Abstract
Therapeutic augmentation of collateral artery growth (arteriogenesis) is of tremendous clinical interest. Since monocytes home to areas of arteriogenesis and create a local arteriogeneic milieu by secreting a wide range of growth factors, we followed the idea of utilizing these cells for augmentation of collateral growth. For that purpose, we adoptively transferred both syngeneic (same strain) and allogeneic (different strain) bone marrow derived monocytes (BMDMs) into balb/c mice 24 h after femoral artery ligation. Restoration of hind-limb perfusion was determined by Laser Doppler Perfusion Imaging and histological workup. While syngeneic cell transplantation did not augment arteriogenesis in comparison to non-transplanted animals (PI = 0.56 ± 0.06 vs. 0.48 ± 0.09, respectively, ns), allogeneic monocytes massively promoted the collateralization (PI = 0.85 ± 0.14, p < 0.001). Homed monocytes were visualized near growing collateral vessels by staining the cells with the lipophil fluorochrome DiI prior to transplantation. To analyze whether the effect of allogeneic BMDM transplantations is due to local inflammation triggered by a host-versus-graft reaction, transplant recipients were pre-treated with the immunosuppressive drug cyclosporine A, which completely prevented the effect of allogeineic monocyte transplantation (PI = 0.45 ± 0.06, p < 0.001). Here, we have demonstrated murine allogeneic monocytes to be an attractive way to trigger local inflammatory responses near growing collateral vessels and stimulate their adaption, overcoming the endogenous restriction of collateral vessel growth.
Keywords: Monocyte, cell transplantation, collateral circulation, immune system, vascular biology
Introduction
Patients suffering from coronary artery disease have been described to develop less severe symptoms when they exhibit a well developed collateral network [1,2]. The collateralization, however, is rarely adequate to fully compensate for the lost conductance of the occluded vessel. The process of collateral vessel growth is called arteriogenesis and describes the functional adaption of pre-existing arterioles into larger conductive vessels spanning a side of occlusion [3].
As early as back in 1979, Schaper et al reported the adhesion and transmigration of monocytes and the accumulation of perivascular macrophages to be one of the first events occurring during collateral growth [4]. This was later on contributed to the secretion of monocyte chemoattractant MCP-1 [5-7] and the increased expression of adhesion molecules [8,9] by endothelial cells, both associated with an increased attraction and adhesion of monocytes and thereby with perivascular monocyte accumulation. Mice lacking the MCP-1 chemokin receptor (CCR2) display a complete abolition of monocyte recruitment, as well as arteriogenesis in general [10]. Depletion of circulating monocytes also lead to decreased arteriogenesis, which was restored by the adoptive transfer of monocytes in a dose depending manner [11].
The actual role of monocytes in arteriogenesis, however, remains largely unknown. The cytokine milieu created by accumulating monocytes, resident vascular smooth muscle cells and endothelial cells represents a local inflammatory environment, resulting in proliferation and growth of the collateral vasculature [3,12,13].
The range of adaption, however, is limited – the endogenous arteriogenesis stops prematurely, and functional impairment remains in most settings, leading to efforts concerning the stimulation of collateral growth, especially featuring local or systemic delivery of cytokines and growth factors associated with arteriogenesis, i.e. TGFb1 [14], G-CSF [15-19], M-CSF [20], GM-CSF [21,22] or FGF2 [23-29]. The infusion of proinflammatory lipopolysaccarides has been previously described to promote collateral growth, as well [12].
Here, we sought to augment the local inflammatory cytokine milieu by exploiting the homing behavior of monocytes as key players in collateral growth, using their ability to produce growth factors in the complex temporal and spatial pattern required for arteriogenesis. We aimed to describe the potential of monocytes as targets of cellular therapy by adoptively transferring both syngeneic and allogeneic monocytes in a murine model of femoral artery ligation.
Materials and methods
Isolation and differentiation of murine bone marrow derived monocytes (BMDMs)
Monocytes were differentiated from adult bone marrow cells as previously described [30]. Briefly, bone marrow cell suspensions were obtained by flushing femurs and tibias of 8-12 week old syngeneic balb/c and allogeneic C57Bl/6 (Charles River, Sulzfeld, Germany) with complete RPMI1640 (supplemented with 10% FCS, 1% Pen/Strep; PAA, Pasching, Austria). Aggregates were dislodged by gentle pipetting and debris was removed by passaging the suspension through a 70 μm cell strainer. Cells were washed twice with medium, adjusted to give a suspension of 106 cells/ml and seeded onto 6-well ultra-low-attachment surface plates (Corning Costar, Schiphol-Rijk, NL). Cultures were supplemented with 20 ng/ml rmM-CSF (Strathman/Miltenyi, Bergisch Gladbach, Germany) and cultured in a humidified incubator at 37°C and 5% CO2. Cells were harvested at indicated time points by gentle pipetting and repeated washing of the wells with PBS, 0.5% BSA and 2 mM EDTA for detachment of adherent cells. Before transplantation, cell suspensions were depleted of CD117+ cells by MACS technology (Miltenyi, Bergisch Gladbach, Germany) according to standard procedures. Syngeneic monocytes were derived from mice of the same inbred strain as the recipients (i.e. balb/c, MHC haplotype H2d), allogeneic monocytes were derived from MHC incompatible mice of a different strain (i.e. C57Bl/6, MHC haplotype H2b).
Surgical procedures: femoral artery ligation and splenectomy
Balb/c and C57Bl/6 mice were bought from Charles River (Sulzfeld, Germany) at the age of 7 weeks and underwent surgical femoral artery ligation at 8 weeks of age. Briefly, mice were anaesthetized with a combination of 120 mg/kg ketamine and 2 mg/kg xylazine. The femoral nerve, artery and vein were exposed by a small inguinal incision. The femoral artery was separated from the nerve and vein and occluded distal to the branching of the profunda femoris artery by a double ligation with silk suture (Ethicon, Norderstedt, Germany). The skin was closed with 2-3 stitches and sealed with liquid band-aid. Animals were left under red light for recovery from anesthesia. Success of the operation was immediately verified using Laser Doppler Perfusion Imaging (PeriMed, Stockholm, SE).
