Abstract
Low levels of hypoxia have been suggested to be a mechanism of retinal damage in glaucoma. To test the hypothesis that the activation of the hypoxia-responsive transcription factor hypoxia inducible factor-1α (HIF-1α) is involved in the pathophysiology of glaucoma, we used a rat model of glaucoma to study (1) HIF-1α retinal protein levels by immunoblot analysis, (2) cellular localization of HIF-1α in the retina by immunohistochemistry, and (3) expression of retinal HIF-1 gene targets by quantitative real-time polymerase chain reaction. Glaucoma was unilaterally induced in rats by injecting hypertonic saline in episcleral veins. We find that HIF-1α protein was increased in the retina following elevation of intraocular pressure, specifically in Müller glia and astrocytes but not in activated microglia. Eight established HIF-1 target genes were measured in experimental glaucoma. Retinal Epo, Flt-1, Hsp-27, Pai-1, and Vegfa mRNA levels were increased and Et-1, Igf2, and Tgfβ3 levels were decreased in the glaucomatous retinas. Thus, the increase in HIF-1α levels in Müller glia and astrocytes is accompanied by a marked up regulation of some, but not all, HIF-1 transcriptional targets. These data support the hypothesis that HIF-1α becomes transcriptionally active in astrocytes and Müller cells but not microglia or neurons in glaucoma, arguing against a global hypoxia stimulus to the retina.
Keywords: Retinal ganglion cells, Glaucoma, HIF-1α, Müller cells, Astrocytes, qRT-PCR
Introduction
Dysregulation of blood flow with subsequent hypoxia has been suggested to contribute to retinal ganglion cell (RGC) death in glaucoma (Harris et al. 2005; Feke and Pasquale 2008). Stabilization of the transcription factor hypoxia inducible factor-1α (HIF-1α) is known to be an early tissue response to hypoxia and other stresses (Semenza 2000; Bilton and Booker 2003; Dery et al. 2005), leading to molecular events important in tissue responses including cell protective factors, proliferation, angiogenesis, and glucose metabolism (Semenza 2001; Ke and Costa 2006). HIF-1α/HIF-1β heterodimers form the active transcription factor HIF-1, whose activity is tightly controlled by HIF-1α availability. Previous immunohistochemical (IHC) studies showed that HIF-1α levels were elevated in human postmortem glaucomatous retinal tissue (Tezel and Wax 2004) and in the retinas of dogs with glaucoma (Savagian et al. 2008). However, whether hypoxia induces HIF-1α in glaucoma and whether HIF-1α then supports a HIF-1-mediated transcriptional cascade are unknown. Therefore, we investigated retinal HIF-1α levels in a rat model of experimental glaucoma by immunoblotting, identified the specific cell types in which HIF-1α is elevated in glaucoma by immunohistochemistry, and studied the gene expression of eight HIF-1 transcriptional targets by quantitative real-time polymerase chain reaction (qRT-PCR). Our results suggest that HIF-1 mediates a cascade of transcriptional activation, especially in Müller cells and astrocytes. However, the pattern observed in experimental glaucoma does not match that anticipated for global hypoxia, since neither RGC nor microglia show elevated HIF-1α, and the pattern of transcriptional upregulation differs from that expected for hypoxia.
Methods
Animals
Twenty-two male adult Brown Norway retired breeder rats (300–450 g; Charles River Laboratories) were employed for the study. Rats were kept on a 12-h illumination cycle and had ad libitum access to food and water. All animal-related procedures were carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Backlabeling of RGC
A subgroup of rats (n=4) was anesthetized intraperitoneally with a solution containing 1.5 mg/kg of acepromazine maleate, 7.5 mg/kg of xylazine, and 75 mg/kg of ketamine (all from Webster Veterinary Supply, Sterling, MA). Two microliters of 3% Fluoro-Gold solution in phosphate-buffered saline with 10% dimethyl sulfoxide (Fluorochrome, Denver, CO) was injected 4.8 mm ventral to the skull surface into the superior colliculus bilaterally. At the end of the surgery, the rats were allowed to recover for 7 days.
Induction of Experimental Glaucoma and Measurement of Intraocular Pressure
Baseline intraocular pressures (IOPs) were determined. IOP was then unilaterally elevated in rats by injecting 1.9 M hypertonic saline solution in the episcleral veins as previously described (Morrison et al. 1997). The contralateral eye served as the control. Following glaucoma-inducing surgery, IOPs were measured three times a week in conscious rats with a TonoPen XL tonometer (Medtronic Ophthalmics, Jacksonville, FL; Moore et al. 1993). At each time point, 15 readings were taken for each eye and averaged. To assess IOP exposure, we calculated the area under the pressure–time curve (AUC) beginning with the day of the first saline injection (experimental eye–control eye). This metric reflects both the length and the degree of IOP exposure.
Tissue Preparation
Rats were killed by CO2 after 5 or 10 days of elevated IOP. For IHC analysis, eyes were fixed with 4% paraformaldehyde, cryoprotected, and sectioned 16-μm thick.
For immunoblot analysis, retinas were immediately isolated and sonicated. After the samples were spun at 21,000 rpm at 4°C for 30 min, the protein content was quantified by spectrophotometry using the Bio-Rad Dc Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Immunoblotting
Retinal proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, MA). Blocked membranes were incubated at 4°C overnight with the following primary antibodies: mouse monoclonal anti-HIF-1α (1.5 μg/ml; R&D Systems), anti-HIF-1α (1:200; Abcam), and anti-α-tubulin (1:100,000; Sigma). The next day, membranes were incubated for 1 h at room temperature with peroxidase-conjugated goat anti-mouse secondary antibody (1:20,000–1:100,000; Jackson ImmunoResearch) and processed with the ECL Advance Western Blotting Detection Kit (Amersham Biosciences, Piscataway, NJ) and exposed to Kodak BioMax Light Film (Crestream Health, Inc., Rochester, NY).
