Abstract
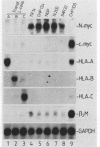
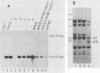
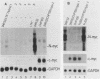
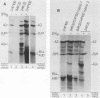
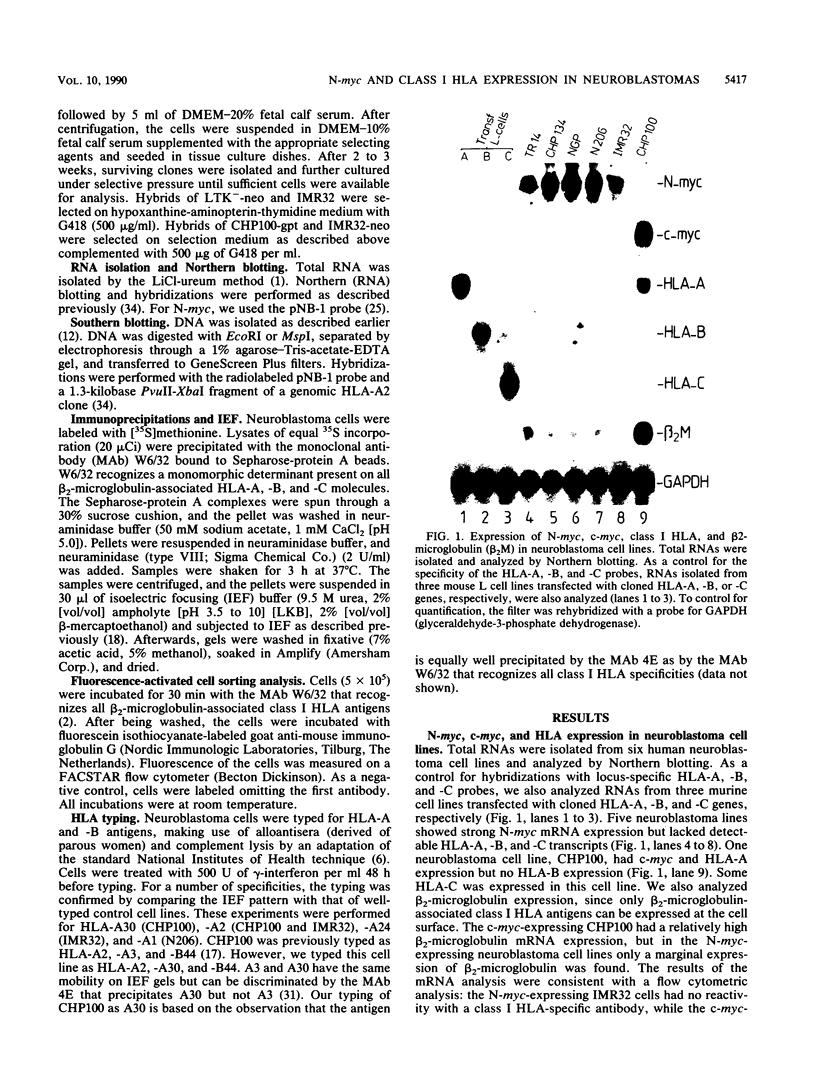
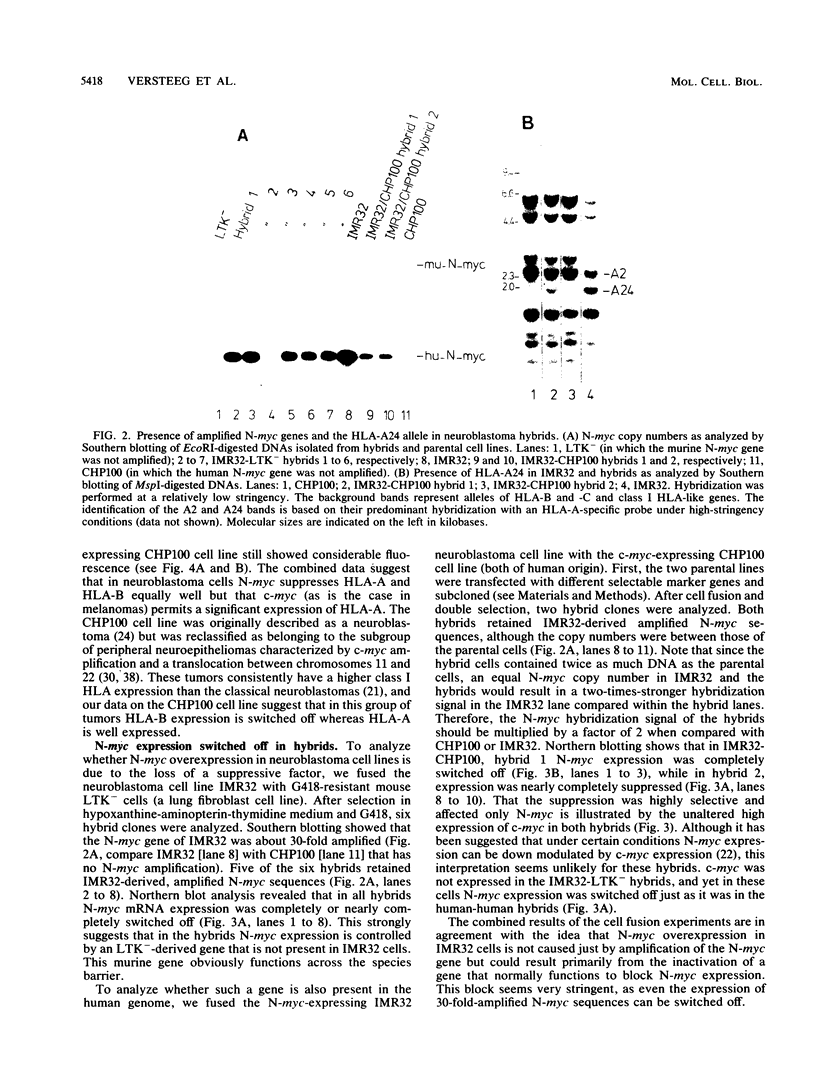
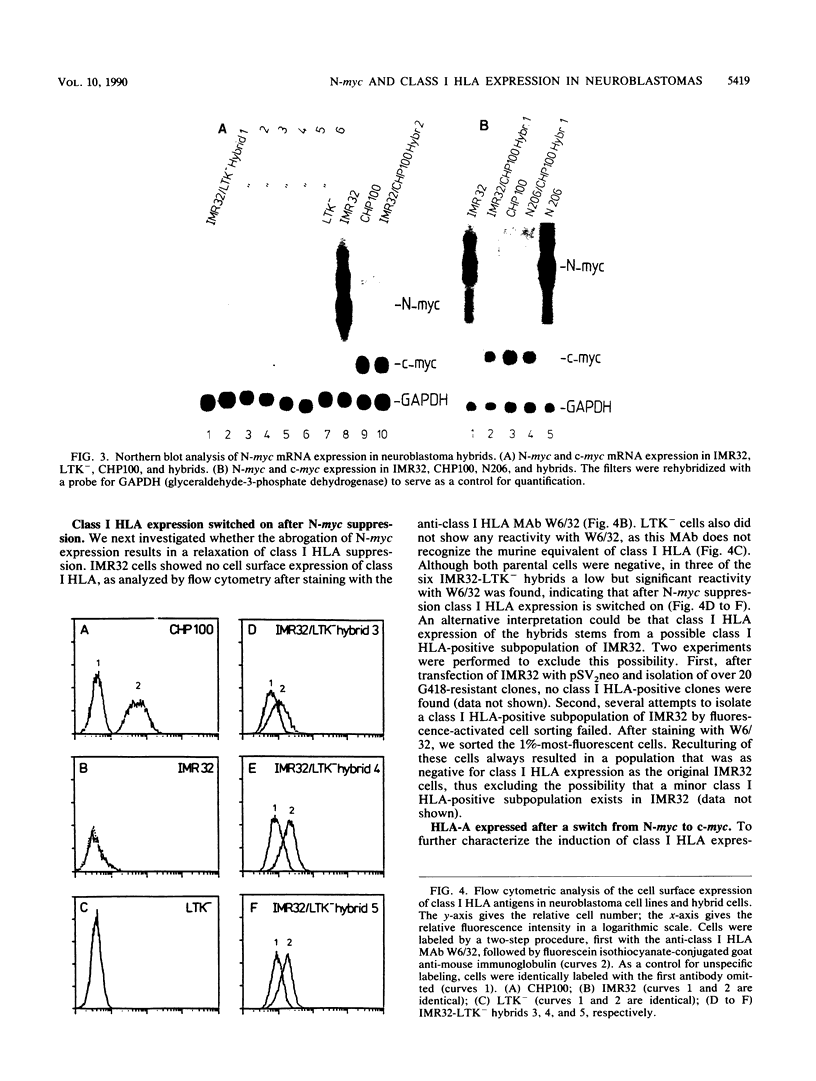
Neuroblastomas often show amplification and high expression of the N-myc oncogene. N-myc expression could be explained as a consequence of gene amplification, but an alternative possibility is that expression primarily results from the inactivation or loss of some factor that normally represses the N-myc gene. To test this idea, we fused N-myc-overexpressing neuroblastoma cell lines with lines that do not express N-myc. In the resulting hybrids, N-myc expression turned out to be switched off, although amplified N-myc copies were still present. This suggests that N-myc overexpression in neuroblastomas results, at least in part, from the inactivation of a suppressor gene that is present in normal cells. In rat neuroblastomas, it has been found that N-myc can switch off class I major histocompatibility complex (MHC) expression. Therefore, we analyzed in our hybrid cells whether suppression of N-myc results in reexpression of human class I MHC genes. Because this was found to be the case, the picture emerges of a hierarchic pathway that connects a putative tumor-suppressor gene with the expression of N-myc and consequently of class I MHC, thus affecting the potential immunogenic properties of neuroblastomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bernards R., Dessain S. K., Weinberg R. A. N-myc amplification causes down-modulation of MHC class I antigen expression in neuroblastoma. Cell. 1986 Dec 5;47(5):667–674. doi: 10.1016/0092-8674(86)90509-x. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Brodeur G. M., Sekhon G., Goldstein M. N. Chromosomal aberrations in human neuroblastomas. Cancer. 