Abstract
Purpose
To determine the in vivo expression of neurotrophins (NTs) and nerve regeneration-associated genes (RAGs) after surgically creating a hinged lamellar corneal flap in thy1-YFP mice.
Methods
Lamellar corneal flaps with multiple hinges were created in thy1-YFP mice. Mice were sacrificed weeks 2, 4, and 8. Quantitative PCR was performed to determine the expression of NTs and RAGs in the corneas following lamellar transection. Nerve growth factor (Ngf), Brain-derived neurotrophic factor (Bdnf), Glial cell-derived neurotrophic factor (Gdnf), Neurotrophin-3 (Ntf3), Neurotrophin 5 (Ntf5), Small proline-rich repeat protein 1A (Sprr1a), Growth-associated protein 43 (Gap43) and Beta III tubulin (Tubb3) gene expressions were analyzed. Whole-mount confocal immunofluorescence and Western analyses were performed for localization and abundance of robustly expressed genes.
Results
Sprouts of fine YFP positive fronds emanating from transected (injured) nerve bundles were seen in the flap area at 2 weeks onwards. Bdnf and Sprr1a were robustly and significantly expressed at 2 weeks postoperatively (> 2 folds increase in expression and p < 0.05). Bdnf localized to thy1-YFP+ cells in operated corneas. Sprr1a localized to corneal epithelial cell membranes. At 8 weeks, none of the NTs and RAGs had increased expression. Bdnf (ρ = 0.73, p = 0.001) and Sprr1a (ρ = 0.76, p = 0.001) showed a significant positive correlation with Tubb3.
Conclusion
The neurotrophin Bdnf and regeneration-associated gene Sprr1a are robustly and significantly expressed during corneal nerve regeneration in vivo.
Keywords: Regeneration-associated gene, neurotrophin, corneal nerve, nerve regeneration, lamellar flap
Introduction
Sensory innervation to the cornea from the trigeminal ganglion is important for the perception of stimuli, maintenance of hydration and avoidance of injury. Corneal nerve dysfunction is the pathophysiological basis of ocular diseases that cause considerable morbidity (e.g. neurotrophic keratitis and dry eye disease).1, 2 Several ophthalmic surgical procedures such as corneal transplantation, radial keratotomy, photorefractive keratectomy (PRK), and laser-assisted in situ keratomileusis (LASIK), cause corneal nerve disruption that can lead to neurotrophic epitheliopathy.3 Despite the clinical need to promote corneal nerve regeneration in neurotrophic corneas, there are relatively few specific therapeutic interventions.4 The overall goal of our research is to identify molecular targets that are expressed in the cornea during nerve regeneration, as these molecular targets may have therapeutic potential in neurotrophic corneas.
The robust regenerative response following nerve injury is thought to correspond with coordinated expression of a number of neurotrophins (NTs) and regeneration-associated genes (RAGs) that aid rapid regeneration.5 Although expression of NTs and RAGs has been reported in the cornea, their expression in the setting of nerve regeneration is not known. Neurotrophins represent a family of neurotrophic growth factors comprising nerve growth factor (Ngf), brain-derived neurotrophic factor (Bdnf), neurotrophin-3 (Ntf3), and neurotrophin-4/5 (NT-4/5).6,7 A number of these RAGs have now been identified and they encode a diverse group of molecules.8 Two widely known RAGs are the growth-associated protein-43 (Gap43)9 and small proline-rich repeat protein 1A (Sprr1a).10 Growth-associated protein 43 (Gap43) is a prototypical regeneration-associated gene that is constitutively expressed in the cornea.11 It has been shown to increase in HSV infections in the cornea.12 Small proline-rich repeat protein 1A (Sprr1a) is a regeneration-associated gene that is expressed in primary sensory neurons and spinal cord of the adult mouse following peripheral and central injury.13 Sprr1a has been localized to the corneal epithelium and investigated in the context of corneal envelope proteins.14
In the studies reported herein our purpose is to determine neurotrophin and nerve regeneration-associated genes that are expressed during corneal reinnervation. To achieve that we created a lamellar flap in the mouse cornea and determined the expression of NTs and RAGs and validated the gene expression data with protein localization and abundance. We have used thy1-YFP transgenic mice in this study. In thy1-YFP mice corneal nerves can be visualized using stereofluorescent microscope, making in vivo investigations of corneal nerves feasible.15, 16
Methods
Animals
Neurofluorescent homozygous adult mice of the thy1-YFP line 16 were purchased from Jackson Laboratories (Bar Harbor, ME). For in vivo experiments, mice were anesthetized with intraperitoneal injections of a combination of ketamine (20 mg/kg; Phoenix Scientific, St. Joseph, MO) and xylazine (6 mg/kg; Phoenix Scientific). For terminal experiments, mice were sacrificed with a lethal dose of intraperitoneal pentobarbital (100 mg/kg; Abbott Laboratories, North Chicago, IL). All animals were managed and experiments were conducted according to the ARVO Statement for the Use of Animal in Ophthalmic and Vision Research.
