Abstract
BACKGROUND
Cocaine has long been known to increase blood pressure, but the degree and mechanism of vasoconstricting action remain poorly understood. Here we examine the interaction between cocaine and alpha-adrenoceptor agonists, with the action of reuptake inhibition minimized.
METHODS
Cocaine was administered to isolated rings of rat thoracic aorta, alone and in combination with three different adrenoceptor agonists: phenylephrine, methoxamine, and norepinephrine. Synergy analysis begins with the predicted additive effect of the combination of two agonists, based upon dose equivalence theory. This case where one agonist (cocaine) has no effect when administered alone requires only a t-test to demonstrate that a departure from additivity has occurred.
RESULTS
At doses where cocaine alone produced no vasoconstriction, it potentiated the vasoconstriction produced by all three alpha agonists, a clear indication of synergism between cocaine and these agents. Higher doses of cocaine in combination with alpha adrenoceptor agents gave an inverted-U shaped (hormetic) dose-effect curve, i.e., dose-related relaxation at higher doses. The hormetic dose-effect relation was analyzed using computational methodology based on dose equivalence to derive the unknown second component of action that causes relaxation.
CONCLUSIONS
Cocaine exhibits both vasoconstricting and vasorelaxant effects. This relaxing component, possibly related to activation of myosin light chain phosphatase, was quantified as a dose-effect curve. Most important is the synergism between cocaine and alpha-adrenoceptor stimulation which cannot be explained as an action due to reuptake inhibition, and has not been previously described.
Keywords: synergy, dose equivalence, isobole, cocaine, alpha adrenoceptor
1. INTRODUCTION
Cocaine abuse is a major public health issue, responsible for 488,101 emergency department visits (Drug Abuse Warning Network [DAWN], 2010) and around 148,000 admissions to addiction treatment facilities (Treatment Episode Data Set [TEDS], 2010) each year in the US. Also, cocaine intoxication is the most frequent cause of drug-related death reported by US medical examiners (Phillips et al., 2009). Cocaine produces sympathomimetic actions via neuronal reuptake inhibition of norepinephrine at central and peripheral sites. It also inhibits the reuptake of dopamine and serotonin, blocks cation channels, and activates sigma-1 receptors (Knuepfer, 2003).
The cardiovascular system is particularly sensitive to cocaine and even acute use can cause myocardial infarction, stroke, and aneurysm. Chronic use impairs endothelium-dependent vasodilation in rat vasculature (Pradhan et al., 2005) and increases the incidence of atherosclerosis in humans (Patrizi et al., 2006), a condition associated with endothelial dysfunction. However, despite the importance, there is a paucity of studies on the direct effects of cocaine on the vasculature.
Cocaine is well known as a vasoconstrictor, and numerous studies have demonstrated its constrictor effects on cerebral (Kaufman et al., 1998) and coronary arteries (Moliterno et al., 1994). However, Li, et al. (Li et al., 2004) demonstrated a dose-dependent endothelium-independent vasodilatory effect of cocaine in rat aorta. The effect was attributed to a cocaine-dependent activation of myosin light chain phosphatase.
As part of our interest in cocaine’s effects on the vasculature and its potential interactions with endogenous adrenoceptor agonists, we examined the responses of isolated rat thoracic aorta to cocaine alone and to cocaine in combination with three different alpha adrenoceptor agonists. Specifically, our aim was to examine cocaine’s effect on isometric tension development over a range of concentrations and to quantitatively assess cocaine’s interaction with norepinephrine, as well as with the alpha-selective agonists phenylephrine and methoxamine. In addition, we extended the cocaine dose range in combination with each adrenoceptor agonist in order to obtain a broader view of these combinations and, in so doing, utilized a novel computational method of analysis that is applicable to both monotone increasing dose effect curves and to “inverted-U shaped” (hormetic) dose effect curves (Tallarida et al., 2010). This quantitative methodology is based on the concept of dose equivalence (Tallarida and Raffa, 2010), a fundamental principle that is the basis of the well-known isobolographic approach for examining drug-drug interactions for synergistic and antagonistic interactions. Details of the isobolar approach may be found in several reviews (Raffa et al., 2007; Tallarida, 2001, 2006; Tallarida and Raffa, 2010), but the main points applicable to the current communication are briefly summarized here in a section 2.4. Cocaine has been reported to have an action on the endothelium that results in the release of the potent vasoconstrictor peptide endothelin-1 (Wilbert-Lampen et al., 1998), and decreased nitric oxide release, via inhibition of Ca2+-ATPase (Togna et al., 2001). For this reason, both endothelium intact and denuded rings were studied.
2. MATERIALS AND METHODS
2.1. Chemicals and animals
All chemicals were obtained from Sigma Aldrich (St. Louis, MO). Cocaine HCl was obtained from the National Institute for Drug Abuse (Bethesda, MD). Male Sprague Dawley rats weighing 250 – 350 g were obtained from SAGE (Boyertown, PA). They were housed and treated in accordance with Temple University Institutional Animal Care and Use procedures.
