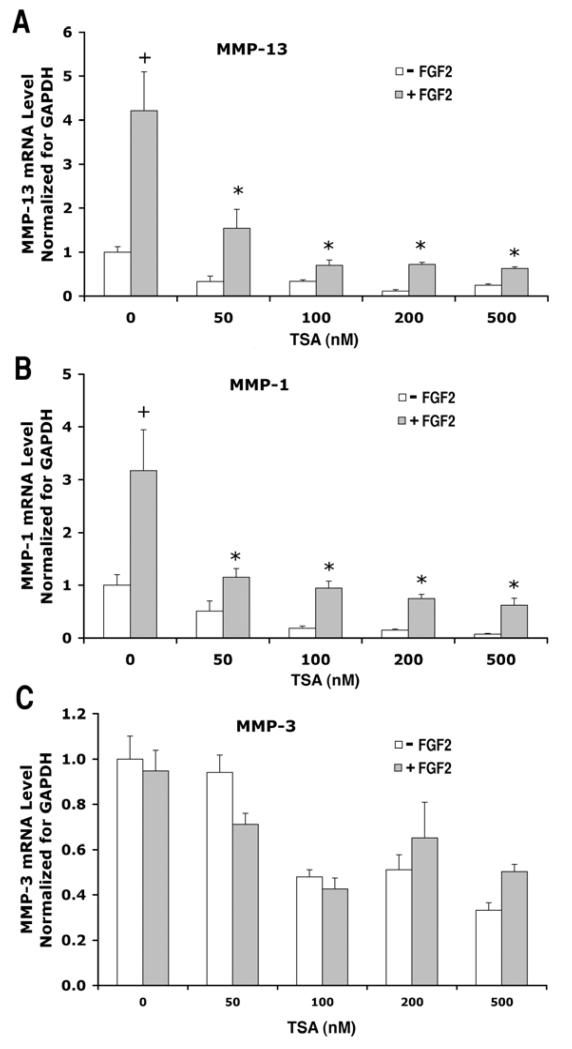
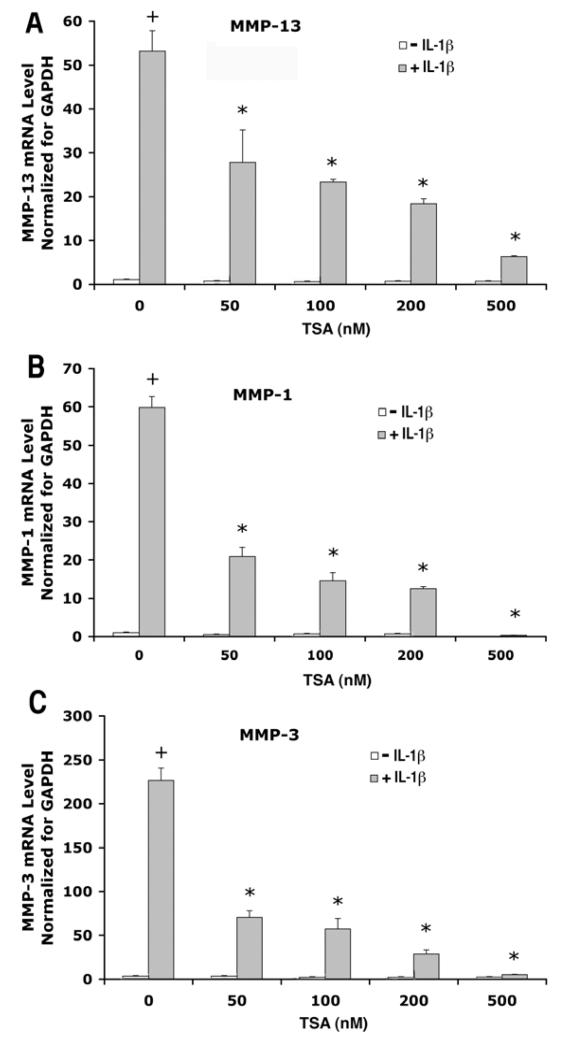
We further investigated the dose dependence of TSA treatment on FGF2 and IL1β effects on MMP expression. We observed that TSA at a concentration as low as 50 nM reduced FGF2 effects on MMP-13 (Fig. 6A) and MMP-1 expression (Fig. 6B), as well as IL-1β effects on MMP-13, MMP-1 and MMP-3 expression (Fig. 7A, B, C). This antagonistic effect of TSA increased in a dose dependent manner.