Animal Surgery
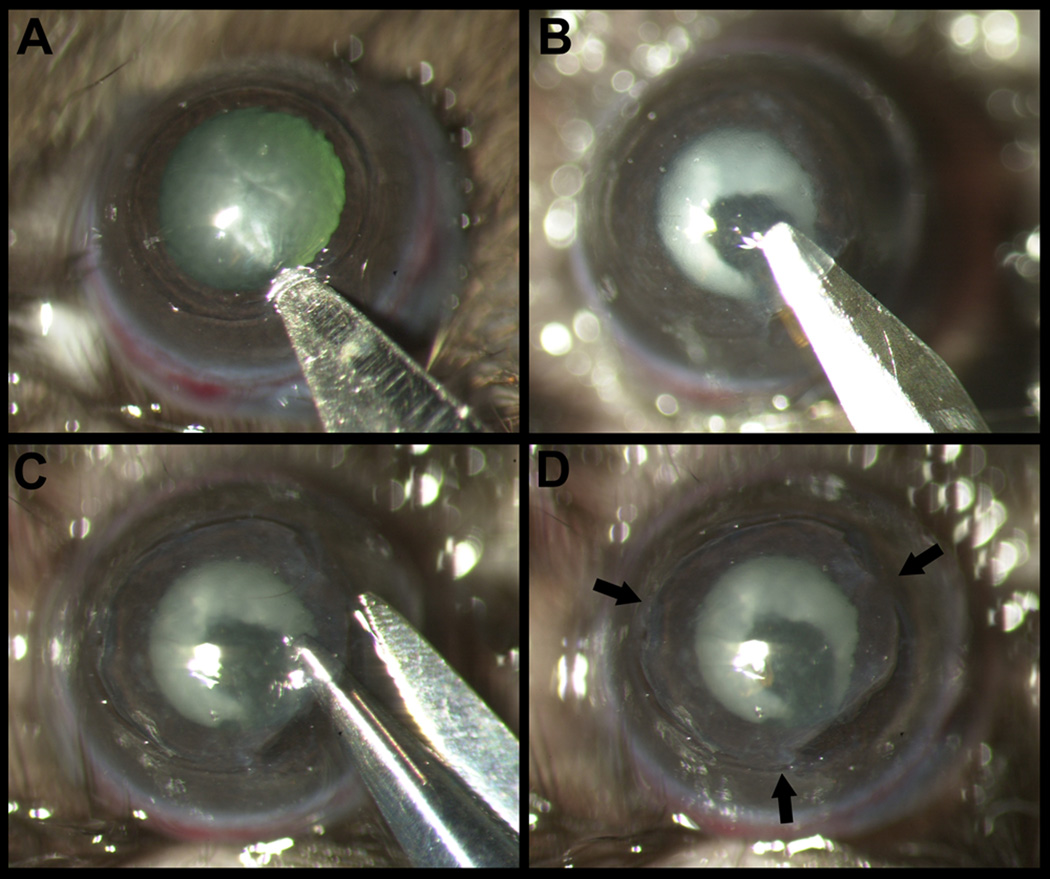
The central cornea was marked with a 2mm diameter disposable trephine (VISIPUNCH, Huot instruments, Menomonee Falls, WI). A partial thickness incision 0.5mm in length was made perpendicular to the corneal surface, tangential to the circular trephine mark, using a 40um preset custom diamond blade. The peripheral lip of the corneal incision was depressed to enter the stroma centripetally using a 15°, 5.0mm standard angle knife (I-KNIFE model # 8065401501, Alcon, Fort Worth, TX), thus creating a corneal pocket (Figure 1 A). A 1.0 mm paracentesis knife (CLEARCUT Sideport model # 8065921540, Alcon, Fort Worth, TX) was used to expand the corneal pocket to the 2 mm diameter trephine mark (Figure 1 B). Next, a 45° 1.75mm subretinal spatula (GRIESHABER UltraSharp model # 682.11, Alcon Fort Worth, TX) was used to enter the corneal pocket and incise it from within (ab-interno) to exit out at the 2 mm diameter trephine mark. A Vannas scissor was used to extend the circumferential incision along the trephine mark (Figure 1 C). At three points (each approximately 0.5 clock hour) the corneal pocket was left unincised, thus forming three hinges for the flap to remain attached to the cornea (Figure 1 D). Finally, an antibiotic ointment was applied to the eye and a suture tarsorrhaphy was performed, which was opened in 3 days.
Figure 1.
Dissection of hinged lamellar corneal flap. A: After marking the central cornea with a 2mm trephine, an initial cut is made with a diamond blade and a stromal pocket is created using 15°, 5.0mm standard angle knife. B: The stromal pocket is extended by lamellar dissection using a 1.0 mm paracentesis knife. C: The stromal pocket is opened with scissors circumferentially, except at the 3 hinges to leave the flap attached to the stromal bed. D: Cornea with the hinged lamellar flap. Arrows point to the hinges.
In vivo Stereofluorescence Imaging
Animals were photographed with a fluorescence stereoscope (StereoLumar V.12) equipped with a digital camera (Axiocam MRm) and software (Axiovision 4.7) as described by Namavari et al.16 An anesthetized thy1-YFP mouse was placed on the stage of a stereoscope (Carl Zeiss Meditec GmbH). The pupil was constricted with topical drops of carbachol intraocular solution (Miostat; Alcon, Fort Worth, TX) and the cornea was anesthetized with 0.5% proparacaine (Bausch & Lomb, Tampa, FL). Z-stack images were obtained at 5 µm intervals, and compacted into one image using Zeiss Axiovision software.
Real-Time quantitative PCR
Mice were sacrificed at weeks 2 (n=6), 4 (n=5) and 8 (n=5). Cornea from each mice was excised and processed for qPCR separately. Therefore, at each time point gene expression has been determined in 5 or more corneas. The corneas were homogenized in extraction reagent (Trizol; Invitrogen, Carslbad, CA) at 4°C, and total RNA was extracted according to the manufacturer's protocol. RNA quantity and quality were assessed by spectrophotometric analysis. Reverse transcription (RT) was performed with 50ng total RNA using RT2 First Strand cDNA Synthesis kit (SABiosciences, Frederick, MD). Reverse transcription control (an artificial RNA template) included in the RT kit was used to confirm the efficiency of RTsteps. The resulting cDNA was preamplified using RT2 Nano PreAMP Kit (SABiosciences, Frederick, MD) according to the manufacturer’s instructions (12 cycles of 95°C, 15 sec; and 60 °C, 2 min). Relative expression level of neurotrophins and regeneration-associated genes was assessed by SYBR green real-time qPCR (7900 HT; Applied Biosystems, Foster, CA). Primers for the genes analyzed were obtained from SABiosciences (Table 1). Samples were assayed in triplicate in a total volume of 25 µL, using thermal cycling conditions of 10 minutes at 95°C followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Artificial DNA template was used as a positive PCR control. No template controls were run in each assay to confirm lack of DNA contamination in reagents used for amplification. In order to reduce the confounding error due to corneal wound healing, samples were included in the analysis only if the wound healing genes, Aldehyde dehydrogenase (Aldh3a1) and Alpha-smooth muscle actin (Acta2) did not show significant change in expression.
