Abstract
Tendon is one of the least understood tissues of the musculoskeletal system in terms of development and morphogenesis. Collagen fibrillogenesis has been the most studied aspect of tendon development, focusing largely on the role of matrix molecules such as collagen type III and decorin. While involvement of matrix molecules in collagen fibrillogenesis during chick tendon development is well understood, the role of growth factors has yet to be elucidated. This work examines the expression patterns of TGF-ß1, -ß2, and -ß3, and their receptors with respect to expression patterns of collagen type III, decorin, and fibronectin. We focus on the intermediate stages of tendon development in the chick embryo, a period during which the tendon micro- and macro-architecture are being established. Our findings demonstrate for the first time that TGF-ß1, -ß2, and -ß3 have distinct spatiotemporal developmental protein localization patterns in the developing tendon and strongly suggest that these isoforms have independent roles in tendon development.
Keywords: tendon, tendon development, chick, TGF-ß, collagen type III, decorin, fibronectin
INTRODUCTION
Tendon is one of the least understood tissues of the musculoskeletal system in terms of development and morphogenesis. The extracellular matrix of mature tendon, fibrous connective tissue that transmits load from muscle to bone, consists primarily of collagen type I, with smaller amounts of other collagen types, elastin, proteoglycans, and glycoproteins (Goh et al., 2003). Collagen type I molecules are hierarchically organized into structural units: molecules aggregate to form fibrils; bundles of fibrils form fibers; fibers group into primary fiber bundles or fascicles; and fascicles group together to form tertiary fiber bundles. Endotendinous connective tissue (endotenon) surrounds the fiber bundles, and contains blood vessels, lymphatics, and nerves. The whole tendon, composed of multiple bundles and endotenon, is surrounded by the epitenon, a thin layer of connective tissue that is contiguous with the endotenon (Figure 1).
Figure 1.
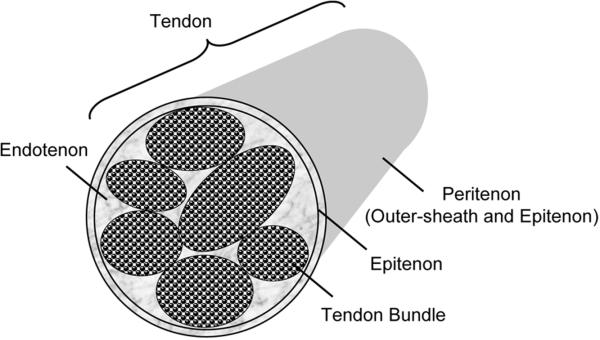
Schematic of tendon architecture.
The biomechanical function and biochemical composition of tendon in its mature form are well characterized, but its embryonic development, unlike other musculoskeletal tissues, has only been minimally addressed. The regulation of tendon formation during development is largely unknown (reviewed in Edom-Vovard and Duprez, 2004; Tozer and Duprez, 2005). Only in recent years has a highly specific marker for tendons, basic helix-loop-helix transcription factor scleraxis, been identified (Schweitzer et al., 2001; Brent et al., 2003). The function of scleraxis during tendon development has yet to be elucidated. However, a necessary role for scleraxis is suggested by the disruption in development of force-transmitting and intermuscular tendons in scleraxis-null mice (Murchison et al., 2007). Ultimately, the bulk of our understanding of tendon development derives from collagen fibrillogenesis studies.
The vast majority of published work investigating collagen fibrillogenesis has been conducted with chick embryonic tendon (reviewed by Zhang et al., 2005). Three distinct steps of fibrillogenesis during tendon development have been described: (1) immature fibril assembly (corresponding to days 12-16 of chick embryonic metatarsal tendon development); (2) linear growth (days 16 and 17); and (3) lateral growth (days 17 and 18) (Birk et al., 1995; Zhang et al., 2005). Collagen type III and decorin have been shown to be involved in the regulation of collagen fibrillogenesis (Vogel et al., 1984; Thieszen and Rosenquist, 1995; Birk et al., 1997; Danielson et al., 1997; Hakkinen et al., 2000; Zhang et al., 2006). Collagen type III is a member of the fibrillar collagen family and the second most abundant collagen molecule in tendon. Studies in skin, tendon, and other tissues indicate a role for collagen type III in regulating fibrillogenesis of collagen type I (Fleischmajer et al., 1988; Fleischmajer et al., 1990; Liu et al., 1997; Birk and Mayne, 1997; Ros et al., 1995). Decorin is a small chondroitin-dermatan sulfate proteoglycan consisting of a core protein and a single glycosaminoglycan chain, and is the most abundant proteoglycan in mature tendon (Goh et al., 2003). It is through the core protein that decorin binds collagen types I and III, presumably to regulate fibrillogenesis (Vogel et al., 1984; Thieszen and Rosenquist, 1995).
While involvement of decorin and collagen type III in chick tendon development has been investigated, the role of growth factors has yet to be elucidated. The transforming growth factor (TGF)-ß superfamily encompasses structurally-related proteins that include TGF-ß isoforms, activins, inhibins, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs). Isoforms TGF-ß1, -ß2, and -ß3 regulate processes of development, cell proliferation, and extracellular matrix production, typically via binding to three high-affinity cell surface receptors: TGF-ß type I (TGF-ßRI), type II (TGF-ßRII) and type III receptors (Massague, 1998; Massague and Gomis, 2006). ECM proteins under TGF-ß regulation include collagens, decorin, and fibronectin (Balza et al., 1988; Saed et al., 1999; Klein et al., 2002; Fu et al., 2005). Both decorin and fibronectin are capable of binding TGF-ß, presumably to regulate its activity (Schmidt et al., 1987; Mooradian et al., 1989; Yamaguchi et al., 1990; Hildebrand et al., 1994; Schonherr et al., 1998). Decorin, in particular, has been shown to possess independent binding sites for TGF-ßs and collagen type I (Schonherr et al., 1998).
