Abstract
Purpose
Adoptive T cell immunotherapy (ACT) with tumor infiltrating lymphocytes or genetically-modified T cells has yielded dramatic results in some cancers. However, T cells need to traffic properly into tumors in order to adequately exert therapeutic effects.
Experimental Design
The chemokine CCL2 was highly secreted by malignant pleural mesotheliomas (MPM) (a planned tumor target), but the corresponding chemokine receptor (CCR2) was minimally expressed on activated human T cells transduced with a chimeric antibody receptor (CAR) directed to the MPM tumor antigen mesothelin (mesoCAR T cells). The chemokine receptor CCR2b was thus transduced into mesoCAR T cells using a lentiviral vector and the modified T cells were used to treat established mesothelin-expressing tumors.
Results
CCR2b transduction led to CCL2-induced calcium flux and increased transmigration, as well as augmentation of in vitro T cell killing ability. A single intravenous injection of 20 million mesoCAR + CCR2b T cells into immunodeficient mice bearing large, established tumors (without any adjunct therapy) resulted in a 12.5-fold increase in T cell tumor infiltration by Day 5 compared to mesoCAR T cells. This was associated with significantly increased anti-tumor activity.
Conclusions
CAR T cells bearing a functional chemokine receptor can overcome the inadequate tumor localization that limits conventional CAR targeting strategies and can significantly improve anti-tumor efficacy in vivo.
Introduction
Adoptive T cell immunotherapy (ACT) denotes the transfer of T lymphocytes for the treatment of malignant or infectious diseases. This approach has resulted in dramatic clinical responses in different cancer types including melanoma and Epstein-Barr virus lymphomas. (1-3) Classically, ACT involved the transfer of tumor infiltrating lymphocytes (TILs) that were expanded ex vivo from tumor biopsies. However, only 30-40% of tumor biopsy specimens yield satisfactory T cell populations, the expansion time required for patient therapy is long, and the approach has been primarily useful in malignant melanoma. (4) One reason for this may be that the immunosuppressive environment of many tumors induces tolerance by the deletion or functional inactivation of TIL T cell receptors (TCRs). (5-7)
A major advance in ACT has been the ability to rapidly generate large numbers of genetically redirected T cells that target specific tumor antigens using peripheral blood lymphocytes. One approach has used insertion of recombinant T cell receptors (TcRs). (8, 9) Another approach has been the creation of “chimeric antibody receptors” (CARs). (10) CARs are cell surface molecules in which the VH and VL regions of a monoclonal antibody are expressed as a single-chain variable fragment (scFv) and linked to the signal transduction domain of the CD3-zeta chain. More recent versions have added additional costimulatory domains, such as CD28 and 4-1BB, fused to the CD3-zeta chain. CAR-mediated ACT has several advantages over TcR-based ACT including high affinity recognition of the tumor antigen, MHC-independent activity, as well as the ability to link additional signaling modules to one antigen recognition event in order achieve optimal T cell activation. (11, 12) Our group has focused on introducing CARs using lentiviral vectors (13) and has initiated a clinical trial using this technology.
Although ACT appears to be one of the most robust forms of immunotherapy for the treatment of tumors, the strategy requires further optimization to overcome some significant hurdles. (11, 12) An overarching issue is safety, especially in light of lethal toxicities in recent attempts at applying ACT strategy clinically. (14, 15) However, there are also a number of issues related to increasing the efficacy of the infused T cells including: i) augmenting T cell trafficking to tumor, ii) increasing survival within tumor, and iii) making sure that the T cells retain anti-tumor activity within the intra-tumoral milieu of immunosuppressive cytokines and cells (e.g. T regulatory cells and myeloid derived suppressor cells). (16-21) In patients with metastatic melanoma, persistence of tumor antigen-specific T cells after adoptive transfer correlates with tumor regression. (22)
T cell trafficking involves a complex four-step interaction between circulating lymphocytes and endothelial cells that requires initial T cell attachment to and rolling on endothelium, T cell activation on the endothelial surface, secondary adhesion, and T cell extravasation. (17) All three of these latter steps involve chemokines and chemokine receptors (CCRs). T cell trafficking can be enhanced through binding of the tumor-produced chemokines to the appropriate CCRs on the activated T cells injected. Secretion of chemokines from the tumor that do not match the expression of the appropriate CCRs on the T cells will result in suboptimal trafficking. (23) Given that the adoptively transferred T cells are being genetically modified by insertion of optimized T cell receptors or CARs, a reasonable hypothesis is that additional modifications to change chemokine receptor expression could be advantageous.
Our group is developing ACT employing T cells transduced with lentiviral vectors encoding a chimeric antibody receptor recognizing the protein mesothelin (mesoCAR T cells). Mesothelin is a surface protein that is expressed at low levels on serosal cells (i.e. on the pleura, pericardium, peritoneum, tunica vaginalis), but is highly expressed on a number of malignancies, including malignant pleural mesothelioma (MPM), ovarian cancer and pancreatic cancer (24, 25). Due to the restricted low basal levels of expression on non-malignant tissue and the ubiquitous over-expression on various tumor types, mesothelin appears to be an attractive tumor antigen target. Clinical trials are underway using unmodified or exotoxin-conjugated anti-mesothelin antibodies and have demonstrated safety and some efficacy. (26-28) We have recently demonstrated anti-tumor activity of adoptively transferred mesoCAR T cells in preclinical mouse models of MPM (29, 30) and have initiated a pilot and feasibility clinical trial using mesoCAR T cells for patients with MPM. The hypothesis for this study is that optimization of this approach requires the CCR repertoire on the injected activated mesoCAR T cells to be “matched” to the chemokines most abundantly secreted by MPM tumors. Accordingly, we determined: 1) which chemokines are consistently secreted at high levels by MPM tumor cells, 2) which CCRs are expressed in T cells activated using clinically compliant conditions, 3) if introduction of an appropriate “matching” CCR into T cells augments trafficking to tumor, 4) and if enhanced trafficking translates into improved anti-tumor efficacy. Given the clinical prospects of treating MPM patients with extensive disease, our priority was to address these issues using large, established tumors with only a single dose of CAR T cells, and without adjunct cytokine/cellular therapy.