Densitometry
Exposed films were scanned with the Personal Densitometer SI (Molecular Dynamics). The amount of protein for each band was measured with densitometry using Image-Quant 1.2 (Molecular Dynamics). After the background densitometry was subtracted from densitometry for each band, the reading for each protein was normalized to α-tubulin levels. For each retina pair, the normalized densitometric reading from the glaucomatous retina was divided by the reading from the control retina. Then, the ratios from different pairs of retinas were averaged.
Immunohistochemistry
Following blocking, slides with retinal sections were incubated at 4°C overnight with the mouse HIF-1α primary antibody (4.5 μg/ml; R&D Systems). Double staining was performed for HIF-1α with three cell-type specific markers: antivimentin (1:40,000; Sigma) for Müller cells, antiglial fibrillary acidic protein (GFAP; 1:500; Dako) for astrocytes, and anti-ionized calcium-binding adaptor molecule 1 (IBA1; 0.5 μg/ml; Wako) for activated microglia. The next day, sections were incubated with a secondary antibody for 1 h at room temperature: Alexa Fluor 488- or 594-conjugated goat antimouse (1:500; Invitrogen) and Alexa Fluor 488- or 594-conjugated goat antirabbit (1:250; Invitrogen). Staining was visualized using an Olympus BX51 microscope 1 day after the sections were treated with Prolong Gold antifade reagent. Equal exposure times were used when acquiring images for each antibody in figures comparing expression levels in normal retinas versus glaucomatous retinas.
Primer Design
The Brown Norway rat sequence for the mRNA of interest was obtained from the National Center for Biotechnology Information website. Primers for the cDNA of interest were selected using the Primer 3 software (Table 1). Genomic DNA amplification was avoided by choosing primers that span at least an intron in the genomic sequence. Vegfa primers were designed to amplify all of Vegfa mRNA isoforms in the rat.
Table 1.
Oligonucleotide information
| NCIB gene ID # |
Gene name | Official gene symbol |
Other designations | Primers (5′→3′) (F, forward; R, reverse) |
Annealing temperature (°C) |
Product size (bp) |
|---|---|---|---|---|---|---|
| 24323 | Endothelin 1 | Edn1 | Et1 | F agaactccgagcccaaagta R aggtcttgatgctgttgctg |
60 | 155 |
| 24335 | Erythropoietin | Epo | F aaaatgtcacaatgggctgt R atggcttctgagagcagaga |
60 | 154 | |
| 54251 | FMS-like tyrosine kinase 1 | Flt-1 | Vegfr-1 | F ttctgtcctccagaaagtgc R atccattttaggggaagtcg |
60 | 163 |
| 24383 | Glyceraldehyde-3-phospate dehydrogenase |
Gapdh | Gapd | F ccaatgtatccgttgtggat R catcaaaggtggaagaatgg |
60 | 177 |
| 24471 | Heat shock protein 1 | Hspb1 | Hsp25; Hsp27 | F ctacatctctcggtgcttcac R gcttctacttggctccagact |
58 | 218 |
| 29560 | Hypoxia inducible factor 1, alpha subunit |
Hif-1α | MOP1 | F attcaagatcagccagcaag R gccagcatttccaagtctaa |
59 | 209 |
| 24483 | Insulin-like growth factor 2 | Igf2 | IGFII; RNIGF2 | F gtcgatgttggtgcttctca R aagcagcactcttccacgat |
58 | 195 |
| 25589 | Kinase insert domain protein receptor |
Kdr | Vegfr-2; MGC93590 | F agagccacatggtctctctg R tgcttcttctagctgccagt |
58 | 170 |
| 24617 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 |
Serpine 1 | Pai1; Pal1A; Planh; Pai1aa; RATPAl1A |
F gtacgacatcctggaactgc R ctgggttgagctgaagatgt |
61 | 250 |
| 25717 | Transforming growth factor, beta 3 |
Tgfβ3 | MGC105479 | F ggtcctggcactttacaaca R aggacacattgaaacggaaa |
58 | 209 |
| 83785 | Vascular endothelial growth factor A |
Vegfa | Vegf | F gtctaccagcgcagctattg R ctatgctgcaggaagctcat |
59 | 242 |
Quantitative Real-Time PCR
Initially, RNA from normal and glaucomatous whole retinas from each rat was reverse transcribed into cDNA using random primers and SuperScript™ II RT (Invitrogen, Carlsbad, CA). Next, qRT-PCR was performed using the iCycler IQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The expression level of each target was calculated based on the standard curve using the ΔCt method (Meijerink et al. 2001). The target levels in each retina were normalized with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase. Agarose gel electrophoresis and direct sequencing confirmed the identity of the amplicons.
Statistical Analysis
Data were analyzed using the OriginPro 8 software (OriginLab, Northampton, MA). Shapiro–Wilk normality test was initially used to determine whether or not the protein or the mRNA data followed a normal distribution. In normally distributed data, Student’s t test was employed to determine the significance of the ratios (glaucomatous/control retina) for a given protein or mRNA. Data are reported as the mean ± standard error of the mean (SEM). For the two samples for which the distribution was not normal, the data were analyzed using a nonparametric one-sample Wilcoxon signed rank test.
