Hippocampal injury typically leads to mood and memory impairments associated with reduced and aberrant neurogenesis in the dentate gyrus. This study examined whether subventricular zone-neural stem cell (SVZ-NSC) grafting after hippocampal injury would counteract impairments in mood, memory, and neurogenesis. Analyses through forced swim, water maze, and novel object recognition tests revealed significant impairments in mood and memory function in animals that underwent injury and sham-grafting surgery, but animals that received NSC grafts after injury exhibited mood and memory function comparable to those of naïve control animals. The results suggest that grafting of SVZ-NSCs into the hippocampus is a useful approach for alleviating mood and memory dysfunction in neurological disorders associated with hippocampal lesions or damage.
Keywords: Nervous system, Neural stem cell, Cell transplantation, Cellular therapy, Neural differentiation, Rat model, Stem cell transplantation, Tissue-specific stem cells
Abstract
The hippocampus is vital for functions such as mood and memory. Hippocampal injury typically leads to mood and memory impairments associated with reduced and aberrant neurogenesis in the dentate gyrus. We examined whether neural stem cell (NSC) grafting after hippocampal injury would counteract impairments in mood, memory, and neurogenesis. We expanded NSCs from the anterior subventricular zone (SVZ) of postnatal F344 rat pups expressing the human placental alkaline phosphatase and grafted them into the hippocampus of young adult F344 rats at 5 days after an injury inflicted through a unilateral intracerebroventricular administration of kainic acid. Analyses through forced swim, water maze, and novel object recognition tests revealed significant impairments in mood and memory function in animals that underwent injury and sham-grafting surgery. In contrast, animals that received SVZ-NSC grafts after injury exhibited mood and memory function comparable to those of naïve control animals. Graft-derived cells exhibited excellent survival and pervasive migration, and they differentiated into neurons, subtypes of inhibitory GABAergic interneurons, astrocytes, oligodendrocytes, and oligodendrocyte progenitors. Significant fractions of graft-derived cells also expressed beneficial neurotrophic factors such as the glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, fibroblast growth factor, and vascular endothelial growth factor. Furthermore, SVZ-NSC grafting counteracted the injury-induced reductions and abnormalities in neurogenesis by both maintaining a normal level of NSC activity in the subgranular zone and providing protection to reelin+ interneurons in the dentate gyrus. These results underscore that early SVZ-NSC grafting intervention after hippocampal injury is efficacious for thwarting mood and memory dysfunction and abnormal neurogenesis.
Introduction
The hippocampus is vital for cognitive and mood function [1–3]. It is also a brain area well known for postinjury plasticity [4]. Hippocampal injury associated with neurodegeneration can ensue through multiple causes, including head injury [5], ischemia [6], acute seizures [7], and severe stress [8]. Among hippocampal alterations in the early postinjury period, increased neurogenesis from neural stem cells (NSCs) and upregulation in the concentration of neurotrophic factors are conspicuous [9–13]. Although the implications of these plastic changes are still being examined, it is believed that these changes signify compensatory mechanisms to lessen the overall hippocampal dysfunction. Nonetheless, hippocampal injury leads to mood and memory impairments months after injury [14–16], which are allied with reduced NSC proliferation in the neurogenic subgranular zone (SGZ) of the dentate gyrus (DG) and aberrant hippocampal neurogenesis. Abnormal hippocampal neurogenesis is typified by both reduced incorporation of newly born neurons into the dentate granule cell layer (GCL) and abnormal migration of newly born neurons into the dentate hilus [10, 14]. These changes are associated with reduced concentration of multiple neurotrophic factors important for neurogenesis [10, 13] and loss of DG interneurons secreting reelin, an extracellular matrix protein that controls newly born dentate granule cell migration [17, 18].
From the above perspectives, interventions that are competent for averting the evolution of initial hippocampal injury into mood and memory impairments have significance. In particular, therapeutic strategies that have promise for maintaining the normal extent and pattern of neurogenesis in the injured hippocampus are of great interest. This is because hippocampal neurogenesis is considered to be vital for functions such as mood and memory [2, 19, 20], and the aberrant neurogenesis that ensues after injury is believed to contribute to mood and memory dysfunction, as well as dentate hyperexcitability [21, 22]. In this context, NSC transplantation therapy appears to be a good candidate for ameliorating hippocampal injury-induced impairments, as these cells have the ability to survive, migrate, and engraft into brain regions exhibiting neuron loss [23]. Furthermore, NSCs can contribute new neurons, including the inhibitory GABAergic interneurons, introduce new astrocytes that are capable of secreting neurotrophic factors [24], and improve neurogenesis through stimulation of the proliferation of endogenous NSCs in the neurogenic SGZ [25]. Although NSCs can be obtained from a variety of sources, we chose subventricular zone (SVZ)-derived NSCs as donor cells because of the feasibility of their expansion in culture for extended periods without losing multipotency owing to their self-renewal ability [26, 27]. Moreover, harvesting of SVZ-NSCs from autopsied postnatal or adult human brains and live human brain is feasible [28–30].
We ascertained the efficacy of SVZ-NSC grafting into the hippocampus shortly after an injury for counteracting the injury-induced impairments in mood and memory function and neurogenesis. We expanded and characterized NSCs in vitro from the anterior SVZ of postnatal day 2 rats (F344) expressing the transgene alkaline phosphatase (AP). The AP+ NSCs exhibiting robust expression of multiple neurotrophic factors were then labeled with 5′-bromodeoxyuridine (BrdU) and grafted into the hippocampus of young adult F344 rats at 5 days after an injury inflicted through a unilateral administration of kainic acid (KA) into the posterior lateral ventricle. At 1.5 months postgrafting, animals were evaluated for functions such as mood, recognition memory, and spatial memory and compared with animals that received sham-grafting surgery after injury and age-matched control animals. Animals were then euthanized to measure the yield, migration, and phenotypic differentiation of graft-derived cells and the extent and pattern of hippocampal neurogenesis. To understand the potential mechanisms of grafting-mediated normalization of neurogenesis, both NSC proliferative activity in the SGZ and the survival of reelin+ interneurons in the DG were quantified. A flowchart summary of the experimental design is shown in Figure 1.
Figure 1.
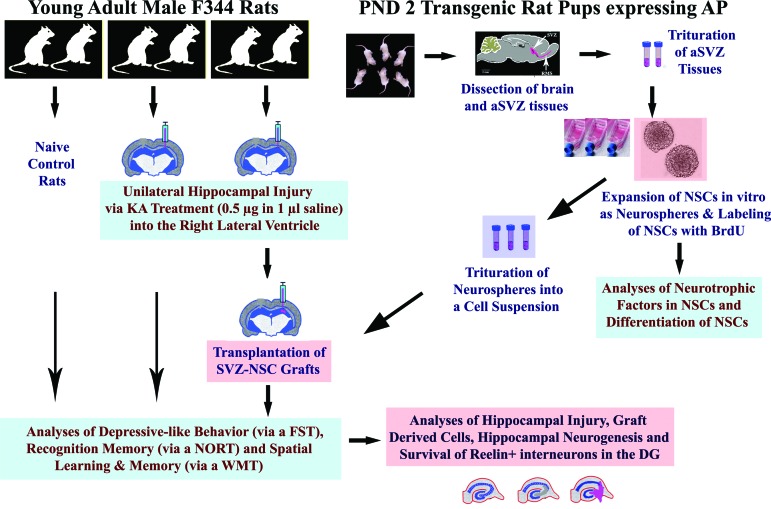
An overview of the experimental design. The right side of the figure shows dissection of the anterior SVZ tissues from the PND 2 rat pups expressing the transgene alkaline phosphatase, trituration of SVZ tissues and expansion of NSCs as neurospheres, labeling of NSCs with BrdU, trituration of neurospheres into a cell suspension for grafting studies, and in vitro analyses. The left side of the figure depicts the induction of unilateral hippocampal injury and transplantation of SVZ-NSC grafts into the hippocampus; evaluation of animals for depressive-like behavior using an FST, recognition memory using a NORT, and spatial learning and memory using a WMT; and histological analyses of the host hippocampus for various analyses. Abbreviations: aSVZ, anterior subventricular zone; BrdU, 5′-bromodeoxyuridine; DG, dentate gyrus; FST, forced swim test; KA, kainic acid; NORT, novel object recognition test; NSC, neural stem cell; PND, postnatal day; SVZ, subventricular zone; WMT, water maze test.
