Abstract
Background
The development of asthma after respiratory syncytial virus (RSV) bronchiolitis has been demonstrated in case-control studies, although the determinants of post-RSV asthma remain undefined.
Objectives
We sought to evaluate the potential determinants of physician-diagnosed asthma after severe RSV bronchiolitis during infancy.
Methods
We enrolled 206 children during an initial episode of severe RSV bronchiolitis at 12 months of age or less in a prospective cohort study and followed these children for up to 6 years. In a subset of 81 children, we analyzed CCL5 (RANTES) mRNA expression in upper airway epithelial cells.
Results
Forty-eight percent of children had physician-diagnosed asthma before the seventh birthday. Independent determinants significantly associated with increased risk for physician-diagnosed asthma by the seventh birthday included maternal asthma (odds ratio [OR], 5.2; 95% CI, 1.7-15.9; P = .004), exposure to high levels of dog allergen (OR, 3.2; 95% CI, 1.3-7.7; P = .012), aeroallergen sensitivity at age 3 years (OR, 10.7; 95% CI, 2.1-55.0; P = .005), recurrent wheezing during the first 3 years of life (OR, 7.3; 95% CI, 1.2-43.3; P = .028), and CCL5 expression in nasal epithelia during acute RSV infection (OR, 3.8; 95% CI, 1.2-2.4; P < .001). White children (OR, 0.19; 95% CI, 0.04-0.93; P = .041) and children attending day care (OR, 0.18; 95% CI, 0.04-0.84; P = .029) had a decreased risk of physician-diagnosed asthma.
Conclusions
Approximately 50% of children who experience severe RSV bronchiolitis have a subsequent asthma diagnosis. The presence of increased CCL5 levels in nasal epithelia at the time of bronchiolitis or the development of allergic sensitization by age 3 years are associated with increased likelihood of subsequent asthma.
Keywords: Bronchiolitis, respiratory syncytial virus, asthma, prospective cohort, CCL5
Respiratory syncytial virus (RSV) infection is nearly ubiquitous in early life, with nearly all children seropositive by age 2 years.1 Although most children are asymptomatic or have only mild illness, some children require hospitalization because of severe respiratory symptoms. RSV bronchiolitis was the most common diagnosis for hospitalized infants less than 1 year old from 1998 to 2001, accounting for more than 70,000 hospitalizations annually.2
Long-term prospective case-control and cohort studies have also linked RSV bronchiolitis to the development of wheeze3-6 and asthma5,7-9 later in childhood. Studies by Sigurs et al7 have demonstrated a 7.2-fold increased risk of asthma 18 years after severe RSV bronchiolitis,7 although this might be related in part to a very low incidence of asthma in the control subjects. Epidemiologic studies suggest that the risk of asthma after bronchiolitis is related to episode severity, with children receiving hospital-based care experiencing a 2.8-fold increased risk of asthma by 4.5 to 5.5 years of age.10
CCL5, previously known as RANTES, is a β-chemokine chemoattractant for inflammatory cells, including T-lymphocyte subsets associated with allergic inflammation,11 as well as eosinophils.12 Previous studies have demonstrated increased CCL5 expression in airway epithelial and submucosal inflammatory cells of subjects with asthma.13,14 CCL5 expression and transcription in human tracheal epithelial cells is induced by RSV in vitro.15 Furthermore, mice infected with a related paramyxovirus, Sendai virus, demonstrate increased CCL5 expression along with greater airway resistance and methacholine hyperresponsiveness.16 Therefore we sought to evaluate whether CCL5 expression in airway epithelial cells obtained from children with severe RSV bronchiolitis was predictive of the subsequent development of asthma.
We have assembled a cohort of 206 infants during an episode of severe RSV bronchiolitis and followed these children closely through their seventh birthdays to describe the prevalence of asthma and evaluate potential determinants of asthma after a severe RSV-related lower respiratory tract infection during this vulnerable time period.
METHODS
Study population
From 1998 to 2001, we enrolled 206 infants 12 months of age or less in the RSV Bronchiolitis in Early Life (RBEL) prospective cohort study. Selection of the study population and characteristics of the cohort at study entry are described in detail elsewhere.17 Included infants were required to have bronchiolitis severe enough to require emergency department care or hospitalization, a positive nasopharyngeal swab result confirming infection with RSV, and physician-documented wheezing during the acute illness. Exclusion criteria were a history of previous wheezing or a diagnosis of asthma, congenital abnormalities of the heart and lung, cystic fibrosis diagnosed in the patient or immediate family, regular use of anti–gastroesophageal reflux medication, bronchodilators, or anti-inflammatory medications. A study coordinator collected detailed information about demographic factors, environmental exposures, and family history of respiratory and atopic disease from the child's parent or parents. This study was approved by the Washington University School of Medicine Institutional Review Board. Informed consent was obtained from parents or guardians.
Parent/guardian interviews
Telephone interviews of parents or guardians were conducted every 3 months to monitor children's respiratory symptoms (wheeze, cough, chest tightness, and shortness of breath), physician-diagnosed respiratory disease, and any change in environmental exposure or family history.
Allergy evaluation
We performed percutaneous allergy skin tests during the third year of follow-up (n = 150; mean age, 3.6 ± 0.7 years of age) using the MultiTest device. Additional details are included in the Methods section in this article's Online Repository at www.jacionline.org.
Total serum IgE levels were measured at study entry by using nephelometry. The percentage of eosinophils among peripheral white blood cells was measured at study entry with automated cell count methodology.
Nasal epithelial CCL5 expression
We obtained nasal epithelial cells (>90% cytokeratin positive) from nasal brushings from 81 subjects based on their consent to provide samples at entry and at 1 month. Cytospin preparations were prepared and hybridized to anti-sense and sense 35S-labeled CCL5 cRNA. The samples were then exposed to a Phosphor screen and quantified for 35S-labeled anti-sense RNA by using ImageQuant (Molecular Dynamics Storm, Sunnyvale Calif). A CCL5 ratio was calculated by subtracting sense RNA from antisense RNA 35S signal and correcting for the total number of cells as follows:
Environmental assessment
Dust samples (n = 174) were collected from enrolled infants’ homes within the first year of enrollment and analyzed, as described previously (see the Methods section in this article's Online Repository).17
Asthma outcomes
We defined the occurrence of a wheezing episode each time a parent/guardian answered yes to either “Has your child had wheezing with colds?” or “Has your child had wheezing without colds?” during each follow-up contact every 3 months.
We examined 2 asthma outcomes of differing stringencies: physician-diagnosed asthma and active asthma. A child was classified as having physician-diagnosed asthma if the parent/guardian answered yes to “Has your child ever been diagnosed with asthma by a physician?” at any time before the child's seventh birthday. A persistent negative response defined children without physician-diagnosed asthma. All children with at least 1 follow-up contact (201/206) were included in analyses by using data obtained at baseline as predictors of physician-diagnosed asthma. To analyze factors associated with physician-diagnosed asthma after age 3 years, we excluded children given a diagnosis of asthma before age 3 years, as well as those children with no follow-up contact after age 3 years.
We defined active asthma as physician-diagnosed asthma at any time along with parent-reported wheezing during the last year of follow-up between the child's third and seventh birthdays. For example, for subjects with a follow-up at age 5 years, active asthma was defined based on the active wheezing records for the 12 months preceding that last follow-up. Children without follow-up contact between the third and seventh birthdays were excluded from analyses examining predictors of asthma outcomes obtained at either baseline or by age 3 years.
Statistical considerations and data analysis
Data are reported as means ± SDs or percentages (frequencies), as appropriate. Univariate and multivariate logistic regression models were used to explore potential relationships among demographic factors, CCL5 expression, environmental exposures, and the 2 asthma outcomes (physician-diagnosed asthma and active asthma). Variables with P values of less than .1 in univariate analysis were entered into multivariate models, and stepwise procedures were used to identify the specific factors that were independently associated with the 2 asthma outcomes. P values of .05 or less were considered statistically significant in the multivariate predictive models. Odds ratios (ORs) are presented with their 95% CIs. The Hosmer and Lemeshow goodness-of-fit test was applied to all of the logistic regression models and established the fit of all models we present. Preliminary analysis found a strong association between nonwhite race and baseline annual household income of $20,000 or less (P < .0001); we thus included only nonwhite race in the multivariate model. IgE levels and PC20 values were log-transformed.