Figure 6.
Dose response of TSA effects on gene expression of MMP-13, MMP-1, MMP-3 in human articular chondrocytes treated with FGF2. Cells from a single patient were cultured as described in Figure 1 and treated with or without FGF2 (25 ng/ml) in the presence of TSA at various concentrations (0, 50, 100, 200 and 500 nM) for 24 hours. mRNA levels were quantified by real time RT-PCR and normalized to GAPDH. The mean of the group without FGF2 and TSA is adjusted to 1. Data are presented as mean ± SEM. + (p<0.05) indicates significant FGF2 effects after comparison of the two group means with and without FGF2 in the absence of TSA; * (p<0.05) indicates that TSA significantly blocked FGF2 effects by comparing data from FGF2-treated cultures with or without TSA.
Figure 7.
Dose response of TSA effects on gene expression of MMP-13, MMP-1, MMP-3 in human articular chondrocytes treated with IL-1β. Cells from a single patient were cultured as described in Figure 1 and treated with/without FGF2 (25ng/ml) in the presence of TSA at various concentrations (0, 50, 100, 200 and 500 nM) for 24 hours. mRNA levels were quantified by real time RT-PCR and normalized to GAPDH. The mean of the group without IL-1β and TSA is adjusted to 1. Data are presented as mean ± SEM. + (p<0.05) indicates significant IL-1β effects after comparison of the two group means with and without IL-1β in the absence of TSA; * (p<0.05) indicates that TSA significantly blocked IL-1β effects comparing data from IL-1β-treated cultures with or without TSA.
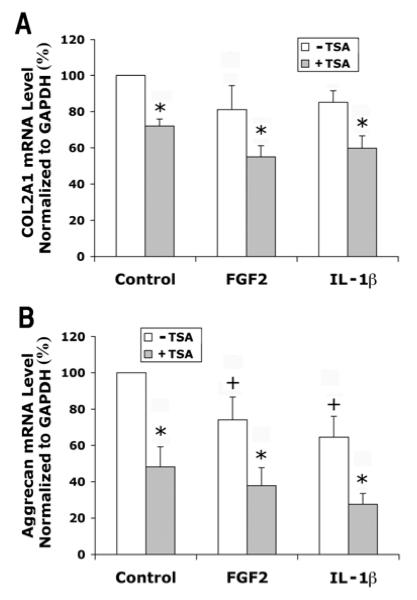
In contrast to the effects on MMPs, FGF2 and IL-1β decreased gene expression levels of aggrecan expression (Fig. 8B). TSA treatment significantly inhibited the expression of both COL2A1 (Fig. 8A) and aggrecan (Fig. 8B).
Figure 8.
Effects of FGF2 and IL-1β with or without TSA on gene expression of COL2A1 and aggrecan in human articular chondrocytes. Cells were cultured and treated with IL-1β and TSA for 24 hours as described in Figure 1. mRNA levels were quantified by real time RT-PCR and normalized to GAPDH. Chondrocytes from three patients were examined in separate experiments. Data were combined from these experiments after the group mean of the control without TSA is adjusted to 100. Data are presented as mean ± SEM. + (p<0.05) indicates significant effects of FGF2 or IL-1β after comparison of the two group means with and without FGF2 or IL-1β in the absence of TSA; * (p<0.05) indicates significant TSA effects by comparing data with and without TSA at the same level of FGF2 or IL-1β.
Discussion
This study examined the role of HDAC in the signaling pathways of FGF2 or IL-1β related to the expression of cartilage degrading enzymes (MMP-1, -3, and -13, and ADAMTS5) in human articular chondrocytes in vitro. The results showed for the first time that the HDAC inhibitor, TSA, attenuated FGF2 induction of MMP-1 and MMP-13. TSA also reduced the stimulatory effect of IL-1β on the expression of MMP-13, MMP-1 and MMP-3.