Results
Rat IOP History
The peak IOP for animals used for immunoblot analysis was 40±1.9 mmHg (n=6) for animals with 5 days of elevated IOP and 43.1±0.8 mmHg (n=6) for animals with 10 days of elevated IOP. The AUC for these two groups was 111.1±15.1 mmHg-days for 5-day animals and 275.3± 32.2 mmHg-days for 10-day animals. For animals used for IHC, the peak IOP was 43.4±0.82 mmHg (n=4) and the AUC was 138.4±24.4 mmHg-days. For animals used for qRT-PCR, the peak IOP was 44.5±0.57 mmHg (n=6) and the AUC was 302.7±11.5 mmHg-days.
Elevated IOP Leads to an Increase in HIF-1α Protein Levels in the Retina
We initially identified HIF-1α in control and glaucomatous retinas by immunoblotting using a well-characterized HIF-1α antibody (R&D Systems). The observed band corresponded to a single band also seen in the brain at the same size (Fig. 1a, b). The levels of HIF-1α were significantly increased in glaucomatous retinas compared to controls after 5 days (1.37±0.11-fold, P=0.009, n=6) and 10 days of IOP elevation (1.34±0.13-fold, P=0.021, n=6; Fig. 1c).
Figure 1.
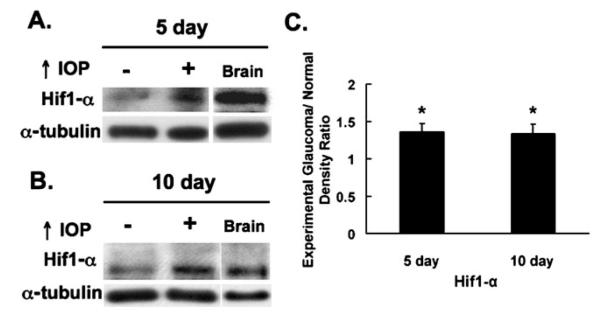
Western blotting: HIF-1α levels are increased in the glaucomatous retina compared to controls. a HIF-1α expression after 5 days of elevated IOP (n=6). A band for HIF-1α was detected between 100 and 150 kDa in the control and glaucomatous retinas as well as in the positive control brain. HIF-1α levels were elevated in the glaucomatous retinas compared to controls. b HIF-1α expression after 10 days of elevated IOP (n=6). Similarly, HIF-1α was detected between 100 and 150 kDa in the control and glaucomatous retinas. HIF-1α expression remained elevated in the glaucomatous retinas compared to controls. c Glaucomatous/control ratios of HIF-1α levels in the retina. HIF-1α levels were significantly increased in the glaucomatous retinas compared to controls (P=0.009 for the 5-day group and P=0.021 for the 10-day group). Fold increase was (mean ± SEM) 1.37±0.11 and 1.34±0.13 after 5 and 10 days of elevated IOP, respectively. Positive control: brain, loading control: α-tubulin
HIF-1α is Expressed in Müller Glia in the Glaucomatous Retina
IHC was employed to determine the retinal cell types accountable for the increase in HIF-1α protein expression in rats with experimental glaucoma. In the initial experiment, IHC was performed in retinal sections of animals that previously had their RGC backlabeled with Fluoro-Gold (Fig. 2). Figure 2b shows the lack of staining in a negative control stained only with secondary antibody. Figure 2e, h shows the increase in HIF-1α staining in the retinal section from an animal with high IOP (Fig. 2h) when compared with normal IOP (Fig. 2e). When the same histologic section was imaged for Fluoro-Gold to identify RGC and HIF-1α, the merged image demonstrates the lack of colocalization of Fluoro-Gold and HIF-1α staining. In normal and glaucomatous retinas, HIF-1α staining was localized to the inner layers of the retina and did not colocalize with the RGC marker Fluoro-Gold in either group (Fig. 2f, i). Consistent with our immunoblot findings, HIF-1α staining was stronger in the glaucomatous retina compared to the weak staining observed in the normal retina (Fig. 2e, h). Additional IHC to look for colocalization with glial cell markers (Fig. 3) included the previous staining with the HIF-1α primary antibody (red) followed by staining with an antivimentin antibody (green). In the normal and glaucomatous retina, HIF-1α staining colocalized (yellow) primarily with the Müller glia marker vimentin (Fig. 3f, i).
Figure 2.
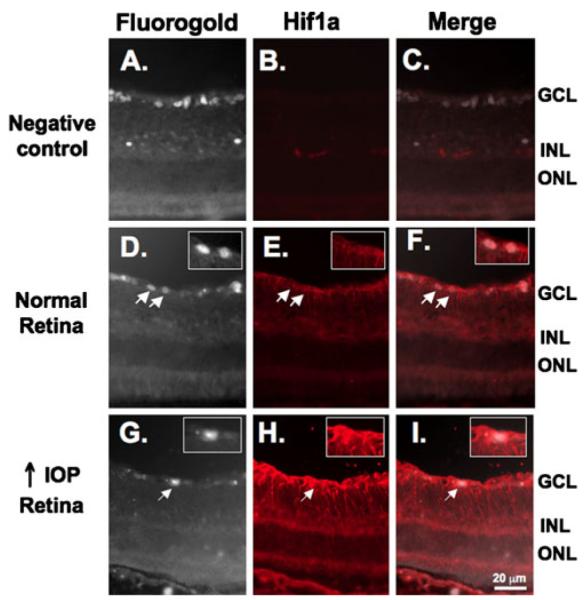
HIF-1α staining is not present in RGC. RGC were previously backlabeled with Fluoro-Gold (a, d, and g). Fluoro-Gold images were converted to gray scale to optimize visualization to determine colocalization with HIF-1α. b No nonspecific staining was observed when sections were incubated with secondary antibody alone. Subtle HIF-1α staining was observed in the inner layers of the normal retina (e) and was increased under conditions of high IOP (h). HIF-1α staining (red) did not colocalize with the RGC marker Fluoro-Gold (white) in the normal retina (f) or in the retina under conditions of high IOP (i). Insets are higher magnification views of the RGC indicated by arrows
Figure 3.
