Abstract
OBJECTIVES
We characterize cannabinoid disposition in oral fluid (OF) after Dronabinol, synthetic oral Δ9-tetrahydrocannabinol (THC), and Sativex, a cannabis-extract oromucosal spray, and evaluate whether smoked cannabis relapse or Sativex compliance can be identified with OF cannabinoid monitoring.
METHODS
5 and 15 mg synthetic oral THC, low (5.4 mg THC, 5.0 mg cannabidiol (CBD)) and high (16.2 mg THC, 15.0 mg CBD) dose Sativex, and placebo were administered in random order (n=14). Oral fluid specimens were collected for 10.5h after dosing and analyzed for THC, CBD, cannabinol (CBN), and 11-nor-9-carboxy-THC (THCCOOH).
RESULTS
After oral THC, OF THC concentrations decreased over time from baseline, reflecting residual THC excretion from previously self-administered smoked cannabis. CBD and CBN also were rarely detected. After Sativex, THC, CBD and CBN increased greatly, peaking at 0.25–1h. Median CBD/THC and CBN/THC ratios were 0.82–1.34 and 0.04–0.06, respectively, reflecting cannabinoids’ composition in Sativex. THCCOOH/THC ratios within 4.5h post Sativex were ≤1.6 pg/ng, always lower than after oral THC and placebo. THCCOOH/THC ratios increased throughout each dosing session.
CONCLUSIONS
Lack of measurable THC, CBD and CBN in OF following oral THC, and high OF CBD/THC ratios after Sativex distinguish oral and sublingual drug delivery routes from cannabis smoking. Low THCCOOH/THC ratios suggest recent Sativex and smoked cannabis exposure. These data indicate that OF cannabinoid monitoring can document compliance with Sativex pharmacotherapy, and identify relapse to smoked cannabis during oral THC medication but not Sativex treatment, unless samples were collected shortly after smoking.
Keywords: Oral fluid, delta9-tetrahydrocannabinol, cannabis, saliva, Sativex
1. INTRODUCTION
Cannabis has a long history of medicinal and psychoactive usage (Aggarwal et al., 2009), and interest in potential therapeutic applications is high (Mechoulam, 2005). More than 100 cannabinoids were identified in the cannabis plant (Mehmedic et al., 2010); Δ9-tetrahydrocannabinol (THC) is the primary psychoactive component that induces a wide spectrum of physiological and behavioral effects including heart rate and blood pressure changes, euphoria, impaired cognition, and psychosis (Huestis, 2002; Moore et al., 2007; Schwope et al., 2012). Cannabidiol (CBD), although not psychoactive, has shown anti-psychotic (Zuardi et al., 2009), anti-inflammatory (De Filippis et al., 2011), anti-epileptic (Cunha et al., 1980; Carlini and Cunha, 1981), and anxiolytic (Crippa et al., 2004) properties.
Cannabis is administered by different routes of administration, the most common being smoking. The 1999 Institute of Medicine report recommended developing alternative administration routes for therapeutic applications, as smoking produces harmful substances associated with respiratory and reproductive risks (Watson et al., 2000). Dronabinol (Marinol), synthetic oral THC, is US Food and Drug Administration (FDA)-approved for treating anorexia in patients with HIV-wasting disease, and chemotherapy-related nausea and vomiting. Cannabinoids are highly lipophilic and subject to extensive first-pass hepatic metabolism; thus, oral THC has low bioavailability (6–20%), and delayed onset compared to cannabis smoke (Huestis, 2007). Sativex (GW Pharmaceuticals), a whole-plant cannabis extract, contains nearly equivalent THC and CBD concentrations delivered via spray onto the oral mucosa to improve bioavailability. Sativex is an approved medication in Canada for multiple sclerosis (MS) neuropathic and opioid-resistant cancer pain, and in the UK, Spain, New Zealand, Germany and Denmark to treat MS-related spasticity. In the US, Sativex is in phase III clinical trials as an adjunct analgesic in cancer patients receiving opioid treatment (Oreja-Guevara, 2012; Portenoy et al., 2012). Besides the approved indications, oral THC and Sativex showed efficacy in treating post-operative, chronic intractable, and rheumatoid arthritis pain, insomnia, epilepsy, glaucoma, and cannabis dependence (Robson, 2001; Di Marzo and Petrocellis, 2006; Russo et al., 2007; Wright, 2007; Weinstein and Gorelick, 2011).
Several studies investigated cannabinoid disposition in oral fluid (OF) after cannabis smoking (Huestis and Cone, 2004; Kauert et al., 2007; Toennes et al., 2010; Lee et al., 2012), two after oral THC or cannabis (Niedbala et al., 2001; Milman et al., 2010), but none evaluated OF cannabinoids after Sativex intake. We and others reported that THC, CBD and cannabinol (CBN) are directly deposited onto the oral mucosa from cannabis smoke, with less contribution from blood (Hawks, 1982; Huestis, 2005; Lee et al., 2012). Greater than 1000 ng/mL OF THC was documented within 15–30 min after cannabis smoking (Huestis and Cone, 2004; Kauert et al., 2007; Lee et al., 2012), whereas ingestion of cannabis-laced brownies produced maximum OF THC ≤7.1 ng/mL after 1–2 h (Niedbala et al., 2001). THC concentrations in OF also never exceeded 8.0 ng/mL following 37 oral 20 mg THC doses administered over 8 days with mean peak concentrations occurring 12h before the first dose (Milman et al., 2010). On the other hand, the inactive THC metabolite, 11-nor-9-carboxy-THC (THCCOOH), continually increased during oral THC dosing; mean peak THCCOOH concentrations (≤1118 pg/mL) occurred on the 6th of 8 days of around-the-clock oral THC (Milman et al., 2010). Maximum OF THCCOOH concentrations were ≤763 pg/mL within 1–2 h after smoking a single 6.8% THC cigarette (Lee et al., 2012). In the present study, THC, CBD, CBN, 11-hydroxy-THC (11-OH-THC) and THCCOOH were quantified in cannabis smokers’ OF after 5 and 15 mg oral THC; low-dose (5.4 mg THC + 5.0 mg CBD) and high-dose (16.2 mg THC + 15.0 mg CBD) oromucosal Sativex; and placebo oral THC and placebo Sativex. We investigated cannabinoid disposition in OF after oral and sublingual administration. Cannabinoid time-course profiles, windows of detection and OF cannabinoid ratios were evaluated to determine if OF cannabinoid testing could document treatment compliance and/or identify relapse to smoked cannabis during oral THC or oromucosal Sativex pharmacotherapy.
