Abstract
The cerebral blood supply is delivered by a surface network of pial arteries and arterioles from which arise (parenchymal) arterioles that penetrate into the cortex and terminate in a rich capillary bed. The critical regulation of cerebral blood flow, locally and globally, requires precise vasomotor regulation of the intracerebral microvasculature. This vascular region is anatomically unique as illustrated by the presence of astrocytic processes that envelope almost the entire basolateral surface of parenchymal arterioles. There are, moreover, notable functional differences between pial arteries and parenchymal arterioles. For example, in pial vascular smooth muscle cells (VSMCs), local calcium release events (“calcium sparks”) through ryanodine receptor (RyR) channels in sarcoplasmic reticulum membrane activate large conductance, calcium-sensitive potassium (BK) channels to modulate vascular diameter. In contrast, VSMCs in parenchymal arterioles express functional RyR and BK channels, but under physiological conditions these channels do not oppose pressure-induced vasoconstriction. Here we summarize the roles of ryanodine receptors in the parenchymal microvasculature under physiologic and pathologic conditions, and discuss their importance in the control of cerebral blood flow.
Keywords: brain parenchymal arteriole, cerebral blood flow, acidosis, ryanodine receptor, potassium channel
Introduction
In vascular smooth muscle cells (VSMCs), a global increase in cytoplasmic calcium concentration ([Ca2+]i) leads to vasoconstriction by activation of Ca2+/calmodulin-dependent myosin light-chain kinase. However Ca2+ is a versatile second messenger, and vasomotor pathways involving local Ca2+ signals, such as Ca2+ sparks encoded by ryanodine receptors (RyRs), or propagated Ca2+ signals in the form of Ca2+ waves, have been the subject of many recent studies. The topic of Ca2+ signaling in the microcirculation is one of growing interest, particularly in the cerebral parenchyma as brain integrity is critically dependent on constantly adapting blood perfusion. Arteriolar tone significantly depends on the Ca2+ entry through ion channels of the VSMC plasma membrane, and also on the Ca2+-dependent vasodilator influences of endothelial cells. However these aspects of microvascular Ca2+ signaling are beyond the scope of the current review. The goal of this review is to provide a synopsis of current knowledge regarding RyR-dependent Ca2+ signals in smooth muscle cells of the brain parenchymal microvasculature and how these signals influence cerebral blood flow (CBF).
Part 1: Regulation of cerebral blood flow by parenchymal arterioles
Within the brain microcirculation, the parenchymal arterioles (PAs) are fundamental regulators of CBF. PAs account for approximately 40% of the total cerebral vascular resistance (33). Thus regulation of their diameter is a central process for (i) maintaining constant perfusion of brain tissue across a range of systemic arterial pressures (cerebrovascular autoregulation), and (ii) during neurovascular coupling (NVC), i.e. the mechanisms by which functional hyperemia occurs in the brain (34, 58).
1.1 Myogenic tone and cerebrovascular autoregulation
A function of arteries and arterioles is to constrict and relax in response to changes in intravascular pressure. An increase in intraluminal pressure constricts cerebral arteries and arterioles. A decrease in intravascular pressure has the opposite effect, inducing vasodilation. This essential regulatory mechanism, known as the vascular myogenic response was first described more than one hundred years ago (9) and ensures that blood flow remains nearly constant during moment-to-moment fluctuations in arterial pressure. PAs are the last smooth muscle-containing vessels upstream of the capillary bed and play a critical role in maintaining appropriate capillary perfusion pressure under both normal and pathologic conditions (33, 55). Additional anatomical features of the brain microcirculation, particularly the limited collateral supply blood flow, which has a so-called “bottleneck” effect on perfusion of the neocortex (87), further emphasize the relative importance of this part of the intracerebral microcirculation and its control mechanisms including those involved in regulation of myogenic tone.