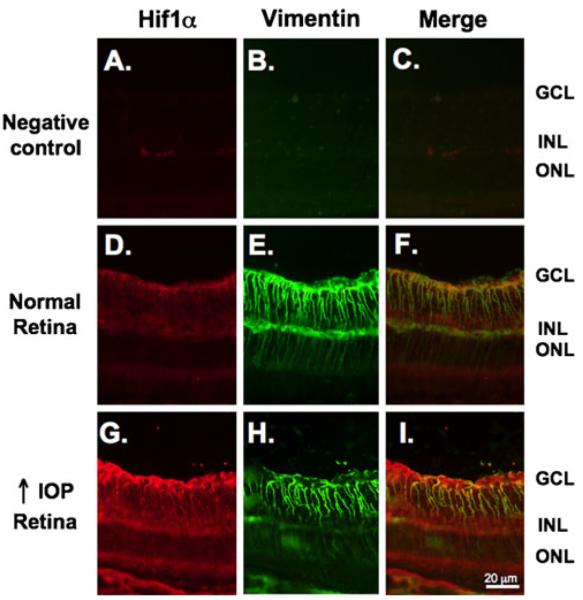
HIF-1α expression is increased in Müller glia in the glaucomatous retina. Sections were double stained with an anti-HIF-1α antibody (red) followed by staining with an antivimentin antibody (green). a, b No nonspecific staining was observed when sections were incubated with their respective secondary antibodies alone. As shown in the same sections in Fig. 2, there is subtle HIF-1α staining in the inner layers of the normal retina (d) that increase under conditions of high IOP (g). d Low levels of HIF-1α staining colocalized (yellow) with the Müller glia marker vimentin under conditions of normal IOP. i Elevated HIF-1α expression was observed primarily in Müller glia as the staining colocalized with vimentin (yellow)
HIF-1α is also Expressed in Astrocytes but not in Activated Microglia or Retinal Ganglion Cells
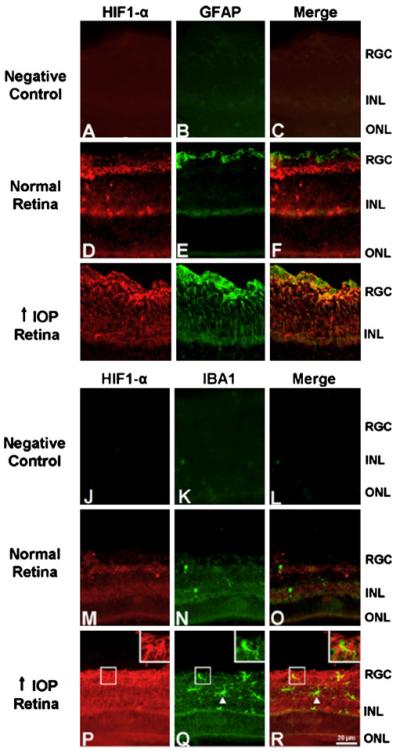
We also investigated whether other glial cell types were positive for HIF-1α in glaucomatous retina (Fig. 4). To test whether astrocytes express HIF-1α, we double stained the retinal sections with anti-HIF-1α (red) and anti-GFAP (green) primary antibodies. Both in the normal and glaucomatous retina, the expression of the astrocyte marker GFAP was observed in the RGC layer and inner nuclear layer (INL) with a stronger staining in the glaucomatous retina (Fig. 4e, h). Whereas GFAP staining did not colocalize with HIF-1α in normal retina (Fig. 4d–f), it over-lapped with HIF-1α staining in the glaucomatous retina (Fig. 4g–i). GFAP is expressed by both astrocytes and Müller glia under pathological conditions (Wakakura and Foulds 1989; Lupien et al. 2004). The distribution of HIF-1α staining in our sections suggests that HIF-1α is present in both astrocytes and Müller glia in the glaucomatous retina.
Figure 4.
HIF-1α is also expressed by astrocytes but not by activated microglia in the glaucomatous retina. a–c and j–l There was no nonspecific staining in the negative control sections which were incubated only with the secondary antibodies. d–i To examine whether astrocytes express HIF-1α, retinal sections were double stained with anti-HIF-1α and anti-GFAP primary antibodies. e In the normal retina, expression of the astrocyte marker GFAP was observed in the RGC layer and INL. d–f GFAP expression (green) did not colocalize with HIF-1α (red) staining in the normal retina. h In the glaucomatous retina, increased GFAP expression was present in the RGC layer and INL. g–i GFAP staining colocalized with HIF-1α expression in the glaucomatous retina. m–r To test whether HIF-1α was expressed by activated microglia, retinal sections were double labeled with anti-HIF-1α and anti-IBA1 primary antibodies. n Expression of the activated microglia marker IBA1 (green) was minimal in the normal retina. m–o IBA1 expression did not colocalize with HIF-1α (red) staining. q versus n In the glaucomatous retina, stronger IBA1 staining was observed in the RGC layer and INL compared with the normal retina. p–r IBA1 staining did not colocalize with HIF-1α staining in the glaucomatous retina (see arrowheads and insets)
To examine whether activated microglia express HIF-1α, retinal sections were double stained for HIF-1α and IBA1. In the normal retina, expression of the activated microglia marker IBA1 was minimal (Fig. 4n). In the glaucomatous retina, IBA1-positive cells were detected in the RGC layer and INL (Fig. 4q) and IBA1 staining did not colocalize with HIF-1α staining (Fig. 4p–r, see arrowheads and insets). These results indicate that Müller glia and astrocytes, but not RGC or activated microglia, express HIF-1α in the glaucomatous retina.