Table 1.
Specifications of the primers.
| Gene Name | Gene Symbol |
RefSeq Accession Number* |
Band Size |
Reference Position** |
|---|---|---|---|---|
| Small proline-rich protein 1a | Sprr1a | NM_009264.2 | 190 | 526 |
| Growth associated protein 43 | Gap43 | NM_008083.2 | 100 | 56 |
| Tubulin, beta 3 | Tubb3 | NM_023279.2 | 89 | 394 |
| Nerve growth factor | Ngf | NM_013609.2 | 81 | 896 |
| Brain-derived neurotrophic factor | Bdnf | NM_007540.4 | 155 | 3671 |
| Neurotrophin 3 | Ntf3 | NM_008742.2 | 154 | 311 |
| Neurotrophin 5 | Ntf5 | NM_198190.1 | 142 | 751 |
| Glial cell derived neurotrophic factor | GDNF | NM_010275.2 | 191 | 3178 |
| Neurturin | Nrtn | NM_008738.2 | 104 | 927 |
| Artemin | Artn | NM_009711.3 | 157 | 1060 |
| Persephin | Pspn | NM_008954.1 | 111 | 179 |
| Ciliary neurotrophic factor | Cntf | NM_170786.2 | 94 | 155 |
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | NM_008084.2 | 140 | 309 |
The RefSeq Accession Number refers to the representative sequence used to design the enclosed primers.
The Reference Position is a position within the sequence of the amplicon relative to the start of the relevant RefSeq sequence.
For data analysis, the comparative threshold cycle (CT) method was used to determine the fold increase in mRNA level in operated corneas over unoperated control corneas.17 To normalize the amount of target gene in operated and unoperated control corneas, we calculated the difference in CT (ΔCT) by subtracting the average CT of the endogenous control (Gapdh) from that of the target gene. The ΔΔCT was calculated by subtracting the ΔCT of genes in the operated cornea from the mean ΔCT of genes in unoperated control cornea. The fold change in mRNA for genes in the operated cornea was determined relative to the amount present in the unoperated control cornea, using the formula 2−ΔΔCT. Results were averaged and the SEM was calculated. We defined robust gene expression using dual criteria: (i) fold change in gene expression > 2 as determined by comparison of 2−ΔΔCT values; and (ii) statistically significant difference between 2−ΔΔCT values (p < 0.05).18 We used the dual criteria in order to identify only the most robustly expressed genes given that the protein abundance of neurotrophins in peripheral tissues, including iris, has been reported to range from very low to undetectable by Western analysis.19 We reasoned that subsequent protein validation studies would be feasible for the genes that show the most robust increase in expression. The use of dual criteria using change in gene expression > 2 has been reported before.20
Corneal Whole-Mount Immunostaining
Excised corneas from operated eyes (2 week time-point, n = 3) and unoperated control eyes (n = 2) were processed for whole-mount immunofluorescence staining. Corneas were fixed in 4% PFA for 1 h at room temperature, and washed four times with PBS (for 15 min each). Corneas were then permeabilized and blocked for 1 h at room temperature in 1% Triton X-100, 1% bovine serum albumin (BSA), and 10% normal donkey serum in PBS. The corneas were incubated in primary antibody diluted in the blocking solution (1:200) for 72 h at 4°C, washed four times in PBS (for 15 min each), and incubated with secondary antibody diluted in the blocking solution (1:350) overnight at 4°C. Corneas were further washed and mounted in mounting medium on glass slides. Primary antibodies were chicken anti-Bdnf (catalog# G164A, Promega, Fitchburg, WI) and rabbit anti-Sprr1a (a kind gift from Prof. Stephen Strittmatter, Yale University). The specificity of these antibodies has been established in murine tissue.21,22 Secondary antibodies were Dylight 594-conjugated AffiniPure donkey anti-chicken and anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). Dylight 594 was chosen to ensure non-overlap with the yellow fluorescent protein (YFP) wavelength and to minimize false positive staining. Primary antibody was omitted for negative control. Z-stack images of corneal whole-mounts were obtained using a LSM 510 META confocal microscope (Carl Zeiss, GmbH, Hamburg, Germany). The operated corneas were imaged first to optimize the fluorescent signal. Immediately thereafter, unoperated control corneas were imaged using the same settings to determine a relative difference in fluorescence intensity.