2. METHODS
2.1 Participants
Cannabis smokers, age 18–45 years, were recruited to a double-blind, double-dummy, within- and between-subject study, evaluating oral THC and Sativex effects on cannabinoid OF pharmacokinetics. Participants self-reported smoking cannabis at least once, but less than daily, during the 3 months before study entry. Additional inclusion criteria were blood pressure ≤140 mm Hg for systolic and 90 mm Hg for diastolic, heart rate ≤100 bpm, electrocardiogram and 3-minute rhythm strip without clinically relevant abnormalities, and estimated IQ ≥85. Exclusion criteria consisted of history or presence of any clinically significant illness or adverse event associated with cannabis intoxication or withdrawal; current physical dependence on any substance other than nicotine or caffeine; donation of >450 mL blood within 30 days; ≥5 alcoholic drinks per day ≥4 times per week; allergy to sesame seed oil (dronabinol capsule ingredients), propylene glycol, ethanol, or peppermint oil (Sativex ingredients); interest or participation in drug abuse treatment within 60 days preceding study enrollment; and, if female, pregnant or nursing. The National Institute on Drug Abuse Institutional Review Board approved the study, and participants provided written informed consent.
2.2 Drug Administration
Participants resided on the Johns Hopkins Bayview Behavioral Pharmacology Research Unit under continuous medical surveillance at least 10h prior to each drug administration session. Female participants were required to have a negative urine pregnancy test result before each session. A standard breakfast was consumed approximately 1h before dosing.
Each participant received, in random order, 1 of 5 treatments: 5 or 15 mg oral synthetic THC; 2 (low dose, 5.4 mg THC and 5.0 mg CBD) or 6 (high dose, 16.2 mg THC and 15.0 mg CBD) actuations of Sativex; or placebo oral THC and 6 placebo Sativex actuations (Figure 1). Placebo capsules contained only lactose, while placebo Sativex contained propylene glycol, ethanol and peppermint oil. Participants swallowed 2 THC or placebo capsules with water and were administered 6 actuations of placebo or active Sativex within 2 min. Sativex actuations were directed sublingually and at the buccal mucosa. Dosing sessions were separated by at least 5 days.
Figure 1.
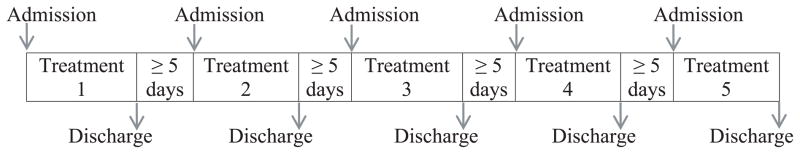
Study design. Participants (n=14) received in random order, 1 of 5 treatments: synthetic 5 mg Δ9-tetrahydrocannabinol (THC); synthetic 15 mg THC; 2 actuations of Sativex (5.4 mg THC and 5 mg cannabidiol (CBD)); 6 actuations of Sativex (16.2 mg THC + 15 mg CBD); or placebo synthetic THC and placebo Sativex.
2.3 OF Specimen collection and analysis
The Quantisal device (Immunalysis, Pomona, CA) was utilized to collect OF at −0.5 (baseline), 0.25, 1, 4.5, 7.5, and 10.5h after dosing. The device collects 1.0±0.1 mL OF with an absorptive cellulose pad. The pad was placed into a plastic tube containing 3 mL elution/stabilizing buffer for at least 24h to elute drugs. The OF-buffer mixture was subsequently decanted into Nunc CryoTubes (Thermo Scientific, Waltham, MA) and stored at −20°C prior to analysis.
Specimens were analyzed for THC, CBD, CBN, 11-OH-THC, and THCCOOH by a previously published, validated two-dimensional gas chromatography-mass spectrometry method (Milman et al., 2010). If analyte concentrations exceeded the upper limit of linearity, participants’ OF specimens were diluted with drug-free OF-Quantisal buffer mixture until concentrations were within the linear dynamic range. Limits of quantification (LOQ) were 0.5 ng/mL for THC, CBD, and 11-OH-THC, 1 ng/mL for CBN, and 7.5 pg/mL for THCCOOH.
2.4 Data Analysis
IBM SPSS Statistics version 19.0 and Microsoft Excel 2007 were utilized for statistical evaluation. Cannabinoid concentrations were non-normally distributed, as determined by the Shapiro-Wilk test and Normal Q-Q plot. Consequently, correlations were analyzed with nonparametric Spearman tests; effects of time, dose, and baseline concentrations on cannabinoid concentrations post-dose were evaluated with Generalized Linear Mixed Models after log transformation of data. Values below LOQ were considered as one tenth the LOQ for all statistical analyses. Results with 2 tailed P <0.05 were considered significant.
3. RESULTS
Eleven male and 3 female cannabis smokers (ages 19–43 years) completed 5 study sessions, providing 6 OF specimens per session (Table 1). Four OF specimens could not be collected due to participant response to drug or extension of the functional magnetic resonance imaging session past the collection time. 11-OH-THC was detected in only 4 specimens collected within 1h after Sativex with concentrations ≤2.8 ng/mL; thus, this analyte was excluded from data analyses. Descriptive statistical data are reported in Table 2. Median cannabinoid concentrations over time after 5 and 15 mg THC, low- and high-dose Sativex, and placebo are illustrated in Figures 2–4.
Table 1.
Demographics and self-reported drug use histories for 14 cannabis smokers.
|
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Cannabis use | ||||||||
|
| ||||||||
| Study participant | Sex | Age, years | Racea | BMI, kg/m2 | Days smoked in past 14 | Average use, joints | Age 1st smoked, years | Duration of longest use, years |
| A | M | 19 | W | 22.9 | 1 | 1/month | 15 | 2 |
| B | F | 24 | W | 22.0 | 6 | 3/week | 16 | 5 |
| C | M | 21 | AA | 30.1 | 1 | 2/month | 21 | 1 |
| D | M | 28 | AA | 22.9 | 1 | 2/3 months | 16 | 9 |
| E | M | 26 | AA | 23.0 | 2 | 1/month | 12 | 7 |
| F | M | 43 | AA | 28.5 | 10 | 30/week | 16 | 10 |
| G | F | 20 | AA | 32.9 | 2 | 2/week | 13 | 2 |
| H | F | 21 | W | 23.6 | 12 | 5–6/week | 17 | 2 |
| I | M | 23 | AA | 25.6 | 3 | 9/week | 14 | 5 |
| J | M | 21 | AA | 22.4 | 11 | 24/week | 16 | 2 |
| K | M | 24 | W | 21.5 | 5 | 2/week | 18 | 2 |
| L | M | 30 | AA | 27.3 | 0 | 9/month | 14 | 3 |
| M | M | 25 | W | 23.0 | 8 | 24/week | 15 | 5 |
| N | M | 42 | AA | 26.4 | 0 | 1/3 months | 18 | 17 |
|
| ||||||||
| Median | 24.1 | 23.3 | 2.5 | 16.0 | 4.0 | |||
| Mean | 26.3 | 25.1 | 4.4 | 15.8 | 5.1 | |||
| SD | 7.5 | 3.4 | 4.3 | 2.3 | 4.4 | |||
W, White; AA, African American
Table 2.