In some cases, mice underwent a splenectomy 24 hours prior to ligation. Mice were anaesthetized and a small lateral left subcostal flank incision was made to access the spleen. The hilum was located and coagulated at two places and the spleen removed en bloc. Animals where monitored for bleeding and underwent wound closure by silk-suture of the musculature and polyurethane suture of the skin (Ethicon, Norderstedt, Germany).
Laser doppler perfusion imaging (LDPI)
Hind limb perfusion was determined by LDPI measurement (PeriMed, Stockholm, SE). For consistent measurements, imaging was performed after an acclimatization period on a 37°C warming plate (Föhr Instruments, Seeheim-Ober, Germany). Regions of interest were defined fitting the foot. Mean values from the occluded and non occluded side were divided to calculate a perfusion index (PI). LDPI was carried out before, immediately after, and subsequently 7, 14 and 21 days after surgery.
Immunofluorescence and morphometrical analysis
Mice were sacrificed at indicated time points, typically 21 days after femoral artery ligation, and muscle specimens were embedded in optimal-cutting-temperature freezing medium and frozen in 2-methylbutane cooled by liquid nitrogen. Samples were stored at -80°C. 5-7 μm cross sections were taken on a CM1900 cryotome (Leica, Wetzlar, Germany) and air tried before being refrozen at -20°C.
Samples were further processed by rehydration and repeated washing in PBS. Fixation and permeabilization were achieved with acetone followed by an incubation with serum free protein block (Dako, Hamburg, Germany) for 20 minutes. Slices where incubated with primary antibodies in recommended dilutions (rabbit anti-CD31, abcam; rabbit anti-Ki67, abcam; rat anti-F4/80, eBioscience; rabbit HIF1α, abcam) for 1h at room temperature, followed by appropriate cross-absorbed secondary antibody conjugated to Cy3 fluorochrome. DAPI and FITC-conjugated α-Actin antibodies (Sigma Aldrich, Hamburg, Germany) where used for counterstaining.
Sections were analyzed on an Axiovert S100 (Carl Zeiss, Jena, Germany) equipped with appropriate filters sets and a RT/SE camera system (Diagnostic Instruments, Sterling Heights, MI USA). Representative images were taken using SpotAdvanced, and analyzed using ImagePro (Media Cybernetics inc., Bethesda, MD USA) and MetaMorph (MolecularDevices, Sunnywale, CA USA). Capillary density was measured in 400x magnification of CD31 stainings, vessel-size and -density were analyzed in 200x magnification of actin-stainings. Only vessels with a media-to-lumen-ratio typical for arteries were taken into consideration. Macrophage infiltration was assessed by counting F4/80+ cells near vessels identified as arteries based on their actin staining.
Cell tracking: BMDM homing
For cell tracking experiments, progenitor cell depleted syngeneic and allogeneic monocytes were stained with red DiI lipophil fluorochrome prior to transplantation. Muscles were harvested for histology 48 h after the transplantation, 72 h after surgical ligation. One hundred fourty 7 μm serial cryosections per muscle were taken across the entire length of the specimens, stained with FITC anti-α-actin and analyzed under 400 fold magnification. Only strong, definitely positive signals (as compared to sections of animals that received unstained cells) were taken into consideration.
Statistics
Statistical analysis was performed using Microsoft Excel and SPSS 20 for PCs. The data were tested using a standard T-test for parametrical data or Mann-Whitney U-test for nonparametrical data. When multiple testing was performed, p values were Bonferroni-adjusted. P values were considered significant when P<0.05 (*), P<0.01 (**), or P<0.001 (***).
Results
Recent reports indicate a mouse-strain specific potential of revascularization for common wild type strains [31]. In order to choose an adequate recipient-strain, both Balb/c and C57Bl6 mice were subjected to femoral artery ligation and subsequent histological workup to demonstrate their regenerative potential.
The hind limb perfusion in Balb/c displayed a dramatically impaired revascularization compared to mice of the C57Bl/6 strain with significantly lower PI values throughout the entire process (post lig: 0.16 ± 0.04 vs. 0.27 ± 0.06 p < 0.01, 7 d: 0.29 ± 0.08 vs. 0.77 ± 0.21 p < 0.001, 14 d: 0.41 ± 0.07 vs. 0.94 ± 0.13 p < 0.001, 21 d: 0.48 ± 0.09 vs. 1.07 ± 0.07 p < 0.001; see Figure 1).
Figure 1.
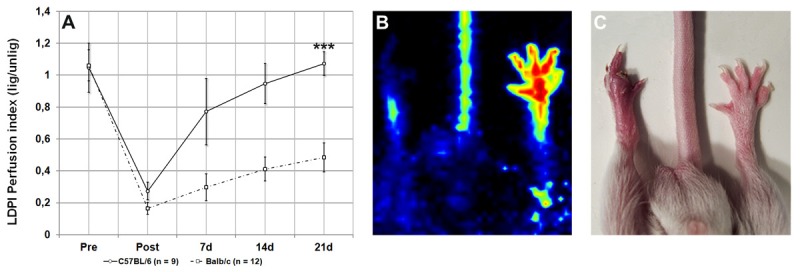
Perfusion recovery in hind limbs of C57Bl/6 and Balb/c after femoral artery ligation (A), representative clinical outcome for Balb/c strain three weeks after ligation (B, C).
Histological workup (see Figure 2) of both the proximal thigh and the distal calf musculature revealed an increasing vascular diameter in the thigh of C57Bl6 mice subsequent to the ligation (unligated: 23.8 ± 1.3 μm vs. 32.8 ± 28 μm after 3 wks, p < 0.001), which was absent in Balb/c mice (unligated: 17.5 ± 2.8 μm vs. 21.1 ± 2.6 μm after 3 wks, n.s.). Concerning the calf musculature, there was no definitive increase in vascular diameters present after 3 weeks in either strain.
Figure 2.