2.2 Isometric tension in aortic rings
Following sacrifice of the rats via CO2 asphyxiation, the thoracic aorta was excised and placed in ice-cold Krebs solution (a bicarbonate-buffered physiological salt solution with pH of 7.4) bubbled with a 5%CO2/95% O2 gas mixture. Salt concentrations were 120 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, and 10 mM dextrose. The thoracic aorta, from just below the aortic arch, was dissected and gently cleaned of extraneous connective tissue. Care was taken to avoid damage to the endothelium. The vessel segment was then cut into rings of approximately 3 mm length. To produce denuded preparations, the lumen of the ring was gently rubbed with a wooden dowel. Next, rings were hung between two stainless steel hooks in a water-jacket 15 mL organ chamber kept at 37°C. One hook was anchored to a fixed position, and the other attached to a force-displacement transducer (model FT03, Grass Instruments) mounted on a micrometer for manual adjustment of tension. Isometric tension developed by the rings in response to drug administration was detected by force transducers, and sent via a signal amplifier to an analog-to-digital converter (PowerLab, ADInstruments) connected to a PC. The data were collected and visualized with Chart software (ADInstruments), and dose response parameters analyzed using KaleidaGraph 4.1 (Synergy Software). After an equilibration period of 30 min, the basal tension was adjusted to 2 g, and 30 min later the tissues were exposed to 120 mM KCl (an equimolar swap for NaCl in Krebs). To ensure consistency, the KCl response was performed a second time, after washing three times and an additional 30 min period of recovery. In cases where the maximum KCl values differ by >10%, rings are discarded. To verify functional status of the endothelium, 1 µM phenylephrine was added to produce contraction, followed by 10 µM carbachol to produce endothelium-dependent vasodilation. Endothelium intact rings routinely relaxed >80% of KCl-induced tension in response to carbachol. Response <10% was the criteria for the endothelium denuded group. Following three additional washes and 30 min recovery time, phenylephrine or methoxamine were added at doses at that gave measurable effect. This dose differed for endothelium intact vs. denuded conditions. Approximately fifteen min after preconstriction with phenylephrine or methoxamine, cocaine was added in a cumulative fashion starting with a dose of 10 µM, in half log increments to 1 mM after the previous the tension had stabilized, or ten min when no change was observed. Because the tension produced by norepinephrine was not stable over time, the dose of cocaine indicated was added prior to a cumulative dose response curve for norepinephrine, from 1 nM to 10 µM.
2.3 Curve-fitting and statistics
Where curve fitting was needed (as used in second component analysis), the standard hyperbolic dose-effect curve given by E= (Emax D)/(D+C) was employed where E is the effect, D is the dose, Emax is the effect upper limit, and C is a constant representing potency (dose of the drug yielding its half-maximal response).
2.4 Dose equivalence and applications of theory
The theory that underlies the analysis is based on the concept of dose equivalence, a topic covered in detail in Tallarida and Raffa (2010), and briefly summarized here. This concept is the basis of isobolographic theory and, thus, we first show here how this applies and how it is used in the current analysis. The concept is best described in terms of two agonist drugs (here termed drug A and drug B) with overtly similar effects, e.g., each capable of producing tension in a muscle ring (Lamarre et al., 2011). At any level of tension that is common to both drugs, one determines the individual doses that produced the effect. Let’s say these are a and b and therefore represent the potencies of the drugs. Thus, the drug-A equivalent of dose a is b, and vice versa. When the individual dose-effect curves are fitted according to the usual model, E = Emax a / ( a + CA) and E = Emax b /(b + CB ), it follows that the drug-B equivalent of dose a of drug A is easily calculated as beq(a) = a CB/CA . This concept of dose equivalence is what leads to the common isobole (for 50% Emax level) as we now show. The dose pair (a,b) that defines the isobole has doses b + beq(a) such that this sum must equal CB; hence, b + beq(a) = b + a CB/CA = CB. When re-arranged this gives the familiar linear form: b/Cb+ a/Ca= 1.
All points on the isobole are dose pairs that give the 50% effect when there is no interaction and these are termed “additive.” Lesser dose pairs that give this effect would plot below the isobole and indicate synergism, whereas those that plot above show antagonism. While the isobolar approach (which uses a specified effect level) is quite common, it is also possible to view the drug combination on the effect scale. In this approach the additive effect of combination (a,b) may be calculated from either the drug-B equation or the drug-A equation that are given above. For example, if the drug B equation is used, we substitute for its dose the total, b + beq(a) and calculate E from this total in the drug B equation. This gives the expected (additive) effect. The additive effect is then compared with the observed effect of the combination and the difference between these effects becomes the basis for assessing synergism. If one of the two drugs is devoid of activity (say drug A) then the expected effect of the combination equals the effect of the active drug’s dose and, thus, a greater observed effect means synergism.