Surprisingly, while the involvement of TGF-ßs in embryonic tendon development has been suggested by a limited number of studies, its role in tendon morphogenesis is largely unknown. TGF-ß2 expression was detected via in situ hybridization in embryonic mouse tendon (Pelton et al., 1989). Detection of TGF-ß1 via subtractive hybridization in chick tendon during days 14 and 19 of development implicated the isoform in collagen fibrillogenesis in tendon morphogenesis (Nurminskaya and Birk, 1998). In contrast, most studies have focused mainly on the role of TGF-ßs in adult tendon and ligament pathology and wound healing. Thus, elevated protein and mRNA levels of TGF-ß have been demonstrated in pathological Achilles tendons and during healing of injured tendons and ligaments (Natsu-ume et al., 1997; Fenwick et al., 2001; Darmani et al., 2004; Tsubone et al., 2004; Dahlgren et al., 2005). Exogenous application of TGF-ß1 and -ß2 significantly increased expression of collagen types I and III, and improved the mechanical properties of healing tendons or ligaments (Spindler et al., 2003; Kashiwagi et al., 2004; Anaguchi et al., 2005). Alternatively, in vivo treatment of injured ligament by antisense decorin oligodeoxynucleotides resulted in larger collagen fibrils and a significant improvement in mechanical properties, compared to control treatments (Nakamura et al., 2000). In vitro, TGF-ß1 has been reported to stimulate mitogenic responses of cultured patellar tendon fibroblasts (Spindler et al., 1996) and mediate proteoglycan synthesis in tendon explants (Robbins et al., 1997). While these studies have served as the basis for developing strategies to improve adult tendon and ligament healing utilizing TGF-ßs, the approaches are largely focused on enhancing mechanical properties of scar tissue rather than regenerating normal, scarless tissue. Furthermore, TGF-ß isoforms in these studies are often used interchangeably.
We propose that a rational approach to enhance adult tissue healing should focus on regenerating normal adult tissue, based on mechanisms of embryonic development. Our interest is whether TGF-ßs are involved in tendon morphogenesis. The aim of the current study is to characterize the spatiotemporal patterns of TGF-ß1, -ß2, and -ß3, and their receptors (TGF-ßRI and TGF-ßRII) with respect to expression patterns of collagen type III, decorin, and fibronectin during the intermediate stages of tendon development in the chick embryo, a period during which the tendon micro- and macro-architecture are being established. In particular, we focus on the intermediate tendon in the mid-substance, and the region near the myotendinous junction (MTJ). We chose to include the region near the MTJ because other growth factors demonstrated to play a role in tendon development have been shown to be present at elevated levels in the tendon close to muscle fibers (Edom-Vovard et al., 2001).
RESULTS
Histology
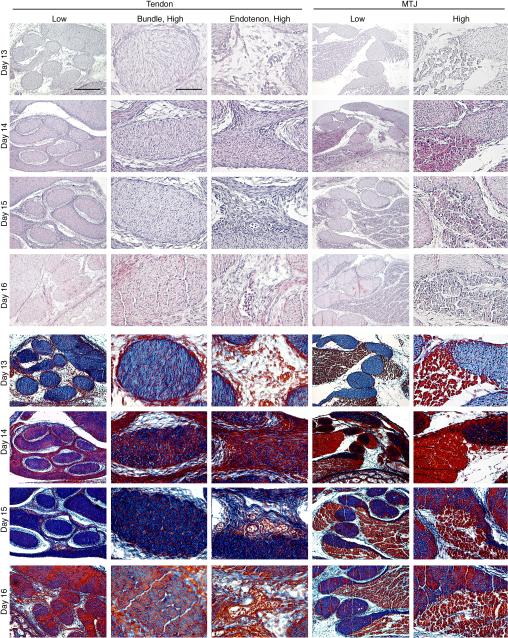
During the embryonic development time period examined (days 13-16), the tertiary bundles increased in size and apparent matrix density, while maintaining high cellularity (Figure 2). In the tendon mid-substance, on day 13, the bundles were round in shape and appeared to be comprised mainly of disorganized collagen (blue in trichrome staining) and uniformly distributed cells (red nuclei in trichrome staining). By day 14, the bundles began to take on a more oval shape. Within each bundle, collagen appeared to be organizing into fascicles, as suggested by dense aggregates of staining. Day 15 bundles stained extremely dark blue for increased collagen content, indicating active matrix accumulation and organization. Cellularity seemed to begin to decrease on day 15. On day 16, clearly defined fascicles were evident with reduced cellularity, as compared to earlier time points.
Figure 2.
Histology of intermediate tendon at developmental days 13,14, 15, and 16. (Top) Hematoxylin-eosin staining; (Bottom) Mallory's trichrome staining. Low magnification, Bar = 200 μm; high magnification, Bar = 50 μm.
Dramatic changes with time were also detected in the endotenon in the tendon mid-substance from days 13 through 16. On day 13, the endotenon was sparse in matrix and cell density. Cell density and matrix and collagen content in the endotenon increased rapidly over the next 24 h. By day 15, collagen fiber alignment was apparent, and immature blood vessels containing erythrocytes were easily identified. Maturation of the endotenon and blood vessels continued through day 16.
At all time points studied, tendon and muscle were easily differentiated. Muscle fibers developed within fascicles adjacent to tendon bundles and contiguous with those that surrounded individual tendon bundles. At day 13, the tendon bundles were histologically similar to those in the midsubstance. Muscle fibers were loosely organized within fascicles at a relatively low density. By day 14, both muscle and tendon stained more intensely, indicating increases in cell and matrix density. Muscle fibers were greater in size and packed together compactly within muscle fascicles. Tendon stained intensely for matrix and cells. Significant reorganization seemed to occur after day 14. Within fascicles, muscle fibers appeared to undergo growth and maturation, become more condensed and morphologically defined. Tendon bundles contained well-defined fascicles as characterized in the mid-substance. Surrounding each tendon bundle were muscle fibers interfacing with the tendon fascicles.