The association between CCL5 expression and asthma outcomes was explored separately because of the smaller sample size. Receiver operating characteristic (ROC) curves were generated for CCL5 expression to evaluate the sensitivity and specificity for predicting the 2 asthma outcomes. All analyses were performed with SAS 9 software (SAS institute, Inc, Cary, NC).
RESULTS
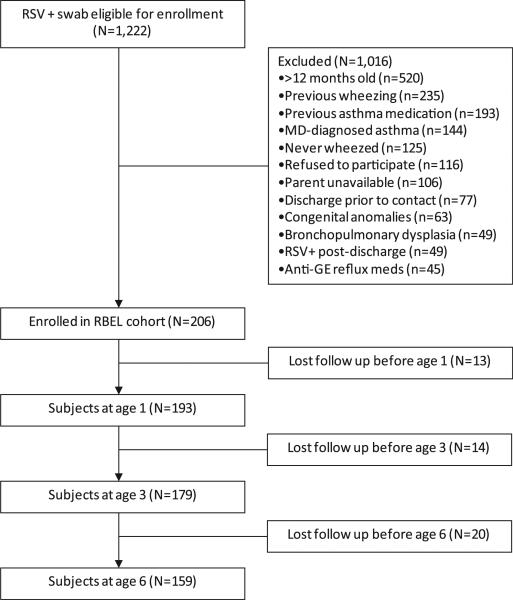
Of 206 children enrolled in the RBEL cohort, 201 (98%) had at least 1 follow-up contact during the study period. Fig 1 and the Results section in this article's Online Repository at www.jacionline.org show recruitment and retention over the course of the study. One hundred fifty-nine (77%) subjects provided data between ages 3 and 6 years. Children who did not provide data between ages 3 and 6 years did not differ from those with data in terms of age, sex, race, bronchiolitis severity, smoke exposure, IgE levels, eosinophil percentages, or nasal epithelial CCL5 levels, although there was a slightly higher, although not statistically significant, proportion of children with maternal asthma in the group followed through 6 years (P = .054, see Table E1 in this article's Online Repository at www.jacionline.org). The baseline demographic characteristics of this population have been previously described (see Table E2 in this article's Online Repository at www.jacionline.org).17
FIG 1.
Enrollment and follow-up of participants through the RBEL study. Patients were enrolled during an episode of severe RSV bronchiolitis and followed prospectively through the first 6 years of life. GE, Gastroesophageal.
TABLE E1.
Clinical characteristics of subjects with and without follow-up between 3 and 6 years of age
| Subjects without follow-up (n = 47) | Subjects with follow-up (n = 159) | P value | |
|---|---|---|---|
| Demographics | |||
| Age at study entry (d) | 122 ± 71 | 139 ± 105 | .976 |
| Male sex (%) | 63.8 (30) | 56.6 (90) | .378 |
| White race (%) | 51.1 (24) | 52.8 (84) | .831 |
| Hospitalization data | |||
| Length of stay in hospital (d) | 2.30 ± 2.53 | 2.47 ± 2.51 | .416 |
| Lowest SaO2 (%) | 92 ± 9 | 92 ± 7 | .239 |
| Family history | |||
| Maternal history of asthma (%) | 8.51 (4) | 21.4 (34) | .054 |
| Maternal history of eczema (%) | 8.51 (4) | 5.66 (9) | .499 |
| Maternal history of allergic rhinitis (%) | 25.5 (12) | 30.8 (49) | .486 |
| Clinical history and other potential exposures | |||
| Smoking in home during first year of life (%) | 76.6 (36) | 64.5 (100) | .122 |
| Dog in home (%) | 51.3 (20) | 43.5 (60) | .387 |
| Laboratory tests at baseline | |||
| Baseline IgE (IU/mL) | 13.7 ± 16.0 | 24.3 ± 46.4 | .452 |
| Baseline eosinophils (%) | 1.67 ± 2.00 | 1.81 ± 2.65 | .604 |
| Nasal CCL5 (ratio) | 4.60 ± 2.19 | 4.39 ± 1.72 | .445 |
SaO2, Lowest oxygen saturation measured during index hospitalization.
TABLE E2.
Baseline characteristics of the RBEL cohort
| Characteristic | Included (n = 206) |
|---|---|
| Demographics | |
| Age at study entry (d) | 135 ± 99 |
| Male sex (%) | 58.3 |
| Race/ethnicity (%) | |
| White | 52.4 |
| Black | 43.7 |
| Other | 3.9 |
| Annual household income ≥$20,000 (%) | 60.8 |
| Pregnancy history | |
| Duration of pregnancy (wk) | 38.5 ± 2.1 |
| Birth weight (kg) | 3.19 ± 0.56 |
| Birth length (cm) | 50.4 ± 3.8 |
| Bronchiolitis severity | |
| Lowest SaO2 (n = 191 [%]) | 91.6 ± 7.3 |
| Length of stay (n = 199 [d]) | 2.5 ± 2.5 |
| Presence of hyperexpansion on CXR (n = 131 [%]) | 63.4 |
| Atopic features | |
| IgE level (n = 186 [IU/mL]) | 22.1 ± 42.1 |
| Eosinophils (n = 152 [%]) | 1.8 ± 2.5 |
| Family history | |
| Maternal history of asthma (%) | 18.5 |
| Maternal history of eczema (%) | 6.3 |
| Maternal history of allergic rhinitis (%) | 20.4 |
| Home environment | |
| Dog in home (%) | 42.1 |
| Cat in home (%) | 26.7 |
| Rodent in home (%) | 6.3 |
| Cockroaches in home (%) | 7.8 |
| Home allergen levels (n = 130) | |
| Fel d 1 >8000 ng/g (%) | 25.8 |
| Can f 1 >8000 ng/g (%) | 29.3 |
| Bla g 1 >1 U/g (%) | 9.5 |
| Der p 1 and Der f 1 >2000 ng/g (%) | 22.4 |
Values are presented as means ± SDs.
CXR, Chest radiograph; SaO2, lowest oxygen saturation measured during index hospitalization.
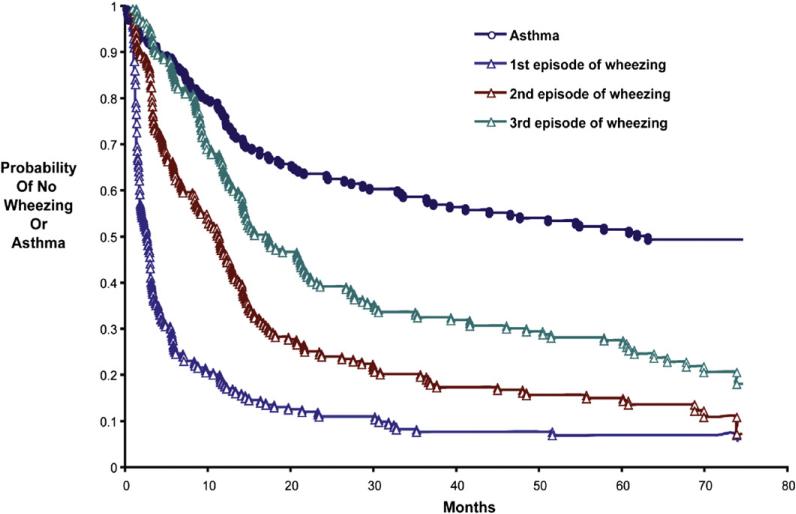
Recurrent wheezing after severe RSV bronchiolitis was common —92% of participants experienced 1 or more additional wheezing episode before 3 years of age (Fig 2), and only 27% of participants did not experience another wheezing episode after 3 years of age. Nearly half (48%) of the study participants were given a diagnosis of asthma by a physician before the seventh birthday, and 35% were classified as having active asthma before the seventh birthday. Compared with children without asthma, children with physician-diagnosed asthma had similar measures of lung function (prebronchodilator FEV1 percent predicted and FEV1/forced vital capacity [FVC] ratio) and bronchodilator response at age 6 years (Table I). Although the prevalence and degree of airways hyperresponsiveness (defined as PC20 ≤3.2 mg/mL) were high in the population as a whole (n = 109 [84%]; mean PC20, 1.80 ± 2.39 mg/mL), the prevalence of airways hyper-responsiveness did not differ between those with and without physician-diagnosed asthma (87.5% vs 81.5%, P =.35), and children with physician-diagnosed asthma demonstrated statistically greater mean airway hyperresponsiveness than those without physician-diagnosed asthma (PC20, 1.42 ± 1.86 vs 2.19 ± 2.78 mg/mL; P =.016). There were no significant differences between participants with or without active asthma in terms of lung function or airway hyperresponsiveness by the seventh birthday (Table I).