Median (range) pharmacokinetic parameters of Δ9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD) and cannabinol (CBN), before and after 5 and 15 mg oral THC and low-dose (5.4 mg THC + 5 CBD) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex in 14 healthy adult cannabis smokers. Participants with no positive results throughout collection in each dosing session were excluded from calculations.
| Analyte | Dose | Na | Cinitialb | Cmaxc | Tmax, hd | Claste | Tlast, hf |
|---|---|---|---|---|---|---|---|
| THC, ng/mL | 5 mg oral THC | 6 | 3.7 (<LOQ-9.0) | 6.0 (0.8–12.7) | 0.6 (−0.5–1.0) | 1.2 (0.7–3.7) | 10.5 (0.25–10.5) |
| 15 mg oral THC | 8 | 0.8 (<LOQ-20.5) | 1.6 (0.7–20.5) | 0.3 (−0.5–4.5) | 0.9 (0.5–5.8) | 4.5 (1–10.5) | |
| Low Sativex | 14 | 0.8 (<LOQ-12.8) | 1815 (266–11424) | 0.25 (0.25–1.0) | 17.3 (1.0–60.0) | 10.5 | |
| High Sativex | 14 | <LOQ (<LOQ-10.1) | 7853 (1323–18216) | 0.25 | 34.5 (2.9–92.0) | 10.5 | |
|
| |||||||
| THCCOOH, pg/mL | 5 mg oral THC | 9 | 32.9 (<LOQ-109) | 39.1 (7.7–191) | 7.5 (−0.5–10.5) | 34.0 (7.7–191) | 10.5 (7.5–10.5) |
| 15 mg oral THC | 11 | 15.9 (<LOQ-274) | 49.3 (8.0–311) | 7.5 (1.0–10.5) | 44.5 (8.0–311) | 10.5 (4.5–10.5) | |
| Low Sativex | 11 | 25.6 (<LOQ-129) | 44.2 (8.8–226) | 4.5 (−0.5–10.5) | 12.6 (8.3–141) | 10.5 (7.5–10.5) | |
| High Sativex | 12 | 11.9 (<LOQ-138) | 35.6 (8.5–184) | 7.5 (−0.5–10.5) | 25.2 (8.5–176) | 10.5 (0.25–10.5) | |
|
| |||||||
| CBD, ng/mL | 5 mg oral THC | 0 | NA | NA | NA | NA | NA |
| 15 mg oral THC | 1 | <LOQ | 0.8 | 0.25 | 0.6 | 1.0 | |
| Low Sativex | 14 | <LOQ | 1975 (196–12120) | 0.25 (0.25–4.5) | 22.6 (0.5–67.8) | 10.5 | |
| High Sativex | 14 | <LOQ | 7129 (1552–18636) | 0.25 | 34.6 (0.8–131) | 10.5 | |
|
| |||||||
| CBN, ng/mL | 5 mg oral THC | 0 | NA | NA | NA | NA | NA |
| 15 mg oral THC | 1 | 1.1 | 1.1 | −0.5 | 1.1 | −0.5 | |
| Low Sativex | 14 | <LOQ (<LOQ-1.1) | 140 (9.5–560) | 0.25 (0.25–1.0) | 2.0 (1.1–3.5) | 7.5 (4.5–10.5) | |
| High Sativex | 14 | <LOQ (<LOQ-1.3) | 414 (74–2232) | 0.25 | 2.4 (1.2–6.9) | 10.5 (7.5–10.5) | |
number of participants with cannabinoid concentrations >limit of quantification (LOQ) before and/or after dosing, and included in calculation of Cinitial, Cmax, Tmax, Clast and TClast
Cinitial, concentrations 0.5h prior to dosing
Cmax, maximum concentration
Tmax, time of Cmax
Clast, last specimen concentration ≥LOQ (0.5 ng/mL for THC and CBD; 7.5 pg/mL for THCCOOH; 1 ng/mL for CBN)
TClast, time of Clast
Figure 2.
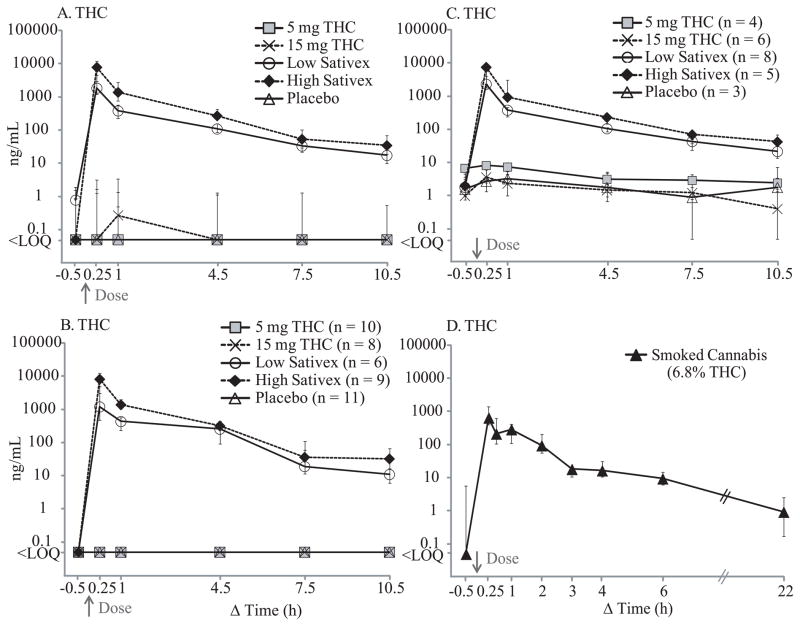
Median Δ9-tetrahydrocannabinol (THC) concentrations after 5 and 15 mg THC, low-dose (5.4 mg THC + 5 mg cannabidiol (CBD)) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex, and placebo. Panel A includes all 14 participants, Panel B includes participants who were THC-negative at baseline, and Panel C includes THC-positive participants; numbers of participants are indicated in parenthesis for Panels B and C. Panel D shows median THC time course after smoking 1 cannabis cigarette containing 6.8% THC, adapted from our previously published data (Lee et al., 2012) for comparison. Error bars indicate interquartile ranges.