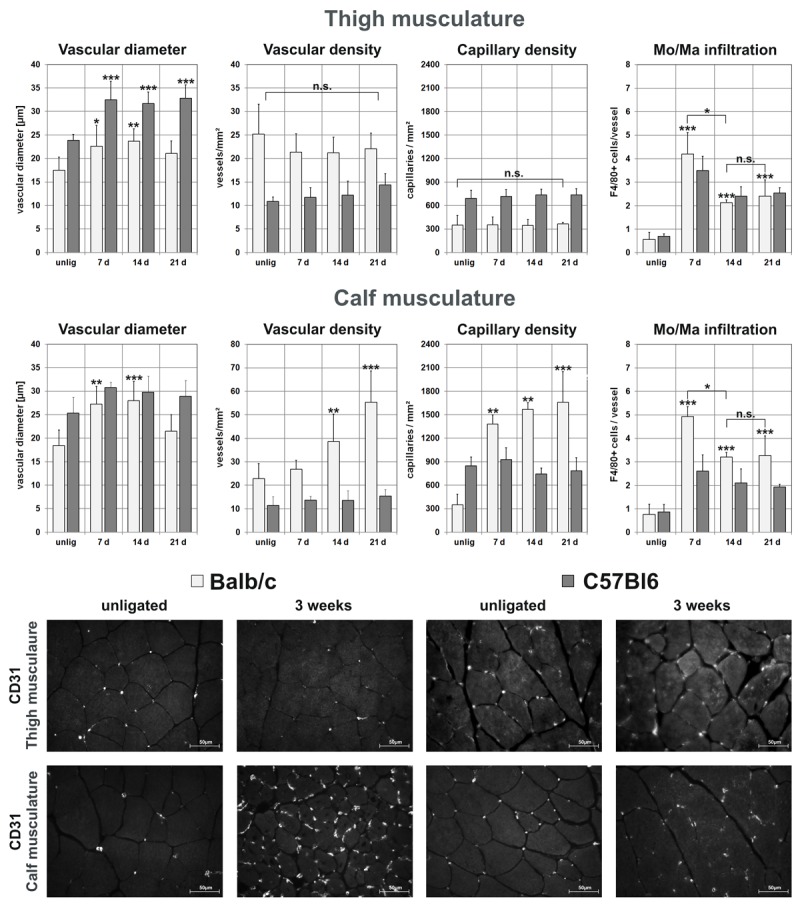
Timeline histological evaluation of thigh (Mm. Adductores) and calf musculature (M. gastrocnemius) of untreated Balb/c and C57Bl6 using α-actin staining to measure mean vascular diameter and –density, CD31 staining to measure capillary density and perivascular Mo/Ma infiltration using F4/80 staining (*p<0.05; **p<0.01; *** p<0.001). Note the constant capillary density in thigh muscles and increasing density in calf musculature of Balb/c, as well as the pronounced Mo/Ma infiltration occurring early during revascularization. Representative CD31-stained sections demonstrate the increase in capillary density in the calf-musculature of Balb/c mice, as well as the general higher capillary density in C57Bl6 (400 fold-magnification).
Capillary density in the thigh area of both Balb/c (unligated: 350 ± 120 vs. ligated: 370 ± 30 capillaries/mm², n.s.) and C57Bl6 (unligated: 690 ± 105 vs. ligated: 740 ± 80 capillaries/mm², n.s.) remained steady throughout the process. Strikingly, capillary density in the Balb/c calf muscles increased almost 4 fold from 350 ± 130 to 1400 ± 120 capillaries/mm² after one (p < 0.01), 1570 ± 90 after two (p < 0.05 vs. one week), and 1660 ± 390 capillaries/mm² three weeks after ligation (n.s. vs. two week), while capillary density in the C57Bl6 lineage remained virtually unaltered (unligated: 840 ± 110 vs. ligated: 783 ± 165 capillaries/mm² after 3 wks, n.s.).
Further workup revealed a significant stabilization of HIF1α in the calf musculature of Balb/c detectable by means of immunofluorescence, even 3 weeks after the ligation, which was absent in the thigh region. This surrogate marker for hypoxia indicated persisting hypoperfusion in the distal musculature of Balb/c mice. Congruent with the concept that angiogenesis (i.e. capillary sprouting) is predominantly triggered by ischemia, HIF1α stabilization was associated with a vast increase in capillary density in the calf of Balb/c mice, which was absent in thigh area of Balb/c and both the thigh- and calf musculature of C57Bl6 (Figure 2).
Consistent with the mechanism of vascular remodeling, we observed a compelling increase in perivascular monocyte infiltration occurring early during the process in both Balb/c and C57Bl6 (unligated thigh: 0.6 ± 0.3 vs. 4.2 ± 0.9 F4/80+ cells/vessel after one week in Balb/c, p<0.001; unligated thigh: 0.7 ± 0.1 vs 3.5 ± 0.6 F4/80+ cells/vessel after one week in C57Bl6, p<0.001).
In summary, C57Bl6 showed a significant increase in vascular diameter in the thigh region, while vascular- and capillary density remained unaltered throughout the process. Balb/c, on the other hand, had a smaller mean vascular diameter in thigh region and demonstrated merely minimal change in diameter, while capillary density in the calf, but not the thigh region, increased 4 fold within one week, which was associated with a significant HIF1α stabilization as a marker of ischemia. Strikingly, capillary density in unligated C57Bl6 was 2 fold larger than in unligated Balb/c in both the thigh and the calf area.
Balb/c and C57Bl6 showed significant differences in their vascular system. Additionally, Balb/c mice displayed compelling functional impairment, including paresis, muscular atrophy, fibrosis, and in severe cases, necrosis and gangrene (Figure 1C), while C57Bl6 reached their preoperative functionality and made a full recovery. This renders Balb/c to be suitable recipients for arteriogenic therapies.
Transplantation model
Syngeneic (synMo) and allogeneic (alloMo) bone marrow derived monocytes were differentiated as previously described [30], depleted of CD117 progenitor cells using MACS-technology and transplanted intravenously 24 hours after femoral artery ligation.
The transplantation of syngeneic cells had no effect on PI values (0.5 mio cells: 0.51 ± 0.09, ns; 2.5 mio cells: 0.56 ± 0.06, ns; Figure 3A). Transfer of allogeneic cells showed a 75% improvement of PI values, but only when 2.5 million cells were injected (0.5 mio cells: 0.55 ± 0.10, n.s; 2.5 mio cells: 0.85 ± 0.14, p<0.001 vs. control; Figure 3B).