FIG 2.
Survival curves for time to development of the first 3 wheezing illnesses and physician's diagnosis of asthma subsequent to the index RSV bronchiolitis episode. The vertical axis represents the probability of not wheezing (for times to first, second, and third wheezing episodes) or not having physician-diagnosed asthma (blue circles). Blue triangles represent the first subsequent parent-reported wheezing episode, brown triangles represent the second subsequent parent-reported wheezing episode, green triangles represent the third subsequent parent-reported wheezing episode, and blue circles represent a physician's diagnosis of asthma.
TABLE I.
Characteristics by outcome group
| Physician-diagnosed asthma by seventh birthday (n = 97) | No physician-diagnosed asthma by seventh birthday (n = 104) | P value | Active asthma by seventh birthday (n = 62) | No active asthma by seventh birthday (n = 117) | P value | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age at RSV (study entry [d]) | 123 ± 90 | 147 ± 107 | .091 | 95 ± 14 | 150 ± 105 | .059 |
| Male sex (%) | 52.6 (51) | 63.5 (66) | .118 | 51.6 (32) | 63.3 (74) | .132 |
| White race (%) | 44.3 (43) | 60.6 (63) | .021 | 37.1 (23) | 63.3 (74) | .001 |
| Hospitalization data | ||||||
| Length of stay in hospital (d) | 2.60 ± 2.46 | 2.32 ± 2.57 | .207 | 2.60 ± 2.34 | 2.35 ± 2.55 | .196 |
| Lowest SaO2 (%) | 92 ± 6 | 91 ± 8 | .271 | 92 ± 6 | 92 ± 7 | .688 |
| Family history | ||||||
| Maternal history of asthma (%) | 28.9 (28) | 8.7 (9) | <.001 | 35.5 (22) | 12.0 (14) | <.001 |
| Maternal history of eczema (%) | 12.4 (12) | 12.5 (13) | .978 | 9.7 (6) | 15.4 (18) | .286 |
| Maternal history of allergic rhinitis (%) | 23.7 (23) | 17.3 (18) | .260 | 30.7 (19) | 18.0 (21) | .052 |
| Clinical history and other potential exposures | ||||||
| Smoking in home during first year of life (%) | 51.0 (49) | 55.5 (56) | .536 | 52.5 (32) | 53.5 (61) | .895 |
| Dog in home (%) | 42.3 (41) | 42.0 (42) | .970 | 38.7 (24) | 46.0 (52) | .351 |
| Any positive skin test response to aeroallergen at 3 y (%) | 44.0 (33) | 18.7 (14) | .001 | 46.3 (25) | 22.9 (22) | .003 |
| Laboratory tests at baseline | ||||||
| Baseline IgE (IU/mL) | 25.2 ± 52.1 | 19.6 ± 30.8 | .917 | 30.5 ± 60.5 | 19.2 ± 32.3 | .306 |
| Baseline eosinophils (%) | 1.75 ± 2.40 | 1.87 ± 2.65 | .587 | 2.03 ± 2.71 | 1.83 ± 2.60 | .944 |
| Nasal CCL5 (ratio) at enrollment (n = 81) | 5.34 ± 1.63 (n = 47) | 3.14 ± 1.05 (n = 34) | <.001 | 5.28 ± 1.87 (n = 29) | 3.89 ± 1.55 (n = 49) | <.001 |
| Lung function at age 6 y | ||||||
| FEV1 (% predicted, before BD) | 98 ± 13 | 100 ± 15 | .439 | 98 ± 13 | 100 ± 15 | .444 |
| FEV1/FVC ratio (before BD) | 0.91 ± 0.09 | 0.89 ± 0.11 | .213 | 0.91 ± 0.09 | 0.90 ± 1.11 | .451 |
| Change in FEV1 after BD (%) | 6.2 ± 15.2 | 4.2 ± 10.3 | .857 | 6.8 ± 16.4 | 4.2 ± 10.5 | .890 |
| Methacholine PC20 (mg/mL) | 1.42 ± 1.86 | 2.19 ± 2.78 | .016 | 1.52 ± 2.02 | 1.95 ± 2.56 | .181 |
Data are expressed as means ± SDs, except as noted.
BD, Bronchodilator; FVC, forced vital capacity; SaO2, lowest oxygen saturation measured during index hospitalization.
Factors related to physician-diagnosed asthma by the seventh birthday
Univariate analyses
We examined the factors associated with a physician's diagnosis of asthma by the seventh birthday based on data available at 2 time points: baseline (enrollment) and age 3 years (Table II). By using unadjusted data obtained at baseline, factors significantly associated with physician-diagnosed asthma included white race (OR, 0.5; P = .022), longer birth length (OR, 0.91; P = .025), day care attendance before study enrollment (OR, 0.50; P = .042), maternal asthma (OR, 4.28; P < .001), and exposure to high levels of dog allergen in the home (OR, 2.05; P = .035). Among this cohort of children who required hospital care for bronchiolitis, neither factors reflective of severity of the initial bronchiolitis episode (lowest oxygen saturation, length of hospital stay, presence of hyperexpansion, or opacity on chest radiography) nor baseline laboratory data (peripheral blood eosinophil numbers or total IgE levels) were significantly associated with physician-diagnosed asthma. The nasal epithelial CCL5 ratio at baseline (in a subset of 81 participants) was significantly associated with physician-diagnosed asthma (OR, 3.81 per unit increase in CCL5 ratio; P < .001). In addition, at age 3 years, the occurrence of repeated wheezing episodes (≥3 episodes; OR, 9.14; P = .005) and evidence of sensitization to 1 or more aeroallergens (OR, 5.81; P = .004) were each significantly associated with physician-diagnosed asthma.
TABLE II.