Western Immunoblot Analysis
Corneas were excised from operated control mice (n=7) as well as from mice that underwent corneal flap surgery (n=7). Corneas in each group were pooled for analyses. Corneas were snap-frozen in liquid nitrogen and homogenized using a Biopulverizer (Biospec Products Inc., Bartlesville, OK) in a modified RIPA cell lysis buffer (20 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% IGEPAL, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, pH 7.4) supplemented with a complete protease inhibitor and a phosphatase inhibitor Cocktail I and II (Sigma Chemical Co., St. Louis, MO). Samples were then centrifuged at 10,000g for 15 minutes at 4°C, and the supernatant (cell lysate) was collected. Total protein was determined using a modified Lowry method (BioRad DC Protein assay, BioRad Laboratories, Hercules, CA). For Western blot analysis, 100 µg total protein was electrophoretically run on 4–12% Tris–Glycine SDS polyacrylamide gel (XCell SureLock® Mini-Cell Electrophoresis System, Invitrogen, Carlsbad, CA). Samples were transferred to 0.2-µm nitrocellulose membranes (Whatman Inc., Florham Park, NJ) by electro-elution. Membranes were blocked in Li-Cor blocking buffer (Li-Cor Biosciences, Lincoln, NE), followed by incubation overnight at 4°C with rabbit anti-Sprr1a (1:500; a kind gift from Prof. Stephen Strittmatter, Yale University) antibody diluted in blocking buffer. Mouse monoclonal anti-GAPDH (1:1000; Santa Cruz Biotech Inc., Santa Cruz, CA) was used as a loading control. After three 10-minute washes in PBS containing 0.1% Tween-20, the blots were incubated for 2 hours at room temperature in the fluorescently-labeled secondary antibody mixture (Rockland Immunoresearch, Gilbertsville, PA) of goat anti-rabbit (IRDye®800CW, 1:15,000) and goat anti-mouse (IRDye®700DX, 1:10,000) antibodies diluted in blocking buffer. Membranes were then imaged using LiCor Odyssey® Infrared imager (Li-Cor Biosciences, Lincoln, NB). The relative intensity of each band was determined with the LiCor Odyssey® application software (LiCor Biosciences, Lincoln, NB). Quantification was performed by subtracting background readings from the relative intensity for each sample band and normalizing it with that of GAPDH. Data are expressed as fold-increase in protein expression of the surgery group versus the untreated control group.
Statistical Analysis
Mean values and their standard errors were computed for operated corneas and unoperated controls at each time point. Student t-test was used for measurement comparisons between groups. Microsoft Excel Office software package was used for correlation analysis descriptive statistics. A p value less than 0.05 was considered statistically significant.
Results
Evidence of Corneal Nerve Regeneration
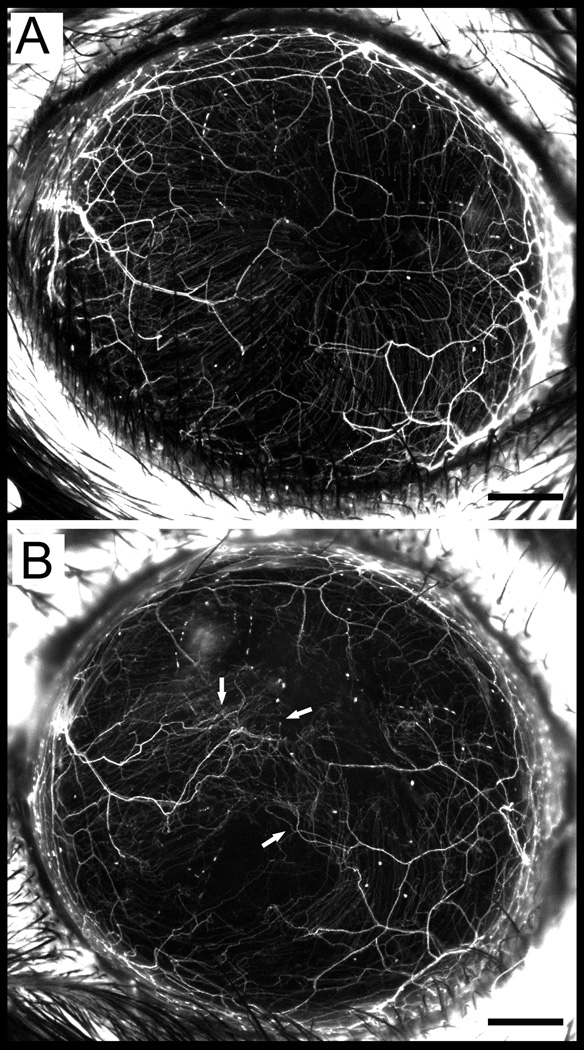
To determine evidence of nerve regeneration within the flap area, we performed wide-field fluorescent imaging in a thy1-YFP neurofluorescent mouse (Figure 2). Sprouting of fine YFP positive fibers emanating from transected (injured) nerve bundles was seen in the flap area at 8 weeks (Fig. 2B). Collateral sprouting from intact (non-transected) nerve bundles was not seen at 8 weeks.
Figure 2.
In vivo stereofluorescent microscope image of Thy1-YFP mice showing fluorescent corneal nerves before surgery (A) and 8 weeks postoperatively (B). A: Preoperative cornea shows innervation by stromal trunks and subbasal nerves. B: The same eye, 8 weeks after the flap surgery. Arrows point towards regenerative sprouts from transected nerve trunk. Scale, 500 µm.
Gene expression during Corneal Nerve Regeneration
The “fold increase” in Neurotrophin (NT) and Regeneration-associated gene (RAG) expression in the operated corneas over unoperated control corneas was determined by using the formula 2−ΔΔCT.