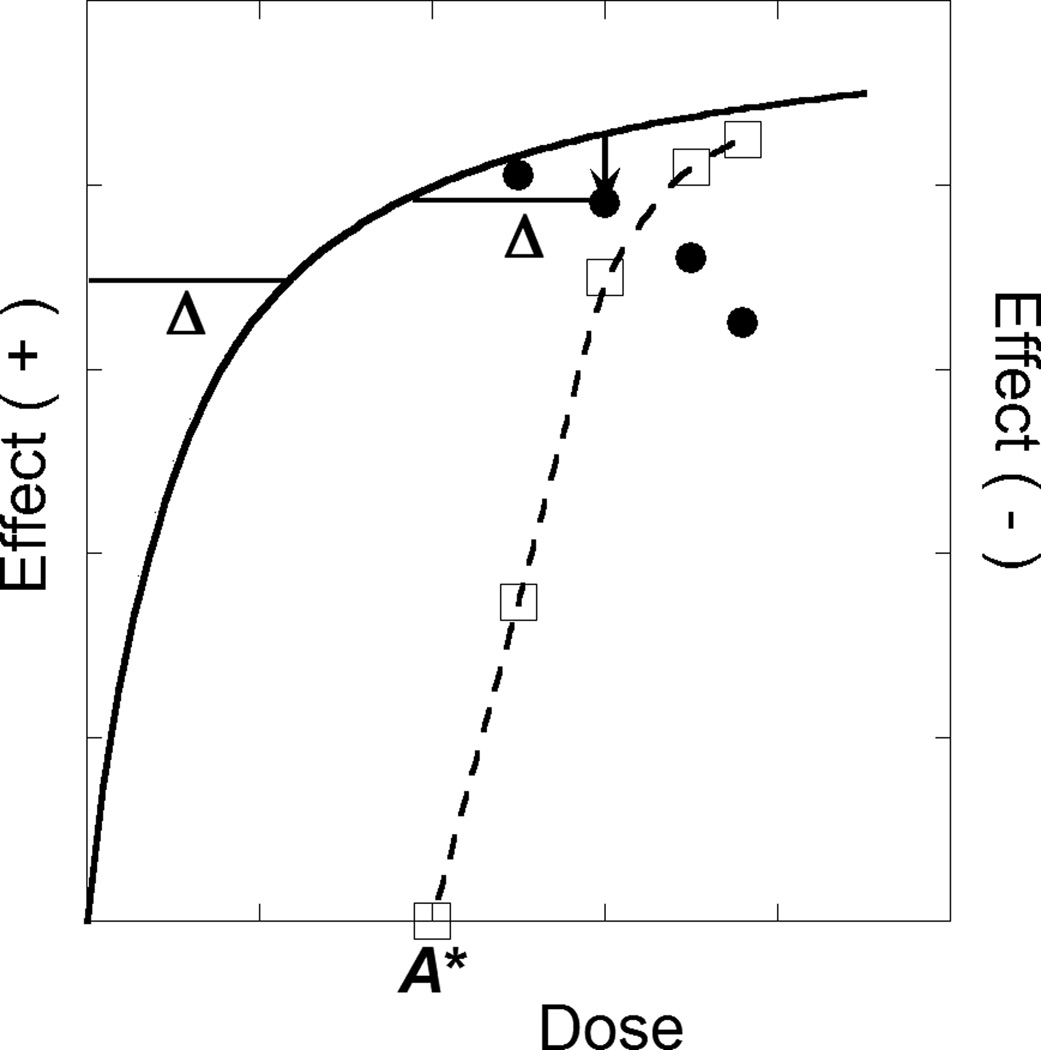
A drug that displays an inverted-U shape dose effect curve allows an analysis that also uses dose equivalence (Lamarre et al., 2011; Tallarida, 2011, 2010). In this case the “drop” in effect is viewed to result from an unknown cause with effects in the opposite direction to those of the primary drug. In this case an effect magnitude and its negative equivalent are such that the effective total dose is b – beq(a). While the beq is not known a priori, it is determinable from the magnitude of the drop and the drug’s total dose-effect curve as illustrated in Figure 1 with specific numerical results of this analysis in Supplementary Material (Figure S1)1.
Figure 1.
This figure illustrates the analysis of an inverted-U dose-effect curve. The dose-effect curve (solid) is shown to be monotone increasing for doses up to A*, whereas for greater doses the effect decreases due to a second component of action. The broken curve, whose effect magnitudes are indicated on the right ordinate scale, show the decreasing effects for doses greater than A*. The combination of the decreasing and increasing components of action produce the inverted U shape. A representative drop (a ordinate its vertical location gives the effect value (open square). All drops are analyzed in the same way and produce the set of open squares through which we constructed the broken curve shown.
3. RESULTS
3.1 Cocaine interactions with alpha adrenoceptor agonists
The addition of cocaine to the isolated tissues produced virtually no change in isometric force over the cocaine concentration range 0 to 100 µM (Figure 2A). For greater doses (333 µM and above) cocaine produced a more variable response, from between 0 and 50% of Emax (not shown in the figure).