Figure 4.
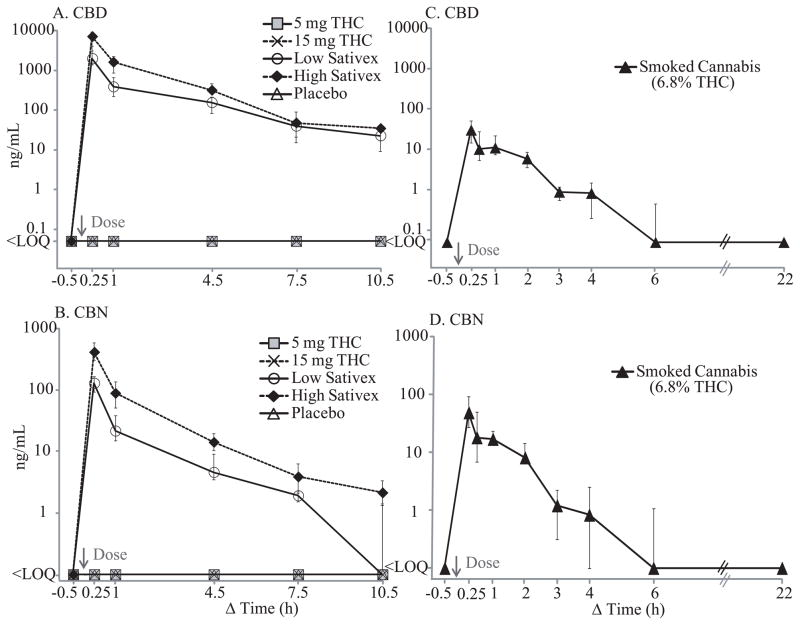
Median cannabidiol (CBD; A) and cannabinol (CBN; B) concentrations after 5 and 15 mg Δ9-tetrahydrocannabinol (THC), low-dose (5.4 mg THC + 5 mg cannabidiol (CBD)) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex, and placebo. Panels C and D show median CBD and CBN time courses, respectively, after smoking 1 cannabis cigarette with 6.8% THC, adapted from our previously published data (Lee et al., 2012) for comparison. Error bars indicate interquartile ranges.
3.1 Cannabinoid disposition after oral THC administration
Following 5 and 15 mg oral THC, THC was generally not detected if baseline specimens were negative (Figure 2), and never exceeded 17.9 ng/mL after dosing. THC remained positive (≤5.8 ng/mL) in about 25% of participants at 10.5h. THC concentrations following low and high THC oral doses were significantly correlated with time [F4,64 = 3.559, P = 0.011 (5 mg); F4,64 = 3.236, P = 0.018 (15 mg)] and baseline concentrations [F1,64 = 489.4, P <0.0001 (5 mg); F1,64 = 165.4 P <0.0001 (15 mg)]. All but 3 specimens were negative for CBD and CBN before and after oral THC dosing (Table 2)
THCCOOH also was significantly influenced by baseline concentrations after oral THC [F1,64 = 1107, P <0.0001 (5 mg); F1,64 = 308.1, P <0.0001 (15 mg)], but did not correlate with time (Figure 3). Maximum THCCOOH concentrations (7.7–311 pg/mL) generally occurred between 4.5–10.5h, indicating possible contribution of oral THC doses. In 7 participants, THCCOOH was detectable 10.5h post 5 and 15 mg oral THC doses.
Figure 3.
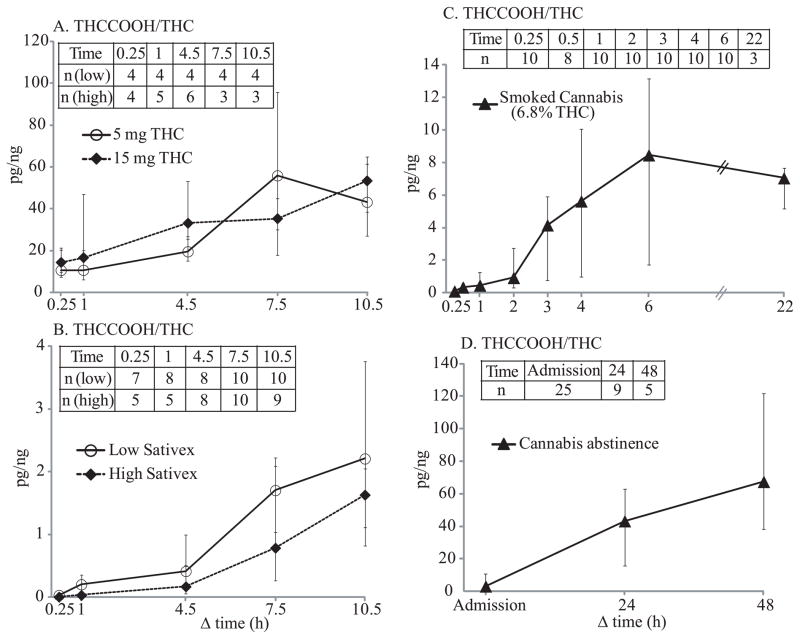
Median 11-nor-9-carboxy-THC (THCCOOH) concentrations after 5 and 15 mg Δ9-tetrahydrocannabinol (THC), low-dose (5.4 mg THC + 5 mg cannabidiol (CBD)) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex, and placebo. Panel A included all 14 participants, Panel B includes participants with negative THCCOOH at baseline, and Panel C includes participants with positive THCCOOH at baseline; numbers of participants are indicated in parenthesis for Panels B and C. Panel D shows median THCCOOH time course after smoking 1 cannabis cigarette with 6.8% THC, adapted from our previously published data (Lee et al., 2012) for comparison. Error bars indicate interquartile ranges.
3.2 Cannabinoid disposition after low Sativex administration
Unlike oral THC, Sativex significantly increased OF THC, CBD, and CBN concentrations immediately following administration (Figures 2 and 4). After low-dose (5.4 mg THC + 5 mg CBD) Sativex dosing, THC concentrations were significantly elevated throughout 10.5h, with medians (range) of 1847 (257–11424), 381 (88.2–1000), 109 (41.7–551), and 33.3 (3.3–83.7) ng/mL at 0.25, 1, 4.5, and 7.5h, respectively; peak THC concentrations occurred at 0.25h for all but one at 1h. At 10.5h post-dose, all OF specimens were still THC-positive (1.0–60.0 ng/mL).