Some, but not All, HIF-1 Gene Targets are Upregulated in Experimental Glaucoma
We next used qRT-PCR to examine the expression of known HIF-1 gene targets Epo, Et-1, Flt-1, Hsp27, Igf2, Pai-1, Tgfβ3, and Vegfa and nontarget genes HIF-1α itself and the VEGF receptor Kdr (Table 2) in the retinas of control eyes and in eyes with increased IOP. mRNA levels of Epo, Flt-1, Hsp27, Pai-1, and Vegfa were increased in the glaucomatous retinas (n=6). As expected, the mRNA levels of Hif-1α and Kdr remained unchanged in the retina following IOP elevation. Surprisingly, however, Et-1, Igf2, and Tgfβ3 gene expression were decreased in the glaucomatous retinas.
Table 2.
qRT-PCR results
| Gene symbol | Glaucomatous/control retina mRNA fold change (n=6) (mean ± SEM) |
P value |
|---|---|---|
| Epo | 21.57±15.19 | 0.03a |
| Et-1 | 0.67±0.15 | 0.039 |
| Flt-1 | 2.79±0.78 | 0.016a |
| Hif-1α | 0.92±0.14 | 0.71 |
| Hsp27 | 10.67±1.51 | 0.0007 |
| Igf2 | 0.64±0.06 | 0.001 |
| Kdr | 1.2±0.13 | 0.09 |
| Pai-1 | 302.22±126.44 | 0.032 |
| Tgfβ3 | 0.56±0.08 | 0.001 |
| Vegfa | 2.37±0.67 | 0.048 |
Nonparametric test result
Discussion
Here, we further examined the observation that HIF-1α immunoreactivity is enhanced in the glaucomatous retina in a canine glaucoma model (Savagian et al. 2008) and in human glaucoma (Tezel and Wax 2004) by quantitatively measuring the extent of HIF-1α increase in glaucoma, determining the specific cell types responsible for that increase, and examining whether the expected consequences of activation of the transcriptional factor HIF-1 ensue as a consequence of the change in HIF-1α. We observe a modest (~1.4-fold) but consistent and statistically significant increase in retinal HIF-1α protein levels after 5 and 10 days of elevated IOP in a rat model of glaucoma. Since this increase is determined over the entire retina, but by immunohistochemistry the cells with elevated HIF-1α appear to be primarily astrocytes and Müller glia, the increase in these specific cell populations is likely substantially greater. Although in our study HIF-1α expression was not detected in RGC, Tezel and Wax (2004) showed that HIF-1α was detected in RGC in glaucomatous human retinas. Notably, that study employed analysis of postmortem retinas from glaucoma patients who had been treated with IOP-lowering agents while alive (Tezel and Wax 2004). It is possible that the chronicity of disease or IOP-lowering treatments led to alterations in protein stabilization that might explain the difference between the two studies.
IHC analysis showed clearly that subpopulations of cells, including primarily astrocytes and Müller glia, but not activated microglia, endothelial cells, or neurons, exhibited an elevation in immunohistochemically detectable HIF-1α, arguing that the increase is due to stabilization of HIF-1α in astrocytes and Müller cells rather than due to global hypoxia which would be expected to impact multiple cell types. Recent advances in understanding the complex relationships that lead to stabilization or degradation of HIF-1a may help explain this observation (Yee Koh et al. 2008). Under nonstressed conditions, HIF-1α is hydroxylated and acetylated, which leads to its rapid degradation by the proteosome pathway (Ke and Costa 2006; Yee Koh et al. 2008; Brahimi-Horn and Pouyssegur 2009). Under hypoxic conditions, HIF-1α is phosphorylated by p42/p44 MAP kinases, which stabilizes HIF-1α (Richard et al. 1999; Ke and Costa 2006). Stabilized HIF-1α translocates to the nucleus and forms the active heterodimeric HIF-1 transcription factor by binding constitutively expressed HIF-1β. HIF-1 then regulates the expression of a wide variety of hypoxia-induced genes (Semenza 2001; Ke and Costa 2006). Thus, HIF-1α is potentially regulated within individual cell types by a variety of stressors.