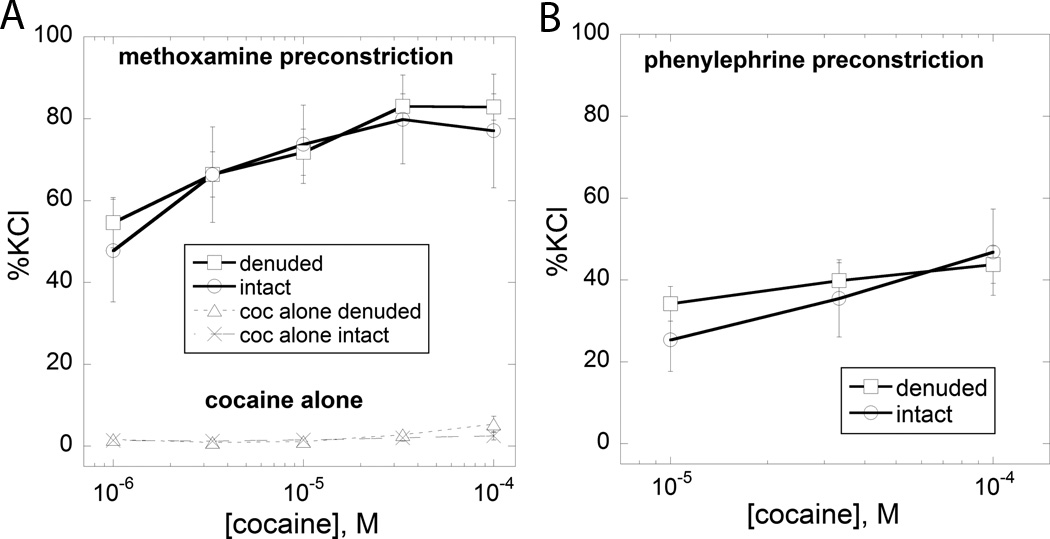
Figure 2.
Each adrenoceptor agonist was given at a fixed dose that produced a measurable force development. For each we show the effect of the dose on the ordinate and also show the magnitude of the increase produced by the addition of cocaine to this fixed dose of agonist. (A) is methoxamine (denuded N=5, intact N=3 at each dose). Also shown is the dose-effect curve for cocaine alone (open triangles, N=7 or greater at each dose) and it is seen that cocaine alone produces no effect for doses up to 100 µM. (B) is phenylephrine (N=7 or greater at each dose). Results are shown for aortic rings with denuded endothelium and for endothelium intact tissues.
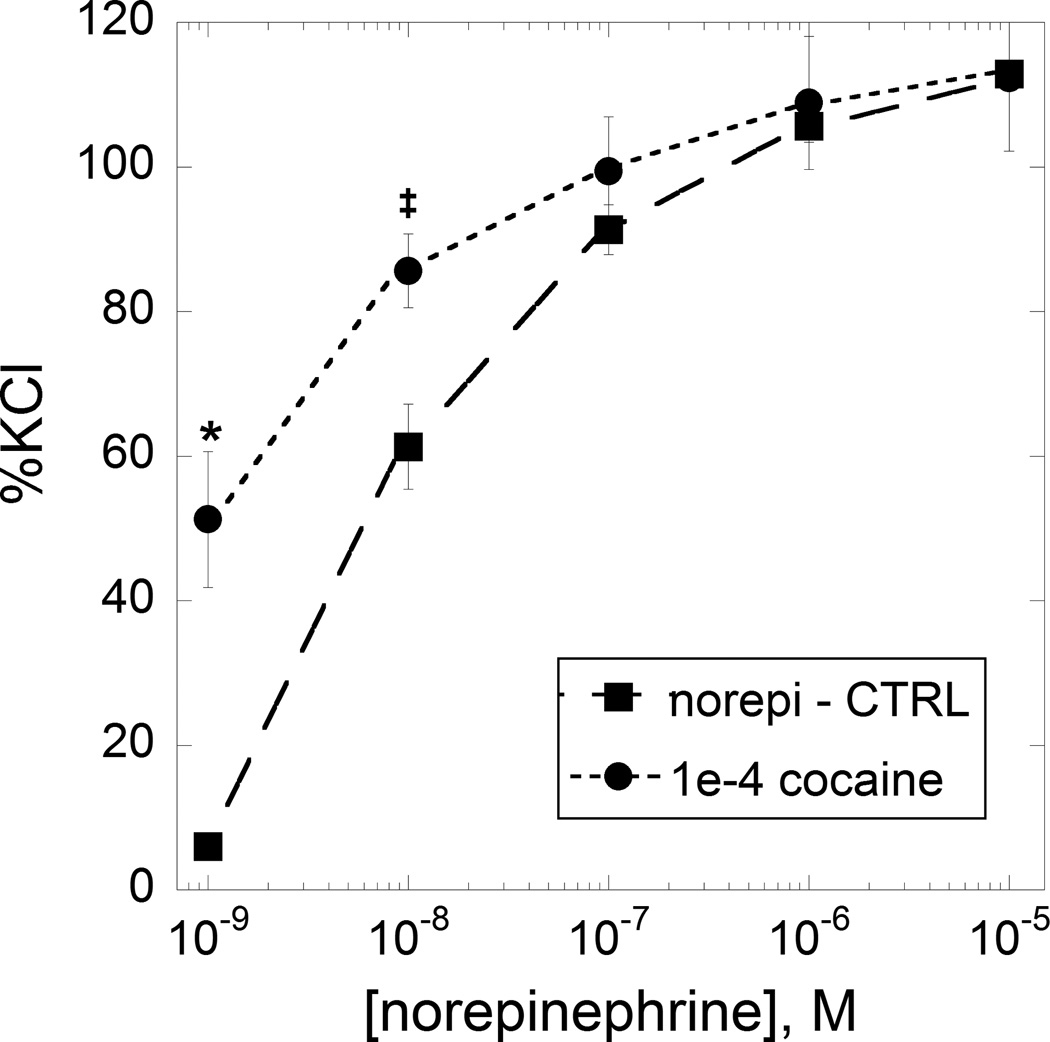
As seen in the Figure 2A, combinations of varying doses of cocaine and a single constricting dose of methoxamine produced clear dose-dependent increases, up to 100 µM. Figure 2A also shows the (lack of) effect of cocaine in the range up to 100 µM. The increased force due to cocaine addition is quite evident. Because cocaine in this dose range is devoid of constrictor activity, this increase in force indicates a synergistic interaction between it and methoxamine. Phenylephrine was also tested in combination with cocaine, but this time at a dose that produced a different tension than that of the methoxamine test. That combination also produced a prominent response as seen in Figure 2B, again demonstrating synergism (with specific calculations given in Appendix A. Synergism analysis). The methoxamine result is especially interesting because methoxamine has very low affinity for reuptake mechanisms (Trendelenburg et al., 1970). We repeated the combination experiment with norepinephrine. Because