Figure 3.

Hind limb revascularization in Balb/c after femoral artery ligation and monocyte transplantation. Various amounts of syngeneic and allogeneic monocytes were injected intravenously 24 h after ligation (A-C). Additionally, 0.5 million allogeneic cells were injected intramuscular (0.5 i.m.) or intravenously after splenectomy (0.5 spl) (D). The augmentation of arteriogenesis was abolished by Cyclosporine A treatment (2.5 mio CyA) (E).
Surprisingly, the transplantation of 0.5 million allogeneic cells had no significant effect on the revascularization. To increase the effect achieved by low cell dosage, we thought to increase the local cell concentration by choosing alternate injection routes. While the local intramuscular delivery in the thigh by injection of 4 x 25 μl cell-suspension only had a limited effect on the PI improvement (alloMo i.m.: 0.62 ± 0.14, p<0.05 vs ctrl; ns vs. intravenous injection; Figure 3D), when 0.5 million BMDMs were injected after splenectomy, reperfusion increased 54% to 0.74 ± 0.14 (p < 0.001 vs. ctrl, ns vs. 2.5 mio allogeneic cells i.v.; see Figure 3D), while splenectomy alone had no impact on PI values (0.59 ± 0.1, ns).
The immunological background of the effect was investigated using immunosuppression with 10 μg/g bodyweight of intraperitoneal cyclosporine A (CyA) per day. While the PI of animals that received cyclosporine A did not change compared to controls receiving no cyclosporine (control: 0.49 ± 0.11 vs. 0.48 ± 0.09 with immuosuppression, n.s.), the effect of the allogeneic cell transplantation as measured by using LDPI was completely abolished and did not alter compared to untreated controls (CyA+alloMo: 0.45 ± 0.06 vs. 0.48 ± 0.09 CyA w/o cells, ns; Figure 3E).
Histo-Morphometrical analysis of the transplantation groups revealed further insight into the events occurring during revascularization (Figure 4). In consistency with the LDPI data, none of the analyzed biometrical parameter changed in animals receiving any amount of syngeneic cells, or 0.5 million allogeneic cells compared to control mice receiving no transplantation. Perivascular monocyte infiltration was not influenced, either (controls: 2.4 ± 0.7 cells/vessel, 0.5 alloMo: 1.7 ± 1.2, 2.5 synMo: 1.6 ± 0.2, 0.5 alloMo: 2.3 ± 1.1; ns vs. control condition).
Figure 4.
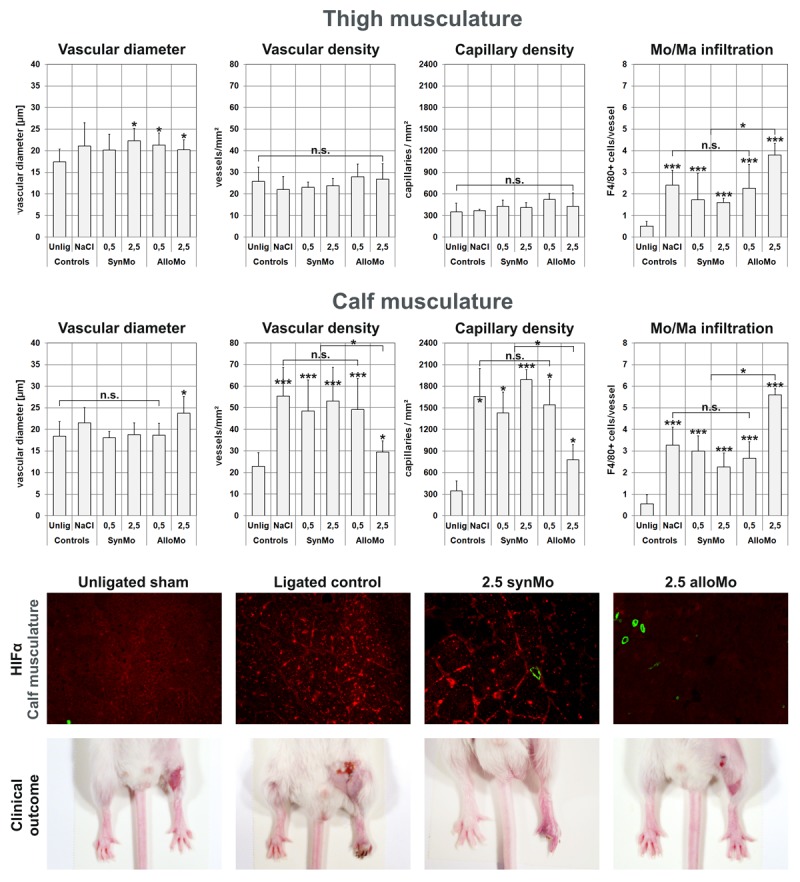
Histological evaluation of thigh (Mm. Adductores) and calf musculature (M. gastrocnemius) of the transplantation experiments using α-actin staining to measure mean vascular diameter and –density, CD31 staining to measure capillary density and perivascular Mo/Ma infiltration using F4/80 staining (*p<0.05; **p<0.01; *** p<0.001). Note the unaltered data in syngeneic cell recipients, the reduction of angiogenesis in calf musculature in allogeneic cell recipients, and the pronounced Mo/Ma infiltration when allogeneic cells were transplanted. Representative immune-histological stainings of normal calf muscles, ischemic calf muscles from animals with that received 2.5 million syngeneic cells, and calf muscles from animals that received proarteriogenic treatment to augment collateralization (2.5 million allogeneic cells). Stainings show HIF1α (red) expression as well as actin-positive vessels (green) in 100x overview magnification of calf musculature. Additionally, the clinical condition of the ligated hind limb was documented one week after femoral artery ligation using a digital stereo microscope.