Characteristics at entry and age 3 years associated with physician-diagnosed asthma after severe RSV bronchiolitis
| Baseline (n = 201) |
Age 3 y (n = 104) |
|||
|---|---|---|---|---|
| OR for physician-diagnosed asthma (95% CI) | P value | OR for physician-diagnosed asthma (95% CI) | P value | |
| Demographics | ||||
| Age at study entry (d) | 0.998 (0.995-1) | .092 | 1.0 (0.995-1.01) | .920 |
| Male sex | 0.64 (0.36-1.12) | .119 | 0.49 (0.16-1.48) | .207 |
| White race | 0.52 (0.30-0.91) | .022 | 0.28 (0.09-0.89) | .031 |
| Annual household income <$20,000 | 1.80 (1.00-3.26) | .051 | 1.09 (0.31-3.89) | .891 |
| Personal history | ||||
| Duration of pregnancy (wk) | 0.96 (0.84-1.11) | .597 | 0.95 (0.87-1.04) | .597 |
| Birth weight (g) | 1.0 (0.99-1.0) | .265 | 1.0 (0.999-1.002) | .511 |
| Birth length (cm) | 0.91 (0.83-0.99) | .025 | 1.04 (0.83-1.29) | .764 |
| Family history | ||||
| Maternal asthma | 4.28 (1.90-9.65) | <.001 | 3.95 (1.12-13.91) | .033 |
| Maternal eczema | 0.91 (0.30-2.82) | .875 | 1.38 (0.45-4.25) | .575 |
| Maternal hay fever | 1.49 (0.74-2.96) | .262 | 1.06 (0.27-4.17) | .935 |
| Exposure to allergens | ||||
| Exposure to ≥1 allergen at high levels | 1.51 (0.81-2.81) | .200 | 1.35 (0.38-4.84) | .648 |
| Fel d 1 >8000 ng/g | 0.81 (0.41-1.61) | .551 | 0.81 (0.20-3.24) | .761 |
| Can f 1 >8000 ng/g | 2.05 (1.05-3.98) | .035 | 3.32 (0.96-11.49) | .059 |
| Bla g 1 >1 U/g | 1.43 (0.51-4.03) | .501 | 1.14 (0.13-10.35) | .910 |
| Der f 1 >2000 ng/g | 1.81 (0.85-3.85) | .123 | 1.06 (0.21-5.42) | .943 |
| Der p 1 >2000 ng/g | 0.70 (0.19-2.57) | .591 | NA for mathematical reasons | |
| Exposure to other potential respiratory irritants | ||||
| Maternal smoking during pregnancy | 0.57 (0.30-1.10) | .096 | 0.37 (0.08-1.77) | .214 |
| Smoking in home at each time point | 1.17 (0.64-2.12) | .617 | 0.85 (0.28-2.55) | .774 |
| Day care at each time point | 0.50 (0.26-0.98) | .042 | 0.24 (0.07-0.77) | .017 |
| Breast-feeding | 0.67 (0.38-1.17) | .158 | 0.78 (0.26-2.33) | .213 |
| Clinical history | ||||
| Wheezing (≥3 reported episodes before age 3 y) | NA | 9.14 (1.94-42.92) | .005 | |
| Physician-diagnosed eczema before age 3 y | 1.38 (0.45-4.25) | .575 | ||
| Sensitivity to allergens at age 3 y | ||||
| Positive response to ≥1 any allergen | NA | 5.81 (1.74-19.43) | .004 | |
| Positive response to ≥1 food allergen | 3.94 (0.60-26.13) | .155 | ||
| Positive response to ≥1 environmental allergen | 5.81 (1.74-19.43) | .004 | ||
| Laboratory tests at baseline | ||||
| IgE | 1.03 (0.74-1.41) | .881 | 1.21 (0.68-2.15) | .517 |
| Blood eosinophils (%) | 0.98 (0.86-1.12) | .778 | 0.94 (0.69-1.27) | .670 |
| CCL5 | 3.81 (2.07-7.01) | <.001 | 6.52 (1.67-25.5) | .007 |
NA, Not applicable.
Multivariate analyses
Two separate multivariate models were developed incorporating patient characteristics available at the time of enrollment (baseline) and age 3 years (Table III). These models demonstrated the following factors to be significantly associated with physician-diagnosed asthma with adjustment for the other factors: white race (baseline and 3-year models), longer birth length (baseline model), and day care attendance (3 year model) were associated with decreased risks of physician-diagnosed asthma, whereas maternal asthma (baseline model), allergic sensitization (3-year model), recurrent wheeze (3-year model), and exposure to high levels of dog allergen (baseline model) were associated with increased risks. In separate models incorporating nasal epithelial CCL5 levels at baseline, higher CCL5 ratios (OR of 3.81 [P < .001] in the baseline model and OR of 6.52 [P =.028] in the 3-year model) were significantly associated with physician-diagnosed asthma by the seventh birthday. In addition, the CCL5 ratio from samples obtained 1 month after RSV bronchiolitis was also significantly associated with physician-diagnosed asthma (OR, 1.89; 95% CI, 1.04-3.4; P = .038).
TABLE III.
Multivariate model for factors associated with physician-diagnosed and active asthma by the seventh birthday after severe RSV bronchiolitis
| Baseline data |
Age 3 y data |
||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | P value | Variables | OR (95% CI) | P value |
| Physician-diagnosed asthma | |||||
| Without CCL5 included | |||||
| n = 201 | n = 104 | ||||
| White race | 0.25 (0.11-0.57) | .001 | White race | 0.19 (0.04-0.93) | .041 |
| Longer birth length per cm | 0.86 (0.77-0.97) | .013 | Day care by age 3 y | 0.18 (0.04-0.84) | .029 |
| History of maternal asthma at entry | 5.21 (1.71-15.9) | .004 | Positive response to ≥1 environmental allergen | 10.7 (2.08-55.1) | .005 |
| Exposure to levels of dog allergen >8000 ng/g dust | 3.15 (1.29-7.68) | .012 | Wheezing (≥3 episodes before age 3 y) | 7.31 (1.24-43.3) | .028 |
| With CCL5 included | |||||
| n = 81 | n = 40 | ||||
| CCL5 | 3.81 (2.07-7.01) | <.001 | CCL5 | 6.52 (1.67-25.5) | .007 |
| Active asthma | |||||
| Without CCL5 included | |||||
| n = 179 | n = 104 | ||||
| White race | 0.39 (0.20-0.76) | .005 | White race | 0.15 (0.03-0.76) | .022 |
| History of maternal asthma at entry | 3.49 (1.59-7.65) | .002 | Day care by age 3 y | 0.14 (0.03-0.70) | .017 |
| Positive response to ≥1 environmental allergen | 9.0 (1.66-48.8) | .046 | |||
| Wheezing (≥3 episodes before age 3 y) | 6.12 (1.03-36.3) | .011 | |||
| With CCL5 included | |||||
| n = 78 | n = 40 | ||||
| CCL5 | 1.67 (1.19-2.36) | .003 | CCL5 | 15.1 (1.51-152) | .021 |
| White race | 0.24 (0.08-0.71) | .010 | |||
Factors related to active asthma by the seventh birthday
Univariate analyses
To increase the stringency of the asthma outcome diagnosis, we also examined factors associated with active asthma by the seventh birthday (Table IV). Characteristics available at enrollment that were significantly associated with active asthma before the seventh birthday in univariate unadjusted analyses included maternal asthma (OR, 4.0; P < .001), a high level of cockroach allergen (Bla g 1) in the home (OR, 3.2; P =.037), day care attendance (OR, 0.5; P =.047), and white race (OR, 0.3; P < .001). Neither the severity of the initial infection nor baseline laboratory data were significantly associated with development of active asthma.
TABLE IV.