We examined by qRT-PCR eight genes, Epo, Et-1, Flt-1, Hsp27, Igf2, Pai-1, Tgfβ3, and Vegfa, which are known to be transcriptionally regulated by HIF-1 (Semenza 2001). Since HIF-1-responsive genes are generally stress response or neuroprotective in nature, several have been previously independently examined in the setting of glaucomatous retinal damage; our current data support and extend these observations, putting them into the context of HIF-1 transcriptional responses. For example, we observe markedly increased retinal Epo expression. Increased retinal EPO protein levels have recently been demonstrated in experimental glaucoma and this increase is seen primarily in Müller cells (Fu et al. 2008). The VEGF receptor Flt-1 (Vegfr1) is known to be upregulated by HIF-1α (Gerber et al. 1997), and we demonstrate that it is also increased in the glaucomatous retina. Hsp27 also is upregulated in the glaucomatous retina. Hsp27 expression has been previously shown to be increased by HIF-1α in the retina under hypoxic stress (Whitlock et al. 2005) and in glaucoma (Ahmed et al. 2004). We and others have demonstrated that retinal HSP27 protein levels are elevated in glaucoma, primarily in Müller cells and astrocytes (Huang et al. 2007; Kalesnykas et al. 2007). Both HSP27 and EPO have been shown to be neuronal survival factors (Benn et al. 2002; Grimm et al. 2005; Latchman 2005; Fu et al. 2008) and we speculate that their upregulation by HIF-1α is part of an endogenous compensatory response to elevated IOP. Our results also show that Pai-1 levels are strongly upregulated in experimental glaucoma. Previously, elevated PAI-1 levels were found in the aqueous humor of glaucoma patients (Dan et al. 2005). Plasminogen activators have been shown to promote excitotoxicity-induced retinal cell apoptosis (Kumada et al. 2005; Mali et al. 2005) and PAI-1 is an endogenous inhibitor of plasminogen activators. Our observation that Pai-1 levels are increased in glaucoma prompts consideration of a neuroprotective role for PAI-1.
Elevated aqueous humor VEGF levels have been shown to be present in glaucoma patients (Hu et al. 2002), and we show a subtle upregulation in overall Vegfa message levels in the glaucomatous retina. Interestingly, FLT-1 has been proposed to mediate the actions of the anti-angiogenic VEGF-A165b (Woolard et al. 2004; Glass et al. 2006), which we have previously shown to be increased in experimental glaucoma (Ergorul et al. 2008). This may help to explain, in part, why no neovascularization of the retina is observed in glaucoma. We also examined a second VEGF receptor, Kdr (Vegfr2), which is not regulated by HIF-1α (Gerber et al. 1997), and find that retina message is unchanged in experimental glaucoma.
In contrast to the upregulation of several HIF-1-responsive genes after elevation of IOP, we find a surprising down-regulation of several transcripts thought to be regulated by HIF-1. For example, our data demonstrate that Et-1 mRNA is downregulated in the glaucomatous retina. ET-1 has been previously shown to be elevated in aqueous humor of primary open-angle glaucoma patients (Tezel et al. 1997), in aqueous humor of glaucomatous dog eyes (Kallberg et al. 2007), and in aqueous humor in a rat model of glaucoma (Prasanna et al. 2005). However, measures of plasma ET-1 levels in glaucoma patients have had inconsistent results (Tezel et al. 1997; Emre et al. 2005). ET-1 is a vasoconstrictor and abnormal vascular responses related with it have been previously shown in patients with glaucoma (Kaiser et al. 1995; Nicolela et al. 2003). It has been suggested that an imbalance between the levels of vasoconstrictors and vasodilators may lead to vascular dysregulation (Nicolela 2008). Therefore, our finding that Et-1 mRNA is downregulated in the glaucomatous retina may either be a contributory or a compensatory factor to vascular dysregulation observed in glaucoma.
Igf2 and Tgfβ3, genes whose expression is thought to be HIF-1α responsive in some systems (Feldser et al. 1999; Caniggia et al. 2000), are also nonetheless downregulated in the retina in experimental glaucoma. An Igf-2 gene polymorphism has been previously shown to be associated with primary open-angle glaucoma (Tsai et al. 2003). TGFβ3 levels were found to be elevated in the aqueous humor of pseudoexfoliation patients (Yoneda et al. 2007). The roles of these factors in glaucoma are unclear and clarification will require additional studies.
Two other HIF-1α target genes have been previously examined by other investigators in the glaucomatous retina. Retinal ceruloplasmin message levels are increased in several models (Miyahara et al. 2003; Farkas et al. 2004; Stasi et al. 2007), and the increase in protein levels was seen to be primarily in Müller cells in humans and mice with glaucoma. Retinal transferrin message is also increased in glaucoma (Farkas et al. 2004). These data reinforce our observations that HIF-1α is elevated primarily in astrocytes and Müller cells and that many HIF-1-responsive genes are upregulated across multiple glaucoma models.
Taken together, seven of 10 known HIF-1α targets that have been examined using quantitative techniques increase their expression and three of 10 targets decrease their expression in experimental glaucoma. Although oxygen-responsive changes include upregulation of all of these factors (Damert et al. 1997; Gerber et al. 1997; Hu et al. 1998; Caniggia et al. 2000; Kietzmann et al. 2003; Whitlock et al. 2005; Mazurek et al. 2006; Stockmann and Fandrey 2006; Peyssonnaux et al. 2008), it is clear that HIF-1α is also activated by nonhypoxic conditions (Yee Koh et al. 2008), and it seems likely that the regulation of individual target genes may vary based upon the stress leading to elevation of HIF-1. Thus, in glaucoma, HIF-1 activation may not necessarily be a specific indicator of hypoxia. Although hypoxia is a major driver of HIF-1α activation, recent evidence indicates that HIF-1α can also be induced under normoxic conditions in other systems by receptor-mediated signals (Bilton and Booker 2003; Dery et al. 2005; Yee Koh et al. 2008).
Whether activated by hypoxia and/or receptor-mediated signals, HIF-1α levels increase and several HIF-1 transcriptional targets are upregulated in the glaucomatous retina in this study. Our data suggest fine-tuning of molecular responses of the HIF-1 transcriptional cascade predominantly in Müller cells and astrocytes, under conditions of elevated IOP, and may reflect cell-specific or tissue-specific HIF-1 transcriptional activity. These support the idea that HIF-1 activation and its downstream consequences play a role in glaucoma pathophysiology.