The precise nature of the mechanisms and modulators of myogenic vasoconstriction is still a matter of some debate. However, myogenic constriction occurs in blood vessels studied in isolation, demonstrating that mechanisms intrinsic to the vascular wall are sufficient to induce this response (90, 119). Disruption of the endothelium does not impair pressure-induced constriction suggesting that both sensor and effector mechanisms responsible for the myogenic response reside within smooth muscle cells (75). In turn, it is broadly accepted that the pressure-induced constriction involves a depolarization of the arterial myocyte cell membrane (52) that activates voltage-dependent Ca2+ channels (VDCC), resulting in Ca2+ influx and subsequent vasoconstriction (62). Interestingly, compared with pial arteries, PAs depolarize and constrict to lower levels of intravascular pressure leading to a high amount of tone (between 30% and 40% at 40 mm Hg) (23, 29, 32, 51, 86, 88). Physiologically, this creates a high “vasodilator reserve”, i.e. a substantial capacity of these arterioles to dilate in response to locally generated vasodilator signals. This is particularly adapted to CBF regulation and the functional hyperemic response, as the vascular resistance is inversely proportional to the fourth power of the arterial radius according to the Hagen-Poiseuille equation.
1.2 The neurovascular unit regulates CBF locally
The ability of the brain to coordinate neural activation state with local CBF has been recognized since the end of the nineteenth century (102). This linkage is called neurovascular coupling. It underlies functional hyperemia in the brain, which ensures that local increases in metabolic demand are satisfied by increased substrate delivery by the blood. Impairment of this coupling between neuronal metabolism and cerebral perfusion, even if brief, generally trigger dramatic consequences. Anatomic and functional observations support a role of capillaries in functional hyperemia (59, 94). However, PAs are thought to mediate a large part of the local CBF increase in response to increased neuronal activity, due to their high level of vascular resistance and ability to dilate rapidly in response to a variety of endogenous substances (34, 37, 44, 110, 133). In contrast to pial arteries, PAs are not supplied with perivascular nerves (49) but are wrapped in astrocytic processes, called endfeet, which cover nearly their entire basolateral surface (58, 106). Studies over the last ten years have illuminated the importance of this anatomical configuration in mediating NVC. Synaptic release of neurotransmitters such as glutamate during brain neuronal activity stimulates metabotropic receptors on astrocytes and generates an increase in cytoplasmic Ca2+ which propagates through astrocytic processes by activation of inositol 1,4,5-trisphosphate receptors (InsP3Rs), ultimately elevating [Ca2+]i in the endfeet. There, this Ca2+ signal activates pathways leading to dilation of the PAs, involving various factors such as locally released K+ ions, adenosine, prostaglandins or epoxyeicosatrienoic acids (EETs) (37, 58, 96, 108). Neurons also release several substances able to regulate CBF such as nitric oxide (NO), prostanoids and vasoactive neurotransmitters (5, 57). Therefore neurons, astrocytes, myocytes and endothelial cells form a neurovascular unit that collectively controls local blood flow. These various pathways leading to vasodilation all act, to some extent, by hyperpolarizing the VSMC membrane which deactivates VDCC and then decreases [Ca2+]i.
Thus, it is clear that the intracerebral microcirculation participates actively and substantially in the global and local regulation of CBF. The control of parenchymal arteriolar diameter by the VSMCs within the vessel wall is a process that is fundamental to this regulation. In turn, regulation of VSMC tone depends on variations in cytoplasmic [Ca2+]i. Therefore, understanding the regulation of [Ca2+]i in smooth muscle cells of PAs is of central importance.
Part 2: Roles of the sarcoplasmic reticulum, inositol 1,4,5-trisphosphate receptors and ryanodine receptors in regulation of parenchymal arterioles
2.1 The sarcoplasmic reticulum and regulation of intracellular Ca2+
The sarcoplasmic reticulum (SR) is constantly involved, through the release and re-uptake of Ca2+ into the cytoplasm, in vascular smooth muscle Ca2+ homeostasis (123). At low intraluminal pressure (e.g. 10 mm Hg), VSMC [Ca2+]i has been estimated to be ~120 nM (62, 88). The sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) transports Ca2+ from the cytoplasm into the SR. The presence in the SR lumen of the Ca2+ binding proteins, calreticulin and calsequestrin (65), also facilitates the active pumping of Ca2+ against its gradient by decreasing the luminal Ca2+ concentration (123). As a result, the available Ca2+ in the SR is significant, and therefore the opening of SR Ca2+ channels leads to the release of Ca2+ into the cytosol (54, 97).