Spatiotemporal Profiles of Matrix Components in the Tendon
Mid-Substance: Tertiary Bundles
Collagen Type III
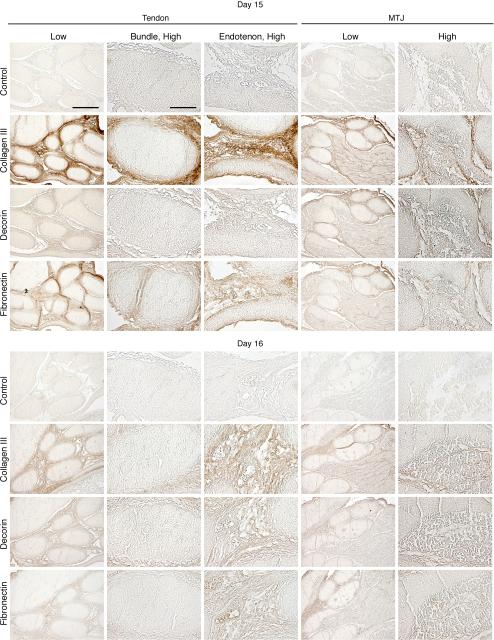
Collagen type III immunostaining was present throughout the tertiary bundles on day 13 (Figure 3). At day 14, collagen type III signal became detectable outward, towards the edges of the tertiary bundles (Figure 3). By day 15, collagen type III was no longer detected in the tertiary bundles (Figure 4).
Figure 3.
Immunohistochemical localization of collagen type III, decorin, and fibronectin in intermediate tendon at developmental days 13 and 14. Low magnification, Bar = 200 μm; high magnification, Bar = 50 μm.
Figure 4.
Immunohistochemical localization of collagen type III, decorin, and fibronectin in intermediate tendon at developmental days 15 and 16. Low magnification, Bar = 200 μm; high magnification, Bar = 50 μm.
Decorin
Decorin was detected on day 13 in the tertiary bundles, with substantially reduced staining intensity on day 14 (Figure 3). Decorin staining was only detected in the lining of the tertiary bundles by day 15, with lower intensity on day 16 (Figure 4).
Fibronectin
On day 13, fibronectin was immunodetected in the tertiary bundles in a similar pattern as collagen type III (Figure 3). On days 14 and 15, fibronectin level was reduced, and became undetectable in the bundle by day 16 (Figures 3 and 4).
Mid-Substance: Endotenon
Collagen Type III
Collagen type III was immunodetected throughout the endotenon and epitenon on days 13 and 14 (Figure 3). At day 15, collagen type III was visible in the endotenon and in the peritenon (Figure 4). Distribution of collagen type III at day 16 was similar to day 15, but lower in intensity (Figure 4).
Decorin
Decorin immunostained the endotenon at all time points, but was detected at low intensity on days 15 and 16 (Figure 4).
Fibronectin
Fibronectin was detected in the endotenon and in the peritenon on day 13 (Figure 3). Immunostaining was diffuse throughout the tissue as well as in developing blood vessels. After day 13, expression levels were reduced in intensity but remained in the endotenon through day 16 (Figures 3 and 4).
MTJ
Collagen Type III
On all days, collagen type III was immunodetected in the connective tissue between muscle fibers and surrounding muscle fascicles. On day 13, collagen type III immunostaining was detected in the tertiary bundles, and more strongly in the endotenon (Figure 3). Signal was lower in the tertiary bundles on day 14, but continued in the endotenon through day 16 (Figures 3 and 4). However, no staining was detectable in the tertiary bundles after day 14.
Decorin
Decorin was detected in the tertiary bundles and endotenon, but not in muscle on day 13 (Figure 3). On days 14-16, staining was present in muscle, but no longer detected in the tertiary bundles (Figures 3 and 4). However, staining intensity continued in the endotenon through day 16.
Fibronectin
Fibronectin immunostaining was diffuse throughout muscle and tendon (tertiary bundles and endotenon) on day 13 (Figure 3). From days 14-16, however, staining was detected in muscle and the endotenon of tendon (Figures 3 and 4).
Spatiotemporal Profiles of TGF-ßs and TGF-ß Receptors in the Tendon
Mid-Substance: Tertiary Bundles
TGF-ß1, -ß2, and -ß3
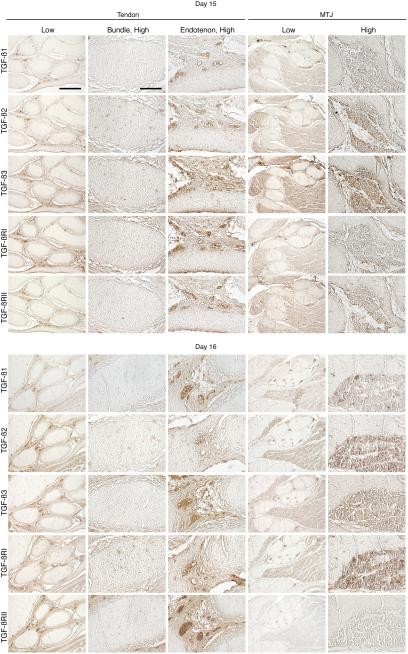
TGF-ß1 was not detected in the tertiary bundles on any of the days studied (Figures 5 and 6). TGF-ß2 staining was detected throughout the tertiary bundles on all days examined, with broadest presence and highest intensity on day 14 (Figures 5 and 6). On day 15, TGF-ß2 became localized to aggregates of staining within the tertiary bundles, maintaining its presence through day 16. TGF-ß3 was detected in the tertiary bundle diffusely on day 13, and in aggregates (Figure 5). TGF-ß3 signal continued to be diffuse throughout the tertiary bundles on day 14 (Figure 5). By day 15, staining was again detected in aggregates within the tertiary bundle, but was nearly undetectable by day 16 (Figure 6).