Methods
Mice
NOD/SCID/γ-chain Knockout (NSG) mice were bred and maintained at the Wistar Institute Animal Facility. All mouse experiments were performed in accordance with the Wistar Institute Animal Care and Use Committee guidelines and were approved by the University of Pennsylvania School of Medicine's IACUC.
Antibodies
Antibodies used in this study are outlined in Supplemental Figure 1.
Flow Cytometry
Flow cytometric analysis (FACS) was performed according to standard protocols using a FACSCanto (Becton Dickinson) flow cytometer and analyzed with FACSDiva (Becton Dickinson) and FlowJo software (TreeStar, San Carlos, CA).
Tumor Cells
The M108 tumor cell line was established from a patient with MPM malignant pleural fluid and has been previously described. (29) M108 cells naturally express mesothelin (Suppl. Fig. 2). M108 was grown in EH media as previously described. (31)
Evaluating MPM tumor chemokine expression
Human MPM cell lines (previously described by Crisanti et al. (32)) were cultured in R10 (L-glutamine supplemented RPMI-1640 medium with 10% FBS, 100-U/ml penicillin, and 100-ug/ml streptomycin sulfate) and supernatant was collected. Fluids were run on a Bio-plex Multiplex Cyokine Assay. Supernatants or malignant pleural fluid samples from subjects with MPM were analyzed by ELISA for cytokine measurements using the manufacturer's instructions.
Evaluating resting and activated T cell chemokine receptor (CCR) expression
CCR expression was measured by FACS in resting peripheral blood mononuclear cells (PBMCs) acquired from the University of Pennsylvania's Human Immunology Core. Purified PBMC T cells were activated by magnetic beads coated with anti-CD3/anti-CD28 (protocol details outlined elsewhere (29)). TILs were acquired from malignant MPM pleural effusion samples or MPM surgical biopsies, and activated by high dose IL-2 (Novartis, Basel, Switzerland) (protocol details outlined elsewhere (4)).
Lentivirus preparation
The human CCR2b construct was synthesized by Integrated DNA Technologies (Coralville, Iowa) in the pIDT.SMART cloning plasmid and flanking 5’ BamHI and 3’ SalI restriction sites allowed subsequent subcloning into the lentiviral vector pELNS bearing the EF1α promoter. (33) The mesoCAR construct contains the single-chain Fv domain of the anti-mesothelin antibody (SS1 scFv) and the CD3z and 4-1BB intracellular signaling domains, and has been previously shown to have good anti-mesothelioma tumor efficacy in a NSG MPM mouse model. (29) Packaging of each plasmid into lentivirus has been previously described. (34)
Transduction of mesoCAR and CCR2b into human T cells
Bulk CD4 and CD8 T cells isolated from PBMC were transduced with mesoCAR lentiviral vectors at an MOI ~3 24 hrs after the start of bead-activation. The following day, the media with lentivirus was discarded by centrifugation, and T cells were split into two populations, with one receiving fresh R10 and the other receiving CCR2b lentiviral vector at an MOI ~3. Cells were expanded for approximately 2 weeks and cryopreserved at -140°C in 90% FBS/10% DMSO until use.
MesoCAR FACS detection
Cells were stained using a biotinylated goat anti-mouse IgG recognizing the F(ab’)2 fragment (Jackson Immunoresearch, West Grove PA) as the primary antibody and PE-conjugated streptavidin (BD) as the secondary antibody.
Confirmation of CCR2b function by calcium flux analysis in the presence of CCL2
CCR2b-transduced T cells were analyzed using a single cell assay as described. (35) Briefly, cells were loaded with 4ug/ml of Fluo-3 AM and 10ug/ml of Fura Red AM at 37°C for 30 minutes. 4mM of probenecid was added to prevent active secretion of loaded dyes from cells. Cells were then analyzed by exciting at 488nm in the BD FACS Canto, and collecting Fluo-3 fluorescence at 515 to 535nm and Fura Red fluorescence at 665 to 685nm. After baseline measurements, CCL2 was added at 100ng/ml and analysis was continued. Untransduced T cells were used as negative controls. Ionomycin was added at 1ug/ml at the conclusion of analysis to confirm proper cell loading with the two calcium-sensitive probes.
Confirmation of CCR2b function by transwell migration in the presence of CCL2
600ul of R10 media alone or with 100ng/ml of CCL2 was placed in triplicate wells of a 24 well plate. Corning 0.5 μm polycarbonate membrane, 65 mm transwell inserts (Corning 3421, Corning, NY) were placed in the wells and 100ul of 5×106/ml transduced or non-transduced T cells were placed in the top chamber. After incubation for 4 hours at 37°C and 5%CO2, the number of T cells that migrated to the bottom chamber was quantified manually using a hemocytometer. This was repeated using M108-conditioned supernatant collected over 24 hours in the presence or absence of anti-CCL2 antibody (Centocor, Inc., Horsham, PA).
In vitro testing of mesothelioma tumor cell killing by mesoCAR bearing T cells
M108 was stably transduced to express firefly luciferase and plated in a 96 well plate at 5000 cells per well in triplicate. After overnight incubation at 37°C and 5%CO2, T cells expressing either mesoCAR or mesoCAR + CCR2b were co-cultured at a 20:1 effector:target (E:T) ratio in the absence or presence of 100ng/ml CCL2. After 4hrs of incubation at 37°C and 5%CO2, the wells were washed, remaining tumor cells were lysed (in BD Luciferase Cell Culture Lysis Buffer), and luminescence determined after addition of 100ul of luciferin reagent (Promega E1501, Madison, WI).