Various smooth muscle cellular functions, including contraction, relaxation, proliferation and differentiation, involve Ca2+ release from the SR (13, 54, 123). Two families of SR Ca2+ release channels have been identified so far: the InsP3R and the RyR.
2.2 Inositol 1,4,5-trisphosphate receptors
The InsP3R consists of 4 subunits with a molecular weight of ~310 kDa each (40). Three distinct genes code three different types of subunits leading to three receptor subtypes (InsP3R1-3). Although all three isoforms appear to be expressed in vascular myocytes, InsP3R1 seems to be the predominant subtype in adult VSMCs, and InsP3R2 and InsP3R3 appear to be expressed primarily during proliferative phases of cellular activity (69, 79, 83, 112, 129). The possible formation of heterotetrameric complexes has been demonstrated by co-immunoprecipitation of InsP3R expressed in cell lines (77).
All InsP3R subtypes are activated by inositol 1,4,5-trisphosphate (InsP3) (38), which is produced by phospholipase C (PLC) activation in response to Gαq/11 protein coupled receptor activation (11, 13, 40). Therefore, InsP3Rs are involved in the responses to several endogenous vasoactive molecules such as endothelin 1, acetylcholine, noradrenaline, and 5-HT (12). InsP3Rs also display a bell shaped Ca2+ sensitivity: InsP3R-Ca2+ release is potentiated at low Ca2+ concentrations (< 500 nM) whereas it is inhibited at higher Ca2+ concentrations (> 1~10 μM), with differences between subtypes (15, 38, 40, 115). These properties appear to be fundamental in the generation of Ca2+ oscillations observed in VSMCs in response to vasoconstrictors (12). Recently, in pial arteries, InsP3R-mediated Ca2+ release has been shown to activate transient receptor potential TRPM4 channels (46) and large conductance Ca2+-activated potassium (BK) channels (130). Activation of transient receptor potential TRPC3 channels by direct physical coupling with InsP3R has also been described (124).
2.3 Ryanodine receptors
Similarly to InsP3Rs, three different genes encode three different RyR subtypes (RyR1-3) and functional channels are formed by the association of four subunits (35, 50). Possible heteromeric RyR2/RyR3 and RyR2/RyR1 formations, but not RyR1/RyR3 formations, have been demonstrated by immunoprecipitation (127). To date, the RyR is the only ion channel discovered by electron microscopy (41), presumably due to the strikingly large size of RyR protein subunits (550–660 KDa) (89). RyR subunits have cytosolic C- and N-termini and between four and twelve transmembrane domains, depending on the four different models proposed (19, 111, 117, 134). The reason(s) for the well-conserved large size of the RyRs, and the associated substantial metabolic cost of production and maintenance, remain unclear but underscores the physiological importance of these proteins.
RyR1 and RyR2 have been extensively studied in skeletal muscle and cardiac muscle, respectively, (66) where they are highly expressed and play a fundamental role in excitation-contraction (E-C) coupling. RyR3 appears to be more broadly expressed, with predominance in neurons (43, 68). The three subtypes are expressed in large cerebral arteries (118) as well as in the cerebral microcirculation (Dabertrand & Brayden, unpublished observations). Their expression in VSMCs also appears to be modulated by pathologic and environmental conditions (25, 26, 30, 65, 78). The channel opening is regulated by several agents including SR Ca2+ load (35, 89). But RyRs seem regulated primarily by binding of Ca2+ on the cytosolic face of the channel. [Ca2+]i ranging from 1 to 10 μM triggers the opening of the channel, whereas millimolar [Ca2+]i inhibits it (35, 60). Consequently, RyRs can be activated by Ca2+ entry through plasma membrane Ca2+ channels or by SR Ca2+ release from adjacent InsP3Rs or RyRs (13). This mechanism, called Ca2+-induced Ca2+ release (CICR), is fundamental in cardiac contraction (66, 98). CICR has been described in several types of smooth muscle including cerebral arteries (61), in which bolus application of caffeine, which simultaneously activates all the RyRs, causes transient increases in [Ca2+]i and vasoconstriction in both pial and parenchymal arterioles (29, 63). However, in contrast to cardiac and skeletal myocytes, global RyR-Ca2+ release appears to be minimal during E-C coupling in VSMCs under physiological conditions (60, 120).