Animals receiving 2.5 million allogeneic cells, however, showed a significantly decreased capillary- (780 ± 210 vs. 1660 ± 390 capillaries/mm² in controls, p < 0.05 vs. control) and vascular-density (29.5 ± 5 vs. 55 ± 14 vessels/mm² in controls, p < 0.05 vs. control) in the calf, and a pronounced perivascular Mo/Ma infiltration (thigh: 3.8 ± 0.5 vs. 2.4 ± 0.7 cells/vessel in controls, p < 0.05, calf: 5.6 ± 0.3 vs. 3,2 ± 0.8 cells/vessel in control animals, p < 0.05 vs. controls) compared to both controls and mice receiving syngeneic cells. The less pronounced increase in vascular- and capillary density in the calf muscles was associated with an abolishment of the HIF1α stabilization, which could not be detected by means of immunohistology (Figure 4). HIF1α stabilization was preserved in animals receiving either syngeneic or 0.5 million allogeneic cells (data not shown). Consistent with the LDPI data, the changes in histological morphometry and macrophage infiltration was abolished by parallel treatment with cylcosporine A (see Table 1), indicating that there was remaining ischemia in the distal hind limb causing capillary sprouting because of a lack of proximal vessel growth. Notably, animals treated with cyclosporine demonstrated less perivascular monocyte infiltration, indicating a lack of perivascular inflammation (Figure 5).
Table 1.
Morphometrical comparism of control (NaCl) and Cyclosporine A treated allogeneic cell recipients
| Mm. ad. | Vas. diam. | Vas. dens. | Cap. dens. | MoMa Inf |
| (Thigh) | [μm] | [ves/mm2] | [cap/mm2] | [cells/ves.] |
|
| ||||
| Contols | 21 ± 5 | 22 ± 6 | 365 ± 20 | 2.4 ± 0.6 |
| CyA+AlloMo | 24 ± 4 | 24 ± 3.5 | 690 ± 80* | 2 ± 0.8 |
|
| ||||
|
| ||||
| M. gas. | Vas. diam. | Vas. dens. | Cap. dens. | MoMa Inf. |
| (Calf) | [μm] | [ves/mm2] | [cap/mm2] | [cells/ves.] |
|
| ||||
| Controls | 21.5 ± 3.5 | 55 ± 13 | 1660 ± 390 | 3.2 ± 0.8 |
| CyA + AlloMo | 18.3 ± 1.5 | 42.8 ± 4.9 | 2070 ± 220 | 1 ± 0.7** |
p < 0.05,
p < 0.01.
Figure 5.
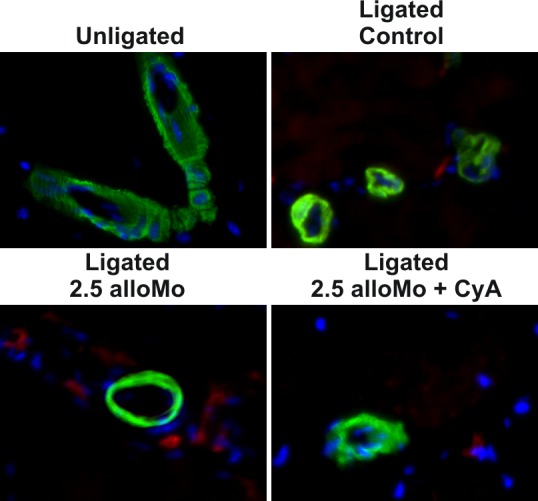
Representative immune-histological detection of perivascular F4/80 positive cells (Actin: green, F4/80: red, DAPI: blue). Note the pronounced increase in animals treated with alloMo and the lack of infiltration in animals co-treated with CyA.
BMDM homing
In order to localize the transplanted donor cells within the recipients, BMDM where stained with DiI fluorochrome. Transplanted, homed syngeneic monocytes were found near vessels with morphometricaly measured lumina between 10 and 30 μm, rarely near vessels larger than 35 μm. They were not found in the peripheral muscles of the unligated hind limb. Stained cells were found in the perivascular connective tissue, but did not incorporate into the structure of the actual vessel (Figure 6). Strikingly, we were unable to detect any allogeneic cells after the transplantation, indicating that they where disintegrated quickly after the injection.
Figure 6.

DiI+ (red) syngeneic monocytes near α-Actin (green, DAPI blue) positive vessels 48h after transplantation.
Discussion
In this study, we sought to use the immunogeneic potential of allogeneic monocytes to augment the local inflammatory milieu. By adoptively transferring monocytes, we are able to manipulate the population of cells recruited into collateral vessels.
Bone marrow derived monocytes (BMDM) were differentiated from adult bone marrow of donor mice by supplementation of M-CSF and cultivation on ultra low attachment surface culture plates for five days. Monocyte purity was enhanced by depleting strongly adherent macrophages during the harvesting process. Cell suspensions were depleted of CD117+ (c-kit) stem cells prior to transplantation to avoid the potential influence of undifferentiated progenitor cells. These cells have been previously described to exhibit pro angiogeneic properties and were associated with the therapeutic effect of bone marrow cell transplantations [32].
FACS phenotyping revealed major populations of the transplants to be 80-90% CD115+Ly6C+F4/80low mature monocytes, and 5-10% CD115+Ly6CnegF4/80high macrophages, all of which were positive for other markers, including CD11b and CD45. Cell viability was determined by propidium iodide uptake of dead cells and reached <5% dead cells. Monocytes where sub-phenotyped using additional markers. Here, we found phenotypes that matched the ones describe by Robbins et al: an inflammatory subset was Ly6ChighCCR2+CD62L+CD11cneg (80% of all BMDMs), another showed lower, jet still positive Ly6C expression and was determined as Ly6ClowCCR2negCD62L+CD11c+ resident monocyte subgroup (20% of all BMDMs). Since the CCR2 MCP-1 receptor is required for recruiting monocytes to sides of arteriogenesis, the contribution of the transplanted cells is most likely restricted to the population of inflammatory monocytes. Cell suspensions were void of lymphocyte- (CD3, CD4, CD8, B220) and granulocyte (CD11b+Gr1+CD115-) contaminations. The functionality of BMDM has been previously described by our group using a wide range of in vitro assays [30].
Histological workup of untreated Balb/c and C57Bl6 mice revealed further insight into the processes occurring after femoral artery ligation. By defining distinctive morphometric parameters based on strong, unique immune staining and processing both the thigh and calf musculature, we were able to distinguish the contribution of angiogenesis and arteriogenesis to the revascularization processes.