In vivo testing of T cell trafficking and anti-tumor activity
Five million M108 cells in a solution of X-Vivo 15 media (Lonza, Basel, Switzerland) and 50% Matrigel (BD Biosciences) were subcutaneously injected in the flanks of NSG mice. After large tumors (200-300mm3) were established (usually after 4 weeks), the mice were grouped and received one of three treatments via tail-vein: 1) 20 million untransduced bead-activated T cells, 2) 20 million bead-activated T cells transduced with mesoCAR, or 3) 20 million bead- activated T cells transduced with mesoCAR and CCR2b (mesoCAR + CCR2b) (Refer to Fig. 1 for transduction efficiencies.) Tumors were harvested five days after T cell injection and digested in a solution of 1:2 DNAse:Collagenase with rotation at 37°C. Digested tumor was then filtered through 70um nylon mesh cell strainers and washed twice with PBS+1%FBS with red blood cell lysis performed with Pharm Lyse (BD Biosciences), if needed. Whole blood was also obtained by retroorbital bleeding into heparinized tubes and subjected to red blood cell lysis. One million cells were placed in standard FACS tubes and were stained for human CD45 or CD3 expression.
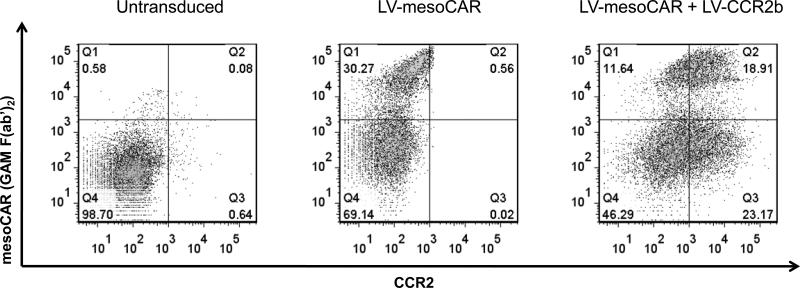
Figure 1. Expression of mesoCAR and CCR2b expression after lentivirus (LV) transduction.
Bead-activated T cells (left panel), bead-activated, LV-mesoCAR transduced T cells (middle panel), or bead-activated, LV-mesoCAR and LV-CCR2b transduced T cells (right panel) were studied using two color FACS analysis. Expression of the mesoCAR is shown on the Y axis. Expression of CCR2 is shown on the X axis.
Statistical Analyses
For the in vitro killing assays, in vivo flank tumor studies, and ex vivo TIL analyses comparing difference between two groups, we used unpaired Student's t tests. When comparing more than two groups, we used one sided ANOVA with appropriate post hoc testing. Differences were considered significant when p < 0.05. Data are presented as mean ± SEM.
Results
Chemokine production by MPM tumors
Our first goal was to assess which chemokines were produced consistently and at elevated levels by MPM tumors. Cell culture supernatants from 11 human MPM cell lines were analyzed by a multiplex cytokine assay. Of the 20 chemokines and cytokines examined, CCL2 was one of the most highly and uniformly expressed. Confirmation using an ELISA, showed that the mean concentration of CCL2 was 3500 ± 2210 pg/ml/106 cells/24 hrs, with 6 of the 11 lines secreting more than 500 pg/ml/106 cells/24 hrs (Table 1, upper panel). To confirm this observation in actual mesothelioma patients, CCL2 levels were measured by ELISA in pre-treatment pleural fluids obtained from 7 MPM patients from an earlier clinical trial. (36) The average concentration of CCL2 was 3640 ± 1030 pg/ml, with 5 samples showing greater than 2400 pg/ml (Table 1, bottom panel).
Table 1.
Supernatants from equal numbers of mesothelioma cell line cultures or pleural fluids from patients with mesothelioma were analyzed by ELISA for CCL2 concentrations (expressed in pg/ml)
| Cell Line # | H2502 | I-45 | LRK | MSTO | M30 | OK7 | OK6 | OK5 | OH4 | M108 | mEMMESO1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CCL2 (pg/ml/106 cells/24 hrs) | 29 | 5,294 | 25,000 | 3,183 | 106 | 35 | 38 | 1,719 | 121 | 2405 | 606 |
| Patient # | 101 | 104 | 105 | 108 | 110 | 111 | 112 |
|---|---|---|---|---|---|---|---|
| Pleural Fluid CCL2 (pg/ml) | 137 | 6864 | 6587 | 4372 | 404 | 2466 | 4635 |
Chemokine receptor expression on resting and activated T cells
We next analyzed which CCRs were expressed on activated T cells, with special attention to the receptor that binds to CCL2 (CCR2). Because different in vitro activation protocols can result in different CCR profiles (see discussion), we compared expression levels on resting T cells from PBMCs, MPM TILs activated by high IL-2 supplementation (600IU/ml), and bead-activated peripheral blood T cells (the method we are using in our clinical trials). FACS was performed by gating on CD4 or CD8 positive lymphocytes (Table 2). Consistent with the literature (37-39), the most highly expressed CCR was CXCR3 (which binds CXCL9, 10, and 11), with expression as high as 83% of the bead-activated cells. In contrast, CCR2 was uniformly expressed at low levels among resting and activated T cells, although IL-2 stimulated TILs had higher expression levels than T cells activated by beads. In the bead-activated cells, on average, only about 7% of cells expressed CCR2. Under all conditions of activation, the intensity of CCR2 expression was low (representative FACS tracings are shown in Supplemental Figure 3).