Characteristics at baseline and age 3 years associated with active asthma after severe RSV bronchiolitis
| Baseline (n = 179) |
Age 3 y (n = 104) |
|||
|---|---|---|---|---|
| OR for active asthma (95% CI) | P value | OR for active asthma (95% CI) | P value | |
| Demographics | ||||
| Age at study entry (d) | 0.997 (0.994-1) | .061 | 1.0 (0.995-1.01) | .940 |
| Male sex | 0.62 (0.33-1.16) | .133 | 0.58 (0.19-1.80) | .344 |
| White race | 0.34 (0.18-0.65) | <.001 | 0.22 (0.06-0.76) | .017 |
| Annual household income <$20,000 | 2.02 (1.05-3.87) | .035 | 1.25 (0.34-4.54) | .735 |
| Personal history | ||||
| Duration of pregnancy (wk) | 1.07 (0.90-1.27) | .439 | 1.09 (0.80-1.48) | .580 |
| Birth weight (g) | 1.00 (0.99-1.00) | .739 | 1.0 (0.999-1.02) | .398 |
| Birth length (cm) | 0.94 (0.85-1.03) | .184 | 1.08 (0.86-1.37) | .505 |
| Family history | ||||
| Maternal asthma | 4.05 (1.89-8.68) | <.001 | 4.45 (1.24-15.9) | .022 |
| Maternal eczema | 0.59 (0.22-1.57) | .291 | 0.79 (0.09-6.83) | .829 |
| Maternal hay fever | 2.02 (0.99-4.14) | .055 | 1.17 (0.29-4.66) | .823 |
| Exposure to allergens | ||||
| Exposure to ≥1 allergen at high levels | 1.16 (0.31-4.26) | .716 | 1.16 (0.31-4.26) | .829 |
| Fel d 1 >8000 ng/g | 0.52 (0.24-1.10) | .100 | 0.52 (0.10-2.56) | .418 |
| Can f 1 >8000 ng/g | 1.19 (0.60-2.39) | .616 | 2.63 (0.72-9.53) | .142 |
| Bla g 1 >1 U/g | 3.20 (1.07-9.51) | .037 | 1.27 (0.14-11.6) | .834 |
| Der f 1 >2000 ng/g | 1.62 (0.75-3.50) | .222 | 1.20 (0.23-6.18) | .830 |
| Der p 1 >2000 ng/g | 0.19 (0.02-1.54) | .120 | NA for mathematical reasons | |
| Exposure to other potential respiratory irritants | ||||
| Maternal smoking during pregnancy | 0.55 (0.26-1.18) | .125 | 0.41 (0.09-1.96) | .264 |
| Smoking in home at each time point | 1.15 (0.59-2.25) | .680 | 0.86 (0.23-3.19) | .818 |
| Day care at each time point | 0.47 (0.22-0.99) | .047 | 0.20 (0.06-0.67) | .009 |
| Breast-feeding | 0.58 (0.31-1.09) | .091 | 0.67 (0.22-2.06) | .482 |
| Clinical history | ||||
| Wheezing (≥3 reported episodes before age 3 y) | NA | 8.21 (1.74-38.8) | .008 | |
| Physician-diagnosed eczema before age 3 y | 1.11 (0.34-3.61) | .861 | ||
| Sensitivity to allergens at age 3 y | ||||
| Positive response to ≥1 any allergen | NA | 4.75 (1.39-16.2) | .013 | |
| Positive response to ≥1 food allergen | 4.36 (0.65-29.1) | .128 | ||
| Positive response to ≥1 environmental allergen | 4.75 (1.39-16.2) | .013 | ||
| Laboratory tests at baseline | ||||
| IgE | 1.21 (0.86-1.70) | .281 | 0.94 (0.48-1.82) | .843 |
| Blood eosinophils (%) | 1.03 (0.90-1.18) | .678 | 0.95 (0.71-1.29) | .756 |
| CCL5 | 1.66 (1.21-2.28) | .002 | 15.1 (1.51-152) | .021 |
NA, Not applicable.
Multivariate analyses
Two separate multivariate models were developed incorporating patients’ characteristics available at the time of enrollment (baseline) and age 3 years (Table III). These models demonstrated the following factors to be significantly associated with active asthma with adjustment for the other factors: white race (baseline and 3-year models) and day care attendance (3-year model) were associated with decreased risks of active asthma, whereas maternal asthma (baseline model), allergic sensitization (3-year model), and recurrent wheeze (3-year model) were associated with increased risks. In separate models incorporating nasal epithelial CCL5 levels at baseline, higher CCL5 ratios (OR of 1.67 [P = .003] in the baseline model and OR of 15.1 [P = .021] in the 3-year model) were significantly associated with active asthma by the seventh birthday. In contrast, CCL5 ratios from samples obtained 1 month after RSV bronchiolitis were not significantly associated with active asthma (OR, 1.29; 95% CI, 0.71-2.34; P = .4).
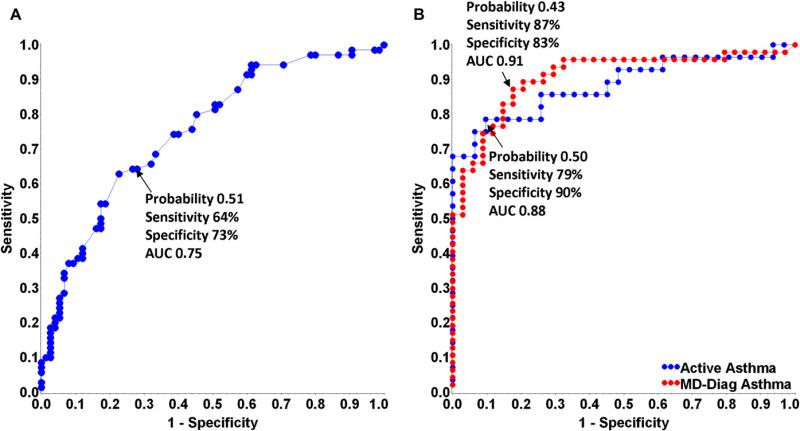
In an effort to develop a post-RSV Asthma Predictive Index with the best overall sensitivity and specificity for predicting asthma outcomes, we analyzed potential predictors using ROC curves. The model that incorporated the clinically derived predictors that were significant in the univariate analyses (race, birth length, maternal asthma history, and exposure to high levels of dog allergen) provided the highest combination of sensitivity (64%) and specificity (73%) for physician-diagnosed asthma (ROC area under the curve [AUC], 0.75; Fig 3, A). However, the model that provided the overall highest combination of sensitivity and specificity for predicting asthma status by the seventh birthday included only the CCL5 ratio at enrollment (physician-diagnosed asthma: sensitivity, 87%; specificity, 84%, ROC AUC, 0.90; positive predictive value [PPV], 89%; negative predictive value, 82%; active asthma: sensitivity, 79%; specificity, 90%; ROC AUC, 0.88; PPV, 88%; negative predictive value, 82%; Fig 3, B).
FIG 3.
ROC curves for a post-RSV Asthma Predictive Index for physician-diagnosed asthma and active asthma. A, The model for physician-diagnosed asthma, incorporating the clinically derived predictors that were significant in the univariate analyses. B, The model for physician-diagnosed asthma (red) and active asthma (blue) incorporating nasal CCL5 levels alone at enrollment.
DISCUSSION
We have demonstrated that severe RSV bronchiolitis in the first year of life is followed by a diagnosis of childhood asthma by the seventh birthday in nearly half of children. Factors assessed around the time of bronchiolitis that were associated with increased risk of physician-diagnosed asthma in our cohort included maternal asthma, exposure to high levels of dog allergen, and increased upper airway expression of CCL5, whereas white race and longer birth length decreased the risk. The subsequent occurrence of repeated wheezing episodes, aeroallergen sensitization by age 3 years, or both was associated with an increased risk of physician-diagnosed asthma, whereas day care attendance by age 3 years was associated with a significantly lower risk of physician-diagnosed asthma. Similar characteristics were associated with increased risk for both active asthma and physician-diagnosed asthma (maternal asthma, nasal CCL5 ratio, and nonwhite race at baseline and repeated wheezing episodes and aeroallergen sensitization by age 3 years), whereas day care attendance by age 3 years was protective for physician-diagnosed and active asthma.
Prior prospective studies5,7,9 of children after severe RSV bronchiolitis have had lengthy intervals between follow-up assessments and have been unable to evaluate within-group differences because of generally small sample sizes. Our prospective cohort of 206 children enrolled during an initial episode of severe RSV bronchiolitis during infancy is the largest such cohort reported to date, affording us the ability to examine prospectively the interrelationships of multiple demographic, familial, environmental, and biological characteristics associated with asthma development after severe RSV bronchiolitis.
Our finding of a high prevalence of asthma diagnosis (48% by age 7 years) after RSV bronchiolitis is consistent with previous studies,3,5,10 although the proportion of RBEL children with physician-diagnosed asthma is higher than previously described in children of similar age and history of severe RSV infection.3,5,18,19 Our prospective data collection with frequent interviews might have increased the identification of children with a reported asthma diagnosis. Additionally, a substantial proportion of the RBEL cohort exhibited known risk factors for asthma, including African American race,20,21 low socioeconomic status,21 and family history of asthma and other atopic diseases,22-24 although some of these factors were less common in earlier study populations.