Figure 5.
Immunohistochemical localization of TGF-ß1, -ß2, and -ß3, and TGF-ßRI and RII in intermediate tendon at developmental days 13 and 14. Low magnification, Bar = 200 μm; high magnification, Bar = 50 μm.
Figure 6.
Immunohistochemical localization of TGF-ß1, -ß2, and -ß3, and TGF-ßRI and RII in intermediate tendon at developmental days 15 and 16. Low magnification, Bar = 200 μm; high magnification, Bar = 50 μm.
TGF-ß Receptors I and II
On day 13, TGF-ßRI was detected in the cells of the tertiary bundles (Figure 5). By day 14, signal was detected diffusely throughout the tertiary bundle and in aggregates of cells (Figure 5). On day 15, aggregates were no longer detected (Figure 6), although on day 16, staining was again localized to aggregates within the tertiary bundle (Figure 6). On days 13 and 14, TGF-ßRII was detected throughout the tertiary bundles, with peak intensity on day 14 (Figure 5). By day 15, TGF-ßRII was no longer detected in the tertiary bundles (Figure 6).
Mid-Substance: Endotenon
TGF-ß1, -ß2, and -ß3
TGF-ß1 was absent from the endotenon on day 13 (Figure 5). On day 14, a low level was detected within the endotenon, near the tertiary bundles (Figure 5). By days 15 and 16, TGF-ß1 was seen in the blood vessels and the edges of the endotenon (Figure 6). On days 13-16 TGF-ß2 was detected in the blood vessels of the endotenon as well as the endotenon itself (Figures 5 and 6). TGF-ß3 had a similar staining pattern to that of TGF-ß2, but was slightly more widespread on all days (Figures 5 and 6).
TGF-ß Receptors I and II
On days 13 and 14, TGF-ßRI was detected diffusely in aggregates in the endotenon (Figure 5). On days 15 and 16, immunostaining was seen throughout the endotenon, and at high level in the blood vessels (Figure 6). On days 13 and 14, TGF-ßRII staining was diffuse through the endotenon, with peak intensity on day 14, but mostly confined to the blood vessels by days 15 and 16 (Figures 5 and 6).
MTJ
TGF-ß1, -ß2, and -ß3
TGF-ß1 was not detected on day 13, but on day 14 was expressed in the endotenon and blood vessels (Figure 5). On days 15 and 16, the signal was localized to the blood vessels in the endotenon (Figure 6). TGF-ß1 immunostaining was seen in muscle on days 14-16. On day 13, TGF-ß2 was detected in aggregates of cells in the tertiary bundles and endotenon (Figure 5). On days 14 and 15, signal was throughout the endotenon, but was absent by day 16 (Figures 5 and 6). TGF-ß3 stained broadly on day 13, and often in aggregates (Figure 5). By day 14, the aggregates had disappeared, but signal persisted throughout the tertiary bundles and endotenon, including blood vessels, and continued through days 15 and 16 (Figures 5 and 6). Both TGF-ß2 and -ß3 immunostaining was detected in muscle on all days examined.
TGF-ß Receptors I and II
During days 13-16, TGF-ßRI was detected in the endotenon and blood vessels, and also in muscle fibers (Figures 5 and 6). On day 13, staining was detected in the tertiary bundles, and often seen in aggregates. From day 14 on, staining in the bundles was diffuse and at times difficult to detect. Distribution of TGF-ßRII in the tendon from days 13-16 seemed to mimic that of TGF-ßRI (Figures 5 and 6). Signal was also detected in muscle fibers from days 13-16.
DISCUSSION
This study presents the spatiotemporal patterns of TGF-ß1, -ß2, and -ß3, and the TGF-ß receptors I and II with respect to extracellular matrix molecules, collagen type III, decorin, and fibronectin, during days 13-16 of tendon development in the chick embryo (summarized in Table 1), a period during which the tendon micro- and macro-architecture are being established (Birk et al., 1989). Immunostaining patterns for collagen type III and decorin were consistent with previous findings demonstrating the localization of these matrix molecules to the periphery of the bundles, a phenomenon reflecting their role in regulating collagen fibrillogenesis during tendon morphogenesis. Interestingly, TGF-ß1, unlike the other two isoforms, was not detected in tendon on any of the days investigated. In contrast, TGF-ß2 and –ß3 were present in patterns that were dynamic with time, and distinct from one another, suggesting independent roles for each growth factor during development. Furthermore, the spatiotemporal patterns of the matrix molecules and growth factors in the tendon at the MTJ did not differ significantly form those at the mid-substance of the tendon.
Table 1.