Table 2.
CD4 and CD8 T cells from resting PBMCs, high IL-2 stimulated TILs, anti-CD3/CD28 bead-activated isolated T cells were analyzed for CCR expression by FACs. CXCR3 was highly expressed among all samples. CXCR1 and CCR2 were expressed at low levels. (Numbers represent % of CD4 or CD8 T cells in each sample that were positive for CCR expression.)
| CD4 T cells | CXCR3(%) | CCR2(%) |
|---|---|---|
| Resting | 32.91±7.32 | 5.43±1.88 |
| High IL-2 | 64.24±8.72 | 10.11±3.06 |
| Anti-CD3/anti-CD28 | 82.72±6.70 | 6.93±0.44 |
| CD8 T cells | CXCR3 (%) | CCR2 (%) |
|---|---|---|
| Resting | 73.74±2.46 | 5.03±1.88 |
| High IL-2 | 60.35±12.00 | 13.32±2.19 |
| Anti-CD3/anti-CD28 | 97.35±1.51 | 6.88±1.01 |
CCR2b can be successfully transduced into activated T cells
In light of the high CCL2 secretion by the MPM tumors but low CCR2 expression on the activated T cells, we hypothesized that T cell trafficking to MPM could be augmented by genetically engineering T cells to express CCR2. Because CCR2b is the most dominant isoform of this receptor (40), a codon-optimized construct of CCR2b was generated and packaged into a lentivirus vector. Lentiviral vector transduction of T cells undergoing ant-CD3/anti-CD28 bead-activation was achieved using the lentiviral vector for mesoCAR alone or transduction using both mesoCAR and CCR2b lentiviral vectors.
Compared to untransduced cells (Fig. 1, left panel), single mesoCAR transduction resulted in expression in ~30% of cells (Fig. 1, middle panel). FACS analysis post-transduction with both vectors revealed that ~12% of T cells expressed mesoCAR only, ~23% of cells expressed CCR2b only, and ~19% of T cells had robust expression of both mesoCAR and CCR2b (Fig. 1, right panel).
Functional Activity of Transduced CCR2b in activated T cells
Since CCR engagement by ligand induces an increase in intracellular calcium (41), we loaded transduced and untransduced T cells with the calcium-sensitive dyes, Fura Red and Fluo-3. Calcium flux was then monitored in real-time using FACS. As shown in Figure 2A, addition of 100ng/ml CCL2 (arrow) to untransduced T cells did not induce a calcium influx, whereas the subsequent addition of the calcum ionophore, ionomycin, (arrowhead), resulted in a large increase in the fluorescence ratio indicating calcium influx. In contrast, as shown in Figure 2B, addition of 100ng/ml CCL2 (arrow) to CCR2b T cells resulted in a clear calcium spike (arrow), as did the subsequent addition of the calcium ionophore (arrowhead), demonstrating that the introduced CCR2b exhibited physiological signal transduction in T cells.
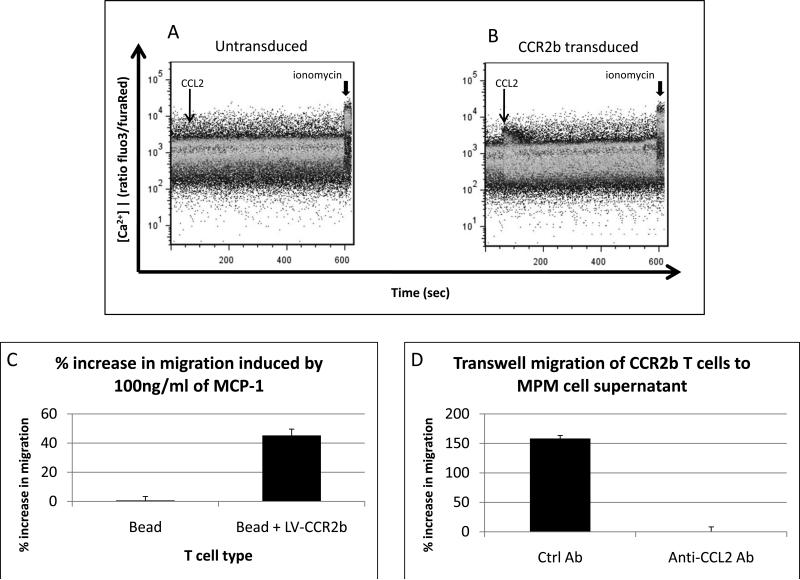
Figure 2. Ability of CCL2 to induce Calcium Flux and Transwell Migration in T cells.
Panels A and B. Bead-activated T cells (Panel A) or bead-activated, LV-CCR2b transduced T cells (Panel B) were loaded with the calcium sensitive dyes (Fluo 3 AM / Fura Red AM) and studied using FACS analysis over time. Cells were first exposed to 100 ng/ml of CCL2 (thin arrow) and then to ionomycin (arrowhead) to confirm equal loading.
Panel C. Bead-activated, LV-CCR2b transduced T cells had 45% greater migration through 0.5um polycarbonate membranes toward R10 with 100ng/ml of CCL2 than untransduced bead-activated T cells.
Panel D. M108 tumor supernatant also induced CCR2b T cell migration in a CCL2 dependent manner. The addition of anti-CCL2 Ab blocked T cell migration. Control Ab was added to media as a negative control.
The chemotactic function of the transduced CCR2b was also tested. Figure 2C shows the results from transwell studies in which transduced or untransduced T cells were allowed to migrate through a 0.5um polycarbonate membrane towards media alone or media containing 100ng/ml CCL2 in the lower well. Whereas CCL2 only increased the migration rate of mesoCAR T cells by 0.7 ± 2.7% compared to media alone, CCL2 induced a 45 ± 4.4% increase in migration of mesoCAR + CCR2b T cells compared to media alone (p<0.001), indicating a robust functional enhancement of the CCR2b modified T cells.