Materials and Methods
Induction of Unilateral Partial Hippocampal Injury
Four-month-old male F344 rats (Harlan Sprague-Dawley) were used in this study. A group of rats (n = 6) received unilateral partial hippocampal injury followed by SVZ-NSC grafting into the injured hippocampus at 5 days postinjury (hereafter referred to as grafted animals). A second group of rats (n = 6) received unilateral partial hippocampal injury followed by sham-grafting surgery at 5 days postinjury (sham-grafted animals). A third group of age-matched rats (n = 6) served as naïve controls (control animals). An unilateral partial hippocampal injury was induced via KA (Tocris) administration into the right lateral ventricle, using procedures detailed in our earlier reports [12, 13, 31] and in the supplemental online data.
Preparation of SVZ-NSC Suspension
Anterior SVZ tissues were dissected from the forebrain, triturated, and expanded as neurospheres in vitro using standard NSC expansion procedures [32]. In order to label the AP+ neurosphere cells with a second marker, 0.25 μM BrdU was added to the proliferation medium. Neurospheres were mechanically triturated, and preparations having >75% cell viability were selected for further study. Samples from the NSC suspension were incubated in culture dishes coated with poly-d-lysine and containing Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 medium (F12) for 1 hour and processed for BrdU immunohistochemistry to calculate the BrdU labeling index [24, 32]. For grafting experiments, the live cells were adjusted to a density of 1.0 × 105 cells per microliter of culture medium containing brain-derived neurotrophic factor (BDNF) (200 ng/ml). The detailed procedures are described in the supplemental online data.
Characterization of Neurotrophic Factors in SVZ-NSCs
Samples from the NSC suspension were washed in DMEM and incubated in culture dishes coated with poly-d-lysine and containing the same medium for 1 hour and then processed for various immunofluorescence methods for detecting the presence of BDNF, glial cell line-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF), and fibroblast growth factor-2 (FGF-2) in NSCs. Details on these procedures are available in the supplemental online data.
Phenotypic Differentiation of SVZ-NSCs in Culture
Samples from the SVZ-NSC suspension were incubated in culture dishes coated with poly-d-lysine and containing DMEM, F12, and B-27 nutrient mixture for 7 days. The cultures were processed for various immunofluorescence methods to quantify fractions of neurons, interneurons, astrocytes, and oligodendrocytes. These procedures are described in detail in the supplemental online data.
Grafting of SVZ-NSCs into the Injured Hippocampus
Grafting was performed on postinjury day 5, using methods described in the supplemental online data. Three grafts (each containing 100,000 live cells in 1 μl of the culture medium including BDNF at a concentration of 200 ng/ml) were placed into the injured hippocampus along its septotemporal axis. Based on the BrdU labeling index (90% of all cells) in the cell suspension, this amounted to grafting of 90,000 BrdU+ cells per graft and 270,000 BrdU+ cells per hippocampus. The injured hippocampus of animals assigned for sham-grafting surgery received injections of the culture medium including BDNF (200 ng/ml) into three sites (1 μl/site).
Analyses of Depressive-Like Behavior Through a Forced Swim Test
Animals were subjected to a forced swim test (FST) at 1.5 months after the grafting/sham-grafting surgery. The forced swim test is one of the most widely used tests for assessing the extent of depressive-like behavior in rodents [33, 34]. A detailed description of this test is available in the supplemental online data.
Characterization of Recognition Memory Function via a Novel Object Recognition Test
Animals were examined using a novel object recognition test (NORT), which is a benchmark test for assessing recognition memory in rodents [34–36]. A greater tendency to explore the novel object more than the familiar object in the memory testing phase reflects the use of learning and recognition memory processes [37, 38]. A detailed description of this test is available in the supplemental online data.
Measurement of Spatial Learning and Memory Function Using a Water Maze Test
Animals were examined using a water maze test (WMT) after the completion of a NORT. A full description of the WMT used in this study is provided in our previous reports [24, 32] and the supplemental online data.
Tissue Processing
Following all behavioral tests (i.e., at ∼2.5 months after grafting), rats were perfused with 4% paraformaldehyde, brain tissues were collected, and 30-μm-thick sections were cut coronally through the entire hippocampus using a cryostat and collected serially in 24-well plates containing phosphate buffer. A set of serial sections (every 15th section) through the entire hippocampus from animals belonging to different groups was processed for neuron-specific nuclear antigen (NeuN) immunostaining [39] to examine neurodegeneration.
Quantification of the Yield of Graft-Derived Cells
A set of serial sections (every 10th section) from grafted animals was first processed for BrdU immunostaining as described in our earlier report [40]. Cells positive for BrdU were then counted in serial sections through the entire anterior-posterior extent of the hippocampus using the optical fractionator counting method in a Stereo Investigator system (MicroBrightField Inc., Williston, VT, http://www.mbfbioscience.com). The counting procedure is detailed in our earlier reports [32, 40] and the supplemental online data. The yield of graft-derived cells in each hippocampus was expressed as the percentage of injected BrdU+ cells.
Analyses of Graft Cell Differentiation and T Lymphocytes in the Host Brain
We quantified the phenotype of graft-derived cells through dual immunofluorescence and confocal microscopy for AP and different neural cell antigens, and BrdU and different neural cell antigens. The neural cell antigens included markers of (a) mature neurons (NeuN), (b) inhibitory interneurons (GABA; the calcium-binding proteins parvalbumin [PV], calretinin [CR], and calbindin [CBN]; and the neuropeptides somatostatin [SS] and neuropeptide Y [NPY]), (c) mature astrocytes (S100β), (d) oligodendrocytes (2′,3′-cyclic nucleotide 3′-phosphodiesterase [CNPase]), and (e) oligodendrocyte progenitors (neuron glia proteoglycan 2 [NG2]). Additionally, we examined the presence of T lymphocytes using a CD4 antibody. The methods are described in the supplemental online data. Dual-labeled cells were quantified through z-section analyses using an Fv10i confocal microscope (Olympus, Tokyo, Japan, http://www.olympus-global.com). One hundred to 150 graft-derived cells from each of the grafted hippocampi (six sections per animal) were analyzed for every neural cell antigen examined.
Characterization of the Expression of Neurotrophic Factors in Graft-Derived Cells
We performed dual immunofluorescence on hippocampal sections passing through grafts using antibodies against BrdU or AP and antibodies for GDNF, BDNF, FGF-2, and VEGF. Using z-section analyses with a FV10i confocal microscope, we then quantified the percentages of graft-derived cells expressing GDNF, BDNF, FGF-2, or VEGF. Approximately 200 graft-derived cells from each of the grafted hippocampus (using four to six sections per animal) were analyzed for every neurotrophic factor examined.
Measurement of the Extent and Pattern of Hippocampal Neurogenesis
A set of serial sections (every 15th section) through the entire hippocampus from all animals was processed for doublecortin (DCX) immunostaining [41]. Using these sections, numbers of newly born (DCX+) neurons in the SGZ-GCL were measured via the optical fractionator counting method using a Stereo Investigator system (MicroBrightField) as detailed in our earlier reports [25, 41]. We also measured percentages of DCX+ newly born neurons in the DG that are located in the dentate hilus, as described in our earlier report [40]. Furthermore, we examined the effect of SVZ-NSC grafting on the occurrence of abnormal basal dendrites [22, 40, 42] via measurement of percentages of relatively mature DCX+ neurons (i.e., DCX+ neurons with vertical dendrites projecting into the molecular layer) exhibiting basal dendrites, as described in our previous report [40].
Analyses of Proliferation of NSCs in the SGZ and Survival of Reelin+ Interneurons in the DG
We quantified fractions of putative NSCs in the SGZ (i.e., glial fibrillary acidic protein [GFAP]+ cells) that are positive for Ki67 (a marker of dividing cells). We first performed dual immunofluorescence on serial sections using antibodies against GFAP and Ki67 [11]. Next, using z-section analyses in a confocal microscope, we quantified the percentage of GFAP+ cells expressing Ki67. At least 200 GFAP+ cells in the SGZ of each hippocampus (using four to six sections per animal) were analyzed for Ki67 expression. Because none of the S100β+ mature astrocytes in the SGZ expressed Ki67 (supplemental online Fig. 1), the above quantification provided an indirect measure of the proliferation of NSCs in the SGZ. We measured the effects of NSC grafting on the survival of reelin+ interneurons via stereological quantification of reelin+ interneurons in the SGZ-GCL and the dentate hilus.