this agent’s tension development (when the sole agent) declines rapidly, we administered the dose of cocaine (100 µM) 10 min before the cumulative additions of norepinephrine. This combination was also synergistic resulting in an upward displacement of the dose-effect curve as shown in Figure 3. It is seen that all three adrenoceptor agonists in combination with cocaine in the range 0 to 100 µM prominently increased developed force. These results are an indication of a synergistic interaction between cocaine and the adrenoceptor agents. Especially interesting is the synergy seen with methoxamine. This is an alpha-1 agonist that is without action on reuptake (Trendelenburg et al., 1970) and yet this agent's combination response to cocaine gave the same result seen with phenylephrine and norepinephrine. In fact, the results with methoxamine were quite similar, in all respects, to the results obtained with phenylephrine and norepinephrine.
Figure 3.
The dose-effect curve of norepinephrine alone is shown (closed squares) and the same norepinephrine doses in the presence of 100 µM cocaine (closed circles). N=4 at each dose. * denotes P < 0.01; ‡ denotes P < 0.05 significance level vs norepinephrine alone.
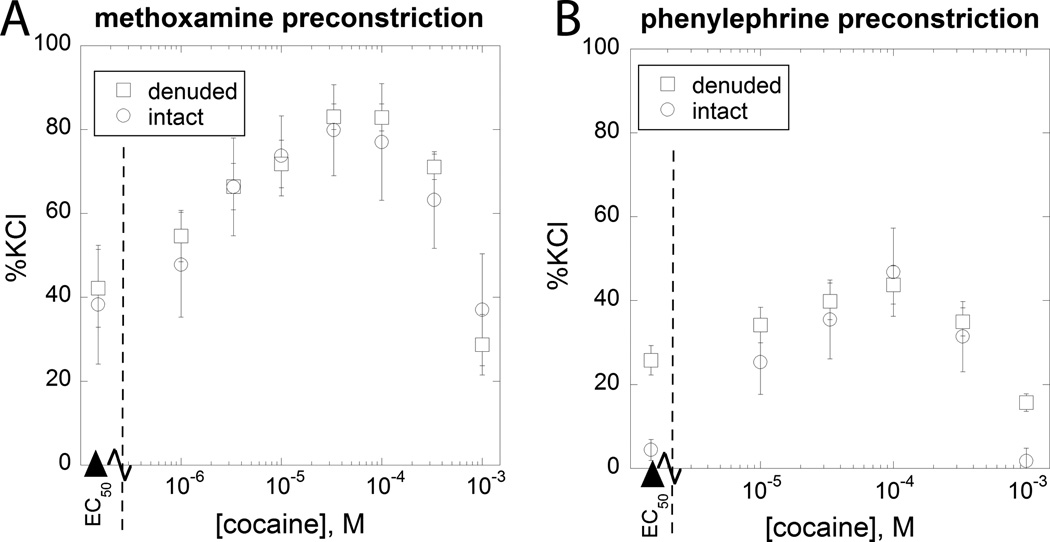
A drop in developed tension at the higher cocaine doses (330 and 1000 µM) occurred, as seen in Figure 4 and these provide an example of an inverted-U shaped dose-effect curve. This shape, most likely due to a second (relaxing) component of action was analyzed for both methoxamine and phenylephrine using our previously developed dose equivalence methodology (Lamarre et al., 2011; Tallarida et al., 2010; Tallarida and Raffa, 2010) and is further discussed below.