CBD OF concentrations also increased to medians (range) of 1990 (186–12120), 386 (71.8–842), 155 (47.3–709), and 39.7 (2.0–124) ng/mL at 0.25, 1, 4.5, and 7.5h after dosing, respectively; all specimens were still positive (0.5–67.8 ng/mL) at 10.5h. Similar to THC and CBD, CBN concentrations increased after oromucosal Sativex dosing with medians (range) of 130 (9.5–560), 21.7 (6.7–199), 4.6 (2.1–47.3), and 1.9 (<LOQ-3.4) ng/mL at 0.25, 1, 4.5, and 7.5h, respectively, more than 10-fold lower than THC or CBD concentrations. While all specimens were positive for CBN until 4.5h, only 86 and 43% of specimens remained positive at 7.5 and 10.5h, respectively.
As with oral THC doses, THCCOOH concentrations significantly correlated with those at baseline [F1,61 = 586.7, P <0.0001], but not with time (Figure 3). Ten participants, THCCOOH-positive at baseline with a median (range) of 29.7 (7.6–129) pg/mL, remained positive [16.9 (8.3–141) pg/mL] until 10.5h with intermittent negatives. Four participants were negative at baseline and remained negative throughout except for one who had 8.8 pg/mL THCCOOH at 7.5h.
3.3 Cannabinoid disposition after high Sativex administration
Increases in THC, CBD, and CBN OF concentration were even more prominent after high-dose Sativex (Figures 2 and 4). THC concentrations increased to medians (range) of 7853 (1323–18216), 1383 (396–3578), 270 (67.1–1337), and 53.4 (5.6–361) ng/mL at 0.25, 1, 4.5, and 7.5h, respectively, with peak concentrations occurring at 0.25h. By 10.5h, concentrations were 34.5 (2.9–92.0) ng/mL.
CBD concentrations followed THC elimination patterns, with medians (range) of 7129 (1552–18636), 1616 (476–4041), 317 (33–1629), 47.7 (1.7–471), and 34.6 (0.8–131) ng/mL at 0.25, 1, 4.5, 7.5, and 10.5h, respectively. As with low-dose Sativex, CBN concentrations after the high dose were more than 10-fold lower than THC or CBD, with medians (range) of 414 (74–2232), 89.0 (24.3–217), 14.2 (3.2–41.3), and 3.9 (1.3–12.8) ng/mL at 0.25, 1, 4.5, and 7.5h, respectively. All OF specimens were CBN-positive for 7.5h, and 79% at 10.5h with a median concentration of 2.5 (1.2–6.9) ng/mL.
After high-dose Sativex, OF THCCOOH increased over time [F4,64 = 4.925, P = 0.002] but was still significantly affected by baseline concentrations [F1,64 = 172.3, P <0.0001] (Figure 3). Eight participants, THCCOOH-positive at baseline with a median (range) of 20.8 (8.2–138) pg/mL remained positive [50.0 (10.9–176) pg/mL] until 10.5h with intermittent negatives. Four participants were THCCOOH-negative at baseline, with only occasional positives from 0.25 to 10.5h with concentrations ≤24.9 pg/mL. Two participants never had a THCCOOH OF positive result.
3.4 Dose-concentration relationships
Controlling for collection time and baseline levels, OF THCCOOH concentrations had a significant dose-dependent increase [F1,133 = 9.351; P = 0.003] from 5 to 15 mg oral THC, while THC concentrations did not. Oral THC doses increased OF THCCOOH but not THC concentrations. In contrast, THC [F1,130 = 32.69, P <0.0001], CBD [F1,131 = 18.03, P <0.0001], and CBN [F1,131 = 45.57, P <0.0001] had significant dose-dependent OF concentration increases after high vs. low Sativex doses, but THCCOOH did not.
The relationship of THC to other cannabinoids in OF reflects the source of THC. After 5 and 15 mg oral THC, THC was significantly correlated with THCCOOH concentrations (n = 70, ρ = 0.722, P <0.0001; n = 70, ρ = 0.604, P <0.0001, respectively), but after low- and high-dose Sativex, THC correlated with CBD (n = 70, ρ = 0.951, P <0.0001; n = 70, ρ = 0.985, P = <0.0001, respectively) and CBN concentrations (n = 70, ρ = 0.983, P <0.0001; n = 70, ρ = 0.950, P = <0.0001, respectively).
3.5 Comparison of active THC dosing with placebo
After controlling for time and baseline levels, THC OF concentrations after placebo were not significantly different from those after either 5 or 15 mg oral THC. However, after active low- and high-dose Sativex (t = 25.09, P <0.0001; t = 30.51, P <0.0001, respectively), THC concentrations were significantly greater than after placebo. THCCOOH concentrations after placebo were significantly different from those after 15 (t = 2.880, P = 0.004) but not 5 mg oral THC, or low- or high-dose Sativex. THC and THCCOOH concentrations were significantly correlated after placebo (n = 69, ρ = 0.774, P <0.0001).
3.6 OF cannabinoid concentration ratios
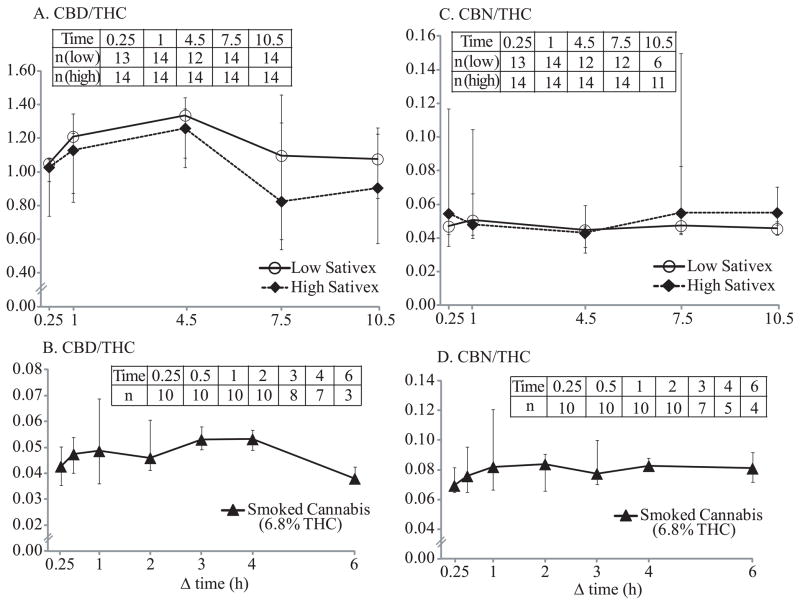
Only positive cannabinoid pairs were included in ratio calculations; numbers of pairs are provided in Figures 5–6. Cannabinoid OF ratios reflected their relative quantities in administered drugs. After low-dose Sativex, median CBD/THC ratios were 1.05–1.34 through 10.5 h. After high-dose Sativex, similar ratios were observed, with medians of 0.82–1.26. After low- and high-dose Sativex, median CBN/THC ratios through 10.5h were 0.04–0.05 and 0.04–0.06, respectively. CBD/THC and CBN/THC ratios were not significantly different over time. However, THCCOOH/THC (pg/ng) ratios significantly increased over time after low- and high-dose Sativex [F4,37 = 12.33, P <0.0001 (low); F4,32 = 19.47, P <0.0001 (high)]; 0.25h median ratios were 0.04 and 0.01, increasing to 2.21 and 1.63 by 10.5h post low- and high-dose Sativex, respectively. THCCOOH/THC ratio ranges after 5 and 15 mg oral THC and placebo (5.27–108, 5.11–180, and 10.5–152, respectively) were much higher than those after Sativex. Median THCCOOH/THC ratios over time after 5 and 15 mg oral THC did not change significantly, but showed an increasing trend from 10.6 and 14.4 at 0.25h to 43.1 and 53.4 at 10.5h, respectively.