1977 Nov;40(5):2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Christiansen H., Lampert F. Tumour karyotype discriminates between good and bad prognostic outcome in neuroblastoma. Br J Cancer. 1988 Jan;57(1):121–126. doi: 10.1038/bjc.1988.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A. E., Chatten J., D'Angio G. J., Gerson J. M., Robinson J., Schnaufer L. A review of 17 IV-S neuroblastoma patients at the children's hospital of philadelphia. Cancer. 1980 Mar 1;45(5):833–839. doi: 10.1002/1097-0142(19800301)45:5<833::aid-cncr2820450502>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Feltner D. E., Cooper M., Weber J., Israel M. A., Thiele C. J. Expression of class I histocompatibility antigens in neuroectodermal tumors is independent of the expression of a transfected neuroblastoma myc gene. J Immunol. 1989 Dec 15;143(12):4292–4299. [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guin G. H., Gilbert E. F., Jones B. Incidental neuroblastoma in infants. Am J Clin Pathol. 1969 Jan;51(1):126–136. doi: 10.1093/ajcp/51.1.126. [DOI] [PubMed] [Google Scholar]
- Gusella J., Varsanyi-Breiner A., Kao F. T., Jones C., Puck T. T., Keys C., Orkin S., Housman D. Precise localization of human beta-globin gene complex on chromosome 11. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5239–5242. doi: 10.1073/pnas.76.10.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güssow D., Rein R. S., Meijer I., de Hoog W., Seemann G. H., Hochstenbach F. M., Ploegh H. L. Isolation, expression, and the primary structure of HLA-Cw1 and HLA-Cw2 genes: evolutionary aspects. Immunogenetics. 1987;25(5):313–322. doi: 10.1007/BF00404424. [DOI] [PubMed] [Google Scholar]
- Kohl N. E., Gee C. E., Alt F. W. Activated expression of the N-myc gene in human neuroblastomas and related tumors. Science. 1984 Dec 14;226(4680):1335–1337. doi: 10.1126/science.6505694. [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Main E. K., Lampson L. A., Hart M. K., Kornbluth J., Wilson D. B. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985 Jul;135(1):242–246. [PubMed] [Google Scholar]