Statistical Analyses
All data are expressed as mean ± SEM. Data were analyzed using one-way analysis of variance followed by Student-Newman-Keuls multiple-comparison post tests.
Results
SVZ-NSCs Express Neurotrophic Factors and Produce All Three Central Nervous System Phenotypes and Subtypes of GABAergic Neurons
The neurosphere cells generated from SVZ-NSCs displayed robust expression of BDNF, GDNF, VEGF, and FGF-2 (Fig. 2A1–2E3). Additional analyses demonstrated the ability of SVZ-NSCs to produce all three central nervous system cell types, including the GABAergic neurons (Fig. 2F–2K). Significant fractions of SVZ-NSCs differentiated into β-III-tubulin-positive (Tuj-1+) neurons (24%), GABA+ interneurons (22%), GFAP+ cells (70%), S100β+ mature astrocytes (53%), and oligodendrocytes positive for O4 (12%) and receptor-interacting protein (18%) (Fig. 2Q). The vast majority of Tuj-1+ neurons (90%) derived from SVZ-NSCs expressed GABA (Fig. 2F–2H), and the GABAergic neuronal population comprised subclasses of neurons expressing PV, CR, CBN, SS, or NPY (Fig. 2L–2P).
Figure 2.
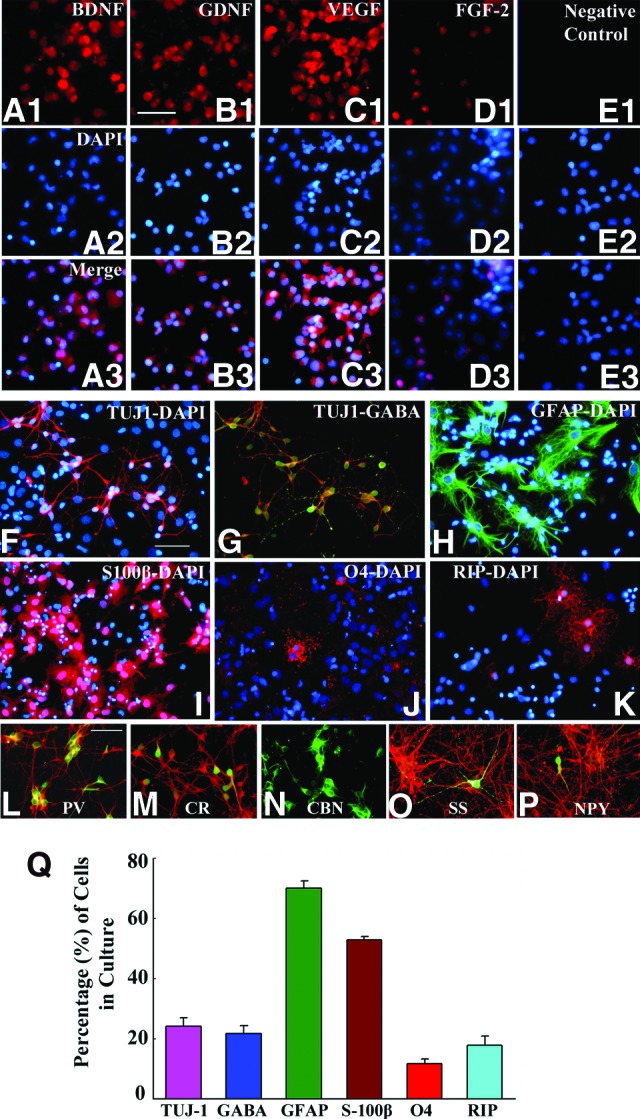
Subventricular zone-neural stem cells (SVZ-NSCs) expanded in vitro demonstrate the expression of multiple neurotrophic factors and produce different central nervous system phenotypes, including the GABAergic interneurons. (A1–E3): NSCs showing the expression of BDNF (A1–A3), GDNF (B1–B3), VEGF (C1–C3), and FGF-2 (D1–D3). (A2–E2): NSC nuclei expressing DAPI. (E1–E3): Negative control samples of NSCs. Scale bar = 50 μm. (F–K): Differentiation of SVZ-NSC derived cells (following 7 days of incubation in a differentiation medium) into Tuj-1+ neurons (F), GABA+ neurons (G), GFAP+ astrocytes (H), S100β+ astrocytes (I), O4+ immature oligodendrocytes (J), and RIP+ mature oligodendrocytes (K). (L–P): Differentiation of SVZ-NSC cells in vitro into subtypes of GABAergic interneurons (shown in green) expressing parvalbumin (L), calretinin (M), calbindin (N), somatostatin (O), and neuropeptide Y (P). Scale bar = 50 μm. The bar chart in (Q) illustrates percentages of different types of neurons and glia derived from SVZ-NSCs following 7 days of incubation in a differentiation medium. Abbreviations: BDNF, brain-derived neurotrophic factor; CBN, calbindin; CR, calretinin; DAPI, 4′,6-diamidino-2-phenylindole; FGF-2, fibroblast growth factor-2; GDNF, glial cell line-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; NPY, neuropeptide Y; PV, parvalbumin; RIP, receptor-interacting protein; SS, somatostatin; TUJ1, β-III-tubulin; VEGF, vascular endothelial growth factor.
Neurodegeneration After KA Administration
Neurodegeneration in the hippocampus ipsilateral to KA administration was typified by a partial loss of neurons in the dentate hilus and extensive loss of neurons in the cornu ammonis 3 (CA3) pyramidal cell layer spanning the CA3b and CA3c regions (supplemental online Fig. 2). Although neurons were spared in the CA3a region, some neuron loss was evident in the CA1 pyramidal cell layer. Furthermore, cell layers in the hippocampus contralateral to KA administration did not exhibit any sign of neuron loss (data not illustrated). This pattern of neurodegeneration was evident in both grafted and sham-grafted animals, which is consistent with our earlier studies using this injury model [31].
SVZ-NSC Grafting Eases Hippocampal Injury-Mediated Mood Dysfunction
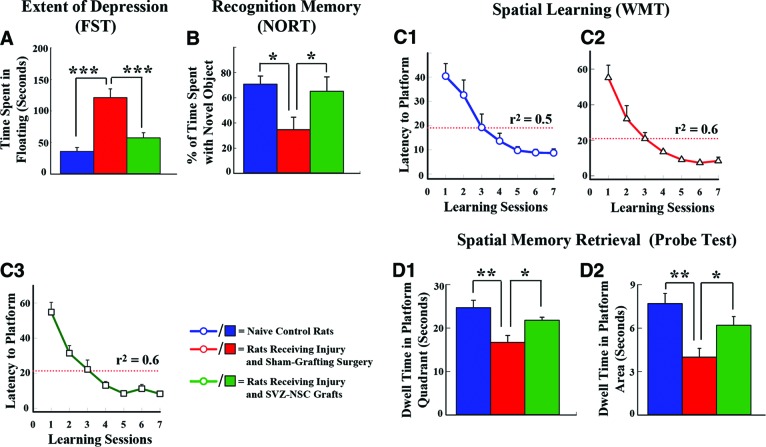
Sham-grafted animals displayed increased depressive-like behavior, which was revealed by an increased time spent in immobility (or floating) in an FST [38] in comparison with control animals (Fig. 3A). Immobility time in these animals was 240% greater than the immobility time exhibited by control animals (p < .001; Fig. 3A). In contrast, grafted animals exhibited mood function that was equivalent to that of control animals and superior to that of sham-grafted animals (p < .001; Fig. 3A).
Figure 3.
Grafting of SVZ-NSCs into the hippocampus at 5 days postinjury maintains normal mood and memory function. (A): Results of an FST, which used the time spent in floating as a measure of depression. Rats receiving sham-grafting surgery after hippocampal injury (red) displayed increased depressive-like behavior, whereas rats receiving SVZ-NSC grafts after hippocampal injury (green) exhibited mood function similar to that of naïve control rats (blue). ***, p < .001. (B): Findings in a NORT, which used percentage of the exploration time spent with novel object as a measure of the object recognition memory. Rats receiving sham-grafting surgery exhibited impaired object recognition memory, whereas rats receiving SVZ-NSC grafts demonstrated object recognition ability comparable to that of naïve control rats. *, p < .05. (C1–D2): Results of a WMT. Note that based on changes in the mean latency values to reach the hidden platform over seven learning sessions (C1–C3), rats in all three groups exhibited ability for spatial learning (r2 = 0.5–0.6). (D1, D2): Comparison of memory retrieval function among the three groups, based on the amounts of time spent within the platform quadrant (D1) and the platform area (D2) in a probe test conducted at 24 hours after the seventh learning session. Note that rats receiving sham-grafting surgery after hippocampal injury exhibited impaired memory retrieval ability, whereas rats receiving SVZ-NSC grafts after hippocampal injury displayed memory retrieval ability similar to that of naïve control rats. *, p < .05; **, p < .01. Abbreviations: FST, forced swim test; NORT, novel object recognition test; SVZ-NSC, subventricular zone-neural stem cell; WMT, water maze test.