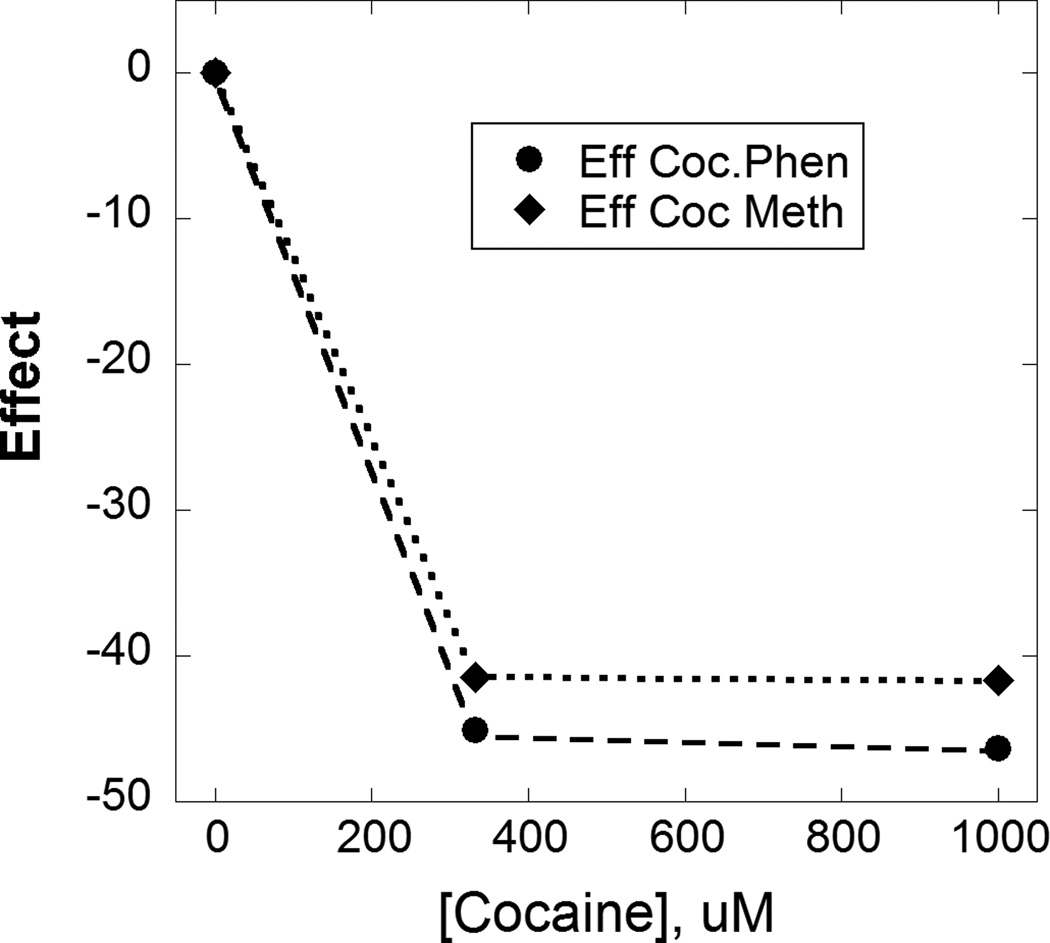
Figure 4.
The effect of a more extended dose range of cocaine is shown for two different cocaineagonist combinations in both endothelium denuded and intact aortic tissue, (A) phenylephrine (N=7 or greater at each dose) and (B) methoxamine (N=3 or greater at each dose). In both cases it is seen that this extended range of cocaine, in combination with the fixed dose of the adrenoceptor agent, produces relaxation at the higher cocaine doses. These U-shaped dose effect curves, presumably due to activation of a second component of action, were analyzed further and the results of that analysis are shown in Fig 5.
3.2 Cocaine U-shaped dose-response: Second component analysis
For cocaine concentrations greater than 100 µM, its combined use with either fixed phenylephrine or methoxamine dosing showed a prominent decrease in force that was analyzed with the application of dose equivalence methodology. It is seen that above 100 µM this “second component” of relaxing action is evident. When analyzed with methodology for inverted U-shaped dose effect curves, this second component’s dose effect curve was derived and is shown in Figure 5. This relaxation may be due to activation of MLC phosphatase by cocaine (Li et al., 2004). The role of MLC phosphatase is well known and is summarized in Webb (2003). MLC phosphatase removes the high-energy phosphate from the light chain of myosin to promote smooth muscle relaxation. The analysis provided here, previously described and applied inLamarre et al. (2011), provides the dose-effect relation of this postulated MLC phosphatase action (which is not derived by simple subtraction of the parent dose-effect curves). The observed effects in endothelium denuded rings was similar in endothelium intact rings, however the magnitude of the contractile responses were attenuated.
Figure 5.