We hypothesized that a lack of arteriogenesis in thigh region would lead to severe ischemia in the calf, contributing to a hypoxia driven angiogenesis. By augmenting collateral growth, tissue oxygenation of the calf might increase, influencing the degree of ischemic vessel sprouting and salvage hind limb functionality.
As previously described [33], we did not observe the stabilization of HIF1α-subunits in the thigh muscles, consistent with the unaltered capillary density in theses muscles. We concluded that angiogenesis did not occur in the thigh region of either Balb/c or C57Bl6. However, we observed a quiet compelling 4-fold increased capillary density in the calf muscles of Balb/c, consistent with the stabilization of HIF1α as verified by immunohistological staining, even three weeks after surgical femoral artery occlusion. The remaining hypoxia is underlined by functional impairment of the hind limb, in severe cases associated with the loss of structural integrity as observed by the development of gangrene and self amputations. We also observed a compelling increase in vascular density in calf muscles, which, however, occurred delayed compared to the increase in capillary density, as demonstrated by timeline histological analysis. The development of primitive endothelium-lined channels in angiogenesis and their maturation via recruitment of smooth muscle cells has been extensively reviewed by Carmeliet et al [13], underlining our histological findings.
In consistency with the reduced range of adaption in Balb/c mice compared to the C57Bl6 strain, as previously demonstrated by Helisch et al [31], we are able to contribute the massive hypoxia driven angiogenesis in the calf of Balb/c mice to the inadequate arteriogenesis in the thigh. Besides the obvious increase in capillary- and vessel density, Balb/c still suffered from a quiet compelling functional impairment and very low LDPI values compared to the C57Bl6 strain, suggesting an inadequate perfusion of the lower limb. We conclude that the calf vessels formed by hypoxia driven angiogenesis suffer from an inadequate feed through large conductive vessels located in the thigh region, i.e. collaterals, which fail to develop in the Balb/c strain.
These findings rendered balb/c to be an attractive target for novel pro-arteriogenic therapeutic approaches, since their endogenous capacities apparently are insufficient to secure tissue homeostasis, but also question the concept of angiogenesis in the contribution to hind limb revascularization, since pure angiogenesis apparently did not salvage hind limb functionality.
Although syngeneic monocytes did not improve collateralization, we were able to proof that they home to sides of vessel growth by staining the transplanted cells with DiI fluorochromes prior to injection. Congruent with previous findings [34], we did not observe any incorporation of the transplanted cells into the structure of the actual vessels, which would have been immanent in the case of the differentiation into smooth muscle or endothelial cells that might have occurred in the case of stem- or progenitor cell contaminations, especially concerning the proposed potential of epithelial progenitor cells [35-38].
Transplantation of allogeneic cells was well tolerated by the animals. Besides the compelling increase in PI values, the animals demonstrated a full recovery of hind limb functionality. Unlike their untreated littermates, transplanted Balb/c did not show any signs of muscular atrophies, paresis, fibrosis or gangrene.
Histological analysis revealed a destabilization of the HIF1α subunit, suggesting normoxic conditions associated with significantly less angiogenesis occurring in the calf musculature. We also observed an increased macrophage infiltration near small arteries, suggesting an augmentation of perivascular inflammation triggered by the allogeneic cells. Monocytes and macrophages have been previously described to secrete a repertoire of cytokines, including GM-CSF, MCP-1, TNFα, bFGF, TGF, PDGF and VEGF [39-41] and matrix-metallo-proteases (MMP). The latter degrade the extracellular matrix and thereby additionally release bound growth factors, creating space for the growing vessels and easing cell migration [42]. Their accumulation might increase topical cytokine concentrations and thereby stimulate collateral growth.
We previously hypothesized that transplanted allogeneic monocytes home to collaterals and are subsequently destroyed by the immunologic host-versus-graft reaction followed by the recruitment of a large number of recipient leucocytes [43], in particular monocytes as indicated by the increased perivascular monocyte infiltration observed in this study. The hypothesis is further underlined by the inhibition of the effect by cylcosporine A and the fact that we were unable to locate allogeneic cells within the recipient, even two days after the transplantation. To improve the efficiency of monocyte homing, we also investigated alternative methods of cell delivery. Strikingly, local intramuscular injection had no major effect on revascularization, while intravenous transplantation of a reduced cell dosage in animals that previously underwent splenectomy to decrease cell retention in the spleen yielded a significant improved revascularization. This might indicate that transplanted cells actually need to be in the intravasal space and have to undergo endothelial transmigration to unfold their proarteriogenic effects.
Noteworthy, the inhibition of the proarteriogeneic effect by cyclosporine A underlines the importance of allo-responsive lymphocyte activation in the concept of allogeneic cell transfers. Cyclosporine treated control mice, however, did not show an impaired revascularization, suggesting that the activation of lymphocytes itself might not play a role in arteriogenesis.
Besides the obvious effect on arteriogenesis, monocyte attraction is a major step in atherosclerotic plaque development [45], especially in events leading to plaque destabilization [46]. Local MCP-1 delivery was not only shown to improve monocyte recruitment into growing collateral vessels [47], but also exhibits systemic plaque destabilizing properties in apo-e-/- knockout mice [48]. Regarding the similarities in attracting and accumulating monocytes, this may also apply for the approach described in this study. Concerning the safety of our approach, further research is required.
The data gathered in this study imply a compelling role of arteriogenesis in the conservation of hind limb integrity. We were able to demonstrate the abolishment of ischemia driven angiogenesis, which was unable to restore functionality, by sufficient proarteriogenic treatment. Strikingly, in this setting, the LDPI values did not correspond to the histological findings in the calf region. This indicates that the mere application of LDPI measurements to quantify the success of proangioenic or proarteriogeneic treatment might be deceptive, since this technique does not discern the contribution of arteriogenesis and angiogenesis to the process of revascularization. To do so, the LDPI data need to be complemented by postmortem histology, x-ray or computer tomography imaging [49,50] of the vascular system. Still, LDPI remains the gold standard of in vivo perfusion assessment due to the possibility to perform cost-effective longitudinal measurements on one and the same animal. To overcome these issues, we have recently described a novel approach for in vivo quantification of collateral flow based on indocyanine-green (ICG) angiography [51].