SVZ-NSC Grafting Prevents Hippocampal Injury-Related Recognition Memory Dysfunction
Sham-grafted animals spent an average of 35% of the total object exploration time with the novel object, which is 51% less than the novel object exploration time observed in control animals (Fig. 3B). In contrast, grafted animals spent 65% of the total object exploration time with the novel object, which is at par with control animals (Fig. 3B) and greater than sham-grafted animals (p < .05; Fig. 3B).
SVZ-NSC Grafting Thwarts Hippocampal Injury-Mediated Spatial Memory Impairment
The mean latency to reach the submerged platform over seven training sessions decreased progressively in all three animal groups (p < .001 to p < .0001; Fig. 3C1–3C3). However, a probe test conducted at 24 hours after the last training session revealed considerable memory retrieval dysfunction in sham-grafted animals but not in grafted animals. The sham-grafted animals spent less of the total probe test time in the platform quadrant and the platform area than control animals (p < .01, 37%–48% reduction; Fig. 3D1, 3D2), which is consistent with the spatial memory impairment observed in patients and animals with a unilateral hippocampal injury [43, 44]. In contrast, times spent in the platform quadrant and platform area by grafted animals were comparable to that of control animals (p > .05; Fig. 3D1, 3D2).
Cells Derived from SVZ-NSC Grafts Survive and Exhibit Pervasive Migration
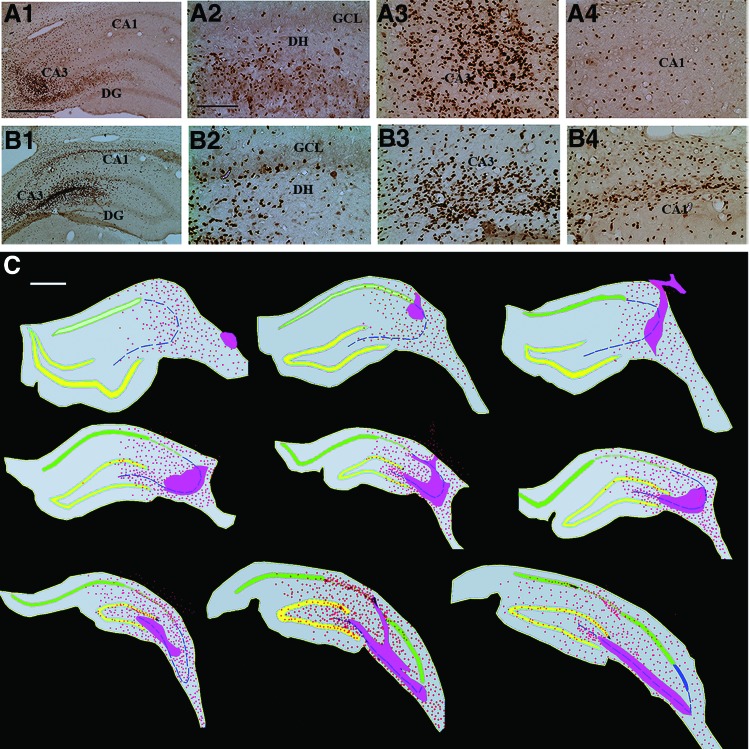
Immunostaining for BrdU revealed the presence of graft cores in and around the injured CA3 region and migration of graft-derived cells into the DG and CA1 regions of the injured hippocampus (Fig. 4A1–4B4). Figure 4C depicts the migration of graft-derived cells into different regions of the hippocampus in representative serial sections along the septotemporal axis. Occasionally, a few graft-derived cells were also found outside the hippocampal area in the adjoining entorhinal cortex and the corpus callosum. Stereological quantification of the surviving graft-derived cells revealed that SVZ-NSC grafts gave rise to an average of 349,129 new cells (349,129 ± 46,303) in each injured hippocampus. We injected three grafts, each containing 100,000 live cells, amounting to 300,000 live cells per hippocampus. However, based on the 90% BrdU labeling index at the time of grafting, the number of BrdU+ cells injected per hippocampus was 270,000. Based on these injected and recovered numbers of BrdU+ cells, the overall yield is equivalent to ∼116% of injected cells.
Figure 4.
Cells derived from the subventricular zone-neural stem cell grafts migrate profusely into different regions of the injured hippocampus. (A1, A2): Examples of injured hippocampi that demonstrated pervasive migration of 5′-bromodeoxyuridine-labeled graft-derived cells. (A2–A4, B2-B4): Respectively, graft-derived cells in magnified regions of the dentate gyrus and the CA3 and CA1 subfields from (A1) and (A2). (C): Tracings of every 15th section through the hippocampus (performed using Neurolucida [MicroBrightField]) to show the distribution of graft-derived cells in one of the grafted animals. Note that the graft core regions (solid pink areas) are located in the lesioned CA3 subfield, whereas the graft-derived cells migrated into all three regions of the hippocampus. Scale bars = 500 μm (A1, B1, C), 200 μm (A2–A4, B2–B4). Abbreviations: CA, cornu ammonis; DG, dentate gyrus; DH, dentate hilus; GCL, granule cell layer.
SVZ-NSC Grafts Contribute Significant Numbers of NeuN+ and GABA+ Neurons
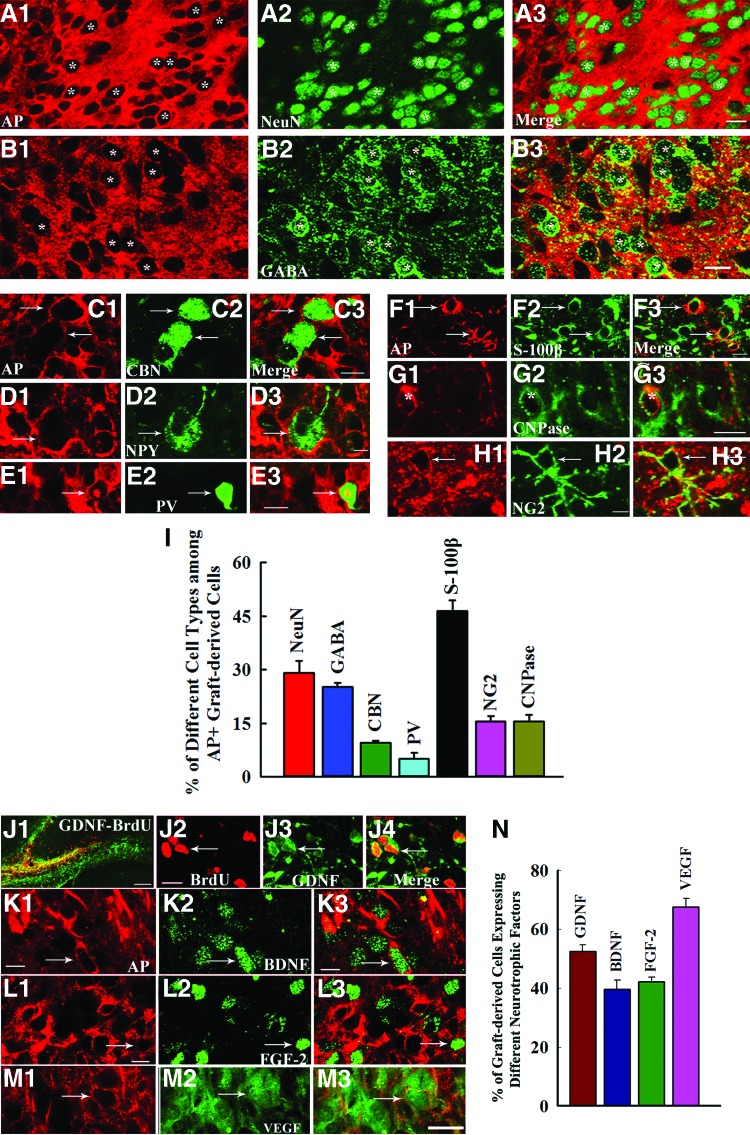
Cells derived from SVZ-NSC grafts differentiated into NeuN+ neurons, GABA+ neurons, and subclasses of GABAergic neurons positive for CBN, NPY, and PV (Fig. 5A1–5E3). Neuronal differentiation was conspicuous in the graft core (Fig. 5A1–5A3). Quantification revealed that 29% of graft-derived cells differentiated into NeuN+ neurons, 25% into GABA+ neurons, 10% into CBN+ interneurons, and 5% into PV+ interneurons (Fig. 5I). Extrapolation of the total yield of graft-derived cells with percentages of neuronal types suggested that SVZ-NSC grafting contributed ∼101,247 NeuN+ neurons and ∼88,330 GABA+ neurons into each injured hippocampus. Analyses of BrdU+ graft-derived cells revealed a similar trend (supplemental online Fig. 3). Additionally, 44% of graft-derived cells that migrated into the SGZ-GCL differentiated into NeuN+ neurons (supplemental online Fig. 3).