We found that participants without physician-diagnosed asthma exhibited a heightened degree of airway hyperresponsiveness approximately 6 years after severe RSV bronchiolitis, with mean PC20 to methacholine approximating 2 mg/mL. These findings are consistent with a previous study showing that 62% of children at 8 to 9 years of age after hospitalization for bronchiolitis during the first 2 years of life exhibited airway hyperresponsiveness (defined as a 20% decrease in peak expiratory flow with <4.9 mg/mL methacholine).25 In contrast, an earlier study of mild bronchiolitis demonstrated no evidence of heightened airway hyperresponsiveness 8 to 12 years later.26 Therefore it appears that severe bronchiolitis is associated with subsequent airway hyperresponsiveness but not necessarily related to the diagnosis of asthma. The long-term consequences of this finding might be the development of asthma in additional subjects because airway hyperresponsiveness at age 6 years has been demonstrated to be a risk factor of newly diagnosed asthma in early adulthood.27
Many children in the RBEL cohort had a family history of asthma and other atopic diseases, and a maternal history of asthma at the time of RSV bronchiolitis was associated with persistence in the study, as well as a significantly increased risk of physician-diagnosed asthma, which is consistent with previous studies.23,24,28 Mothers with asthma could have greater awareness of asthma symptoms, leading to earlier diagnosis of asthma in their children. This effect is specific to maternal asthma because maternal atopy (other than asthma) was not significantly associated with study retention or asthma diagnosis.
Longer birth length was associated with a lower rate of asthma, potentially related to larger airway caliber at birth, and is consistent with recent studies demonstrating a lower risk of persistent wheezing among children with longer length at birth29 and lower risk of asthma at age 10 years among children with longer first-trimester fetal size.30
Aeroallergen sensitization at age 3 years was associated with the diagnosis of asthma after severe RSV bronchiolitis, which is consistent with the findings of 2 other prospective cohort studies.31,32 Previous research regarding environmental allergens and asthma has found no consistent relationship between pet or pet allergen exposure and later asthma, with some studies demonstrating that early dog ownership might be protective against subsequent recurrent wheezing32,33 and allergic sensitization but not against asthma by age 6 to 734 and 1835 years. Our finding of dog allergen exposure augmenting the risk of physician-diagnosed asthma might be due to exposure to allergen in the setting of lower airway injury associated with severe RSV bronchiolitis, suggesting that there might be an interaction between RSV infection and dog allergen exposure. We could not determine whether the effect of dog exposure was mediated through an increase in allergic sensitization to dog because only 5 subjects had evidence of allergic sensitization to dog at age 3 years, resulting in very low statistical power. However, other studies have reported the absence of a relationship between dog exposure in the home during infancy and allergic sensitization to dog during the first 6 years of life.33,34,36,37 Alternatively, our differing results might be due to our definition of dog exposure (increased allergen level in household dust), whereas others have used the presence of a pet in the home to define exposure. However, this intriguing link between exposure to allergen during a time of immune system development and later respiratory disease deserves further attention.
We observed that day care attendance by 3 years of age was associated with significantly reduced likelihood of physician-diagnosed asthma and active asthma, which is consistent with previous studies,38,39 although others have demonstrated increased airway symptoms in early life but no difference in asthma at age 8 years.40 This observation is supportive of the hygiene hypothesis, which argues that repeated exposure to infections, as in day care institutions, might promote TH1 immune response early in life that counterbalances a TH2 propensity, leading to the development of atopic disease.41
Repeated wheezing (≥3 episodes before age 3 years) was significantly associated with both asthma diagnosis and active asthma in our cohort. Asthma is a clinical diagnosis usually ascribed after repeated wheezing episodes, and we observed this to be the case because the asthma diagnosis lagged behind the first several wheezing episodes (Fig 2). In addition, after severe RSV bronchiolitis, these children continue to experience recurrent respiratory symptoms because two thirds have had at least 3 wheezing episodes by the end of their second year of follow-up.
Airway inflammation, as reflected by CCL5 mRNA expression in upper airway epithelial cells from children during RSV bronchiolitis, was strongly predictive for the development of physician-diagnosed asthma and active asthma, whereas CCL5 expression 1 month after resolution of the bronchiolitis episode was only associated with physician-diagnosed asthma but not active asthma. The expression of CCL5 during RSV bronchiolitis is likely genetically determined, and thus it is possible that a predisposition to an exuberant CCL5 response to RSV results in acute RSV bronchiolitis of greater severity acutely and with risk of symptomatology throughout early life. A case-control study provided evidence supporting a hereditary basis for susceptibility to severe lower respiratory tract infection,42,43 whereas Janssen et al44 reported genes related to innate immunity (vitamin D receptor, JUN, NOS2A, and IFNA5) that were strongly associated with bronchiolitis. Whether genetic variations explain the increased risk of asthma in those children who expressed the highest levels of CCL5 after severe RSV bronchiolitis is being evaluated. We have previously reported that peripheral blood T-cell production of the cytokines IL-4, IFN-γ, and IL-2 at study entry and at ages 2, 4, and 6 years did not differ between those children who had physician-diagnosed asthma and those without asthma, whereas those with physician-diagnosed asthma did demonstrate lower IL-13 expression at 6 years.45 These findings, along with the current finding of nasal epithelial CCL5 expression, confirm the complex interplay of immunologic pathways that contribute to post-RSV asthma.
In an effort to provide a clinically useful tool for predicting asthma after severe RSV bronchiolitis, we developed an index using both clinical measures and the biomarker nasal epithelial CCL5 ratio. Maternal history of asthma alone was poorly predictive of physician-diagnosed asthma (ROC AUC, 0.55). The models developed include factors that have not been previously reported (epithelial CCL5) and are unexpected (dog allergen). The model incorporating the biomarker of nasal epithelial CCL5 during the acute bronchiolitis episode alone (Fig 3, B) exhibited very strong performance characteristics for predicting physician-diagnosed asthma (sensitivity, 87%; specificity, 84%; and PPV, 89%) compared with the model incorporating clinical features alone (Fig 3, A). The addition of clinical features (including maternal asthma) to the CCL5 level–containing model did not improve the performance of the model (ROC AUC of 0.9 for models of CCL5 with or without maternal asthma). The PPV of 89% approaches a potentially useful clinical model for pediatricians to use for children hospitalized with RSV bronchiolitis. These findings suggest that a single biomarker, nasal epithelial CCL5 at the time of acute bronchiolitis, is a more robust predictor of physician-diagnosed asthma and active asthma after severe RSV bronchiolitis than other often-cited risk factors for childhood asthma. Prospective validation of this model is necessary, as is exploration of other biomarkers that have greater availability to practicing physicians.
A limitation that could affect the interpretation of our findings includes the absence of a control group of infants without severe RSV infection, making us unable to firmly conclude whether severe RSV bronchiolitis causes asthma or whether RSV bronchiolitis is seen more frequently in children at high risk of asthma later in life. This is a prospective cohort study following subjects with a common initial exposure (severe RSV bronchiolitis) and assessing for the development of the primary outcome (asthma and related phenotypes). Because nearly all children are infected with RSV by age 2 years, establishing a control group of unexposed children is not practical. A case-control study has been previously done.3,7-9,46 Furthermore, a prospective cohort study is a more robust epidemiologic method to establish causation of disease and is not subject to many of the limitations of case-control studies. The directionality of the relationship between RSV infection and asthma has been questioned for many years, and a recent study of twins concluded that severe RSV infection that leads to hospitalization does not cause asthma but serves as an indicator of genetic predisposition to asthma,47 athough the definitions of RSV infection and asthma in this study were less stringent than in the RBEL cohort. The major focus of this research is to determine the significant factors for the development of asthma once severe RSV bronchiolitis has occurred; thus we cannot determine whether these findings are specific to asthma after RSV infection or represent risk factors for asthma in general. Although the fact that the children were hospitalized for bronchiolitis might indeed indicate a predisposition to recurrent wheezing and subsequent asthma, we were careful to exclude children with clinically relevant prebronchiolitis respiratory tract morbidity. However, it is possible that a greater (presumably genetic) susceptibility to severe bronchiolitis, including CCL5 response to severe RSV infection, contributed to the episode's severity. Additionally, we relied on parental report followed by medical record confirmation of physician-diagnosed asthma, which might have introduced bias into our results because parental report of diagnosed asthma is only moderately concordant with a physician's diagnosis obtained from records.48,49 Asthma is a clinical diagnosis, and physicians’ criteria to diagnose pediatric asthma varies by ethnicity,50,51 with African American children with recent wheezing being more likely to receive a diagnosis of asthma by a physician than white children.52 This could account for some of the lower rates of physician-diagnosed asthma and active asthma seen in white children in the RBEL cohort.