Summary of Immunostaining Levels of Collagen Type III, Decorin, Fibronectin, TGF-ß1, -ß2, -ß3, and TGF-ßRI and -ßRII in Chick Embryonic Tendon*
| Tertiary Bundle | Col III | DCN | FN | TGF-ß1 | TGF-ß2 | TGF-ß3 | TGF-ßRI | TGF-ßRII |
|---|---|---|---|---|---|---|---|---|
| Day 13 | +++ | +++ | +++ | - | + | ++ | + | + |
| Day 14 | + | +++ | + | - | +++ | +++ | +++ | +++ |
| Day 15 | - | - | - | - | ++ | + | + | - |
| Day 16 | - | - | - | - | ++ | + | + | - |
| Endotenon | Col III | DCN | FN | TGF-ß1 | TGF-ß2 | TGF-ß3 | TGF-ßRI | TGF-ßRII |
|---|---|---|---|---|---|---|---|---|
| Day 13 | +++ | ++ | ++ | - | + | +++ | + | + |
| Day 14 | + | +++ | ++ | + | ++ | +++ | +++ | +++ |
| Day 15 | +++ | + | ++ | ++ | ++ | +++ | ++ | ++ |
| Day 16 | ++ | + | + | +++ | ++ | +++ | ++ | ++ |
| Tertiary Bundle (MTJ) | Col III | DCN | FN | TGF-ßl | TGF-ß2 | TGF-ß2 | TGF-ßRI | TGF-ßRII |
|---|---|---|---|---|---|---|---|---|
| Day 13 | ++ | ++ | +++ | - | + | + | + | + |
| Day 14 | + | - | - | - | + | ++ | ++ | ++ |
| Day 15 | - | - | - | - | + | + | + | + |
| Day 16 | - | - | - | - | - | + | + | - |
| Muscle (MTJ) | Col III | DCN | FN | TGF-ßl | TGF-ß2 | TGF-ß3 | TGF-ßRI | TGF-ßRII |
|---|---|---|---|---|---|---|---|---|
| Day 13 | ++ | - | +++ | - | +++ | + | ++ | + |
| Day 14 | + | + | ++ | ++ | +++ | +++ | +++ | +++ |
| Day 15 | + | + | + | + | +++ | +++ | + | +++ |
| Day 16 | + | + | + | + | +++ | ++ | +++ | - |
Collagen type III, Col III; decorin, DCN: fibronectin, FN; MTJ, myotendinous junction
In view of the incomplete understanding of embryonic tendon development, it is noteworthy that our results show that many of the matrix molecules involved in mature tendon healing, including collagen type III, decorin, and fibronectin, are also present in the developing tendon. However, the adult healing process results in scar tissue with aberrant properties, and is unlikely to recapitulate tendon development. The current strategies in tendon injury research using animal models are to either apply or suppress growth factors amplified during wound healing to tendon injury sites (Nakamura et al., 2000; Spindler et al., 2003; Kashiwagi et al., 2004; Anaguchi et al., 2005). This approach has been successful in skin, in which a scarless embryonic or scar-forming adult healing response has been demonstrated to be a function of TGF-ß isoform (Krummel et al., 1988; Shah et al., 1992; Shah et al., 1994; Shah et al., 1995). In tendon, altered healing response and induction of matrix molecules in response to abnormally high or low concentrations of growth factors, such as TGF-ßs, have been reported (Nakamura et al., 2000; Spindler et al., 2003; Kashiwagi et al., 2004; Anaguchi et al., 2005); however, rational therapy depends on knowledge of where and when these growth factors are present during embryonic tendon development and wound healing, which is critically lacking. These studies often use the two isoforms, TGF-ß1 and -ß3, interchangeably, while our data suggest that the two isoforms have distinct roles during morphogenesis. Whether tendon follows the same rubric as skin is of interest as this knowledge will be beneficial to regenerating tendon or enhancing scarless adult tendon healing. We are currently investigating how TGF-ß isoforms are involved in collagen fibrillogenesis during prenatal tendon healing (Kuo et al., in preparation). Our aim here is to first establish which TGF-ßs are present during fibril assembly during development.
Decorin and fibronectin have both been shown to associate with collagens, and play critical roles in regulating fibrillogenesis of collagen types I and III (Engvall et al., 1978; Kleinman et al., 1981; Fleischmajer and Timpl, 1984; Speranza et al., 1987; Birk et al., 1995; Danielson et al., 1997; Graham et al., 2000; Velling et al., 2002). In connective tissues, including blood vessels and skin, collagen type III co-localizes with collagen type I within the same fibril, presumably to regulate the size of collagen type I fibrils (Fleischmajer et al., 1988; Fleischmajer et al., 1990). In Col3a1-/- mutant mice, electron microscopy detected collagen fibrils irregular in size in the skin and the adventitia of the aorta, indicating the necessity for collagen type III for normal collagen fibrillogenesis (Liu et al., 1997). Ultrastructural analysis of mice harboring a targeted disruption of the decorin gene revealed abnormal collagen morphology in decorin-deficient tendon, with coarser fibrils and irregularities in size and shape when compared to wild-type tissue (Danielson et al., 1997). In the chick embryo, collagen fibrils isolated from metatarsal leg tendons and treated to digest surface-bound proteoglycans, exhibited lateral association to create large-diameter structures, while undigested fibrils remained separate and showed no aggregation (Graham et al., 2000).
We observed that collagen type III, decorin, and fibronectin are all expressed in the tertiary bundles on day 13, and are localized to the periphery of the bundles and to the endotenon by day 16. These results are in agreement with previous findings that hypothesize decorin and collagen type III regulate collagen fiber diameter by inhibiting the lateral fusion of fibrils. Birk et al. (1995) observed an incremental increase in mean segment length of collagen fibrils isolated from embryonic chick metatarsal tendons between days 13 and 16 of development (Birk et al., 1995), and that fibrils isolated during these days were bound by significant amounts of decorin. After day 16, a significant increase in segment length was detected, accompanied by extensive lateral associations of fibril segments, and was correlated with a significant decrease in fibril-associated decorin. This drop in decorin content correlated with the onset of mature fibril formation that begins at day 17, an event attributed to fusion of fibril segment precursors (Birk et al., 1995). Furthermore, decorin protein and mRNA levels both decreased during the same period. Birk and Mayne (1997) demonstrated an inverse relationship between collagen type III level and fibril diameter in the developing metatarsal tendon (Birk and Mayne, 1997). At day 14, collagen type III was strongly expressed in the fascicles, where it co-distributed with collagen type I. However, by day 17, collagen type III immunoreactivity, as well as small diameter fibrils, was limited to the endotendenium. Similar observations have been reported by Fleischmajer et al. (1988). Birk et al. characterized metatarsal (distal) tendons, while our studies focused on intermediate tendons; thus the similarity in trend but difference in specific developmental stage could be a function of the tendons studied. Taken together, our results, coupled with published studies reporting similarly observed changes in decorin and collagen type III protein distribution and mRNA levels, support the hypothesis that decorin and collagen type III are involved in collagen fibrillogenesis.