Neurotrophin Expression
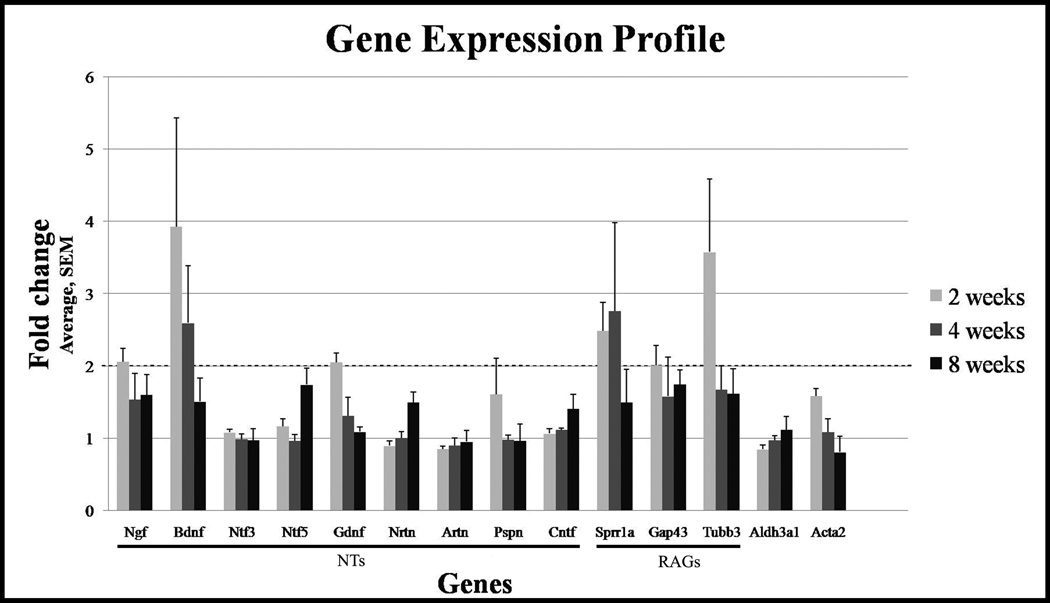
At 2 weeks, Ngf (2.06 ± 0.19 fold, p = 0.001), Bdnf (3.93 ± 1.5 fold, p = 0.05) and Gdnf (2.05 ± 0.13 fold, p = 0.002) showed robust and significant increase in gene expression in operated corneas as compared with unoperated control corneas (Figure 3). Ntf3, Ntf5, Nrtn, Artn, Pspn and Cntf showed less than two fold change in gene expression in operated corneas as compared with unoperated control corneas.
Figure 3.
Expression of neurotrophins and regenerations-associated genes. Quantitative PCR was performed on corneas after lamellar flap surgery and gene expression compared to fellow eye was determined at weeks 2, 4 and 8. More than 2 fold increase was considered significant. Among neurotrophins, Bdnf is most significantly expressed. Among regeneration-associated genes, Sprr1s is most significantly expressed. Gene expression was determined in triplicate/time point.
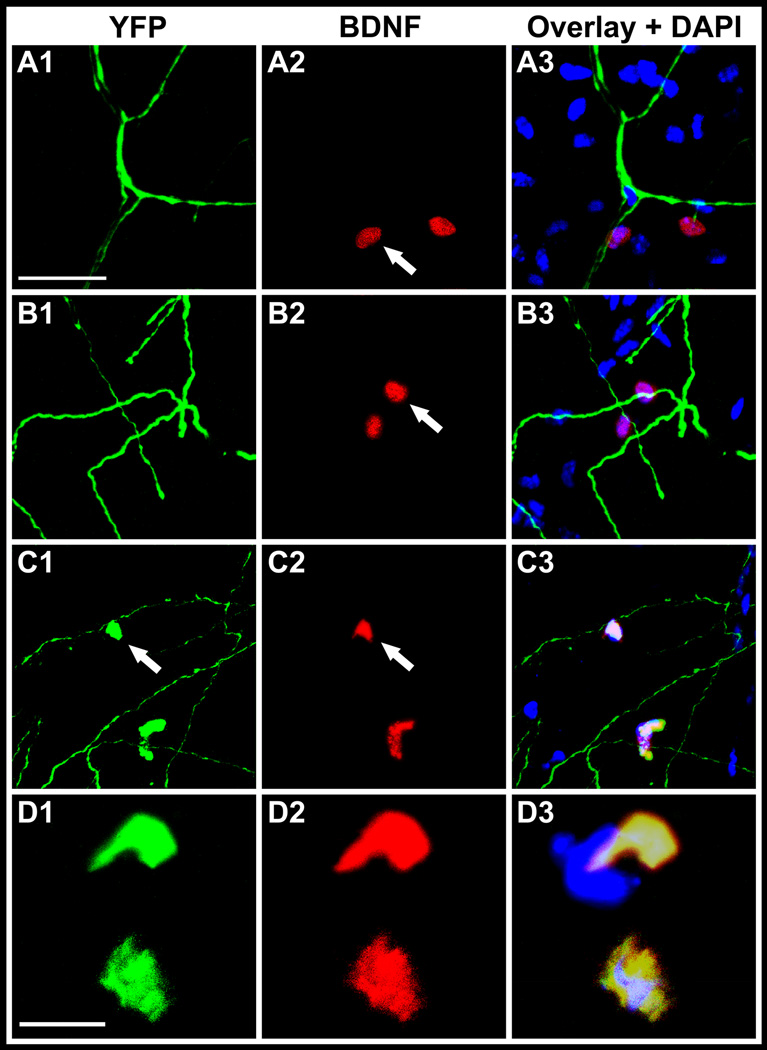
Whole-mount immunostaining of excised corneas at 2 weeks showed localization of Bdnf within stromal cells in the vicinity of nerve trunks in unoperated as well as operated corneas (Figure 4, A1 and B1). The fluorescence of stromal cells was similar in the two groups. Thy1 is a pan T-cell marker in mouse as well as a neuronal marker,15,23 therefore nerves as well as inflammatory cells show YFP fluorescence in thy1-YFP+ mouse. We observed YFP+ cells in the stroma of operated corneas which co-localized with Bdnf (Figure 4, C1 and D1). These YFP+ and Bdnf+ cells were not observed in unoperated control corneas or in negative controls. This finding suggests that YFP+ cells (inflammatory cells or fibroblasts) are a source of Bdnf during corneal reinnervation. Bdnf was not detected in Western analyses in both groups (data not shown).