OA is characterized by the pathology of articular cartilage destruction, which results from elevated activities of MMPs and aggrecanses (Arner 2002; Bramono et al. 2004). Traumatic injury to the joint is considered a significant risk factor for OA. Interestingly, Vincent et al. have demonstrated that cartilage damage releases matrix-bound FGF2 (Vincent et al. 2002), resulting in stimulation of the production of MMP-1 and MMP-3 proteins. It is also established that FGF2 increases MMP-13 expression in human chondrocytes (Tardif et al. 1999; Wang et al. 2004; Im et al. 2007). FGF2 levels are elevated in synovial fluids of OA (Orito et al. 2003; Im et al. 2007) and rheumatoid arthritic joints and correlated with MMP-13 levels (Im et al. 2007). Therefore, in addition to the pro-inflammatory cytokines (such as IL-1 and TNFα, FGF2 could also contribute towards cartilage breakdown in OA. The findings that HDAC inhibitors can block the MMP-inducing effects of pro-inflammatory factors (Young et al. 2005) and of FGF2 suggest their potential pharmacological use for treatment of OA.
It is not clear how HDAC inhibitors antagonize the effects of FGF2 and IL-1β on MMP induction. Both FGF2 and IL-1β stimulate the activity of activating protein 1 (AP-1) to increase MMP-1 and MMP-13 expression (Varghese et al. 2000; Vincenti and Brinckerhoff 2002; Im et al. 2007). c-Jun is one of the major transcription factors in the AP-1 complexes, which are composed of homodimer and heterodimer of proteins from Jun (e.g. c-Jun, JunB, JunD) and Fos (e.g. c-Fos, FosB) families. It was reported that HDAC inhibitors suppress the induction of c-Jun transcription, leading to reduced expression of AP-1-dependent genes in cancer cells (Yamaguchi et al. 2005). We also investigated c-Jun expression in this study, but the results failed to show any correlation between c-Jun mRNA levels and MMP expression (data not shown). Furthermore, TSA did not changed c-Jun mRNA levels, which is consistent with a recent report that HDAC inhibitors do not affect c-Jun expression in chondrocytes (Huh et al. 2007).
HDACs have been shown to directly interact with intracellular signaling molecules, such as NF-κB in fibroblasts (Ashburner et al. 2001) and RUNX2 in osteoblasts (Westendorf et al. 2002; Schroeder et al. 2004). Both FGF2 and IL-1β activate RUNX2, which leads to transactivation of MMP-13 in chondrocytes (Mengshol et al. 2001; Wang et al. 2004). Similarly, NFκB is one of the major signaling targets of IL-1, and mediates IL-1 induced MMP-1 and MMP-13 expression (Vincenti and Brinckerhoff 2002). However, interaction of HDACs with NF-κB (Ashburner et al. 2001) and RUNX2 (Westendorf et al. 2002; Schroeder et al. 2004) by itself has been shown to be associated with gene repression. Further studies are thus needed to clarify how HDACs play a role in MMP expression in a stimulatory manner. Huh et al. have shown that HDAC inhibition increases histone acetylation in Wnt-5a promoter region and induces Wnt-5a expression (Huh et al. 2007), which in turn down-regulates type II collagen expression. Therefore, HDAC inhibition could indirectly suppress MMP expression through upregulating an inhibitory regulator.
We observed that FGF2 increased chondrocyte growth; FGF2 stimulation of proliferation of human articular chondrocytes has been previously reported (Guerne et al. 1994; Loeser et al. 2005). Our results also showed that HDAC inhibition with TSA blocked FGF2 stimulated chondrocyte growth. HDAC inhibitors have been shown to cause G1/G2 growth arrest in a number of normal and transformed cell lines originated from fibroblastic and epithelial cells (Docmanovic and Marks 2005), and suppress proliferation of rheumatoid arthritic synovial fibroblasts by inducing inhibitors of cyclin-dependent kinases (Nishida et al. 2004). Similar mechanisms could be responsible for TSA blocking FGF2-induced chondrocyte proliferation.