Acknowledgments
The authors thank Hisatomo Kowa and Bradley Hyman at MassGeneral Institute for Neurodegeneration, Charlestown, MA for the discussions and for the equipment used in qRT-PCR. The authors also thank Charles Vanderburg, Rachel Diamond, and Ozge Cagsal-Getkin at the Advanced Tissue Resource Center/MGH, Charlestown, MA for their technical help. This work was supported by R01-EY13399 (CLG), T32-EY07145 (CE), Massachusetts Lions Grant (CLG), and MEEI Vision-Core Grant EY014104.
Contributor Information
Ceren Ergorul, Howe Laboratory of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Arjun Ray, Howe Laboratory of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Wei Huang, Howe Laboratory of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Dan Yi Wang, Howe Laboratory of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Yixin Ben, Howe Laboratory of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA.
Ippolita Cantuti-Castelvetri, Department of Neurology, Massachusetts General Hospital, 114 16th Street, CNY114-2250, Charlestown, MA 02129, USA.
Cynthia L. Grosskreutz, Howe Laboratory of Ophthalmology, Massachusetts Eye and Ear Infirmary, Harvard Medical School, 243 Charles Street, Boston, MA 02114, USA
References
- Ahmed F, Brown KM, Stephan DA, Morrison JC, Johnson EC, Tomarev SI. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45(4):1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- Benn SC, Perrelet D, Kato AC, et al. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36(1):45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- Bilton RL, Booker GW. The subtle side to hypoxia inducible factor (HIFalpha) regulation. Eur J Biochem. 2003;270(5):791–798. doi: 10.1046/j.1432-1033.2003.03446.x. [DOI] [PubMed] [Google Scholar]
- Brahimi-Horn MC, Pouyssegur J. HIF at a glance. J Cell Sci. 2009;122(Pt 8):1055–1057. doi: 10.1242/jcs.035022. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105(5):577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damert A, Ikeda E, Risau W. Activator-protein-1 binding potentiates the hypoxia-induciblefactor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem J. 1997;327(Pt 2):419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan J, Belyea D, Gertner G, Leshem I, Lusky M, Miskin R. Plasminogen activator inhibitor-1 in the aqueous humor of patients with and without glaucoma. Arch Ophthalmol. 2005;123(2):220–224. doi: 10.1001/archopht.123.2.220. [DOI] [PubMed] [Google Scholar]
- Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37(3):535–540. doi: 10.1016/j.biocel.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Emre M, Orgul S, Haufschild T, Shaw SG, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005;89(1):60–63. doi: 10.1136/bjo.2004.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergorul C, Ray A, Huang W, Darland D, Luo ZK, Grosskreutz CL. Levels of vascular endothelial growth factor-A165b (VEGF-A165b) are elevated in experimental glaucoma. Mol Vis. 2008;14:1517–1524. [PMC free article] [PubMed] [Google Scholar]
- Farkas RH, Chowers I, Hackam AS, et al. Increased expression of iron-regulating genes in monkey and human glaucoma. Invest Ophthalmol Vis Sci. 2004;45(5):1410–1417. doi: 10.1167/iovs.03-0872. [DOI] [PubMed] [Google Scholar]
- Feke GT, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology. 2008;115(2):246–252. doi: 10.1016/j.ophtha.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59(16):3915–3918. [PubMed] [Google Scholar]
- Fu QL, Wu W, Wang H, Li X, Lee VW, So KF. Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension. Cell Mol Neurobiol. 2008;28(2):317–329. doi: 10.1007/s10571-007-9155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272(38):23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J Physiol. 2006;572(Pt 1):243–257. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Hermann DM, Bogdanova A, et al. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005;16(4–5):531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Harris A, Rechtman E, Siesky B, Jonescu-Cuypers C, McCranor L, Garzozi HJ. The role of optic nerve blood flow in the pathogenesis of glaucoma. Ophthalmol Clin North Am. 2005;18(3):345–353. doi: 10.1016/j.ohc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245(3):894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- Hu DN, Ritch R, Liebmann J, Liu Y, Cheng B, Hu MS. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J Glaucoma. 2002;11(5):406–410. doi: 10.1097/00061198-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Filippopoulos T, Ray A, Dobberfuhl A, Grosskreutz CL. Hsp27 phosphorylation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2007;48(9):4129–4135. doi: 10.1167/iovs.06-0606. [DOI] [PubMed] [Google Scholar]
- Kaiser HJ, Flammer J, Wenk M, Luscher T. Endothelin-1 plasma levels in normal-tension glaucoma: abnormal response to postural changes. Graefes Arch Clin Exp Ophthalmol. 1995;233(8):484–488. doi: 10.1007/BF00183429. [DOI] [PubMed] [Google Scholar]
- Kalesnykas G, Niittykoski M, Rantala J, et al. The expression of heat shock protein 27 in retinal ganglion and glial cells in a rat glaucoma model. Neuroscience. 2007;150(3):692–704. doi: 10.1016/j.neuroscience.2007.09.078. [DOI] [PubMed] [Google Scholar]