Figure 5.
Significant fractions of cells derived from the subventricular zone-neural stem cell (SVZ-NSC) grafts differentiate into different types of neurons and glia, and express several neurotrophic factors. (A1–H3): The AP-positive graft-derived cells (depicted in red) differentiated into neurons expressing NeuN (A1–A3); interneurons expressing GABA (B1–B3), calbindin (C1–C3), NPY (D1–D3), or PV (E1–E3); S100β+ astrocytes (F1–F3); CNPase+ oligodendrocytes (G1–G3); and NG2+ oligodendrocyte progenitors (H1–H3). The bar chart in (I) illustrates the percentages of different types of neurons and glia derived from SVZ-NSC grafts. (J1–M3): Cells derived from (BrdU+/AP+) SVZ-NSC grafts expressed GDNF (J1–J4), BDNF (K1–K3), FGF-2 (L1–L3), and VEGF (M1–M3). The bar chart in (N) illustrates the percentages of graft-derived cells expressing GDNF, BDNF, FGF-2, and VEGF. Scale bars = 10 μm (A1–H3, J2–M3), 100 μm (J1). Abbreviations: AP, alkaline phosphatase; BDNF, brain-derived neurotrophic factor; BrdU, 5′-bromodeoxyuridine; CBN, calbindin; CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase; FGF-2, fibroblast growth factor-2; GABA, γ-aminobutyric acid; GDNF, glial cell line-derived neurotrophic factor; NeuN, neuron-specific nuclear antigen; NG2, neuron glia proteoglycan 2; NPY, neuropeptide Y; PV, parvalbumin; VEGF, vascular endothelial growth factor.
SVZ-NSC Grafts Add Substantial Numbers of Astrocytes, Oligodendrocytes, and Oligodendrocyte Progenitors but Do Not Trigger Host Immune Response or Tumors
Cells derived from SVZ-NSC grafts also differentiated into astrocytes, oligodendrocytes, and oligodendrocyte progenitors (Fig. 5F1–5H3). Forty-six percent of graft-derived cells differentiated into S100β+ astrocytes, 16% into CNPase+ oligodendrocytes, and 16% into NG2+ oligodendrocyte progenitors. Based on the total yield of graft-derived cells and percentages of glial cell types, this amounts to an addition of ∼161,996 S100β+ mature astrocytes, ∼52,369 oligodendrocytes, and ∼52,369 oligodendrocyte progenitors into each injured hippocampus. Analyses of BrdU+ graft-derived cells revealed a similar trend (supplemental online Fig. 3). Furthermore, the injured hippocampus in both grafted and sham-grafted animals revealed only occasional CD4+ T lymphocytes. Importantly, graft areas did not attract CD4+ lymphocytes, implying that SVZ-NSC allografting does not trigger host immune response in the injured hippocampus. Additionally, none of the grafted hippocampi displayed tumors.
Cells Derived from NSC Grafts Express GDNF, BDNF, FGF-2, and VEGF
Considerable fractions of graft-derived cells expressed GDNF, BDNF, and FGF-2 (Fig. 5J1–5M3). Quantification revealed the occurrence of GDNF in 52% of graft-derived cells, BDNF in 40%, FGF-2 in 42%, and VEGF in 68% (Fig. 5N).
SVZ-NSC Grafting Positively Influences Neurogenesis in Both Injured and Contralateral Hippocampi
Neurogenesis in the injured hippocampus of sham-grafted animals exhibited 37% reduction at ∼2.5 months postinjury compared with control animals (p < .05; Fig. 6A1–6D). However, the grafted injured hippocampus exhibited neurogenesis that was comparable to that of the control hippocampus (p > .05; Fig. 6A1–6D). We also measured the extent of neurogenesis in the dorsal and ventral segments of the hippocampus, as it is believed that neurogenesis in the dorsal segment is important for memory, whereas neurogenesis in the ventral segment is vital for mood function [45]. In comparison with the hippocampus of control animals, the dorsal segment of the injured hippocampus exhibited an ∼40% decrease in neurogenesis in sham-grafted animals (p < .001; Fig. 6D) but no changes in grafted animals (p > .05; Fig. 6D). The ventral segment also showed a similar trend, although differences were not statistically significant (Fig. 6D). Furthermore, the hippocampus contralateral to the injury displayed increased neurogenesis in grafted animals but no changes in sham-grafted animals (details are given in supplemental online Fig. 4).
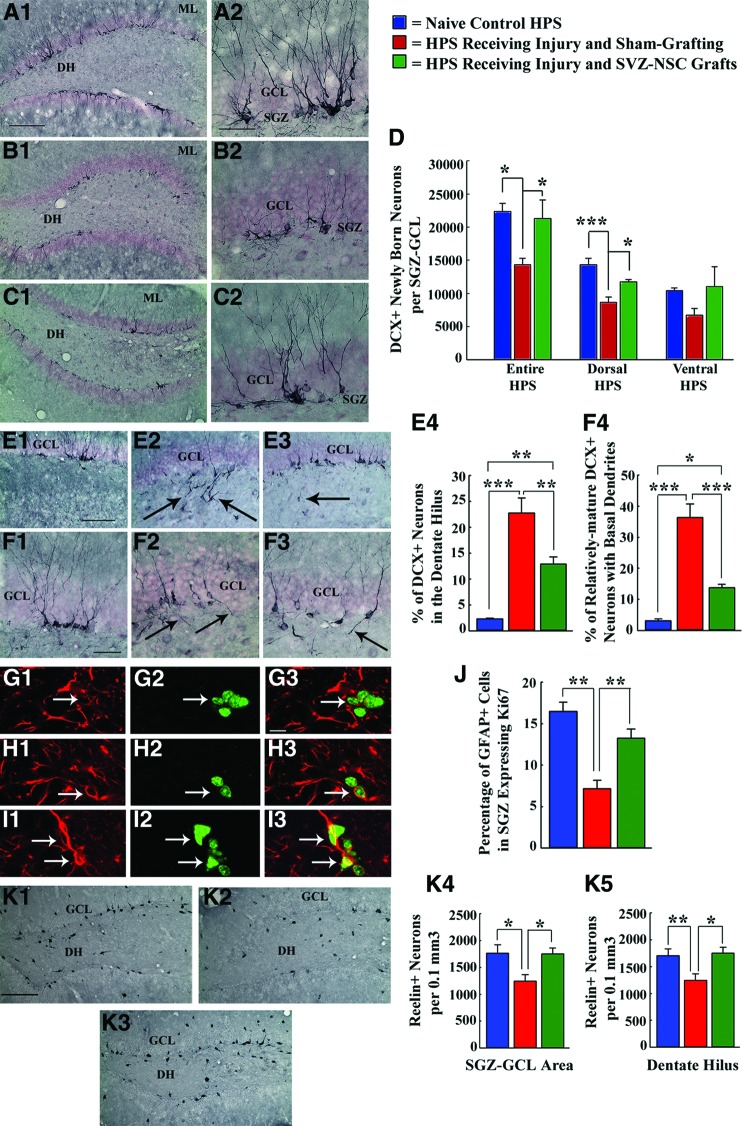
Figure 6.