Figure 4.
Confocal Immunofluorescent localization of Bdnf in whole-mount corneas from thy1-YFP mice. Images show confocal sections in normal unoperated corneas (A1 – A3) and corneas at 2 weeks after lamellar transection (B1 – B3, C1 – C3, D1 – D3). In unoperated corneas, YFP+ fluorescent nerves (green) are seen (A1) and Bdnf expressing stromal cells (red) are seen in the vicinity of nerves (A2). Image A3 shows an overlay of A1 and A2 with DAPI stained nuclei (blue). Similarly, in operated corneas, YFP+ fluorescent nerves (green) are seen (B1) and Bdnf expressing stromal cells (red) are seen in the vicinity of nerves (B2). Image B3 shows an overlay of B1 and B2 with DAPI stained nuclei (blue). YFP+ cells (green) are seen in operated corneas only (C1 and D1). These YFP+ cells are likely inflammatory cells or fibroblasts. Bdnf (red) also localizes to the same YFP+ cells (C2 and D2). The overlay with DAPI (C3 and D3) shows that YFP fluorescence (green) and Bdnf (red) colocalize. Scale bar, 50 µm for panels A – C and 10 µm for the panel D.
At 4 weeks, Bdnf (2.60 ± 0.79, p = 0.05) was the only neurotrophin that showed robust and significant increase in gene expression. Ngf, Gdnf, Ntf3, Ntf5, Nrtn, Artn, Pspn and Cntf gene expression showed less than two fold change. At 8 weeks, none of the neurotrophins showed significant increase in gene expression.
Regeneration-associated gene Expression
At 2 weeks, Sprr1a (2.49 ± 0.39, p = 0.006), Gap43 (2.01 ± 0.27, p = 0.006) and Tubb3 (3.58 ± 1.01, p = 0.02) showed robust and significant increase in gene expression. Whole-mount immunostaining of excised corneas at 2 weeks showed that Sprr1a localized to epithelial cell membranes in unoperated as well as operated corneas (Figure 5, A1 and B1); however, using identical confocal imaging settings, Sprr1a fluorescence was observed to be greater in the epithelial cells of operated group. Western analyses showed that the abundance of Sprr1a was 2.48 folds greater in the operated corneas as compared to unoperated control corneas (Figure 5, C1 and C2).
Figure 5.
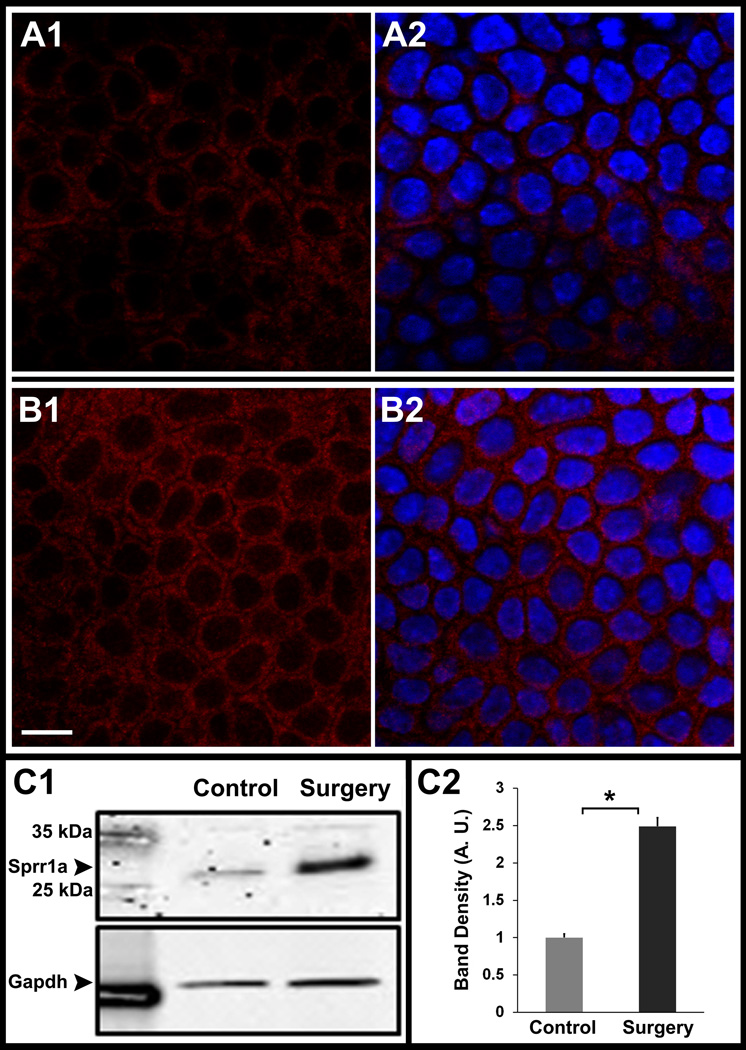
Confocal Immunofluorescent localization of Sprr1a in whole-mount corneas from thy1-YFP mice. (A,B) and Western analyses of corneal lysates for Sprr1a (C). Images show confocal sections in normal unoperated corneas (A1 – A2) and corneas at 2 weeks after lamellar transection (B1 – B2). In unoperated corneas, Sprr1a (red) is localized to corneal epithelial cell membranes (A1). Similarly, in operated corneas Sprr1a (red) is also localized to corneal epithelial cell membranes (B1). Image A2 and B2 shows an overlay with DAPI stained nuclei (blue). Using the same settings on the confocal microscope, the Sprr1a fluorescence in epithelium of operated corneas (B1) was greater than the epithelial fluorescence in unoperated control corneas (A1). Western analyses for Sprr1a (34 kDa) showed a denser band in operated corneas (C1, surgery lane) that was 2.48 fold higher than in unoperated corneas (C2). Gapdh (37 kDa) was used as a loading control. Scale bar, 10 µm. Asterisk, p < 0.05. Error bars, standard error of mean (SEM).