While TGF-ßs have been shown to induce production of collagen type III (Balza et al., 1988; Saed et al., 1999; Klein et al., 2002), decorin production is not affected (Robbins et al., 1997). However, binding of TGF-ß by decorin and fibronectin has been reported (Mooradian et al., 1989; Yamaguchi et al., 1990; Schonherr et al., 1998), and blocking decorin gene expression results in suppression of TGF-ß1 production (Hosaka et al., 2005). Interestingly, it has been reported that TGF-ß does not bind to collagen directly (Schonherr et al., 1998). Despite numerous reports investigating the effects of TGF-ßs on matrix molecule production and negative regulation of the growth factors by these matrix molecules, few studies have focused on the endogenous spatiotemporal distribution of TGF-ßs, particularly specific TGF-ß isoforms, in the tendon proper during development. Notably, our results show that decorin expression is strongest during days 13 and 14, and becomes localized to the endotenon starting on day 15, whereas TGF-ß1 expression is not detected at day 13, but becomes strongly expressed in the endotenon by day 16. Interestingly TGF-ß2 and -ß3 are most broadly expressed on day 14. The temporal profiles and the distinct isotype-specific localizations suggest different roles for the TGF-ß isoforms. In contrast, the majority of previous TGF-ß studies have focused on TGF-ß1, with the assumption that the effects are representative of all TGF-ß isoforms. This assumption may not be valid, given our observations.
TGF-ß has been shown to induce fibronectin synthesis (Hocevar et al., 1999). Interestingly, we detected a very similar expression pattern for fibronectin to that of collagen type III. Fibronectin is a dimeric glycoprotein reported to be present during early tendon development (Hurle et al., 1989). In mice, the homozygous fibronectin mutant allele causes early embryonic lethality (George et al., 1993). However, in vitro experiments have demonstrated specific functions of fibronectin in terms of interactions with collagen. Fibronectin has been shown to have a greater affinity for collagen type III than type I (Engvall et al., 1978). Binding of collagen fibrils by fibronectin regulates fibrillogenesis in vitro, presumably due to the high affinity of fibronectin to collagen type III, which competes with other collagen molecules to result in inhibited fibrillogenesis (Speranza et al., 1987). On the other hand, a recent study has provided evidence that an existing fibronectin matrix is essential for collagen deposition and network formation (Velling et al., 2002). While the specific function of fibronectin during tendon development has yet to be elucidated, our observations, taken together with those from previous studies, strongly suggest that this particular matrix molecule plays an important role in fibrillogenesis during tendon development.
Also expressed in the developing blood vessels are TGF-ßs and their receptors. By day 16, all three isoforms and their receptors are strongly detected along with fibronectin. In normal and pathological Achilles tendons, TGF-ßs and RI and RII are expressed at sites of vascularization (Fenwick et al., 2001). VEGF is expressed during development in both tertiary bundles as well as in the endotenon (unpublished data). A number of studies have reported the ability of VEGF to induce TGF-ß1 mRNA expression both in healing tendon and in tendon fibroblasts in vitro (Zhang et al., 2003; Wang et al., 2005). Alternatively, TGF-ßs have also been demonstrated to induce VEGF secretion in cultures of human retinal pigment epithelial cells (Nagineni et al., 2003). TGF-ßs are known to induce expression of fibronectin, which is involved in angiogenesis (Balza et al., 1988; Nicosia et al., 1993). Taken together, our data support the possibility that in the developing tendon, VEGF and TGF-ß act in a positive feedback loop, involving TGF-ß signaling through receptors RI and RII, to regulate matrix production and angiogenesis.
In conclusion, we have demonstrated distinct spatiotemporal developmental protein expression patterns of TGF-ß1, -ß2, and –ß3, and their receptors RI and RII with respect to collagen type III, decorin, and fibronectin in the developing tendon at the mid-substance and at the MTJ. These findings are strongly supportive of the postulate that TGF-ßs are involved in tendon development, and that TGF-ß1, -ß2, and –ß3 have independent roles. In addition, these results, taken with previous studies, suggest that the actions of TGF-ßs during tendon development are likely to be regulated by the matrix molecules, collagen type III, decorin, and fibronectin, which may, in turn, modulate TGF-ß activity in a feedback loop.
EXPERIMENTAL PROCEDURES
Fertilized white leghorn chicken eggs (CBT Farms, Chestertown, MD) were incubated at 38°C in a humidified incubator. On incubation days 13, 14, 15, and 16, embryos were sacrificed via decapitation, and legs were removed at the knee joint and fixed in phosphate-buffered 4% paraformaldehyde overnight at 4°C. After decalcification in Immunocal (Decal Chemical Corp, Tallman, NY), the leg was trimmed to isolate the ankle with the intermediate tendon (Figure 7). Tissue specimens were dehydrated through serially graded ethanol washes, and paraffin-embedded. Transverse sections were obtained at 5 μm thickness, and stained with hematoxylin-eosin (H&E) and Mallory trichrome.
Figure 7.

Location of intermediate tendon (red circle) of day 14 chick embryo.