Our results demonstrate that children who experience severe RSV bronchiolitis in infancy are at significantly increased risk for asthma during the first 6 years of life and that symptoms generally persist as children grow toward school age. Aeroallergen sensitization, repeated wheeze, and maternal asthma were strongly associated with the development and persistence of asthma in our cohort, whereas white race and day care attendance were associated with decreased likelihood of asthma. The presence of increased CCL5 expression in the nasal epithelia during bronchiolitis is strongly predictive of asthma at school age, with much greater sensitivity and specificity than clinical features, including maternal asthma. Physicians are encouraged to closely monitor children after severe RSV bronchiolitis, to provide additional guidance to children and families with additional risk factors (genetic and environmental) for asthma, and to test at a young age for sensitization to common allergens. Additional research is necessary to evaluate whether these factors remain significant as these children grow older because our data and those of several previous studies3,6,7,9 have shown that characteristics associated with persistence of asthma after RSV bronchiolitis vary with the child's age.
METHODS
Allergy evaluation
We performed percutaneous allergy skin tests during the third year of follow-up (n = 150; mean age, 3.6 ± 0.7 years of age) using the MultiTest device. Children were instructed to avoid antihistamine medications for 72 hours before skin testing was performed. Allergens tested included tree mix, grass mix, weed mix, mold mix, dust mite mix, cockroach, cat and dog, and 3 common food allergens (egg, milk, and peanut; Greer Laboratories, Lenoir, NC). We evaluated for allergic sensitization in 4 children who were unable to undergo skin testing with ImmunoCAP (Phadia, Uppsala, Sweden). An allergen-specific IgE level of greater than 0.35 kU/L was considered a positive response.
Environmental assessment
We examined levels of 5 allergens: cat (Fel d 1), dog (Can f 1), house dust mite (Der p 1 and Der f 1), and cockroach (Bla g 1). If the child moved in the first year, a repeat dust sample was collected, and average allergen levels were calculated. High levels of measured allergen were defined as a Fel d 1 level of greater than 8000 ng/g of dust, a Can f 1 level of greater than 8000 ng/g of dust, a Bla g 1 level of greater than 1 U/g of dust, and a Der f 1 or Der p 1 level of greater than 2000 ng/g of dust.E1
Procurement of upper airway samples
Eighty-one subjects consented to provide nasal epithelial cell samples at entry and 1 month. A cytobrush was used to obtain a sample from below the middle turbinate by using direct visualization. The brush was then agitated to remove cells in 10% formalin for permanent fixation. The cells were then washed, and cytospin preparations performed. The formalin-fixed slides were dehydrated and fixed in 70% ethanol. The identity of epithelial cells from the brushings was confirmed by means of immunohistochemical staining with cytokeratin antibody (R&D Systems, Minneapolis, Minn).
Measurement of CCL5 from upper airway epithelial cells
CCL5 riboprobe was synthesized by using a 0.45-kb human cDNA fragment (nt 1-410, ATCC) positionally cloned into the EcoRI and HindIII sites of pBluescript (Promega, Madison, Wis). Radiolabeled 35S-UTP sense and anti-sense cRNA transcripts were in vitro transcribed by T3 and T7 RNA polymerases, respectively, with the Gemini Riboprobe system (Promega). Riboprobes were then subsequently precipitated with ethanol/acetate, washed, and counted, and an average of 3.0 × 106 cpm/μL was generated for each probe. In situ hybridization was performed by incubating fixed cytospin slides with 50 μL of hybridization solution containing 35S-labeled CCL5/pBluescript riboprobe (2 × 104 cpm/μL) at 60°C for 18 hours in a humidified chamber. Slides were subsequently washed and processed for quantitation.
Quantitation of in situ RNA signal
Slides were exposed to phosphor screens for 1 week and subsequently read on a Storm PhosphorImager system (Molecular Dynamics, Sunnyvale, Calif). ImageQuant software was used to measure 35S-labeled anti-sense and sense RNA signal from each slide. A CCL5 ratio was calculated by subtracting sense RNA from antisense RNA 35S signal and correcting for the total number of cells as follows:
RESULTS
Details of inclusion of data from subjects who did not contribute data at the seventh birthday
Two hundred six subjects were enrolled in the RBEL cohort. Five subjects did not contribute any data after enrollment, and these subjects were removed from all analyses. If asthma was diagnosed at any time, the participant was considered to have physician-diagnosed asthma (n = 16). If asthma was not diagnosed before the participant was lost to follow-up, the participant was defined as nonasthmatic (n = 26). For active asthma (n = 62), only participants with data after age 3 years were included. For the analyses using predictors obtained at age 3 years, only participants with follow-up data after age 3 years were included.
Key messages.
Nearly 50% of children have physician-diagnosed asthma 6 years after severe RSV bronchiolitis.
Asthma after severe RSV bronchiolitis is positively associated with maternal asthma, exposure to high levels of dog allergen, aeroallergen sensitization, and recurrent wheezing, whereas day care attendance and white race are associated with decreased asthma risk.
The biomarker CCL5 in nasal epithelium during RSV bronchiolitis is strongly predictive of physician-diagnosed asthma.
Acknowledgments
We thank Lisa Robertson, RN, Lynette Tegtmeier, RN, Amy Rahm, RN, Michelle Jenkersen, RN, RRT, JoAnn Bonfiglio, RN, MSN, and Gayla Gregory, RN, for their assistance in recruiting children and data collection for the RBEL study. We also thank the children and their families who graciously provided their time and effort to participate in the RBEL study.
Supported by National Institutes of Health grant HL 61895.
Abbreviations used
- AUC
Area under the curve
- OR
Odds ratio
- PPV
Positive predictive value
- RBEL
RSV Bronchiolitis in Early Life
- ROC
Receiver operating characteristic
- RSV
Respiratory syncytial virus
Footnotes
Disclosure of potential conflict of interest: L. B. Bacharier is on the advisory board for AstraZeneca, has received honoraria from GlaxoSmithKline, and is on the advisory board and has received honoraria from Merck. M. Castro is a consultant and speaker for Asthmatx; is on the advisory board and is a speaker for Genentech; is a speaker for AstraZeneca, Merck, and GlaxoSmithKline; has received research support from Asthmatx, Amgen, Ception/Cephalon, Genentech, MedImmune, Merck, the National Institutes of Health, Novartis, and GlaxoSmithKline; and has received royalties from Elsevier. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–6. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 2.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21:629–32. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 4.Schauer U, Hoffjan S, Bittscheidt J, Kochling A, Hemmis S, Bongartz S, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. Eur Respir J. 2002;20:1277–83. doi: 10.1183/09031936.02.00019902. [DOI] [PubMed] [Google Scholar]
- 5.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–92. doi: 10.1111/j.1399-3038.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 7.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 8.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–5. [PubMed] [Google Scholar]
- 9.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 10.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–61, e1. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 12.Alam R, Stafford S, Forsythe P, Harrison R, Faubion D, Lett-Brown MA, et al. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. 1993;150:3442–8. [PubMed] [Google Scholar]