Shown here are the derived dose-effect relations for phenylephrine (circles) and methoxamine (diamonds) as shown in Figure 4 (for simplicity of presentation we show here only the results from the endothelium denuded aortic rings). The dose-effect relation for each is quite similar and is indicative of a second component of action that becomes evident at higher cocaine doses. (See text for description of the quantitative analysis)
4. DISCUSSION
The aim of our study was to examine cocaine in combination with several different alpha agonists and that effort revealed a synergistic interaction between cocaine and each agonist. But also interesting is the observation that cocaine, in the dose range used here, is synergistic but it alone does not produce tension in the rat aorta in the dose range used. In this regard, it is noted that the rat aorta preparation (used here) is devoid of adrenergic nerves, a finding known from the histochemical studies ofPatil et al. (1972). They made a thorough examination and found medio-adventitial adrenergic nerves in the aorta from rabbit, cat and guinea pig; however, no adrenergic nerves were seen in the rat thoracic aorta. This absence of adrenergic nerves implies that cocaine’s well-known action (inhibition of reuptake) would not result in constriction, an observation that is also consistent with the findings of Webb and Vanhoutte (1981) who showed that cocaine does not constrict arteries when they are acutely denervated in a cocaine dose range comparable to ours and that cocaine produces only modest alpha mediated constriction at greater cocaine doses. Thus, while reuptake is certainly a major mechanism for tension in vivo, our findings (especially the results with methoxamine) reveal an additional, and previously unknown component of cocaine action, viz., a synergism with alpha adrenergics in the rat aortic preparation. As is often the case when synergism is first detected, we do not know the precise mechanism but the very detection of synergism is an important first step in exploring mechanism. In that regard we theorize that the alpha receptor is an important target of cocaine and that this synergy might be a clue in aiding our understanding of the phenomenon of cocaine-induced vasoconstriction in coronary arteries.
There are several ways to examine synergism; some use several doses of each compound, an approach that is often used when isobolographic methods are employed. However, when one of the drugs (in this case cocaine) shows no effect over the dose range used it is simpler (and clearer to most readers) to view the combination in terms of the effect (Tallarida, 2006; 2007; 2012, 2012a; Tallarida and Raffa, 2010). In this approach, which is theoretically equivalent to the isobolar approach, the expected combination effect is compared to the actual combination effect and, because one drug (viz., cocaine) produced no effect, the expected effect is of magnitude produced by the single adrenergic agent for the dose used. In this regard we used a single dose of phenylephrine that produced a measurable and sustained effect and then we added graded doses of cocaine which are expected to maintain the same effect of this adrenergic agonist. In our other experiment, we used a single dose of methoxamine that was chosen to give a measurable and steady effect that was even greater than that of phenylephrine and then again administered graded cocaine doses, expecting the same effect as the sustained methoxamine effect. But in both cases the addition of cocaine produced an effect that was greater than the expected, and this case (when one of the drugs lacks efficacy) is a clear indicator of synergism. We altered the experimental procedure with norepinephrine only because a single dose of this agent does not maintain a steady tension (as mentioned in RESULTS) so that we demonstrated the synergistic effect of cocaine’s single dose by showing the elevation in the norepinephrine dose-effect curve (Figure 3). Different experimental designs are used in drug combination analyses and these are dictated by the attributes of the drug pair being tested. Further details on the synergism analysis used here are given in the Supplementary Material2 and more comprehensive discussions of the mathematical analysis may be found in the reviews cited above. The principle aim of this study was the quantitative examination of the interaction between cocaine and the several alpha-adrenergic agents, and our results showed a synergistic interaction that occurs in normal aortic rings and in rings with denuded endothelium. But also interesting is the decline in effect that comes with combinations of cocaine in the higher dose range.
At the higher cocaine dosing range the cocaine addition to the fixed dose adrenoceptor agonist caused a prominent decline in developed force. This decline, analyzed here with the quantitative methodology of dose equivalence, yielded a dose-effect relation for the relaxing effect which is postulated to be due to MLC phosphatase (Li et al., 2004). The relaxation dose-effect curve for this second component was obtained for cocaine addition to phenylephrine and also for methoxamine, and these yielded similar curves (Figure 5).
Our derivation of the second (relaxing) component’s dose-effect relation is a new result and follows from analysis of the inverted-U dose effect curve. Also, we know of no quantitative prior studies of this kind that demonstrated synergism between cocaine and alpha adrenoceptor compounds, although Kalsner and Nickerson (1969) studied adrenergic combinations with cocaine in rabbit aorta, and found a prominent enhancement of the effect. Our analysis of the itneraction, like all findings that show synergistic interactions, is based on the specific end point (effect) examined, in this case isometric force development and the lack of efficacy by cocaine alone in the dose range studied. This endpoint (isometric force) is quite easy to measure and that fact allowed the quantitative analysis showing synergism. Synergism is not concluded here for any other joint action for cocaine and these alpha agents. While we emphasize that this detection of synergism is based only on this one effect, it