For immunohistochemical staining, primary antibodies used in this study were against collagen type III, decorin, fibronectin, TGF-ß1, -ß2, and -ß3, and RI and RII (see below). The sections were deparaffinized in xylene and rehydrated, then treated with 5% hydrogen peroxide for 5 min to inactivate endogenous peroxidase, digested with 300 units/mL of hyaluronidase for 15 min at 37°C, and blocked for 1 h with 10% non-immune rabbit or goat serum. This was followed by an overnight incubation with primary antibody at pre-determined optimal dilutions or phosphate buffered saline (PBS) as negative control. The Histostain-SP kit from Zymed Laboratories Inc. (South San Francisco, CA) was used for the remainder of the immunohistochemical staining protocol. Briefly, sections were incubated in biotinylated secondary antibody for 10 min, incubated for 10 min with streptavidin-peroxidase, then developed with diaminobenzidine tetrahydrochloride for 5 min. Slides were serially dehydrated through graded ethanol washes, cleared through xylene, and mounted using Clarion (Sigma, St. Louis, MO). Sections were examined with a Leica DMIL microscope (Leica Microsystems Inc., Bannockburn, IL) and imaged with a Leica DFC320 camera. Three investigators independently performed assessment of staining patterns and intensity in a blinded manner.
The antibodies were against: (1) collagen type III (Developmental Studies Hybridoma Bank (DSHB), 1:500); (2) decorin (DSHB, 1:75); (3) fibronectin (DSHB, 1:10); (4) TGF-ß1 (Santa Cruz, 1:100); (5) TGF-ß2 (Santa Cruz, 1:50); (6) TGF-ß3 (Santa Cruz, 1:40); (7) TGF-ßRI (Santa Cruz, 1:50); and (8) TGF-ßRII (Santa Cruz, 1:20).
ACKNOWLEDGEMENTS
We thank Ms. Karen Clark for technical advice, Dr. Peter Alexander and Dr. Amanda Boyce for their discussions and helpful comments, and Ms. Kristen L. Marcum for valuable assistance. Support: Intramural Research Program of the NIH, National Institute of Arthritis, and Musculoskeletal and Skin Diseases (Z01 AR41131).
REFERENCES
- Anaguchi Y, Yasuda K, Majima T, Tohyama H, Minami A, Hayashi K. The effect of transforming growth factor-beta on mechanical properties of the fibrous tissue regenerated in the patellar tendon after resecting the central portion. Clin Biomech (Bristol, Avon) 2005;20:959–965. doi: 10.1016/j.clinbiomech.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Balza E, Borsi L, Allemanni G, Zardi L. Transforming growth factor beta regulates the levels of different fibronectin isoforms in normal human cultured fibroblasts. FEBS Lett. 1988;228:42–44. doi: 10.1016/0014-5793(88)80580-5. [DOI] [PubMed] [Google Scholar]
- Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur J Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. doi: 10.1002/aja.1002020303. [DOI] [PubMed] [Google Scholar]
- Birk DE, Southern JF, Zycband EI, Fallon JT, Trelstad RL. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- Birk DE, Zycband EI, Woodruff S, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev Dyn. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005;23:84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani H, Crossan J, McLellan SD, Meek D, Adam C. Expression of nitric oxide synthase and transforming growth factor-beta in crush-injured tendon and synovium. Mediators Inflamm. 2004;13:299–305. doi: 10.1155/S0962935104000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edom-Vovard F, Bonnin M, Duprez D. Fgf8 transcripts are located in tendons during embryonic chick limb development. Mech Dev. 2001;108:203–206. doi: 10.1016/s0925-4773(01)00483-x. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Duprez D. Signals regulating tendon formation during chick embryonic development. Dev Dyn. 2004;229:449–457. doi: 10.1002/dvdy.10481. [DOI] [PubMed] [Google Scholar]
- Engvall E, Ruoslahti E, Miller EJ. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978;147:1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick SA, Curry V, Harrall RL, Hazleman BL, Hackney R, Riley GP. Expression of transforming growth factor-beta isoforms and their receptors in chronic tendinosis. J Anat. 2001;199:231–240. doi: 10.1046/j.1469-7580.2001.19930231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R, Perlish JS, Burgeson RE, Shaikh-Bahai F, Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci. 1990;580:161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Perlish JS, Timpl R, Olsen BR. Procollagen intermediates during tendon fibrillogenesis. J Histochem Cytochem. 1988;36:1425–1432. doi: 10.1177/36.11.3049791. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Timpl R. Ultrastructural localization of fibronectin to different anatomic structures of human skin. J Histochem Cytochem. 1984;32:315–321. doi: 10.1177/32.3.6693760. [DOI] [PubMed] [Google Scholar]
- Fu SC, Wong YP, Cheuk YC, Lee KM, Chan KM. TGF-beta1 reverses the effects of matrix anchorage on the gene expression of decorin and procollagen type I in tendon fibroblasts. Clin Orthop Relat Res. 2005:226–232. doi: 10.1097/01.blo.0000145887.48534.6f. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Goh JC, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9(Suppl 1):S31–44. doi: 10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- Graham HK, Holmes DF, Watson RB, Kadler KE. Identification of collagen fibril fusion during vertebrate tendon morphogenesis. The process relies on unipolar fibrils and is regulated by collagen-proteoglycan interaction. J Mol Biol. 2000;295:891–902. doi: 10.1006/jmbi.1999.3384. [DOI] [PubMed] [Google Scholar]
- Hakkinen L, Strassburger S, Kahari VM, Scott PG, Eichstetter I, Lozzo RV, Larjava H. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80:1869–1880. doi: 10.1038/labinvest.3780197. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. Embo J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y, Kirisawa R, Mafune N, Takehana K. Downregulation of decorin and transforming growth factor-beta1 by decorin gene suppression in tendinocytes. Connect Tissue Res. 2005;46:18–26. doi: 10.1080/03008200590935510. [DOI] [PubMed] [Google Scholar]