Grafting of SVZ-NSCs into the HPS maintains neurogenesis and neural stem cell (NSC) activity at levels comparable to those of the intact control HPS and preserves reelin+ interneurons in the dentate gyrus. *, p < .05; **, p < .01; ***, p < .001. (A1–D): Extent of neurogenesis measured through DCX immunostaining. (E1–F4): Pattern of neurogenesis. (G1–J): NSC activity in the SGZ examined through GFAP-Ki67 dual immunofluorescence. (K1–K5): Reelin+ interneurons in the SGZ-GCL and the DH examined through reelin immunostaining. Note that in comparison with naïve control rats (A1, A2, D; E1, E4; F1, F4; G1–G3, J; K1, K4, K5), rats receiving sham-grafting surgery after hippocampal injury exhibited (a) decreased neurogenesis (B1, B2, D); (b) greater fractions of newly born neurons migrating abnormally into the dentate hilus (E2, E4); (c) increased occurrences of aberrant basal dendrites from newly born neurons (F2, F4); (d) decreased NSC activity (H1–H3, J); and (e) decreased numbers of reelin+ interneurons (K2, K4, K5). However, in, rats receiving SVZ-NSC grafts after hippocampal injury, the extent of neurogenesis (C1, C2, D), NSC activity (I1–I3, J), and surviving reelin+ interneuron numbers (K3, K4, K5) were comparable to those observed in naïve control rats. Additionally, both abnormal hilar migration of newly born neurons (E3, E4) and occurrences of aberrant basal dendrites (F3, F4) were greatly reduced in these rats. Scale bars = 200 μm (A1, B1, C1), 50 μm (A2, B2, C2, F1–F3), 100 μm (E1–E3), 10 μm (G1–I3), 200 μm (K1–K3). Abbreviations: DCX, doublecortin; DH, dentate hilus; GCL, granule cell layer; GFAP, glial fibrillary acidic protein; HPS, hippocampus; ML, molecular layer; SGZ, subgranular zone; SVZ-NSC, subventricular zone-neural stem cell.
SVZ-NSC Grafting Reduces Aberrant Pattern of Neurogenesis in the Injured Hippocampus
Although the normal hippocampus exhibits apt migration of virtually all newly born neurons into the GCL (Fig. 6E1), the injured hippocampus exhibits abnormal migration of significant fractions of newly born neurons into the dentate hilus (Fig. 6E2). Consistent with this, the sham-grafted animals exhibited abnormal migration of ∼23% of newly born neurons into the dentate hilus, in comparison with ∼2% of newly born neurons exhibiting such migration in control animals (∼12-fold increase, p < .001; Fig. 6E4). Interestingly, SVZ-NSC grafting after hippocampal injury reduced the aberrant migration of newly born neurons to ∼13% (44% reduction, p < .01; Fig. 6E3, 6E4). Moreover, NSC grafting diminished the occurrence of basal dendrites in relatively mature newly born neurons (i.e., DCX+ neurons with vertically oriented dendrites projecting into the molecular layer; Fig. 6F1–6F3). This was evidenced by the occurrence of basal dendrites in only 14% of such neurons in grafted animals, in comparison with 36% of such neurons exhibiting basal dendrites in sham-grafted animals (62% reduction, p < .001; Fig. 6F4).
SVZ-NSC Grafting Conserves Proliferative Behavior of NSCs in the SGZ
We examined putative NSCs (GFAP+ cells) in the SGZ expressing Ki67 (Fig. 6G1–6I3). Proliferative activity of NSCs was ∼17% in control animals, which reduced to ∼7% in sham-grafted animals (56% reduction, p < .001; Fig. 6J). However, in grafted animals, proliferative activity of NSCs (∼14%) was similar to that of control animals and greater than that of sham-grafted animals (p < .01; Fig. 6J).
SVZ-NSC Grafting Preserves Reelin+ Interneurons in the SGZ-GCL and the Dentate Hilus
Both SGZ-GCL and dentate hilar areas in sham-grafted animals displayed significantly reduced numbers of reelin+ neurons compared with control animals and grafted animals (25%–35% reduction, p < .05 to p < .01; Fig. 6K1–6K5). In contrast, both areas in grafted animals displayed numbers of reelin+ interneurons similar to those of control animals (p > .05; Fig. 6K1–6K5).
Discussion
This study provides novel evidence that grafting of NSCs derived from the postnatal SVZ into the hippocampus is a highly efficacious approach for counteracting the hippocampal injury-induced mood and memory dysfunction. Preservation of normal mood and memory function in animals receiving SVZ-NSC grafts after hippocampal injury was associated with (a) robust survival and pervasive migration of graft-derived cells; (b) differentiation of substantial percentages of graft-derived cells into various subtypes of GABAergic interneurons, astrocytes, oligodendrocytes, and oligodendrocyte progenitors; (c) expression of the beneficial neurotrophic factors GDNF, BDNF, FGF-2, and VEGF in significant fractions of graft-derived cells; (d) normalization of the extent and pattern of neurogenesis in the injured hippocampus with maintenance of NSC proliferation in the SGZ at levels similar to those of the age-matched intact hippocampus; (e) protection of reelin+ interneurons in the DG of the injured hippocampus; and (f) increased neurogenesis in the hippocampus contralateral to injury. Additionally, SVZ-NSC grafting into the injured hippocampus did not trigger host immune response or tumor formation.
Mechanisms Underlying SVZ-NSC Grafting Mediated Preservation of Mood and Memory Function After Hippocampal Injury
On the basis of the differential structural plasticity of the hippocampus detected after injury with SVZ-NSC grafting or sham-grafting surgery, it is credible that SVZ-NSC grafting preserved mood and memory function through several mechanisms. Nonetheless, conservation of hippocampal neurogenesis in the injured hippocampus and enhancement of neurogenesis in the hippocampus contralateral to injury are the most perceptible possibilities. It is well known that a biphasic neurogenic response occurs in the injured hippocampus, which is typified by increased and anomalous neurogenesis in the acute phase and by decreased and abnormal neurogenesis in the chronic phase [10]. The aberrant neurogenesis is perceived from both migration of substantial fractions of newly born neurons into the dentate hilus and increased occurrences of basal dendrites from newly born neurons that are integrated into the dentate GCL [22, 42]. Our results validate that sham-grafting surgery after injury did not prevent such abnormal response. In contrast, early SVZ-NSC grafting after injury preserved both the extent and the pattern of neurogenesis in the injured hippocampus at par with the control hippocampus. This was evinced by the following observations. Numbers of newly born neurons in the SGZ-GCL and numbers of proliferating NSCs in the SGZ of the injured grafted hippocampus were comparable to numbers observed in the respective regions of the control hippocampus. Furthermore, abnormal migration of newly born neurons into the dentate hilus and incidences of basal dendrites from newly born neurons were considerably reduced in the injured grafted hippocampus, in comparison with the injured sham-grafted hippocampus.
Our proposition that preserved neurogenesis in the injured hippocampus and enhanced neurogenesis in the hippocampus contralateral to injury underlie the conserved mood and memory function in grafted animals is centered on the following. First, a close association between neurogenesis and mood function has been recognized. For instance, a recent study demonstrates that induced neurogenesis deficiency results in an increased depressive-like behavior [20]. Besides, multiple previous studies have shown that recovery from mood dysfunction through antidepressant medications is intermediated by improved hippocampal neurogenesis. This was shown by findings that selective ablation of neurogenesis blocks behavioral responses to antidepressants [46, 47] and chronic antidepressant treatment enhances hippocampal neurogenesis [48, 49]. Second, even though a few earlier studies suggested uncertainties regarding the role of neurogenesis in certain memory functions [50, 51], a series of recent studies supports the idea that hippocampal neurogenesis plays a crucial role in maintaining both spatial and object recognition memories [27, 37, 52–54]. However, contributions from other factors may also be important. These may include the release of beneficial neurotrophic factors by graft-derived cells, protection of reelin+ interneurons, and addition of new GABAergic neurons.
Pertaining to neurotrophic factors, our analyses suggest robust expression of GDNF, BDNF, FGF-2, and VEGF in significant fractions of graft-derived cells. Besides their well-known ability for maintaining neurogenesis [55–57], these neurotrophic factors can directly impact mood and memory function. For example, studies show that major depressive disorder is associated with decreased concentration of BDNF [58], peripheral BDNF administration improves mood function [59], chronic intracerebroventricular FGF-2 treatment decreases depressive-like behavior [60], peripherally administered FGF-2 is highly effective for blunting anxiety [61], and chronic antidepressant treatment increases FGF-2 concentration in the hippocampus [62]. Furthermore, studies support the understanding that GDNF, BDNF, FGF-2, and VEGF have major roles in memory. These include the observations that expression of GDNF transgene in astrocytes ameliorates cognitive deficits in aged rats [63], mice lacking GDNF receptors exhibit significant memory dysfunction [64], forebrain-restricted deficiency of BDNF causes learning deficits [65], NSC grafts improve cognitive function in a mouse model of Alzheimer's disease through BDNF [23], systemic FGF-2 administration enhances long-term memory [66], FGF-2 gene transfer into the hippocampus improves memory function in a mouse model of Alzheimer's disease [67], and suppression of VEGF expression in the hippocampus by RNA interference causes memory impairments [68].