Figure 5.
Median THCCOOH/THC ratios (pg/ng) after 5 and 15 mg oral THC (A), and low-dose (5.4 mg THC + 5 mg CBD) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex (B). Median THCCOOH/THC ratios after smoking 1 cannabis cigarette with 6.8% (mean 54 mg) THC (C) and median THCCOOH/THC ratios in chronic, daily cannabis smokers during abstinence (D) were calculated from our previously published data (Lee et al., 2011; Lee et al., 2012) for comparison. Error bars indicate interquartile ranges. Data inserts indicate total numbers of participants included in ratio calculation at each time point. Δ9-tetrahydrocannabinol (THC), 11-nor-9-carboxy-THC (THCCOOH), and cannabidiol (CBD).
Figure 6.
Median CBD/THC (A) and CBN/THC (C) ratios after low-dose (5.4 mg THC + 5 mg CBD) and high-dose (16.2 mg THC + 15 mg CBD) oromucosal Sativex. Median CBD/THC (B) and CBN/THC (D) ratios after smoking 1 cannabis cigarette with 6.8% (mean 54 mg) THC were calculated from our previously published data (Lee et al., 2012) for comparison. Error bars indicate interquartile ranges. Data inserts indicate total numbers of participants included in ratio calculation at each time point. Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (CBN).
Cannabinoid ratios 0.25–22h after smoking a single 6.8% (54 mg) THC and 0.3% (2.0 mg) CBD cigarette were calculated from previously published data from our laboratory (Lee et al., 2012). CBD/THC ratios after smoking were more than 10 times lower than after Sativex. Median CBD/THC and CBN/THC ratios 0.25 to 6h after smoking were 0.04–0.05 and 0.07–0.08, respectively. CBD and CBN were not detected 22h post-smoking. THCCOOH/THC (pg/ng) ratios significantly changed over time (0.25–22h) [F7,63 = 18.78, P <0.0001] after smoking, with median ratios increasing from 0.10 at 0.25h to 8.46 six h post-dose. Inter-subject variability in THCCOOH/THC ratios was much larger than CBD/THC and CBN/THC variabilities. Median cannabinoid ratio time courses after oral THC, Sativex and smoked cannabis are illustrated in Figures 5–6. In Figure 6, median THCCOOH/THC ratios at admission and after 24 and 48h of monitored cannabis abstinence in chronic daily cannabis smokers also are illustrated for comparison (Lee et al., 2011), showing that THCCOOH/THC ratios increased over time and by 24h abstinence, no participant had a THCCOOH/THC ratio less than 4 pg/ng.
4. DISCUSSION
Cannabinoid OF concentrations and time course were systematically evaluated after two therapeutic administration routes, oral THC and oromucosal Sativex, and compared to results after cannabis smoking and during monitored abstinence in chronic daily cannabis smokers. Doses were within the therapeutic daily dose range; recommended oral THC dosage for appetite stimulation and antiemetic effect is 2.5–20 mg per day (Ben Amar, 2006), and a maximum daily dose of 12 Sativex sprays (32.4 mg THC + 30 mg CBD) is recommended. THC, CBD and CBN OF concentrations at baseline and following oral THC doses primarily reflected self-administered smoked cannabis prior to admission. Encapsulated oral THC does not deposit onto the oral mucosa unless the capsule coating is breached (Milman et al., 2010). As expected, THC concentrations significantly decreased over time, co-varied with baseline concentrations and were not significantly different from those after placebo. The participants negative at baseline remained THC-negative throughout the session. Thus, higher maximum THC concentrations after 5 than 15 mg oral THC were due to higher baseline concentrations (Table 2). Our results agree with previous research documenting continuously decreasing THC OF concentrations during 37 oral THC doses (Milman et al., 2010). Low concentrations and detection rates of THC, CBD, and CBN in OF following oral THC doses support our contention that the main source of those cannabinoids in OF specimens was deposition from cannabis smoking prior to study entry. Residual THC excretion was observed until the last collection time (10.5h) with concentrations ≤5.8 ng/mL (Table 2). THC is highly lipophilic, with body burden increasing with frequency and amount of smoked cannabis (Johansson et al., 2009). Consequently, low OF THC concentrations can persist in chronic cannabis smokers over an extended period (Lee et al., 2011).
Immediately following Sativex administration, THC and CBD concentrations greatly increased along with 10-fold lower CBN concentrations. Median CBD/THC ratios were 0.82–1.34 through 10.5h, significantly higher than those after cannabis smoking, 0.04–0.05 (Figure 6). Median CBN/THC ratios were 0.04–0.06 through 10.5h, similar to what we observed after cannabis smoking, 0.07–0.08 (Figure 6). The results are not surprising as Sativex is a whole cannabis plant extract with approximately 1:1 THC and CBD composition, accounting for the observed CBD/THC ratios, as well as presence of lower CBN concentrations. Sativex is an oromucosal spray that contaminates the oral cavity during use.