At 4 weeks, Sprr1a (2.76 ± 1.22) was the only RAG that showed more than two fold increase in gene expression. Gap43 and Tubb3 (p = 0.02) gene expression showed less than two fold change. At 8 weeks, none of the RAGs showed significant increase in gene expression.
We determined whether NT and RAG expression was correlated with the expression of Tubb3 (structural protein) at all time-points. Bdnf (ρ = 0.73, p = 0.001) and Sprr1a (ρ = 0.76, p = 0.001) showed a significant positive correlation with Tubb3. Bdnf and Sprr1a showed a significant positive correlation with each other also (ρ = 0.65, p = 0.004).
Discussion
The central finding of this manuscript is that neurotrophins and regeneration-associated genes are expressed in the cornea after corneal nerve transection, particularly Bdnf and Sprr1a which are robustly expressed. Our data points to YFP+ cells (inflammatory cells or fibroblasts) in the stroma as a source for Bdnf in the cornea and epithelium as a source of Sprr1a. To our knowledge this is the first report of neurotrophins and regeneration-associated genes expression in the context of corneal nerve regeneration in vivo. Our results point to Sprr1a as a physiologically relevant molecular marker for corneal nerve regeneration as its expression significantly correlated with Tubb3 expression. Assaying Sprr1a expression may prove useful when assessing interventions aimed at enhancing corneal reinnervation.
Gap43 is a rapidly transported axonal protein that is highly induced after nerve injury. It is localized primarily in the axonal growth cone.9 The growth cone localization of Gap43 may be the reason for higher expression levels in the flap as opposed to the bed. Unlike Gap43, which is expressed during development and constitutively by some cells in the adult,11 there is no expression of Sprr1a during development or in naive adult nervous tissue.10 Since Sprr1a is not normally present in sensory neurons, but following peripheral nerve injury becomes de novo expressed, and when overexpressed can increase neurite outgrowth, it has been proposed as a truly regeneration-specific protein.13 Our gene expression and protein abundance data shows that Sprr1a is robustly expressed in the corneal epithelium during reinnervation. It is noteworthy that Sprr1a has been identified in this study as a nerve regeneration-associated gene whereas previously it has been localized in the corneal epithelium in the context of envelope proteins, but its expression remained unchanged in response to epithelial stress injury.14 Sprr1b, but not Sprr1a, has been proposed to be a biomarker for corneal epithelium squamous metaplasia and keratinization.24 Given that not only do the corneal epithelial cells undergo continuous turnover,25 the sensory endings in the corneal epithelium also undergo continual rearrangement,26 it is intriguing to posit that different members of the small proline-rich proteins (SPRRs) may differentially regulate epithelial and nerve remodeling. In the rat keratinocyte, Sprr1a is induced during epithelial differentiation is thought to contribute to the permeability barrier function of the cornified epithelium.27 The common properties of Sprr1a with other cornified epithelium genes (e.g. upregulation with UV light exposure) raise the possibility that peripheral axonal regeneration uses a gene program shared with epithelial differentiation.10
Using mouse strains in which the reporter gene lacZ, encoding the enzyme beta-galactosidase was targeted to either Bdnf or Ntf3 locus, Bdnf and Ntf3 expression was reported in the corneal epithelium.28 The transcription of neurotrophic factors (Ngf, Ntf3 and Bdnf) and Glial derived neurotrophic factor (Gdnf) has been detected in freshly harvested human corneal epithelium.29,30 Ngf has been investigated in the context of wound healing and has been found to promote corneal epithelial healing and improve corneal allograft survival as well as lead to faster corneal nerve regeneration.31 In addition to confirming earlier reports of neurotrophin expression in the cornea, our data shows that Bdnf expression is significantly upregulated during nerve regeneration. Our data suggests that inflammatory cells may be a source of Bdnf in corneas during nerve regeneration. This finding is in conformity with prior published reports that activated human T cells and monocytes secrete bioactive Bdnf32 and reports that these inflammatory cells are increased during corneal inflammation.33
We expect future research in other animal models will confirm and build upon the results of this study. Recently, the use of neurofluorescent thy1-YFP mice has been reported in investigations of incisional injury to nerve trunks in the cornea.15 Our method of a hinged corneal flap has a critical advantage over the incisional model: it allows identification of molecular targets that are differentially expressed during corneal nerve regeneration. Our method of corneal flap creation with hinges obviates the need for placing sutures. We have avoided the use of sutures as they can invite vascularization and incite inflammation.34 The use of Thy1-YFP mice is particularly well suited for corneal nerve regeneration as the corneal neuroflourescence can be followed sequentially in vivo.
Corneal nerve regeneration may involve the expression of several as yet unknown diffusible nerve guidance proteins and neurotrophic factors. This study provides a foundation to be able to study corneal nerve regeneration in murine models in a way we have not been able to in the past. The most direct application of this technique and data is for future research in neurotrophic corneas associated with disease or following surgery. Our techniques in this study are sensitive enough to differentially quantify molecular changes at the transcriptional level allowing for this technique to form the basis of future investigations to measure the effect of interventions designed to promote nerve regeneration.
Acknowledgments
Supported by National Eye Institute (NEI) Grant EY018874 (SJ), NEI core grant EY001792 and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest : None.