With reference to reelin+ interneurons, our results demonstrate that SVZ-NSC grafting greatly reduces the hippocampal injury-mediated loss of reelin+ interneurons in both SGZ-GCL and the dentate hilus. Reelin, a conserved extracellular glycoprotein, is believed to play an important role in both mood and memory function [69]. The role of reelin in mood function is evidenced by observations that (a) reelin gene expression is downregulated in postmortem brain samples of schizophrenia, bipolar disorder, autism, major depression, and Alzheimer's disease patients [70]; (b) transgenic mice having reduced levels of reelin are more vulnerable to developing depressive-like behavior [71]; and (c) transgenic mice overexpressing reelin are protected against developing depressive-like behavior [72]. On the other hand, the significance of reelin in memory function is documented by findings that a single intracerebroventricular injection of reelin is sufficient to increase the activation of cAMP-response element-binding protein, hippocampal long-term potentiation, and spatial learning and memory [73].
As to the addition of new GABAergic neurons, our analyses showed that SVZ-NSC grafting added ∼88,330 new GABAergic neurons into each injured hippocampus. Because hippocampal injury is associated with decreased numbers of GABAergic interneurons in the chronic phase [39], this addition is substantial and could also have positive effects on mood and memory function. This suggestion is substantiated by findings that major depressive disorders are associated with a reduced concentration of GABA in brain regions [74], GABAA receptor activation reverses memory deficits in an animal model of schizophrenia [75], and administration of GABA-enhancing drugs reverses memory deficits in aged rats [76]. Moreover, the addition of substantial numbers of new GABAergic neurons through grafts could also contribute to improved memory function through suppression of hippocampal hyperexcitability that occurs in the chronic phase after hippocampal injury [77], as excess neural activity can impair memory function [76]. Collectively, our observations suggest that SVZ-NSC grafting preserved mood and memory function after hippocampal injury via several mechanisms.
Potential Mechanisms Underlying the Beneficial Effects of SVZ-NSC Grafts on Hippocampal Neurogenesis
One of the mechanisms likely involves the release of beneficial neurotrophic factors by graft-derived cells. This is supported by our findings that significant fractions of graft-derived cells express GDNF, BDNF, FGF-2, and VEGF. These neurotrophic factors are well known to enhance hippocampal neurogenesis through NSC proliferation and differentiation [56–58, 78]. Depleted concentration of most of these proteins in the chronic phase after hippocampal injury also supports this possibility [10, 12, 13, 79]. Addition of significant numbers of GABAergic neurons by SVZ-NSC grafts might also have contributed to normal NSC proliferation in the SGZ, as GABA has an important role in NSC proliferation and differentiation and integration of newly generated neurons [80]. Another possibility may be the addition of new NSCs to the SGZ of the injured hippocampus by SVZ-NSC grafts. However, this possibility is unlikely to be among the major mechanisms, as virtually all AP+/BrdU+ graft-derived cells that migrated into the SGZ differentiated into NeuN+ neurons, S100β+ astrocytes, or NG2+ oligodendrocyte progenitors. Among the potential factors that contributed to the maintenance of the normal pattern of neurogenesis in the injured hippocampus by SVZ-NSC grafts, considerable protection of reelin+ interneurons observed in the DG stands out. This is because reelin is known to regulate the migration of newly born neurons into the GCL, and aberrant chain migration of newly born neurons into the dentate hilus after hippocampal injury has been suggested to be due to a reduced concentration of reelin occurring through a substantial loss of reelin+ interneurons in the DG [18].
Suitability of SVZ-NSCs for Cell Therapy in Neurodegenerative Disorders
The postnatal SVZ-NSCs appear to be ideal for grafting into the hippocampus after injury, as placement of these cells shortly after injury results in excellent yield and differentiation of graft-derived cells and maintenance of mood and memory function and neurogenesis at normal levels. These cells may also be suitable for transplantation in neurodegenerative disorders, where restoration of mood and memory function and neurogenesis are some of the goals. Although NSCs can be obtained from a variety of sources, the selection of a particular type of NSCs is challenging for clinical application [80]. Even though both pluripotent human embryonic stem cells and induced pluripotent stem cells are efficient for providing an unlimited supply of NSCs, their utility for routine clinical application is still unclear because of their potential for forming teratoma, as the presence of even a single pluripotent stem cell in the graft material can give rise to teratoma [81]. Therefore, rigorous testing of the therapeutic utility of NSCs with restricted fate potential (such as NSCs derived from the SVZ/hippocampus of fetal, postnatal, and adult brain) for promoting functional recovery in animal models of neurological disorders will be necessary.
Conclusion
The findings presented here demonstrate that grafting of NSCs expanded from the postnatal SVZ into the hippocampus early after an injury is highly efficacious for preventing injury-induced impairments in mood and memory function and hippocampal neurogenesis. Interestingly, the beneficial effects of grafting were associated with excellent survival and pervasive migration of graft-derived cells into different regions of the hippocampus. Furthermore, significant fractions of graft-derived cells differentiated into GABAergic interneurons, astrocytes, oligodendrocytes, and oligodendrocyte progenitors and expressed useful neurotrophic factors such as GDNF, BDNF, FGF-2, and VEGF. Thus, grafting of SVZ-NSCs into the hippocampus is a useful approach for alleviating mood and memory dysfunction in neurological disorders associated with hippocampal lesions or damage.
Acknowledgments
We thank Dr. Mahendra S. Rao (National Institutes of Health) for providing breeding pairs of transgenic AP+ rats for our studies. We also thank Dr. Bing Shuai for providing outstanding technical assistance for this study. This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01-NS054780 to A.K.S.) and the Department of Veterans Affairs (VA Merit Award to A.K.S.).