- 13.Humbert M, Ying S, Corrigan C, Menz G, Barkans J, Pfister R, et al. Bronchial mucosal expression of the genes encoding chemokines RANTES and MCP-3 in symptomatic atopic and nonatopic asthmatics: relationship to the eosinophil-active cytokines interleukin (IL)-5, granulocyte macrophage-colony-stimulating factor, and IL-3. Am J Respir Cell Mol Biol. 1997;16:1–8. doi: 10.1165/ajrcmb.16.1.8998072. [DOI] [PubMed] [Google Scholar]
- 14.Berkman N, Krishnan VL, Gilbey T, Newton R, O'Connor B, Barnes PJ, et al. Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am J Respir Crit Care Med. 1996;154:1804–11. doi: 10.1164/ajrccm.154.6.8970374. [DOI] [PubMed] [Google Scholar]
- 15.Koga S, Novick AC, Toma H, Fairchild RL. CD81 T cells produce RANTES during acute rejection of murine allogeneic skin grafts. Transplantation. 1999;67:854–64. doi: 10.1097/00007890-199903270-00012. [DOI] [PubMed] [Google Scholar]
- 16.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–75. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 18.Koponen P, Helminen M, Paassilta M, Luukkaala T, Korppi M. Preschool asthma after bronchiolitis in infancy. Eur Respir J. 2012;39:76–80. doi: 10.1183/09031936.00040211. [DOI] [PubMed] [Google Scholar]
- 19.Juntti H, Kokkonen J, Dunder T, Renko M, Niinimaki A, Uhari M. Association of an early respiratory syncytial virus infection and atopic allergy. Allergy. 2003;58:878–84. doi: 10.1034/j.1398-9995.2003.00233.x. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117:e868–77. doi: 10.1542/peds.2005-1721. [DOI] [PubMed] [Google Scholar]
- 21.Simon PA, Zeng Z, Wold CM, Haddock W, Fielding JE. Prevalence of childhood asthma and associated morbidity in Los Angeles County: impacts of race/ethnicity and income. J Asthma. 2003;40:535–43. doi: 10.1081/jas-120018788. [DOI] [PubMed] [Google Scholar]
- 22.Alford SH, Zoratti E, Peterson EL, Maliarik M, Ownby DR, Johnson CC. Parental history of atopic disease: disease pattern and risk of pediatric atopy in offspring. J Allergy Clin Immunol. 2004;114:1046–50. doi: 10.1016/j.jaci.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Bjerg A, Hedman L, Perzanowski MS, Platts-Mills T, Lundback B, Ronmark E. Family history of asthma and atopy: in-depth analyses of the impact on asthma and wheeze in 7- to 8-year-old children. Pediatrics. 2007;120:741–8. doi: 10.1542/peds.2006-3742. [DOI] [PubMed] [Google Scholar]
- 24.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176–81. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- 25.Korppi M, Kuikka L, Reijonen T, Remes K, Juntunen-Backman K, Launiala K. Bronchial asthma and hyperreactivity after early childhood bronchiolitis or pneumonia. An 8-year follow-up study. Arch Pediatr Adolesc Med. 1994;148:1079–84. doi: 10.1001/archpedi.1994.02170100077015. [DOI] [PubMed] [Google Scholar]
- 26.McConnochie KM, Mark JD, McBride JT, Hall WJ, Brooks JG, Klein SJ, et al. Normal pulmonary function measurements and airway reactivity in childhood after mild bronchiolitis. J Pediatr. 1985;107:54–8. doi: 10.1016/s0022-3476(85)80614-4. [DOI] [PubMed] [Google Scholar]
- 27.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–64. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dik N, Tate RB, Manfreda J, Anthonisen NR. Risk of physician-diagnosed asthma in the first 6 years of life. Chest. 2004;126:1147–53. doi: 10.1378/chest.126.4.1147. [DOI] [PubMed] [Google Scholar]
- 29.Jedrychowski W, Perera FP, Maugeri U, Mroz E, Flak E, Mrozek-Budzyn D, et al. Length at birth and effect of prenatal and postnatal factors on early wheezing phenotypes. Krakow epidemiologic cohort study. Int J Occup Med Environ Health. 2008;21:111–9. doi: 10.2478/v10001-008-0013-0. [DOI] [PubMed] [Google Scholar]
- 30.Turner S, Prabhu N, Danielian P, McNeill G, Craig L, Allan K, et al. First and second trimester fetal size and asthma outcomes at age ten years. Am J Respir Crit Care Med. 2011;184:407–13. doi: 10.1164/rccm.201012-2075OC. [DOI] [PubMed] [Google Scholar]
- 31.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–10. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38:1635–43. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 34.Ownby D, Johnson C, Peterson E. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 35.Wegienka G, Johnson CC, Havstad S, Ownby DR, Nicholas C, Zoratti EM. Lifetime dog and cat exposure and dog- and cat-specific sensitization at age 18 years. Clin Exp Allergy. 2011;41:979–86. doi: 10.1111/j.1365-2222.2011.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Remes ST, Castro-Rodriguez JA, Holberg CJ, Martinez FD, Wright AL. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol. 2001;108:509–15. doi: 10.1067/mai.2001.117797. [DOI] [PubMed] [Google Scholar]
- 37.Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29:611–7. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 38.Celedon JC, Litonjua AA, Ryan L, Weiss ST, Gold DR. Day care attendance, respiratory tract illnesses, wheezing, asthma, and total serum IgE level in early childhood. Arch Pediatr Adolesc Med. 2002;156:241–5. doi: 10.1001/archpedi.156.3.241. [DOI] [PubMed] [Google Scholar]
- 39.Ball TM, Castro-Rodriguez JA, Griffith KA, Holberg CJ, Martinez FD, Wright AL. Siblings, day-care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med. 2000;343:538–43. doi: 10.1056/NEJM200008243430803. [DOI] [PubMed] [Google Scholar]
- 40.Caudri D, Wijga A, Scholtens S, Kerkhof M, Gerritsen J, Ruskamp JM, et al. Early daycare is associated with an increase in airway symptoms in early childhood but is no protection against asthma or atopy at 8 years. Am J Respir Crit Care Med. 2009;180:491–8. doi: 10.1164/rccm.200903-0327OC. [DOI] [PubMed] [Google Scholar]
- 41.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goetghebuer T, Isles K, Moore C, Thomson A, Kwiatkowski D, Hull J. Genetic predisposition to wheeze following respiratory syncytial virus bronchiolitis. Clin Exp Allergy. 2004;34:801–3. doi: 10.1111/j.1365-2222.2004.1947.x. [DOI] [PubMed] [Google Scholar]
- 43.Goetghebuer T, Kwiatkowski D, Thomson A, Hull J. Familial susceptibility to severe respiratory infection in early life. Pediatr Pulmonol. 2004;38:321–8. doi: 10.1002/ppul.20069. [DOI] [PubMed] [Google Scholar]
- 44.Janssen R, Bont L, Siezen CL, Hodemaekers HM, Ermers MJ, Doornbos G, et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. J Infect Dis. 2007;196:826–34. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 45.Castro M, Schweiger T, Yin-Declue H, Ramkumar TP, Christie C, Zheng J, et al. Cytokine response after severe respiratory syncytial virus bronchiolitis in early life. J Allergy Clin Immunol. 2008;122:726–33. e3. doi: 10.1016/j.jaci.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigurs N. Epidemiologic and clinical evidence of a respiratory syncytial virus-reactive airway disease link. Am J Respir Crit Care Med. 2001;163(suppl):S2–6. doi: 10.1164/ajrccm.163.supplement_1.2011109. [DOI] [PubMed] [Google Scholar]
- 47.Thomsen SF, van der Sluis S, Stensballe LG, Posthuma D, Skytthe A, Kyvik KO, et al. Exploring the association between severe respiratory syncytial virus infection and asthma: a registry-based twin study. Am J Respir Crit Care Med. 2009;179:1091–7. doi: 10.1164/rccm.200809-1471OC. [DOI] [PubMed] [Google Scholar]
- 48.Miller JE. Predictors of asthma in young children: does reporting source affect our conclusions? Am J Epidemiol. 2001;154:245–50. doi: 10.1093/aje/154.3.245. [DOI] [PubMed] [Google Scholar]
- 49.Yoo KH, Johnson SK, Voigt RG, Campeau LJ, Yawn BP, Juhn YJ. Characterization of asthma status by parent report and medical record review. J Allergy Clin Immunol. 2007;120:1468–9. doi: 10.1016/j.jaci.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Werk LN, Steinbach S, Adams WG, Bauchner H. Beliefs about diagnosing asthma in young children. Pediatrics. 2000;105:585–90. doi: 10.1542/peds.105.3.585. [DOI] [PubMed] [Google Scholar]
- 51.Finkelstein JA, Lozano P, Shulruff R, Inui TS, Soumerai SB, Ng M, et al. Self-reported physician practices for children with asthma: are national guidelines followed? Pediatrics. 2000;106:886–96. [PubMed] [Google Scholar]
- 52.Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115:1254–60. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- E1.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]