- Neefjes J. J., Doxiadis I., Stam N. J., Beckers C. J., Ploegh H. L. An analysis of class I antigens of man and other species by one-dimensional IEF and immunoblotting. Immunogenetics. 1986;23(3):164–171. doi: 10.1007/BF00373817. [DOI] [PubMed] [Google Scholar]
- Nisen P. D., Waber P. G., Rich M. A., Pierce S., Garvin J. R., Jr, Gilbert F., Lanzkowsky P. N-myc oncogene RNA expression in neuroblastoma. J Natl Cancer Inst. 1988 Dec 21;80(20):1633–1637. doi: 10.1093/jnci/80.20.1633. [DOI] [PubMed] [Google Scholar]
- Rein R. S., Seemann G. H., Neefjes J. J., Hochstenbach F. M., Stam N. J., Ploegh H. L. Association with beta 2-microglobulin controls the expression of transfected human class I genes. J Immunol. 1987 Feb 15;138(4):1178–1183. [PubMed] [Google Scholar]
- Reynolds C. P., Tomayko M. M., Donner L., Helson L., Seeger R. C., Triche T. J., Brodeur G. M. Biological classification of cell lines derived from human extra-cranial neural tumors. Prog Clin Biol Res. 1988;271:291–306. [PubMed] [Google Scholar]
- Rosenbaum H., Webb E., Adams J. M., Cory S., Harris A. W. N-myc transgene promotes B lymphoid proliferation, elicits lymphomas and reveals cross-regulation with c-myc. EMBO J. 1989 Mar;8(3):749–755. doi: 10.1002/j.1460-2075.1989.tb03435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger H. R., Gerson J. M., Moorhead P. S., Maguire H., Hummeler K. Establishment and characterization of human neuroblastoma cell lines. Cancer Res. 1976 Sep;36(9 PT1):3094–3100. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Wada R., Brodeur G. M., Moss T. J., Bjork R. L., Sousa L., Slamon D. J. Expression of N-myc by neuroblastomas with one or multiple copies of the oncogene. Prog Clin Biol Res. 1988;271:41–49. [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol. 1989 Mar;19(3):447–451. doi: 10.1002/eji.1830190306. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Dawson J. R., Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C. J., McKeon C., Triche T. J., Ross R. A., Reynolds C. P., Israel M. A. Differential protooncogene expression characterizes histopathologically indistinguishable tumors of the peripheral nervous system. J Clin Invest. 