Author Contributions
B.H.: design, collection, assembly, analysis and interpretation of data, manuscript writing; A.K.S.: conception, design, collection, assembly, analysis and interpretation of data, manuscript writing, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Kryukov VI. The role of the hippocampus in long-term memory: Is it memory store or comparator? J Integr Neurosci. 2008;7:117–184. doi: 10.1142/s021963520800171x. [DOI] [PubMed] [Google Scholar]
- 2.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuels BA, Hen R. Neurogenesis and affective disorders. Eur J Neurosci. 2011;33:1152–1159. doi: 10.1111/j.1460-9568.2011.07614.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen AS, Pfister BJ, Schwarzbach E, et al. Injury-induced alterations in CNS electrophysiology. Prog Brain Res. 2007;161:143–169. doi: 10.1016/S0079-6123(06)61010-8. [DOI] [PubMed] [Google Scholar]
- 5.Huh JW, Widing AG, Raghupathi R. Differential effects of injury severity on cognition and cellular pathology after contusive brain trauma in the immature rat. J Neurotrauma. 2011;28:245–257. doi: 10.1089/neu.2010.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyawaki T, Ofengeim D, Noh KM, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao MS, Hattiangady B, Reddy DS, et al. Hippocampal neurodegeneration, spontaneous seizures, and mossy fiber sprouting in the F344 rat model of temporal lobe epilepsy. J Neurosci Res. 2006;83:1088–1105. doi: 10.1002/jnr.20802. [DOI] [PubMed] [Google Scholar]
- 8.Uno H, Tarara R, Else JG, et al. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 10.Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Hattiangady B, Rao MS, Shetty AK. Plasticity of hippocampal stem/progenitor cells to enhance neurogenesis in response to kainate-induced injury is lost by middle age. Aging Cell. 2008;7:207–224. doi: 10.1111/j.1474-9726.2007.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty AK, Rao MS, Hattiangady B, et al. Hippocampal neurotrophin levels after injury: Relationship to the age of the hippocampus at the time of injury. J Neurosci Res. 2004;78:520–532. doi: 10.1002/jnr.20302. [DOI] [PubMed] [Google Scholar]
- 13.Shetty AK, Zaman V, Shetty GA. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: A lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J Neurochem. 2003;87:147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- 14.Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jorge RE, Acion L, Starkstein SE, et al. Hippocampal volume and mood disorders after traumatic brain injury. Biol Psychiatry. 2007;62:332–338. doi: 10.1016/j.biopsych.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Potvin O, Allen K, Thibaudeau G, et al. Performance on spatial working memory tasks after dorsal or ventral hippocampal lesions and adjacent damage to the subiculum. Behav Neurosci. 2006;120:413–422. doi: 10.1037/0735-7044.120.2.413. [DOI] [PubMed] [Google Scholar]
- 17.Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex. 2003;13:634–640. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- 18.Gong C, Wang TW, Huang HS, et al. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehl M, Abrous DN. A new chapter in the field of memory: Adult hippocampal neurogenesis. Eur J Neurosci. 2011;33:1101–1114. doi: 10.1111/j.1460-9568.2011.07609.x. [DOI] [PubMed] [Google Scholar]
- 20.Snyder JS, Soumier A, Brewer M, et al. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jessberger S, Nakashima K, Clemenson GD, et al. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci. 2007;27:5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48(suppl 2):33–41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldau B, Hattiangady B, Kuruba R, et al. Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy. Stem Cells. 2010;28:1153–1164. doi: 10.1002/stem.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattiangady B, Shuai B, Cai J, et al. Increased dentate neurogenesis after grafting of glial restricted progenitors or neural stem cells in the aging hippocampus. Stem Cells. 2007;25:2104–2117. doi: 10.1634/stemcells.2006-0726. [DOI] [PubMed] [Google Scholar]
- 26.Gritti A, Parati EA, Cova L, et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlenius H, Visan V, Kokaia M, et al. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard BW, Mastroeni D, Grover A, et al. Subventricular zone neural progenitors from rapid brain autopsies of elderly subjects with and without neurodegenerative disease. J Comp Neurol. 2009;515:269–294. doi: 10.1002/cne.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanai N, Tramontin AD, Quinones-Hinojosa A, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- 30.Ayuso-Sacido A, Roy NS, Schwartz TH, et al. Long-term expansion of adult human brain subventricular zone precursors. Neurosurgery. 2008;62:223–229. doi: 10.1227/01.NEU.0000311081.50648.4C. discussion 229–231. [DOI] [PubMed] [Google Scholar]
- 31.Shetty AK, Turner DA. Enhanced cell survival in fetal hippocampal suspension transplants grafted to adult rat hippocampus following kainate lesions: A three-dimensional graft reconstruction study. Neuroscience. 1995;67:561–582. doi: 10.1016/0306-4522(95)00025-e. [DOI] [PubMed] [Google Scholar]
- 32.Hattiangady B, Shetty AK. Neural stem cell grafting in an animal model of chronic temporal lobe epilepsy. Curr Protoc Stem Cell Biol. 2011 doi: 10.1002/9780470151808.sc02d07s18. Chapter 2:Unit2D.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 34.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broadbent NJ, Gaskin S, Squire LR, et al. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17:5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jessberger S, Clark RE, Broadbent NJ, et al. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parihar VK, Hattiangady B, Kuruba R, et al. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2011;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shetty AK, Hattiangady B, Rao MS. Vulnerability of hippocampal GABA-ergic interneurons to kainate-induced excitotoxic injury during old age. J Cell Mol Med. 2009;13:2408–2423. doi: 10.1111/j.1582-4934.2008.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao MS, Hattiangady B, Abdel-Rahman A, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21:464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- 41.Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro LA, Ribak CE. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69:53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Clark RE, Broadbent NJ, Squire LR. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus. 2005;15:340–346. doi: 10.1002/hipo.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glikmann-Johnston Y, Saling MM, Chen J, et al. Structural and functional correlates of unilateral mesial temporal lobe spatial memory impairment. Brain. 2008;131:3006–3018. doi: 10.1093/brain/awn213. [DOI] [PubMed] [Google Scholar]
- 45.Kheirbek MA, Hen R. Dorsal vs ventral hippocampal neurogenesis: Implications for cognition and mood. Neuropsychopharmacology. 2011;36:373–374. doi: 10.1038/npp.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 47.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 48.Khawaja X, Xu J, Liang JJ, et al. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: Implications for depressive disorders and future therapies. J Neurosci Res. 2004;75:451–460. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- 49.Perera TD, Park S, Nemirovskaya Y. Cognitive role of neurogenesis in depression and antidepressant treatment. Neuroscientist. 2008;14:326–338. doi: 10.1177/1073858408317242. [DOI] [PubMed] [Google Scholar]
- 50.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 51.Shors TJ, Townsend DA, Zhao M, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 53.Sahay A, Scobie KN, Hill AS, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aimone JB, Deng W, Gage FH. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee AL, Ogle WO, Sapolsky RM. Stress and depression: Possible links to neuron death in the hippocampus. Bipolar Disord. 2002;4:117–128. doi: 10.1034/j.1399-5618.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Ai Y, Slevin JR, et al. Progenitor proliferation in the adult hippocampus and substantia nigra induced by glial cell line-derived neurotrophic factor. Exp Neurol. 2005;196:87–95. doi: 10.1016/j.expneurol.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Rai KS, Hattiangady B, Shetty AK. Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci. 2007;26:1765–1779. doi: 10.1111/j.1460-9568.2007.05820.x. [DOI] [PubMed] [Google Scholar]
- 58.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner CA, Gula EL, Taylor LP, et al. Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res. 2008;1224:63–68. doi: 10.1016/j.brainres.2008.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez JA, Clinton SM, Turner CA, et al. A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bachis A, Mallei A, Cruz MI, et al. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55:1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pertusa M, Garcia-Matas S, Mammeri H, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 64.Võikar V, Rossi J, Rauvala H, et al. Impaired behavioural flexibility and memory in mice lacking GDNF family receptor alpha2. Eur J Neurosci. 2004;20:308–312. doi: 10.1111/j.1460-9568.2004.03475.x. [DOI] [PubMed] [Google Scholar]
- 65.Gorski JA, Balogh SA, Wehner JM, et al. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- 66.Graham BM, Richardson R. Acute systemic fibroblast growth factor-2 enhances long-term memory in developing rats. Neurobiol Learn Mem. 2009;91:424–430. doi: 10.1016/j.nlm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Kiyota T, Ingraham KL, Jacobsen MT, et al. FGF2 gene transfer restores hippocampal functions in mouse models of Alzheimer's disease and has therapeutic implications for neurocognitive disorders. Proc Natl Acad Sci USA. 2011;108:E1339–E1348. doi: 10.1073/pnas.1102349108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mauceri D, Freitag HE, Oliveira AM, et al. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron. 2011;71:117–130. doi: 10.1016/j.neuron.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 69.Knuesel I. Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog Neurobiol. 2010;91:257–274. doi: 10.1016/j.pneurobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 70.Fatemi SH. Reelin, a marker of stress resilience in depression and psychosis. Neuropsychopharmacology. 2011;36:2371–2372. doi: 10.1038/npp.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lussier AL, Romay-Tallon R, Kalynchuk LE, et al. Reelin as a putative vulnerability factor for depression: Examining the depressogenic effects of repeated corticosterone in heterozygous reeler mice. Neuropharmacology. 2011;60:1064–1074. doi: 10.1016/j.neuropharm.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Teixeira CM, Martin ED, Sahun I, et al. Overexpression of Reelin prevents the manifestation of behavioral phenotypes related to schizophrenia and bipolar disorder. Neuropsychopharmacology. 2011;36:2395–2405. doi: 10.1038/npp.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogers JT, Rusiana I, Trotter J, et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18:558–564. doi: 10.1101/lm.2153511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Damgaard T, Plath N, Neill JC, et al. Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 2011;214:403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- 76.Koh MT, Haberman RP, Foti S, et al. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner DA, Wheal HV. Excitatory synaptic potentials in kainic acid-denervated rat CA1 pyramidal neurons. J Neurosci. 1991;11:2786–2794. doi: 10.1523/JNEUROSCI.11-09-02786.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 79.Heinrich C, Nitta N, Flubacher A, et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006;26:4701–4713. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ge S, Pradhan DA, Ming GL, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Marr RA, Thomas RM, Peterson DA. Insights into neurogenesis and aging: Potential therapy for degenerative disease? Future Neurol. 2010;5:527–541. doi: 10.2217/FNL.10.33. [DOI] [PMC free article] [PubMed] [Google Scholar]