The assay was repeated to evaluate migration of CCR2b T cells towards M108-conditioned supernatant (Fig. 2D). Relative to transwell migration of CCR2b T cells towards R10, supernatant from M108 induced over a 100% increase in transwell migration. Anti-CCL2 antibody caused complete inhibition of supernatant-induced migration, confirming the effect as being CCL2 dependent.
Effect of CCR2b transduction on T cell in vitro killing of M108
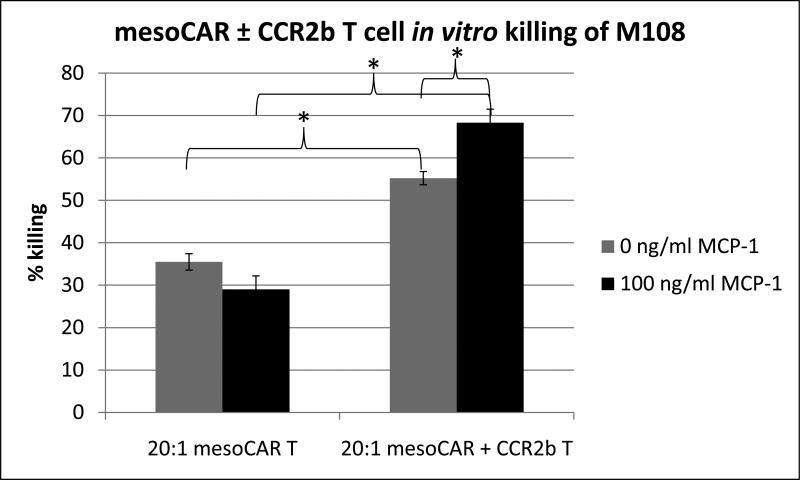
We next explored the susceptibility of M108 to mesoCAR and mesoCAR + CCR2b T cell-mediated lysis using in vitro killing assays. As shown in Figure 3, when we added mesoCAR T cells to luciferase-labeled tumor cells at a constant ratio of 20 lymphocytes to one tumor cell and determined the number of live tumor cells after 4 hours, the T cells were able to kill 35.5 ± 1.9% of M108 cells. Interestingly, mesoCAR + CCR2b T cells were able to kill ~20% more M108 cells than mesoCAR T cells (55.2 ± 1.6% vs. 35.5 ± 1.9%, p<0.01). Additionally, this effect was further augmented by ~13% when mesoCAR + CCR2b T cells were exposed to 100ng/ml of CCL2 during coculture (55.2 ± 1.6% vs. 68.3 ± 3.2%, p=0.02).
Figure 3. Effect of CCR2b on mesoCAR T cell killing of M108 tumor cells.
In vitro killing of tumor cells by T cells was assessed by coculturing mesoCAR ± CCR2b T cells with M108 tumor cells at 20:1 ratio for 4 hours in the presence and absence of CCL2. (* = p<0.05)
Effect of CCR2b transduction on T cell infiltration and anti-tumor activity in NSG mice with M108 flank tumors
We next tested the ability of each type of activated T cell to traffic into established tumors and to exert anti-tumor activity. M108 cells were injected in the flanks of four groups (10 mice per group) of NSG mice and tumors were allowed to grow to between 200 and 300 mm3. Since this typically takes about 4 weeks, the tumors were well established, and previous work has shown that the tumors are highly vascularized by this point. (29) At this time, mice were injected with either: 1) saline, 2) 20 million untransduced, bead-activated T cells, 3) 20 million bead-activated T cells transduced with mesoCAR alone, or 4) 20 million bead-activated T cells transduced with both mesoCAR and CCR2b.
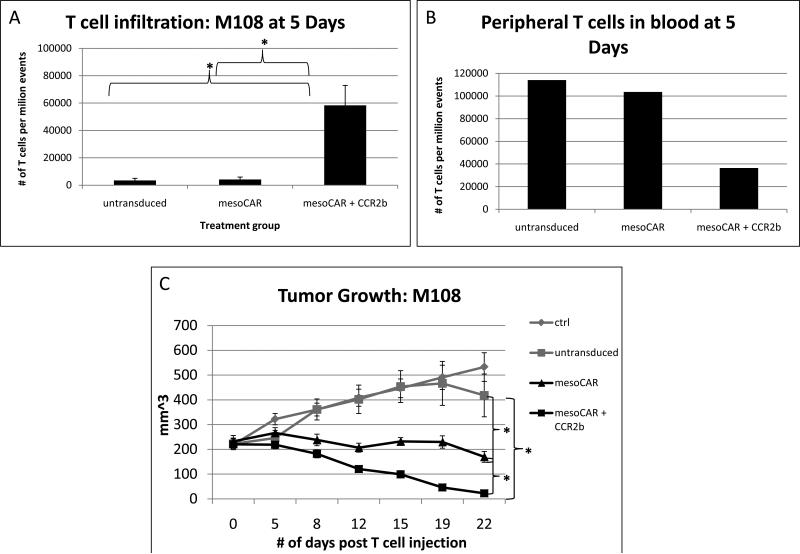
Five days after tail vein injection of T cells, three tumors in each group were harvested, digested, and subjected to FACS analysis (Fig. 4A). Because prior experience revealed that relative to the number of tumor cells, very few T cells traffic to tumor at this early time point, we chose to perform FACS analysis of digested tumor instead of in vivo imaging. The abundance of human T cells present in the tumors injected with untransduced T cells (3,400 per million total cells) or with the mesoCAR T cells (4,100 per million total cells) was very low (~0.3 - 0.4%) and not significantly different. In contrast, there was a large (>12.5 fold) and significant increase in the number of infiltrated T cells in the mesoCAR + CCR2b group (51,700 per million total cells= ~5.2%) compared to the mesoCAR group (p=0.02) and/or the untransduced T cell group (p=0.02). Blood acquired via retroorbital bleeding from the three sacrificed animals was pooled and also subjected to FACS analysis (Fig. 4B). Mice in the untransduced group had 114,100 circulating T cells per million total cells and mice in the mesoCAR T cell group had 103,600 circulating T cells per million total cells. However, mice in the mesoCAR + CCR2b T cell group had only 36,500 circulating T cells per million total cells. We interpret this to indicate that the increased TILs seen in the mesoCAR + CCR2b group tumors were due to enhanced trafficking and not enhanced systemic proliferation.