FGF2 blocked cell death of articular chondroytes in vitro, and HDAC inhibition did not affect the pro-survival effect of FGF2. It is well documented that FGF2 improves the viability of different cell types, including chondrocytes, osteoblasts, stem cells, and ganglion cells, under a variety of conditions (Houchen et al. 1999; Debiais et al. 2004; Rios-Munoz et al. 2005; Song et al. 2005; Schmal et al. 2007). Although HDAC inhibition causes apoptosis of transformed cells, it appears that HDAC inhibitors do not induce cell death of normal cells, a rationale for their use in treating cancers (Docmanovic and Marks 2005). That HDAC inhibition in chondrocytes does not block the FGF2 pro-survival effect, but inhibits MMP expression, suggests that HDAC inhibitors and FGF2 in combination may be beneficial for maintaining cartilage integrity.
FGF2 treatment decreased ADAMTS5 expression levels in cultured articular chondrocytes. ADAMTS5 has been identified to be the major aggrecan-degrading enzyme in mouse models of OA and inflammatory arthritis (Glasson et al. 2005; Stanton et al. 2005). In human cartilage, ADAMTS5 is more abundant than ADAMTS4 (aggrecanase 1) (Bau et al. 2002). We have also detected a very low level of ADAMTS4 in primary human chondrocytes (data not shown). Although it still remains to be established whether ADAMTS-5 is the major aggrecanase in human cartilage, our data suggest that FGF2 could protect proteoglycans from degradation by inhibiting ADAMTS5 expression.
In this study, we also investigated the effects of FGF2, IL-1β and HDAC inhibition on the expression of chondrocyte anabolic genes. We observed a slight decrease in COL2A1 mRNA levels with FGF2 and IL-1β treatments, although the changes were not statistically significant. Schmal et al. reported that FGF2 treatment decreased COL2A1 messages by 49% in human primary articular chondrocytes (Schmal et al. 2007), while Ellman et al. showed that FGF2 up-regulated both collagen types I and II mRNA expression in intervertebral disc (Ellman et al. 2008). The differences in experimental systems and culture conditions may account for this discrepancy in findings. We also observed that TSA decreased COL2A1 expression after 24 hrs of treatment, which is consistent with a previous report by Huh (Huh et al. 2007). However, Furumatsu reported that TSA increased COL2A1 expression at 4 hrs and 8 hrs of treatment with effects decreasing from 4 hrs to 8 hrs (Furumatsu et al. 2005). It is very likely that TSA affects COL2A1 expression via two different mechanisms in a time dependent manner. At early stages, TSA increases acetylation levels of histones in COL2A1 enhancer region and thus activates COL2A1 expression (Furumatsu et al. 2005). At later stages (after 12 hrs), activated Wnt-5a with TSA treatment has a dominant effect and inhibits COL2A1 expression (Huh et al. 2007). Treatment with FGF2, IL-1βor TSA for 24 hrs inhibited expression of aggrecan. Similar to the effects on COL2A1, TSA was shown to increase aggrecan expression at early stages of treatment (4 hrs), but this effect was largely diminished at 8 hrs of treatment (Furumatsu et al. 2005). Therefore, TSA may affect both COL2A1 and aggrecan in a similar time-dependent manner. Interestingly, Young et al. (Young et al. 2005) did not detect any changes in the expression of COL2A1 and aggrecan in human primary chondrocytes after TSA treatment for 6 hrs. This time point could be in the transition period from TSA activation to inhibition of aggrecan expression. Alternatively, conventional RT-PCR was used to obtain these results and could lack sufficient sensitivity to detect changes in gene expression.
In Summary, our findings reported here on the effects of FGF2, IL-1β and HDAC inhibition on cultured human articular chondrocytes provide interesting insights to possible therapeutic intervention of OA. First, FGF2 and IL-1β increase MMP expression, which can be antagonized by inhibiting HDACs. Secondly, FGF2 has pro-survival effects on chondrocytes and inhibits ADAMTS5 expression, which may help to preserve cartilage integrity, and HDAC inhibition did not interfere with these beneficial effects. One of the drawbacks of HDAC inhibitors is that they may decrease expression of cartilage matrix proteins such as COL2A1 (Huh et al. 2007) and aggrecan in long term (Fig. 8). However, inhibition of both anabolic and catabolic processes may in fact slow down cartilage turnover and, therefore, preserve existing cartilage. In vivo studies are clearly needed to further evaluate whether the in vitro effects are potentially applicable to the rational design of approaches to slow the progression of cartilage destruction in degenerative joint diseases such as OA.