THCCOOH OF concentrations were significantly higher after 15 as compared to 5 mg oral THC and placebo doses. THCCOOH also significantly increased over time after the high Sativex dose. However, residual concentrations from previously self-administered smoked cannabis frequently masked THCCOOH concentration changes. Compared to THCCOOH increases after multiple oral THC doses (Milman et al., 2010) and after smoking one cannabis cigarette (Lee et al., 2012), a single, therapeutic oral or sublingual dose in the present study produced less noticeable THCCOOH concentration changes.
THCCOOH concentrations significantly correlated with THC concentrations after oral THC and placebo. However, correlations were qualitative rather than quantitative as ≥63 and 44% of OF specimens were negative for THC and THCCOOH, respectively. We previously reported that THCCOOH/THC ≤4 pg/ng in OF may indicate cannabis smoking within 24h. This cutoff THCCOOH/THC ≤4 pg/ng was empirically selected to eliminate OF specimens positive due to residual THC and THCCOOH excretion in chronic, daily cannabis smokers during prolonged abstinence (Lee et al., 2011). After Sativex and smoked cannabis, all participants had THCCOOH/THC <4 pg/ng for 4.5 and 1h post-dose, respectively, while ratios were never below 5.11 pg/ng following oral THC and placebo doses (Figure 5). These results suggest that OF THCCOOH/THC ≤4 pg/ng indicates recent smoked cannabis or Sativex exposure. However, large inter-subject variability and low detection rates in this cohort of less than daily cannabis smokers renders the THCCOOH/THC ratio ≤4 pg/ng as inclusionary rather than exclusionary to identify recent cannabis intake. Additional research is needed to determine the usefulness of this potential cutoff criterion.
Limitations of this study included presence of THC and/or THCCOOH in up to 71% of baseline OF specimens and variability in participants’ cannabis use histories (Table 1). This diversity expands the applicability of our results, but may have reduced the statistical power in this multi-session study.
When synthetic THC and plant-derived cannabinoids are administered for therapeutic applications, monitoring treatment compliance and/or relapse to cannabis smoking may be necessary. Concurrent use or substitution of non-prescribed drugs can prevent implementation of appropriate treatment, lead to development of abuse, addiction or tolerance, or suggest self-medication of unidentified symptoms. Sixty-four percent of HIV/AIDS patients reported smoking cannabis during dronabinol treatment (Prentiss et al., 2004) while 66% of medical cannabis patients reportedly substituted cannabis for prescription drugs (Reiman, 2009).
Additionally, epidemiological studies reported high prevalence of cannabis use (8.9–59%) in patients with chronic pain, multiple sclerosis, and HIV/AIDS (Ware et al., 2003; Clark et al., 2004; Ware et al., 2005; Cone et al., 2008). Inaccuracy in self-reports was documented in multiple studies (Magura and Kang, 1996; Harrison, 1997; Fishbain et al., 1999; Akinci et al., 2001); biological drug testing provides objective evidence of substance use (Huestis 2007; Schwilke et al., 2011). Katz et al., 2003 showed that urine toxicology improved identification of inappropriate drug-taking behavior in chronic pain patients. While urine is the most common fluid for drug testing, OF is a highly promising alternative matrix (Bosker and Huestis, 2009).
The present data document that oral THC consumption can be distinguished from recent illicit cannabis smoking by low or undetectable OF THC, CBD, and CBN concentrations. While THCCOOH concentration changes were less evident with a single 5 or 15 mg oral THC dose, higher dosage likely increases THCCOOH concentrations. These characteristics imply that OF cannabinoid monitoring could identify relapse to smoked cannabis during oral THC pharmacotherapy for cannabis dependence, but not compliance with medication. Although not an approved medication for cannabis dependence, oral THC can alleviate symptoms of cannabis withdrawal (Levin et al, 2011; Vandrey et al., 2012). In contrast, compliance with Sativex pharmacotherapy for cannabis dependence should be clearly evident by the high OF CBD/THC ratio as compared to that following cannabis smoking; however, additional research is needed to determine if relapse to cannabis smoking can be identified during Sativex pharmacotherapy, as the high OF CBD/THC ratio after Sativex may not be altered sufficiently to identify single smoked cannabis episodes.
In summary, this study is the first to characterize OF cannabinoids after single oral THC or Sativex administration. After oral THC, OF cannabinoid concentrations primarily reflected residual excretion from cannabis smoking. Following Sativex, THC, CBD and CBN were significantly elevated, with CBD concentrations as high and CBN concentrations more than 10 times lower than THC concentrations. THCCOOH concentration increases were less evident and observed only after high-dose Sativex. These data indicate that cannabinoid elimination patterns after oral THC and Sativex are markedly different from those after cannabis smoking. Lack of measurable THC, CBD and CBN in OF following oral THC, and high OF CBD/THC ratios after Sativex distinguish oral and sublingual drug delivery routes from cannabis smoking. Low THCCOOH/THC ratios suggest recent Sativex and smoked cannabis exposure. These data signify that relapse to smoked cannabis during oral THC pharmacotherapy for cannabis dependence, but not compliance with medication, should be evident with OF cannabinoid monitoring. In contrast, compliance with Sativex pharmacotherapy should be clearly observed by the high CBN/THC ratio as compared to that following cannabis smoking.
Acknowledgments
Role of Funding Source
This research was funded by the Intramural Research Program, National Institute on Drug Abuse, NIH.
The authors acknowledge the contributions of the clinical staff of the Intramural Research Program, National Institute on Drug Abuse, and Behavioral Pharmacology Research Unit, Johns Hopkins Bayview Medical Center, as well as the Graduate Partnership Program, NIH. The authors also acknowledge G.W. Pharmaceuticals for providing active Sativex® and placebo, and for Immunalysis Inc. for the Quantisal™ collection devices, but had no role in study design, data analysis or presentation of results.