Figure 4. T cell tumor infiltration and tumor growth after intravenous injection of activated T cells.
Activated T cells were injected intravenously NSG mice with large, established M108 tumors. Groups included no T cells (ctrl), bead-activated, but untransduced T cells (untransduced), bead-activated T cells transduced with mesoCAR (mesoCAR), and bead-activated T cells transduced with both mesoCAR and CCR2b (mesoCAR + CCR2b).
Panel A and B. 5 days after T cell injection, flank tumors (Panel A) and pooled blood (Panel B) from 3 mice in each group were subjected to FACS to determine the number of human T cells. There was a significantly increased number of T cells having infiltrated the tumor in the mesoCAR + CCR2b than the mesoCAR group. (* = p <0.02)
Panel C. Tumor size was measured over time and revealed significantly increased anti-tumor activity after injection of mesoCAR + CCR2b T cells compared to mesoCAR T cells. * indicates statistically significant differences in mean tumor size at day 22 post T cell injection. Difference in mean flank tumor size in the mesoCAR and mesoCAR + CCR2b groups was statistically significant (p=0.003). Tumor volume was calculated using the formula volume = length × width × width / 2.
There were also significant differences in tumor growth over time (Fig. 4C). The tumors in the control and untransduced T cell groups grew steadily at similar rates, reaching a size of approximately 500 mm3 by Day 22 after T cell injection. In contrast, tumor size was significantly smaller (p< 0.01) in both the mesoCAR and mesoCAR + CCR2b groups than in the control or untransduced T cell groups. Importantly, however, tumor size was significantly smaller in the mesoCAR + CCR2b compared to the mesoCAR alone group (p<0.01). In fact, at Day 22 post T cell transfer, virtually all of the tumors in mice injected with the single dose of mesoCAR + CCR2b T cells had completely regressed.
Thus, the presence of CCR2b in the mesoCAR T cells significantly increased the number of intratumoral T cells and significantly enhanced anti-tumor efficacy.
Discussion
This study showed that it was possible to enhance trafficking of adoptively transferred T cells into large, well-established, mesothelin-expressing tumors by matching the chemokine receptors expressed on the activated T-cells with the chemokines secreted by the tumor. This was accompanied by significantly enhanced anti-tumor efficacy, even in large, established tumors.
There are a number of reasons to believe that improving T cell trafficking will be advantageous. The number of injected T cells that actually enter a tumor without targeting is extremely small (in our case ~0.3% of the tumor cells). After transduction with CCR2b, T cell infiltration increased more than 12.5-fold, to represent ~5% of the tumor. Since adoptively transferred T cells must function within tumors (either through direct killing or activation of local immune cells), it seems desirable to have the largest possible number of T cells within the tumor. The rapid localization of T cells to tissues where their targeted antigen is highly expressed may also increase their ability to proliferate and thereby increase the effector to target ratio. Others have shown that early involvement of tumor-reactive T cells is critical to effective ACT. (42)
There may also be a number of important safety advantages to tumor targeting. Focusing T cell activity to tumors could allow lower doses to be administered, thus increasing the therapeutic index, since the CAR cells will traffic to tumor instead of being distributed in the periphery where they could have deleterious “on-target/off-tumor” effects. This can be seen in Figure 4, where CCR2b T cells were found in much lower number in the blood than the T cells lacking CCR2b. Minimizing “on-target/off-tumor” effects remains an important goal in light of case reports of fatalities resulting from CAR T cell treatment toxicity. (14, 15) In the example of mesothelin-targeted T cells, normal pericardial and peritoneal tissues express mesothelin, albeit at lower levels than tumor, and could be potential sources of toxicity. Finally, it is possible that improving T cell trafficking to tumors, where substantial antigen-induced local proliferation could occur, could lessen, if not eliminate, the need for the aggressive lymphodepletion regimens that are now being employed. (43, 44) Lymphodepletion using chemotherapy (e.g. cyclophosphamide, fludarabine) and whole body irradiation presents substantial side-effect risks and markedly increases the costs of current trials. Of course, this hypothesis remains speculative and will need to be studied in a murine system in which animals have an intact immune system and effects of lymphodepletion can be studied.
Our data show that T cells activated by engagement of their T cell receptors using anti-CD3/anti-CD28 beads (the method we plan to use in future clinical trials) upregulate the expression of CCR7, CCR5 (data not shown), and most strongly, CXCR3 (from 15% to 49% of cells). Similar changes in CCRs after activation have been reported in the literature. (45-47) However, the type of T cells used (i.e. TILs versus PBMCs) and the type of stimulation used (i.e. use of IL-2 versus TCR engagement) does seem to make a difference in the expression pattern observed. (23, 48, 49) For example, Brown et al. (23), reported that activation of PBMC's propagated with OKT3 antibody, irradiated allogeneic PBMC, EBV-transformed lymphoblastoid feeder cells, and 25 U/ml IL-2 resulted in T cells with high levels of CCR2 (see their Figure 4C), a result very different than ours.