- Kallberg ME, Brooks DE, Gelatt KN, Garcia-Sanchez GA, Szabo NJ, Lambrou GN. Endothelin-1, nitric oxide, and glutamate in the normal and glaucomatous dog eye. Vet Ophthalmol. 2007;10(Suppl 1):46–52. doi: 10.1111/j.1463-5224.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70(5):1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Samoylenko A, Roth U, Jungermann K. Hypoxia-inducible factor-1 and hypoxia response elements mediate the induction of plasminogen activator inhibitor-1 gene expression by insulin in primary rat hepatocytes. Blood. 2003;101(3):907–914. doi: 10.1182/blood-2002-06-1693. [DOI] [PubMed] [Google Scholar]
- Kumada M, Niwa M, Hara A, et al. Tissue type plasminogen activator facilitates NMDA-receptor-mediated retinal apoptosis through an independent fibrinolytic cascade. Invest Ophthalmol Vis Sci. 2005;46(4):1504–1507. doi: 10.1167/iovs.04-0595. [DOI] [PubMed] [Google Scholar]
- Latchman DS. HSP27 and cell survival in neurones. Int J Hyperthermia. 2005;21(5):393–402. doi: 10.1080/02656730400023664. [DOI] [PubMed] [Google Scholar]
- Lupien C, Brenner M, Guerin SL, Salesse C. Expression of glial fibrillary acidic protein in primary cultures of human Muller cells. Exp Eye Res. 2004;79(3):423–429. doi: 10.1016/j.exer.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Mali RS, Cheng M, Chintala SK. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB J. 2005;19(10):1280–1289. doi: 10.1096/fj.04-3403com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek B, Rheinlander C, Fuchs FU, et al. Influence of ischemia/hypoxia on the HIF-1 activity and expression of hypoxia-dependent genes in the cochlea of the newborn rat. HNO. 2006;54(9):689–697. doi: 10.1007/s00106-005-1371-6. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Mandigers C, van de Locht L, Tonnissen E, Goodsaid F, Raemaekers J. A novel method to compensate for different amplification efficiencies between patient DNA samples in quantitative real-time PCR. J Mol Diagn. 2001;3(2):55–61. doi: 10.1016/S1525-1578(10)60652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara T, Kikuchi T, Akimoto M, Kurokawa T, Shibuki H, Yoshimura N. Gene microarray analysis of experimental glaucomatous retina from cynomologous monkey. Invest Ophthalmol Vis Sci. 2003;44(10):4347–4356. doi: 10.1167/iovs.02-1032. [DOI] [PubMed] [Google Scholar]
- Moore CG, Milne ST, Morrison JC. Noninvasive measurement of rat intraocular pressure with the Tono-Pen. Invest Ophthalmol Vis Sci. 1993;34(2):363–369. [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997;64(1):85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- Nicolela MT. Clinical clues of vascular dysregulation and its association with glaucoma. Can J Ophthalmol. 2008;43(3):337–341. doi: 10.3129/i08-063. [DOI] [PubMed] [Google Scholar]
- Nicolela MT, Ferrier SN, Morrison CA, et al. Effects of cold-induced vasospasm in glaucoma: the role of endothelin-1. Invest Ophthalmol Vis Sci. 2003;44(6):2565–2572. doi: 10.1167/iovs.02-0913. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7(1):28–32. doi: 10.4161/cc.7.1.5145. [DOI] [PubMed] [Google Scholar]
- Prasanna G, Hulet C, Desai D, et al. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol Res. 2005;51(1):41–50. doi: 10.1016/j.phrs.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274(46):32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Savagian CA, Dubielzig RR, Nork TM. Comparison of the distribution of glial fibrillary acidic protein, heat shock protein 60, and hypoxia-inducible factor-1alpha in retinas from glaucomatous and normal canine eyes. Am J Vet Res. 2008;69(2):265–272. doi: 10.2460/ajvr.69.2.265. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7(8):345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Stasi K, Nagel D, Yang X, Ren L, Mittag T, Danias J. Ceruloplasmin upregulation in retina of murine and human glaucomatous eyes. Invest Ophthalmol Vis Sci. 2007;48(2):727–732. doi: 10.1167/iovs.06-0497. [DOI] [PubMed] [Google Scholar]
- Stockmann C, Fandrey J. Hypoxia-induced erythropoietin production: a paradigm for oxygen-regulated gene expression. Clin Exp Pharmacol Physiol. 2006;33(10):968–979. doi: 10.1111/j.1440-1681.2006.04474.x. [DOI] [PubMed] [Google Scholar]
- Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122(9):1348–1356. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- Tezel G, Kass MA, Kolker AE, Becker B, Wax MB. Plasma and aqueous humor endothelin levels in primary open-angle glaucoma. J Glaucoma. 1997;6(2):83–89. [PubMed] [Google Scholar]
- Tsai FJ, Lin HJ, Chen WC, Chen HY, Fan SS. Insulin-like growth factor-II gene polymorphism is associated with primary open angle glaucoma. J Clin Lab Anal. 2003;17(6):259–263. doi: 10.1002/jcla.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakakura M, Foulds WS. Response of cultured Muller cells to heat shock—an immunocytochemical study of heat shock and intermediate filament proteins in response to temperature elevation. Exp Eye Res. 1989;48(3):337–350. doi: 10.1016/s0014-4835(89)80003-x. [DOI] [PubMed] [Google Scholar]
- Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005;46(3):1092–1098. doi: 10.1167/iovs.04-0043. [DOI] [PubMed] [Google Scholar]
- Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64(21):7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33(11):526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Nakano M, Mori K, Kinoshita S, Tashiro K. Disease-related quantitation of TGF-beta3 in human aqueous humor. Growth Factors. 2007;25(3):160–167. doi: 10.1080/08977190701723505. [DOI] [PubMed] [Google Scholar]