Concerning clinical settings, the transplantation of allogeneic cells reveals some striking implications. Transfer of allogeneic cells would require immunosuppressive therapy, which was shown to abolish the effect of the transplantation in this study. For that reason, the host-versus-graft disease would have to be accepted not just as a mere side effect of the therapy, but as part of the actual concept. This draws additional attention to the immunological background of arteriogenesis and underlines the importance of understanding the interaction of proinflammatory cytokines and their influence on proarteriogenic factors.
Here, we have demonstrated murine allogeneic monocytes to be an attractive vehicle to trigger local inflammatory responses near growing collateral vessels and stimulate their adaption, overcoming the endogenous restriction. In terms of future clinical trials, a novel approach featuring syngeneic monocytes triggering local immuneresponses might be more feasible and will have to be addressed in further studies.
References
- 1.Seiler C, Fleisch M, de Marchi SF, Billinger M, Wahl A, Eberli FR, Garachemani AR, Meier B. Functional assessment of collaterals in the human coronary circulation. Semin Interv Cardiol. 1998;3:13–20. [PubMed] [Google Scholar]
- 2.Seiler C, Fleisch M, Garachemani A, Meier B. Coronary collateral quantitation in patients with coronary artery disease using intravascular flow velocity or pressure measurements. J Am Coll Cardiol. 1998;32:1272–1279. doi: 10.1016/s0735-1097(98)00384-2. [DOI] [PubMed] [Google Scholar]
- 3.Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Cardiovasc Res. 1999;43:835–837. doi: 10.1016/s0008-6363(99)00191-1. [DOI] [PubMed] [Google Scholar]
- 4.Schaper J, Konig R, Franz D, Schaper W. The endothelial surface of growing coronary collateral arteries. Intimal margination and diapedesis of monocytes. A combined SEM and TEM study. Virchows Arch A Pathol Anat Histol. 1976;370:193–205. doi: 10.1007/BF00427580. [DOI] [PubMed] [Google Scholar]
- 5.Niiyama H, Kai H, Yamamoto T, Shimada T, Sasaki K, Murohara T, Egashira K, Imaizumi T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol. 2004;44:661–666. doi: 10.1016/j.jacc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 6.Park HJ, Chang K, Park CS, Jang SW, Ihm SH, Kim PJ, Baek SH, Seung KB, Choi KB. Coronary collaterals: The role of MCP-1 during the early phase of acute myocardial infarction. Int J Cardiol. 2008;130:409–13. doi: 10.1016/j.ijcard.2007.08.128. [DOI] [PubMed] [Google Scholar]
- 7.Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, Schaper W, Piek JJ, Buschmann IR. Modulation of collateral artery growth in a porcine hindlimb ligation model using MCP-1. Am J Physiol Heart Circ Physiol. 2003;284:H1422–1428. doi: 10.1152/ajpheart.00506.2002. [DOI] [PubMed] [Google Scholar]
- 8.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis) Virchows Arch. 2000;436:257–270. doi: 10.1007/s004280050039. [DOI] [PubMed] [Google Scholar]
- 9.Hoefer IE, van Royen N, Rectenwald JE, Deindl E, Hua J, Jost M, Grundmann S, Voskuil M, Ozaki CK, Piek JJ, Buschmann IR. Arteriogenesis proceeds via ICAM-1/Mac-1- mediated mechanisms. Circ Res. 2004;94:1179–1185. doi: 10.1161/01.RES.0000126922.18222.F0. [DOI] [PubMed] [Google Scholar]
- 10.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94:671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 11.Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 2002;283:H2411–2419. doi: 10.1152/ajpheart.01098.2001. [DOI] [PubMed] [Google Scholar]
- 12.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 14.van Royen N, Hoefer I, Buschmann I, Heil M, Kostin S, Deindl E, Vogel S, Korff T, Augustin H, Bode C, Piek JJ, Schaper W. Exogenous application of transforming growth factor beta 1 stimulates arteriogenesis in the peripheral circulation. Faseb J. 2002;16:432–434. doi: 10.1096/fj.01-0563fje. [DOI] [PubMed] [Google Scholar]
- 15.Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, Odaka K, Hiroshima K, Tadokoro H, Komuro I. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun. 2004;325:1353–1359. doi: 10.1016/j.bbrc.2004.10.149. [DOI] [PubMed] [Google Scholar]
- 16.Ince H, Petzsch M, Kleine HD, Eckard H, Rehders T, Burska D, Kische S, Freund M, Nienaber CA. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction: final 1-year results of the Front-Integrated Revascularization and Stem Cell Liberation in Evolving Acute Myocardial Infarction by Granulocyte Colony-Stimulating Factor (FIRSTLINE-AMI) Trial. Circulation. 2005;112:I73–80. doi: 10.1161/CIRCULATIONAHA.104.524827. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann MG, Theiss HD, Hennig-Theiss C, Huber A, Wintersperger BJ, Werle-Ruedinger AE, Schoenberg SO, Steinbeck G, Franz WM. Autologous bone marrow stem cell mobilization induced by granulocyte colony-stimulating factor after subacute ST-segment elevation myocardial infarction undergoing late revascularization: final results from the G-CSF-STEMI (Granulocyte Colony-Stimulating Factor ST-Segment Elevation Myocardial Infarction) trial. J Am Coll Cardiol. 2006;48:1712–1721. doi: 10.1016/j.jacc.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, Johnsen HE, Kober L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 19.Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, Meisetschlager G, von Wedel J, Bollwein H, Seyfarth M, Dirschinger J, Schmitt C, Schwaiger M, Kastrati A, Schomig A. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. Jama. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 20.Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, Ichikawa Y, Ikeda Y, Kobayashi H, Ohori K, Shimamoto K. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–634. doi: 10.1016/j.jacc.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Seiler C, Pohl T, Wustmann K, Hutter D, Nicolet PA, Windecker S, Eberli FR, Meier B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: a randomized, double-blind, placebo-controlled study. Circulation. 2001;104:2012–2017. doi: 10.1161/hc4201.097835. [DOI] [PubMed] [Google Scholar]