The expression pattern of CCRs using costimulated T cells indicates that these T cells would mostly likely be attracted to tumors expressing the chemokines CCL5 (binding to CCR5) or CXCL9, CXCL10, or CXCL11 (binding to CXCR3). However, when we examined a large panel of mesothelioma cell lines and pleural fluids from patients with malignant mesothelioma, we found that there was very little secretion of these chemokines (data not shown). Instead we observed substantial amounts of CCL2 expressed by most (but not all) cell lines and patient specimens. The other chemokine produced by many of the cell lines was CXCL8 (interleukin 8) (data not shown). As the receptors for CXCL8 (CXCR1 and CXCR2) are not expressed on our T cells, we are also pursuing similar studies to introduce CXCR1 into T cells.
By introducing the properly matched CCR (i.e. CCR2b) into the T cells using a lentiviral vector, we were able to achieve dual expression of the CAR and the CCR in almost one quarter of the cells (Fig. 1). This is similar to the results published by Di Stasi et al. (50) using retroviral vectors for a CD30 CAR and the CXCR4 receptor. This relatively low percentage of doubly positive cells represents a technical limitation presented by the need for two independent lentiviral transductions, each with less than 100% efficiency. We did not purify these cells after activation, yet still saw impressive responses. We are now modifying our approach to use a biscistronic vector expressing both transgenes and are achieving higher dual expression levels and expect to further augment pre-clinical efficacy.
The concept of matching tumor-secreted chemokines with the appropriate CCR on the transfused, activated T cell, was first proposed by Kershaw et al. in 2002 (48) who conducted in vitro studies matching secretion of the chemokine Groα (CXCL1) by melanoma cells with a retrovirally-transduced CXCR2 receptor in IL-2-activated T cells. Augmented migration of transduced T cells toward tumor supernatant was shown in a transwell assay, but no animal experiments were reported. The importance of chemokine/CCR matching was also shown by Brown et al. (23) in experiments demonstrating that their activated T cells expressed CCR2 (unlike ours) and that these cells trafficked better to tumors from lymphoma, neuroblastoma, and melanoma cell lines that expressed high levels of CCL2. Subsequently, at least three groups have shown augmented trafficking and anti-tumor efficacy of TILs or CARs in which chemokine receptor modification has been accomplished in models of Hodgkins lymphoma (using a human CD30-CAR expressing CCR4) (50), neuroblastoma (using a human GD2-CAR expressing CCR2b) (47), and melanoma (using a mouse transgenic T cell line expressing CXCR2). (51) In these studies, expression and function of the transduced CCRs were confirmed in vitro, followed by demonstration of increased in vivo migration and a resultant slowing in tumor growth. However, in each of these studies, the modified T cells were introduced only one week or less from the time of xenograft tumor cell injection (thus before true “establishment” of the tumors), IL-2 was injected during the period of ACT, and multiple injections of T cells were given. Although IL-2 supplementation may promote survival of introduced T cells, there is now clear data showing that IL-2 also stimulates the expansion of Treg cells (52, 53) – clearly, an undesired effect, especially in MPM (54, 55). Additionally, Peng et al. (51) also injected peptide-pulsed dendritic cells and used flow cytometry-assisted sorting for CAR positive T cells, whereas we did not. We feel that our study significantly extends these previous reports by: 1) being the first report to show an enhancement of actual tumor regression (rather than just slowing of tumor growth), 2) direct enumeration of the efficiency of CAR T cell trafficking rather than using estimates from bio-imaging, 3) demonstrating that increased adoptive T cell migration and anti-tumor activity are possible in a model of large, established tumors, 4) showing clear efficacy with only one dose of genetically modified T cells, and 5) demonstrating that targeted 2nd generation CARs can be highly effective after intravenous injection without the need for additional adjuvant cytokine or cellular therapy. Interestingly, the in vitro data from this paper suggests that CCL2/CCR2 interactions may actually augment T cell effector function independent of its effects on trafficking, and studies to uncover this mechanism are planned.
One of the goals of this study was to provide relevant preclinical data to support optimization of ongoing clinical trials. Our first target population for the mesoCAR T cells will be patients with malignant pleural mesothelioma (MPM). This disease is highly associated with previous asbestos exposure and usually presents in the fifth to seventh decade of life with dyspnea, a pleural effusion, and non-pleuritic chest pain. Current therapies are inadequate, stimulating our group and others to pursue novel immune and immune-gene therapy approaches. (25, 36, 56-59) As mentioned above, most MPM's express high levels of mesothelin (as do ovarian and pancreatic cancers). Once we have collected our baseline safety and efficacy clinical trial data, the findings from this study suggest that we can improve the approach employing tumor-targeting strategies using CCR2b or potentially other CCRs. In addition, our data suggests that the efficacy of ACT, in general, might be improved in clinical trials by “personalizing” the injected T cell chemokine receptor profile with the chemokines produced by the subject's own tumor.
Supplementary Material
Translational Relevance.
In this study, we have provided experimental evidence showing that matching the chemokine receptor (CCR) expression on T cells that bear the mesothelin-reactive chimeric antibody receptor (CAR) to the chemokine secretion by the target tumor leads to enhanced T cell homing to and activity against the tumor. We have further shown that with properly trafficking CAR T cells, it is possible to eradicate large, established tumors, with a single dose of T cells, and without adjunct cellular or cytokine therapy. These discoveries have important implications for optimizing adoptive T cell immune-gene therapy for malignant pleural mesothelioma, a cancer with poor survival that is still minimally affected by current treatment strategies, as well as other tumors.
Acknowledgements
The authors would like to thank John Scholler, Nilam Mangalmurti, and Guan-Jun Cheng for technical assistance in the experiments.