Footnotes
Contributors
Dayong Lee: Analyzed specimens and data, and wrote manuscript
Erin L. Karschner: Designed and performed research, and reviewed manuscript
Garry Milman: Analyzed specimens, and reviewed manuscript
Allan J. Barnes: Reviewed data and manuscript
Robert S. Goodwin: Performed research, and reviewed manuscript
Marilyn A. Huestis: Designed and performed research, and revised manuscript
Conflict of Interest
The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD. Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag. 2009;5:153–168. doi: 10.5055/jom.2009.0016. [DOI] [PubMed] [Google Scholar]
- Akinci IH, Tarter RE, Kirisci L. Concordance between verbal report and urine screen of recent marijuana use in adolescents. Addict Behav. 2001;26:613–619. doi: 10.1016/s0306-4603(00)00146-5. [DOI] [PubMed] [Google Scholar]
- Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. doi: 10.1016/j.jep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55:1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21:417S–427S. doi: 10.1002/j.1552-4604.1981.tb02622.x. [DOI] [PubMed] [Google Scholar]
- Clark AJ, Ware MA, Yazer E, Murray TJ, Lynch ME. Patterns of cannabis use among patients with multiple sclerosis. Neurology. 2004;62:2098–2100. doi: 10.1212/01.wnl.0000127707.07621.72. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Caplan YH, Black DL, Robert T, Moser F. Urine drug testing of chronic pain patients: licit and illicit drug patterns. J Anal Toxicol. 2008;32:530–543. doi: 10.1093/jat/32.8.530. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, Azevedo-Marques PM, Hallak JE, McGuire PK, Filho Busatto G. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175–185. doi: 10.1159/000137430. [DOI] [PubMed] [Google Scholar]
- De Filippis D, Esposito G, Cirillo C, Cipriano M, De Winter BY, Scuderi C, Sarnelli G, Cuomo R, Steardo L, De Man JG, Iuvone T. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One. 2011;6:e28159. doi: 10.1371/journal.pone.0028159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Petrocellis LD. Plant, synthetic, and endogenous cannabinoids in medicine. Annu Rev Med. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Validity of self-reported drug use in chronic pain patients. Clin J Pain. 1999;15:184–191. doi: 10.1097/00002508-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Harrison L. The validity of self-reported drug use in survey research: an overview and critique of research methods. NIDA Res Monogr. 1997;167:17–36. [PubMed] [Google Scholar]
- Hawks RL. The constituents of cannabis and the disposition and metabolism of cannabinoids. NIDA Res Monogr. 1982;42:125–137. [PubMed] [Google Scholar]
- Huestis MA. Cannabis (marijuana) - effects on human behavior and performance. In: Farrell LJ, Logan BK, Dubowski KM, editors. The Effects of Drugs on Human Performance and Behavior. Central Police University Press; Taipei: 2002. pp. 15–60. [Google Scholar]
- Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta-9-tetrahydrocannabinol, cannabidiol and cannabinol. In: Pertwee RG, editor. Handbook of Experimental Pharmacology. Springer; New York: 2005. pp. 657–690. [DOI] [PubMed] [Google Scholar]
- Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–1804. doi: 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Relationship of delta 9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J Anal Toxicol. 2004;28:394–399. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- Johansson E, Noren K, Sjovall J, Halldin MM. Determination of delta-1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed Chromatogr. 1989;3:35–38. doi: 10.1002/bmc.1130030109. [DOI] [PubMed] [Google Scholar]
- Katz NP, Sherburne S, Beach M, Rose JR, Vielguth J, Bradley J, Fanciullo GJ. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097–1102. doi: 10.1213/01.ANE.0000080159.83342.B5. [DOI] [PubMed] [Google Scholar]
- Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288– 293. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- Lee D, Milman G, Barnes AJ, Goodwin RS, Hirvonen J, Huestis MA. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin Chem. 2011;57:1127–1136. doi: 10.1373/clinchem.2011.164822. [DOI] [PubMed] [Google Scholar]
- Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem. 2012;58:748–756. doi: 10.1373/clinchem.2011.177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary delta 9 tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura S, Kang SY. Validity of self-reported drug use in high risk populations: a meta-analytical review. Subst Use Misuse. 1996;31:1131–1153. doi: 10.3109/10826089609063969. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 2005;146:913–915. doi: 10.1038/sj.bjp.0706415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, El Sohly MA. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217:1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56:1261–1269. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systemic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, Robb J, Cone EJ. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25:289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- Oreja-Guevara C. Clinical efficacy and effectiveness of Sativex, a combined cannabinoid medicine, in multiple sclerosis-related spasticity. Expert Rev Neurother. 2012;12:3–8. doi: 10.1586/ern.12.11. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, McQuade R, Wright S, Fallon MT. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13:438–449. doi: 10.1016/j.jpain.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. Patterns of marijuana use among patiens with HIV/AIDS followed in a public health care setting. J Acquir Immune Defic Syndr. 2004;35:38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- Reiman A. Cannabis as a substitute for alcohol and other drugs. Harm Reduct J. 2009;6:1–5. doi: 10.1186/1477-7517-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson P. Therapeutic aspects of cannabis and cannabinoids. Br J Psychiatry. 2001;178:107–115. doi: 10.1192/bjp.178.2.107. [DOI] [PubMed] [Google Scholar]
- Russo EB, Guy GW, Robson PJ. Cannabis, pain, and sleep: lessons from therapeutic clinical trials of Sativex, a cannabis-based medicine. Chem Biodivers. 2007;4:1729–1743. doi: 10.1002/cbdv.200790150. [DOI] [PubMed] [Google Scholar]
- Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA. Psychomotor performance, subjective and physiological effects and whole blood Δ9-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. J Anal Toxicol. 2012;36:405–412. doi: 10.1093/jat/bks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, Pope HG, Huestis MA. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis smokers. Addiction. 2011;106:499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toennes SW, Ramaekers JG, Theunissen EL, Moeller MR, Kauert GF. Pharmacokinetic properties of delta9-tetrahydrocannabinol in oral fluid of occasional and chronic users. J Anal Toxicol. 2010;34:216–221. doi: 10.1093/jat/34.4.216. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Lee D. The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.08.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware MA, Rueda S, Singer J, Kilby D. Cannabis use by persons living with HIV/AIDS: patterns and prevalence of use. J Cannabis Ther. 2003;3:3–15. [Google Scholar]
- Ware MA, Adams H, Guy GW. The medicinal use of cannabis in the UK: results of a nationwide survey. Int J Clin Pract. 2005;59:291–295. doi: 10.1111/j.1742-1241.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- Watson SJ, Benson JA, Jr, Joy JE. Marijuana and medicine: assessing the science base: a summary of the 1999 Institute of Medicine report. Arch Gen Psychiatry. 2000;57:547–552. doi: 10.1001/archpsyc.57.6.547. [DOI] [PubMed] [Google Scholar]
- Weinstein AM, Gorelick DA. Pharmacological treatment of cannabis dependence. Curr Pharm Des. 2011;17:1351–1358. doi: 10.2174/138161211796150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. Cannabinoid-based medicines for neurological disorders--clinical evidence. Mol Neurobiol. 2007;36:129–136. doi: 10.1007/s12035-007-0003-4. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Pinto JP, Chagas MH, Rodrigues GG, Dursun SM, Tumas V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J Psychopharmacol. 2009;23:979–983. doi: 10.1177/0269881108096519. [DOI] [PubMed] [Google Scholar]