- Hurle JM, Hinchliffe JR, Ros MA, Critchlow MA, Genis-Galvez JM. The extracellular matrix architecture relating to myotendinous pattern formation in the distal part of the developing chick limb: an ultrastructural, histochemical and immunocytochemical analysis. Cell Differ Dev. 1989;27:103–120. doi: 10.1016/0922-3371(89)90740-5. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Mochizuki Y, Yasunaga Y, Ishida O, Deie M, Ochi M. Effects of transforming growth factor-beta 1 on the early stages of healing of the Achilles tendon in a rat model. Scand J Plast Reconstr Surg Hand Surg. 2004;38:193–197. doi: 10.1080/02844310410029110. [DOI] [PubMed] [Google Scholar]
- Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg [Am] 2002;27:615–620. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Wilkes CM, Martin GR. Interaction of fibronectin with collagen fibrils. Biochemistry. 1981;20:2325–2330. doi: 10.1021/bi00511a039. [DOI] [PubMed] [Google Scholar]
- Krummel TM, Michna BA, Thomas BL, Sporn MB, Nelson JM, Salzberg AM, Cohen IK, Diegelmann RF. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988;23:647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997;94:1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Mooradian DL, Lucas RC, Weatherbee JA, Furcht LT. Transforming growth factor-beta 1 binds to immobilized fibronectin. J Cell Biochem. 1989;41:189–200. doi: 10.1002/jcb.240410404. [DOI] [PubMed] [Google Scholar]
- Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Hart DA, Boorman RS, Kaneda Y, Shrive NG, Marchuk LL, Shino K, Ochi T, Frank CB. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res. 2000;18:517–523. doi: 10.1002/jor.1100180402. [DOI] [PubMed] [Google Scholar]
- Natsu-ume T, Nakamura N, Shino K, Toritsuka Y, Horibe S, Ochi T. Temporal and spatial expression of transforming growth factor-beta in the healing patellar ligament of the rat. J Orthop Res. 1997;15:837–843. doi: 10.1002/jor.1100150608. [DOI] [PubMed] [Google Scholar]
- Nicosia RF, Bonanno E, Smith M. Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J Cell Physiol. 1993;154:654–661. doi: 10.1002/jcp.1041540325. [DOI] [PubMed] [Google Scholar]
- Nurminskaya MV, Birk DE. Differential expression of genes associated with collagen fibril growth in the chicken tendon: identification of structural and regulatory genes by subtractive hybridization. Arch Biochem Biophys. 1998;350:1–9. doi: 10.1006/abbi.1997.0498. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Nomura S, Moses HL, Hogan BL. Expression of transforming growth factor beta 2 RNA during murine embryogenesis. Development. 1989;106:759–767. doi: 10.1242/dev.106.4.759. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–211. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Saed GM, Zhang W, Chegini N, Holmdahl L, Diamond MP. Alteration of type I and III collagen expression in human peritoneal mesothelial cells in response to hypoxia and transforming growth factor-beta1. Wound Repair Regen. 1999;7:504–510. doi: 10.1046/j.1524-475x.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Robenek H, Harrach B, Glossl J, Nolte V, Hormann H, Richter H, Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987;104:1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherr E, Broszat M, Brandan E, Bruckner P, Kresse H. Decorin core protein fragment Leu155-Val260 interacts with TGF-beta but does not compete for decorin binding to type I collagen. Arch Biochem Biophys. 1998;355:241–248. doi: 10.1006/abbi.1998.0720. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107(Pt 5):1137–1157. doi: 10.1242/jcs.107.5.1137. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Speranza ML, Valentini G, Calligaro A. Influence of fibronectin on the fibrillogenesis of type I and type III collagen. Coll Relat Res. 1987;7:115–123. doi: 10.1016/s0174-173x(87)80003-1. [DOI] [PubMed] [Google Scholar]
- Spindler KP, Imro AK, Mayes CE, Davidson JM. Patellar tendon and anterior cruciate ligament have different mitogenic responses to platelet-derived growth factor and transforming growth factor beta. J Orthop Res. 1996;14:542–546. doi: 10.1002/jor.1100140407. [DOI] [PubMed] [Google Scholar]
- Spindler KP, Murray MM, Detwiler KB, Tarter JT, Dawson JM, Nanney LB, Davidson JM. The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res. 2003;21:245–249. doi: 10.1016/S0736-0266(02)00145-6. [DOI] [PubMed] [Google Scholar]
- Thieszen SL, Rosenquist TH. Expression of collagens and decorin during aortic arch artery development: implications for matrix pattern formation. Matrix Biol. 1995;14:573–582. doi: 10.1016/s0945-053x(05)80006-x. [DOI] [PubMed] [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- Tsubone T, Moran SL, Amadio PC, Zhao C, An KN. Expression of growth factors in canine flexor tendon after laceration in vivo. Ann Plast Surg. 2004;53:393–397. doi: 10.1097/01.sap.0000125501.72773.01. [DOI] [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–37381. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XT, Liu PY, Tang JB. Tendon healing in vitro: modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor beta but minimally affects expression of collagen genes. J Hand Surg [Am] 2005;30:222–229. doi: 10.1016/j.jhsa.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswald TM, Beck J, Dorsett-Martin W, Lineaweaver WC. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg. 2003;112:1613–1619. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005;5:5–21. [PubMed] [Google Scholar]
- Zhang Z, Li XJ, Liang DR, Li YY, Xu WS. [The antagonistic effect of recombinant human decorin on TGF-beta1 stimulation of fibroblasts in collagen lattices]. Zhonghua Shao Shang Za Zhi. 2006;22:207–210. [PubMed] [Google Scholar]