Financial support:
T32-HL07586 (Training Grant in Pulmonary Immunology)
PO1 CA 66726-07 (Immuno-Gene Therapy of Mesothelioma)
REFERENCES
- 1.Gattinoni L, Powell DJ, Jr., Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–75. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–9. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteside TL. Signaling defects in T lymphocytes of patients with malignancy. Cancer Immunol Immunother. 1999;48:346–52. doi: 10.1007/s002620050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monu N, Frey AB. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 2007;67:11447–54. doi: 10.1158/0008-5472.CAN-07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–24. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Spaapen R, van den Oudenalder K, Ivanov R, Bloem A, Lokhorst H, Mutis T. Rebuilding human leukocyte antigen class II-restricted minor histocompatibility antigen specificity in recall antigen-specific T cells by adoptive T cell receptor transfer: implications for adoptive immunotherapy. Clin Cancer Res. 2007;13:4009–15. doi: 10.1158/1078-0432.CCR-07-0286. [DOI] [PubMed] [Google Scholar]
- 9.Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–13. [PubMed] [Google Scholar]
- 10.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–76. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest. 2007;117:1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171:166–74. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 14.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–8. doi: 10.1038/mt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–44. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher DT, Chen Q, Appenheimer MM, Skitzki J, Wang WC, Odunsi K, et al. Hurdles to lymphocyte trafficking in the tumor microenvironment: implications for effective immunotherapy. Immunol Invest. 2006;35:251–77. doi: 10.1080/08820130600745430. [DOI] [PubMed] [Google Scholar]
- 18.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20:1819–28. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 19.Chen DS, Davis MM. Cellular immunotherapy: antigen recognition is just the beginning. Springer Semin Immunopathol. 2005;27:119–27. doi: 10.1007/s00281-005-0200-z. [DOI] [PubMed] [Google Scholar]
- 20.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 22.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–41. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 24.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10:3937–42. doi: 10.1158/1078-0432.CCR-03-0801. [DOI] [PubMed] [Google Scholar]
- 25.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan R, Broaddus VC, Wilson S, Liewehr DJ, Zhang J. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin Cancer Res. 2007;13:7166–71. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 27.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–9. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, et al. Phase I clinical trial of the chimeric anti-mesothelin monoclonal antibody MORAb-009 in patients with mesothelin-expressing cancers. Clin Cancer Res. 2010;16:6132–8. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Moon E, Carpenito C, Paulos C, Liu X, Brennan A, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertozzi CC, Chang CY, Jairaj S, Shan X, Huang J, Weber BL, et al. Multiple initial culture conditions enhance the establishment of cell lines from primary ovarian cancer specimens. In Vitro Cell Dev Biol Anim. 2006;42:58–62. doi: 10.1290/0512084.1. [DOI] [PubMed] [Google Scholar]
- 32.Crisanti MC, Wallace AF, Kapoor V, Vandermeers F, Dowling ML, Pereira LP, et al. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009;8:2221–31. doi: 10.1158/1535-7163.MCT-09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–5. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 35.June CH, Moore JS. Measurement of intracellular ions by flow cytometry. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im0505s64. Chapter 5:Unit 5. [DOI] [PubMed] [Google Scholar]
- 36.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456–66. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 37.Stein JV, Nombela-Arrieta C. Chemokine control of lymphocyte trafficking: a general overview. Immunology. 2005;116:1–12. doi: 10.1111/j.1365-2567.2005.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward SG, Bacon K, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;9:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka S, Green SR, Quehenberger O. Differential expression of the isoforms for the monocyte chemoattractant protein-1 receptor, CCR2, in monocytes. Biochem Biophys Res Commun. 2002;290:73–80. doi: 10.1006/bbrc.2001.6149. [DOI] [PubMed] [Google Scholar]
- 41.Rollins BJ, Walz A, Baggiolini M. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood. 1991;78:1112–6. [PubMed] [Google Scholar]
- 42.Hwang LN, Yu Z, Palmer DC, Restifo NP. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–8. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–50. [PubMed] [Google Scholar]
- 46.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 47.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–8. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–80. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 49.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–85. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16:5458–68. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–30. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–65. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeLong P, Carroll RG, Henry AC, Tanaka T, Ahmad S, Leibowitz MS, et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol Ther. 2005;4:342–6. doi: 10.4161/cbt.4.3.1644. [DOI] [PubMed] [Google Scholar]
- 55.Anraku M, Tagawa T, Wu L, Yun Z, Keshavjee S, Zhang L, et al. Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine malignant mesothelioma. J Immunol. 2010;185:956–66. doi: 10.4049/jimmunol.0900437. [DOI] [PubMed] [Google Scholar]
- 56.Hassan R, Cohen SJ, Phillips M, Pastan I, Sharon E, Kelly RJ, et al. Phase I Clinical Trial of the Chimeric Anti-Mesothelin Monoclonal Antibody MORAb-009 in Patients with Mesothelin-Expressing Cancers. Clin Cancer Res. 2010;16:6132–8. doi: 10.1158/1078-0432.CCR-10-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarzenberger P, Harrison L, Weinacker A, Marrogi A, Byrne P, Ramesh R, et al. The treatment of malignant mesothelioma with a gene modified cancer cell line: a phase I study. Hum Gene Ther. 1998;9:2641–9. doi: 10.1089/hum.1998.9.17-2641. [DOI] [PubMed] [Google Scholar]
- 58.Sterman DH, Treat J, Litzky LA, Amin KM, Coonrod L, Molnar-Kimber K, et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther. 1998;9:1083–92. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 59.Vachani A, Sterman DH, Albelda SM. Cytokine gene therapy for malignant pleural mesothelioma. J Thorac Oncol. 2007;2:265–7. doi: 10.1097/01.JTO.0000263706.23579.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.