Acknowledgements
The authors thank Dr. Paul Manner, George Washington University and University of Washington, for providing human tissue specimens. This research was supported by the Intramural Research Program of NIAMS, NIH (Z01 AR41131).
Support: Intramural Research Program (Z01 AR41131), National Institute of Arthritis, and Musculoskeletal and Skin Diseases, NIH.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aigner T, Zien A, Gehrsitz A, Gebhard PM, McKenna L. Anabolic and catabolic gene expression pattern analysis in normal versus osteoarthritic cartilage using complementary DNA-array technology. Arthritis Rheum. 2001;44:2777–2789. doi: 10.1002/1529-0131(200112)44:12<2777::aid-art465>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Arner EC. Aggrecanase-mediated cartilage degradation. Curr Opin Pharmacol. 2002;2:322–329. doi: 10.1016/s1471-4892(02)00148-0. [DOI] [PubMed] [Google Scholar]
- Ashburner BP, Westerheide SD, Baldwin AS., Jr. The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–7077. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002;46:2648–2657. doi: 10.1002/art.10531. [DOI] [PubMed] [Google Scholar]
- Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, Poole AR. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramono DS, Richmond JC, Weitzel PP, Kaplan DL, Altman GH. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Related Res. 2004;428:272–285. doi: 10.1097/01.blo.0000144166.66737.3a. [DOI] [PubMed] [Google Scholar]
- Chung YL, Lee MY, Wang AJ, Yao LF. A therapeutic strategy uses histone deacetylase inhibitors to modulate the expression of genes involved in the pathogenesis of rheumatoid arthritis. Mol Therapy. 2003;8:707–717. doi: 10.1016/s1525-0016(03)00235-1. [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–749. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiais F, Lefevre G, Lemonnier J, Le Mee S, Lasmoles F, Mascarelli F, Marie PJ. Fibroblast growth factor-2 induces osteoblast survival through a phosphatidylinositol 3-kinase-dependent, -beta-catenin-independent signaling pathway. Exp Cell Res. 2004;297:235–246. doi: 10.1016/j.yexcr.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Docmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. J Cell Biochem. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev of Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- Ellman MB, An HS, Muddasani P, Im HJ. Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 2008;420:82–89. doi: 10.1016/j.gene.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumatsu T, Tsuda M, Yoshida K, Taniguchi N, Ito T, Hashimoto M, Ito T, Asahara H. Sox9 and p300 cooperatively regulate chromatin-mediated transcription. J Biol Chem. 2005;280:35203–35208. doi: 10.1074/jbc.M502409200. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Guerne PA, Sublet A, Lotz M. Growth factor responsiveness of human articular chondrocytes: distinct profiles in primary chondrocytes, subcultured chondrocytes, and fibroblasts. J Cell Physiol. 1994;158:476–484. doi: 10.1002/jcp.1041580312. [DOI] [PubMed] [Google Scholar]
- Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol. 1999;276:G249–258. doi: 10.1152/ajpgi.1999.276.1.G249. [DOI] [PubMed] [Google Scholar]
- Huh YH, Ryu JH, Chun JS. Regulation of type II collagen expression by histone deacetylase in articular chondrocytes. J Biol Chem. 2007;282:17123–17131. doi: 10.1074/jbc.M700599200. [DOI] [PubMed] [Google Scholar]
- Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, van Wijnen AJ, Loeser RF. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J Biol Chem. 2007;282:11110–11121. doi: 10.1074/jbc.M609040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cart. 2002;10:785–791. doi: 10.1053/joca.2002.0823. erratum appears in Osteoarthritis Cartilage 2002 Nov;10(1):905. [DOI] [PubMed] [Google Scholar]
- Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Chubinskaya S, Pacione C, Im HJ. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum. 2005;52:3910–3917. doi: 10.1002/art.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengshol JA, Vincenti MP, Brinckerhoff CE. IL-1 induces collagenase-3 (MMP-13) promoter activity in stably transfected chondrocytic cells: requirement for Runx-2 and activation by p38 MAPK and JNK pathways. Nucleic Acids Res. 2001;29:4361–4372. doi: 10.1093/nar/29.21.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Hideshima T, Akiyama M, Chauhan D, Munshi N, Gu X, Bailey C, Joseph M, Libermann TA, Richon VM, Marks PA, Anderson KC. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: biological and clinical implications. PNAS. 2004;101:540–545. doi: 10.1073/pnas.2536759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Komiyama T, Miyazawa S, Shen ZN, Furumatsu T, Doi H, Yoshida A, Yamana J, Yamamura M, Ninomiya Y, Inoue H, Asahara H. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004;50:3365–3376. doi: 10.1002/art.20709. [DOI] [PubMed] [Google Scholar]
- Orito K, Koshino T, Saito T. Fibroblast growth factor 2 in synovial fluid from an osteoarthritic knee with cartilage regeneration. J Orthop Sci. 2003;8:294–300. doi: 10.1007/s10776-003-0647-6. [DOI] [PubMed] [Google Scholar]
- Peart MJ, Smyth GK, van Laar RK, Bowtell DD, Richon VM, Marks PA, Holloway AJ, Johnstone RW. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. PNAS. 2005;102:3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Munoz W, Soto I, Duprey-Diaz MV, Blagburn J, Blanco RE. Fibroblast growth factor 2 applied to the optic nerve after axotomy increases Bcl-2 and decreases Bax in ganglion cells by activating the extracellular signal-regulated kinase signaling pathway. J Neurochem. 2005;93:1422–1433. doi: 10.1111/j.1471-4159.2005.03129.x. [DOI] [PubMed] [Google Scholar]
- Schmal H, Zwingmann J, Fehrenbach M, Finkenzeller G, Stark GB, Sudkamp NP, Hartl D, Mehlhorn AT. bFGF influences human articular chondrocyte differentiation. Cytotherapy. 2007;9:184–193. doi: 10.1080/14653240601182846. [DOI] [PubMed] [Google Scholar]
- Schroeder TM, Kahler RA, Li X, Westendorf JJ. Histone deacetylase 3 interacts with runx2 to repress the osteocalcin promoter and regulate osteoblast differentiation. J Biol Chem. 2004;279:41998–42007. doi: 10.1074/jbc.M403702200. [DOI] [PubMed] [Google Scholar]
- Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- Song H, Kwon K, Lim S, Kang SM, Ko YG, Xu Z, Chung JH, Kim BS, Lee H, Joung B, Park S, Choi D, Jang Y, Chung NS, Yoo KJ, Hwang KC. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005;19:402–407. [PubMed] [Google Scholar]
- Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Tardif G, Pelletier JP, Dupuis M, Geng C, Cloutier JM, Martel-Pelletier J. Collagenase 3 production by human osteoarthritic chondrocytes in response to growth factors and cytokines is a function of the physiologic state of the cells. Arthritis Rheum. 1999;42:1147–1158. doi: 10.1002/1529-0131(199906)42:6<1147::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Varghese S, Rydziel S, Canalis E. Basic fibroblast growth factor stimulates collagenase-3 promoter activity in osteoblasts through an activator protein-1-binding site. Endocrin. 2000;141:2185–2191. doi: 10.1210/endo.141.6.7504. [DOI] [PubMed] [Google Scholar]
- Vincent T, Hermansson M, Bolton M, Wait R, Saklatvala J. Basic FGF mediates an immediate response of articular cartilage to mechanical injury. PNAS. 2002;99:8259–8264. doi: 10.1073/pnas.122033199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cart. 2004;12:963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Westendorf JJ, Zaidi SK, Cascino JE, Kahler R, van Wijnen AJ, Lian JB, Yoshida M, Stein GS, Li X. Runx2 (Cbfa1, AML-3) interacts with histone deacetylase 6 and represses the p21(CIP1/WAF1) promoter. Mol Cell Biol. 2002;22:7982–7992. doi: 10.1128/MCB.22.22.7982-7992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Lantowski A, Dannenberg AJ, Subbaramaiah K. Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2. J Biol Chem. 2005;280:32569–32577. doi: 10.1074/jbc.M503201200. [DOI] [PubMed] [Google Scholar]
- Young DA, Lakey RL, Pennington CJ, Jones D, Kevorkian L, Edwards DR, Cawston TE, Clark IM. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Therapy. 2005;7:R503–512. doi: 10.1186/ar1702. [DOI] [PMC free article] [PubMed] [Google Scholar]