References
- 1.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003;17:989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 2.Dastjerdi MH, Dana R. Corneal nerve alterations in dry eye-associated ocular surface disease. Int Ophthalmol Clin. 2009;49:11–20. doi: 10.1097/IIO.0b013e31819242c9. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SE. Laser in situ keratomileusis-induced (presumed) neurotrophic epitheliopathy. Ophthalmology. 2001;108:1082–1087. doi: 10.1016/s0161-6420(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 4.He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82:319–325. doi: 10.1016/j.plefa.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huebner EA, Strittmatter SM. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339–351. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartkowska K, Turlejski K, Djavadian RL. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol Exp (Wars) 2010;70:454–467. doi: 10.55782/ane-2010-1816. [DOI] [PubMed] [Google Scholar]
- 7.Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr Neurovasc Res. 2007;4:143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PM, Miao T, Wu D, Zhang Y, Yeh J, Bo X. Responses of the nerve cell body to axotomy. Neurosurgery. 2009;65(4 Suppl):A74–A79. doi: 10.1227/01.NEU.0000352378.26755.C3. [DOI] [PubMed] [Google Scholar]
- 9.Skene JH, Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin RE, Bazan NG. Growth-associated protein Gap43 and nerve cell adhesion molecule in sensory nerves of cornea. Exp Eye Res. 1992;55:307–314. doi: 10.1016/0014-4835(92)90195-x. [DOI] [PubMed] [Google Scholar]
- 12.Martin RE, Henken DB, Hill JM. Altered expression and changing distribution of the nerve growth associated protein Gap43 during ocular HSV-1 infection in the rabbit. J Neurovirol. 1996;2:127–135. doi: 10.3109/13550289609146546. [DOI] [PubMed] [Google Scholar]
- 13.Starkey ML, Davies M, Yip PK, Carter LM, Wong DJ, McMahon SB, Bradbury EJ. Expression of the Regeneration-Associated Protein Sprr1a in Primary Sensory Neurons and Spinal Cord of the Adult Mouse Following Peripheral and Central Injury. J Comp Neurol. 2009;513:51–68. doi: 10.1002/cne.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong L, Corrales RM, Chen Z, Villarreal AL, De Paiva CS, Beuerman R, Li DQ, Pflugfelder SC. Expression and regulation of cornified envelope proteins in human corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:1938–1946. doi: 10.1167/iovs.05-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CQ, Rosenblatt MI. Transgenic corneal neurofluorescence in mice: a new model for in vivo investigation of nerve structure and regeneration. Invest Ophthalmol Vis Sci. 2007;48:1535–1542. doi: 10.1167/iovs.06-1192. [DOI] [PubMed] [Google Scholar]
- 16.Namavari A, Chaudhary S, Sarkar J, Yco L, Patel K, Han KY, Yue BY, Chang JH, Jain S. In Vivo Serial Imaging of Regenerating Corneal Nerves after Surgical Transection in Transgenic Thy1-YFP mice. Invest Ophthalmol Vis Sci. 2011;52:8025–8032. doi: 10.1167/iovs.11-8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan JS, Reed A, Chen F, Stewart CN., Jr. Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 19.Bierl MA, Jones EE, Crutcher KA, Isaacson LG. 'Mature' nerve growth factor is a minor species in most peripheral tissues. Neurosci Lett. 2005;380:133–137. doi: 10.1016/j.neulet.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Muckersie E, Forrester JV, Xu H. Immune activation in retinal aging: a gene expression study. Invest Ophthalmol Vis Sci. 2010;51:5888–5896. doi: 10.1167/iovs.09-5103. [DOI] [PubMed] [Google Scholar]
- 21.Tarsa L, Bałkowiec-Iskra E, Kratochvil FJ, 3rd, Jenkins VK, McLean A, Brown AL, Smith JA, Baumgartner JC, Balkowiec A. Tooth pulp inflammation increases brain-derived neurotrophic factor expression in rodent trigeminal ganglion neurons. Neuroscience. 2010;167:1205–1215. doi: 10.1016/j.neuroscience.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T-cell marker. J Immunol. 2004;173:3581–3588. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Nikulina K, DeVoss J, Wu AJ, Strauss EC, Anderson MS, McNamara NA. Small proline-rich protein 1B (SPRR1B) is a biomarker for squamous metaplasia in dry eye disease. Invest Ophthalmol Vis Sci. 2008;49:34–41. doi: 10.1167/iovs.07-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna C, Bicknell DS, O'Brien JE. Cell turnover in the adult human eye. Arch. Ophthalmol. 1961;65:695–698. doi: 10.1001/archopht.1961.01840020697016. [DOI] [PubMed] [Google Scholar]
- 26.Harris LW, Purves D. Rapid remodeling of sensory endings in the corneas of living mice. J Neurosci. 1989;9:2210–2214. doi: 10.1523/JNEUROSCI.09-06-02210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen CF, Poon R, Drucker DJ. UVB radiation-activated genes induced by transcriptional and posttranscriptional mechanisms in rat keratinocytes. Am J Physiol. 1995;268:C846–C855. doi: 10.1152/ajpcell.1995.268.4.C846. [DOI] [PubMed] [Google Scholar]
- 28.Bennett JL, Zeiler SR, Jones KR. Patterned expression of Bdnf and Ntf3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- 29.You L, Kruse FE, Völcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 30.Qi H, Chuang EY, Yoon KC, de Paiva CS, Shine HD, Jones DB, Pflugfelder SC, Li DQ. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis. 2007;13:1934–1941. [PMC free article] [PubMed] [Google Scholar]
- 31.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of Ngf and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 32.Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, Kolbeck R, Hoppe E, Oropeza-Wekerle RL, Bartke I, Stadelmann C, Lassmann H, Wekerle H, Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamrah P, Liu Y, Zhang Q, Dana MR. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch Ophthalmol. 2003;121:1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 34.Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]