1987 Sep;80(3):804–811. doi: 10.1172/JCI113137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani J. A., Mizuno S., Kang S. H., Yang S. Y., Dupont B. Molecular mapping of a new public HLA class I epitope shared by all HLA-B and HLA-C antigens and defined by a monoclonal antibody. Immunogenetics. 1989;29(1):25–32. doi: 10.1007/BF02341610. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Versteeg R., Krüse-Wolters K. M., Plomp A. C., van Leeuwen A., Stam N. J., Ploegh H. L., Ruiter D. J., Schrier P. I. Suppression of class I human histocompatibility leukocyte antigen by c-myc is locus specific. J Exp Med. 1989 Sep 1;170(3):621–635. doi: 10.1084/jem.170.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg R., Noordermeer I. A., Krüse-Wolters M., Ruiter D. J., Schrier P. I. c-myc down-regulates class I HLA expression in human melanomas. EMBO J. 1988 Apr;7(4):1023–1029. doi: 10.1002/j.1460-2075.1988.tb02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg R., Peltenburg L. T., Plomp A. C., Schrier P. I. High expression of the c-myc oncogene renders melanoma cells prone to lysis by natural killer cells. J Immunol. 1989 Dec 15;143(12):4331–4337. [PubMed] [Google Scholar]
- Wada R. K., Seeger R. C., Brodeur G. M., Slamon D. J., Rayner S. A., Tomayko M., Reynolds C. P. Characterization of human neuroblastoma cell lines that lack N-myc gene amplification. Prog Clin Biol Res. 1988;271:57–69. [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Douglass E. C., Israel M. A. Chromosome translocation in peripheral neuroepithelioma. N Engl J Med. 1984 Aug 30;311(9):584–585. doi: 10.1056/NEJM198408303110907. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Graham F. L. Assay of transforming activity of tumor virus DNA. Methods Enzymol. 1980;65(1):826–839. doi: 10.1016/s0076-6879(80)65077-0. [DOI] [PubMed] [Google Scholar]