is interesting to note that there are reports of cocaine and alpha adrenoceptors based on behavioral endpoints. For example, cocaine sensitization, the increased activity and stereotyped behaviors that result from repeated administration of cocaine, is modulated in different stages of cocaine sensitization by alpha adrenoceptor receptors (Jimenez-Rivera et al., 2006) and it has been shown that cocaine facilitates the effects of alpha-agonists and antagonists on adrenoceptor neurotransmission (Nigro and Enero, 1981). Also, activation of the alpha 1-noradrenoceptor system is associated with increased motivation for cocaine in rats with extended access (Wee et al., 2008). Although there is no clear connection between behavioral, neurotransmission and force development, our finding of synergism on one endpoint suggests a path for further exploration of this drug combination in behavioral studies of cocaine and alpha adrenoceptor compounds.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
REFERENCES
- DAWN. Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality; 2010. [accessed on August 1 2012]. http://www.samhsa.gov/data/DAWN.aspx. [Google Scholar]
- Jimenez-Rivera CA, Feliu-Mojer M, Vazquez-Torres R. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann. N. Y. Acad. Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Kalsner S, Nickerson M. Mechanism of cocaine potentiation of responses to amines. Br. J. Pharmacol. 1969;35:428–439. doi: 10.1111/j.1476-5381.1969.tb08284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Ross MH, Lange N, Rose SL, Kukes TJ, Mendelson JH, Lukas SE, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279:376–380. [PubMed] [Google Scholar]
- Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol. Ther. 2003;97:181–222. doi: 10.1016/s0163-7258(02)00329-7. [DOI] [PubMed] [Google Scholar]
- Lamarre NS, Parry T, Tallarida RJ. On the quantitation of an agonist with dual but opposing components of action: application to vascular endothelial relaxation. Eur. J. Pharmacol. 2011;670:204–207. doi: 10.1016/j.ejphar.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Su J, Sehgal S, Altura BT, Altura BM. Cocaine-induced relaxation of isolated rat aortic rings and mechanisms of action: possible relation to cocaine-induced aortic dissection and hypotension. Eur. J. Pharmacol. 2004;496:151–158. doi: 10.1016/j.ejphar.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Moliterno DJ, Willard JE, Lange RA, Negus BH, Boehrer JD, Glamann DB, Landau C, Rossen JD, Winniford MD, Hillis LD. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N. Engl. J. Med. 1994;330:454–459. doi: 10.1056/NEJM199402173300702. [DOI] [PubMed] [Google Scholar]
- Nigro D, Enero MA. Cocaine facilitates the effects of alpha-agonists and antagonists on adrenergic neurotransmission. Gen. Pharmacol. 1981;12:255–259. doi: 10.1016/0306-3623(81)90054-9. [DOI] [PubMed] [Google Scholar]
- Patil PN, Fudge K, Jacobowitz D. Steric aspects of adrenergic drugs. 18. -Adrenergic receptors of mammalian aorta. Eur. J. Pharmacol. 1972;19:79–87. doi: 10.1016/0014-2999(72)90079-9. [DOI] [PubMed] [Google Scholar]
- Patrizi R, Pasceri V, Sciahbasi A, Summaria F, Rosano GM, Lioy E. Evidence of cocainerelated coronary atherosclerosis in young patients with myocardial infarction. J. Am. Coll. Cardiol. 2006;47:2120–2122. doi: 10.1016/j.jacc.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Phillips K, Luk A, Soor GS, Abraham JR, Leong S, Butany J. Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options. Am. J. Cardiovasc. Drugs. 2009;9:177–196. doi: 10.2165/00129784-200909030-00005. [DOI] [PubMed] [Google Scholar]
- Pradhan L, Dabisch PA, Liles JT, Murthy SN, Baber SR, Simpson SA, Agrawal KC, Kadowitz PJ. Effect of binge cocaine treatment on hindlimb vascular function. J. Appl. Toxicol. 2005;25:479–490. doi: 10.1002/jat.1083. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Stagliano GW, Tallarida RJ. Nonlinear isobologram and superadditive withdrawal from cocaine: cannabinoid combinations in planarians. Eur. J. Pharmacol. 2007;556:89–90. doi: 10.1016/j.ejphar.2006.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism: its detection and applications. J. Pharmacol. Exp. Ther. 2001;298:865–872. [PubMed] [Google Scholar]
- Tallarida RJ. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Interactions between drugs and occupied receptors. Pharmacol. Ther. 2007;113:197–209. doi: 10.1016/j.pharmthera.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Cowan A, Raffa RB. On deriving the dose-effect relation of an unknown second component: an example using buprenorphine preclinical data. Drug Alcohol Depend. 2010;109:126–129. doi: 10.1016/j.drugalcdep.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Raffa RB. The application of drug dose equivalence in the quantitative analysis of receptor occupation and drug combinations. Pharmacol. Ther. 2010;127:165–174. doi: 10.1016/j.pharmthera.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togna GI, Graziani M, Russo P, Caprino L. Cocaine toxic effect on endothelium-dependent vasorelaxation: an in vitro study on rabbit aorta. Toxicol. Lett. 2001;123:43–50. doi: 10.1016/s0378-4274(01)00379-4. [DOI] [PubMed] [Google Scholar]
- Trendelenburg U, Maxwell RA, Pluchino S. Methoxamine as a tool to assess the importance of intraneuronal uptake of l-norepinephrine in the cat's nictitating membrane. J. Pharmacol. Exp. Ther. 1970;172:91–99. [PubMed] [Google Scholar]
- Webb RC. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003;27:201–206. doi: 10.1152/advan.00025.2003. [DOI] [PubMed] [Google Scholar]
- Webb RC, Vanhoutte PM. Cocaine and contractile responses of vascular smooth muscle from spontaneously hypertensive rats. Arch. Int. Pharmacodyn. Ther. 1981;253:241–256. [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur. Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbert-Lampen U, Seliger C, Zilker T, Arendt RM. Cocaine increases the endothelial release of immunoreactive endothelin and its concentrations in human plasma and urine: reversal by coincubation with sigma-receptor antagonists. Circulation. 1998;98:385–390. doi: 10.1161/01.cir.98.5.385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.