- 22.van Royen N, Schirmer SH, Atasever B, Behrens CY, Ubbink D, Buschmann EE, Voskuil M, Bot P, Hoefer I, Schlingemann RO, Biemond BJ, Tijssen JG, Bode C, Schaper W, Oskam J, Legemate DA, Piek JJ, Buschmann I. START Trial: a pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation. 2005;112:1040–1046. doi: 10.1161/CIRCULATIONAHA.104.529552. [DOI] [PubMed] [Google Scholar]
- 23.Deindl E, Hoefer IE, Fernandez B, Barancik M, Heil M, Strniskova M, Schaper W. Involvement of the fibroblast growth factor system in adaptive and chemokine-induced arteriogenesis. Circ Res. 2003;92:561–568. doi: 10.1161/01.RES.0000061181.80065.7D. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J Appl Physiol. 2002;93:2009–2017. doi: 10.1152/japplphysiol.00451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:1100–1105. doi: 10.1161/01.atv.0000023230.17493.e3. [DOI] [PubMed] [Google Scholar]
- 26.Unger EF, Banai S, Shou M, Lazarous DF, Jaklitsch MT, Scheinowitz M, Correa R, Klingbeil C, Epstein SE. Basic fibroblast growth factor enhances myocardial collateral flow in a canine model. Am J Physiol. 1994;266:H1588–1595. doi: 10.1152/ajpheart.1994.266.4.H1588. [DOI] [PubMed] [Google Scholar]
- 27.Yang HT, Deschenes MR, Ogilvie RW, Terjung RL. Basic fibroblast growth factor increases collateral blood flow in rats with femoral arterial ligation. Circ Res. 1996;79:62–69. doi: 10.1161/01.res.79.1.62. [DOI] [PubMed] [Google Scholar]
- 28.Lederman RJ, Mendelsohn FO, Anderson RD, Saucedo JF, Tenaglia AN, Hermiller JB, Hillegass WB, Rocha-Singh K, Moon TE, Whitehouse MJ, Annex BH. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 29.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, Moon T, Chronos NA. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 30.Francke A, Herold J, Weinert S, Strasser RH, Braun-Dullaeus RC. Generation of mature murine monocytes from heterogeneous bone marrow and description of their properties. J Histochem Cytochem. 2011;59:813–825. doi: 10.1369/0022155411416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 32.Li TS, Hamano K, Nishida M, Hayashi M, Ito H, Mikamo A, Matsuzaki M. CD117+ stem cells play a key role in therapeutic angiogenesis induced by bone marrow cell implantation. Am J Physiol Heart Circ Physiol. 2003;285:H931–937. doi: 10.1152/ajpheart.01146.2002. [DOI] [PubMed] [Google Scholar]
- 33.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: insights into the genomic program for collateral vessel development. J Am Coll Cardiol. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 34.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 36.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 37.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010;122:517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volz KS, Miljan E, Khoo A, Cooke JP. Development of pluripotent stem cells for vascular therapy. Vascul Pharmacol. 2012;56:288–296. doi: 10.1016/j.vph.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J Leukoc Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 40.Eubank TD, Galloway M, Montague CM, Waldman WJ, Marsh CB. M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J Immunol. 2003;171:2637–2643. doi: 10.4049/jimmunol.171.5.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84:5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hobeika MJ, Edlin RS, Muhs BE, Sadek M, Gagne PJ. Matrix Metalloproteinases in Critical Limb Ischemia. J Surg Res. 2008;149:148–54. doi: 10.1016/j.jss.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Herold J, Pipp F, Fernandez B, Xing Z, Heil M, Tillmanns H, Braun-Dullaeus RC. Transplantation of monocytes: a novel strategy for in vivo augmentation of collateral vessel growth. Hum Gene Ther. 2004;15:1–12. doi: 10.1089/10430340460732517. [DOI] [PubMed] [Google Scholar]
- 44.Herold J, Tillmanns H, Xing Z, Strasser RH, Braun-Dullaeus RC. Isolation and transduction of monocytes: promising vehicles for therapeutic arteriogenesis. Langenbecks Arch Surg. 2006;391:72–82. doi: 10.1007/s00423-006-0033-9. [DOI] [PubMed] [Google Scholar]
- 45.Girn HR, Orsi NM, Homer-Vanniasinkam S. An overview of cytokine interactions in atherosclerosis and implications for peripheral arterial disease. Vasc Med. 2007;12:299–309. doi: 10.1177/1358863X07083387. [DOI] [PubMed] [Google Scholar]
- 46.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Ito WD, Arras M, Winkler B, Scholz D, Schaper J, Schaper W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ Res. 1997;80:829–837. doi: 10.1161/01.res.80.6.829. [DOI] [PubMed] [Google Scholar]
- 48.van Royen N, Hoefer I, Bottinger M, Hua J, Grundmann S, Voskuil M, Bode C, Schaper W, Buschmann I, Piek JJ. Local monocyte chemoattractant protein-1 therapy increases collateral artery formation in apolipoprotein E-deficient mice but induces systemic monocytic CD11b expression, neointimal formation, and plaque progression. Circ Res. 2003;92:218–225. doi: 10.1161/01.res.0000052313.23087.3f. [DOI] [PubMed] [Google Scholar]
- 49.Wagner S, Helisch A, Bachmann G, Schaper W. Time-of-flight quantitative measurements of blood flow in mouse hindlimbs. J Magn Reson Imaging. 2004;19:468–474. doi: 10.1002/jmri.20025. [DOI] [PubMed] [Google Scholar]
- 50.Wagner S, Helisch A, Ziegelhoeffer T, Bachmann G, Schaper W. Magnetic resonance angiography of collateral vessels in a murine femoral artery ligation model. NMR Biomed. 2004;17:21–27. doi: 10.1002/nbm.859. [DOI] [PubMed] [Google Scholar]
- 51.Wuestenfeld JC, Herold J, Niese U, Tribulova S, Kappert U, Weihnert S, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Indocyanine green angiography: A new method for quantification of collateral flow in mice. J Vasc Surg. 2008;48:1315–21. doi: 10.1016/j.jvs.2008.06.049. [DOI] [PubMed] [Google Scholar]