In contrast to the excitatory role of RyRs in skeletal and cardiac muscle, RyRs mediate local Ca2+-release events (Ca2+ sparks) that activate BK channels to oppose pressure-induced depolarization and contraction of cerebrovascular smooth muscle (60, 63, 85, 120). The serial linkage of RyR and BK in this negative feedback vasodilator mechanism is well established, and clearly demonstrated by the robust and non-cumulative constrictions induced by RyR and BK channel blockers in intact pressurized pial arteries (63, 64). Interestingly, small caliber pial arterioles and PAs do not constrict to BK channel blockers in vivo (91, 109) or in vitro (29, 44, 51, 56), despite functional BK channels in VSMCs of these arterioles (29, 51). This suggests that under basal conditions the functional role of RyRs in the cerebral microcirculation is distinct from pial arteries.
Part 3: Calcium signaling in parenchymal arteriolar myocytes
3.1 Waves rather than sparks in the microcirculation
Under normal conditions of pH and temperature, pressure-induced constriction of intracerebral parenchymal arterioles is poorly counterbalanced by Ca2+ spark-driven BK current (29, 51, 56). This absence of negative feedback likely contributes to the greater myogenic tone observed in PAs compared to pial arteries (23, 24, 88). Using the intracellular fluorescent Ca2+ dye Fluo-4, we found that VSMCs in pressurized PAs exhibited primarily Ca2+ waves (78% of cells) propagating through the cytoplasm over a duration of several seconds, and very few localized Ca2+ events (12% of cells) (29). Ca2+ waves and Ca2+ sparks were not observed in PAs when SR Ca2+ was depleted by cyclopiazonic acid or when the arterioles were exposed to RyR blockers (ryanodine or tetracaine). These results indicate that RyRs mediate both Ca2+ waves and Ca2+ sparks in PAs (29). However, it is premature to conclude that waves rely exclusively on RyRs with no InsP3R involvement (47). Interestingly, a similar Ca2+ signal topography, i.e. Ca2+ sparks in large arteries and Ca2+ waves in the microcirculation, has also been described in cremaster muscle feed arteries and arterioles (121, 122). However, Westcott and Jackson (2011) attributed the waves in the arterioles not to RyR, but to InsP3R activation. Differences in InsP3R and RyR subtype expression between the cremaster and the cerebral microcirculation may account for this divergence (25, 27, 28, 73, 121). Moreover, the presence of perivascular nerves in cremaster arterioles (39) may facilitate the engagement of the InsP3R in the generation of Ca2+ waves by releasing Gαq/11 protein coupled receptor agonists, and thus maintaining a high level of cytoplasmic InsP3. Receptor tyrosine kinase activation also generates InsP3 (13) and possible contributions of this signaling pathway should be considered. In contrast to cremaster arterioles, PAs lack extrinsic innervation (49) and demonstrate elevated cytoplasmic [Ca2+]i (88) which may promote RyR activation. The large variability observed in the mechanisms triggering and propagating Ca2+ waves in smooth muscle makes it difficult to develop a global model, but the variation in RyR function between large and small caliber arteries remains noteworthy and requires further detailed investigation.
In a recent study, Mufti et al. provided interesting insights into the role of the RyR-mediated Ca2+ waves in cerebral arteries. They demonstrated their importance in maintaining myogenic tone under low pressure by increasing phosphorylation of myosin light chain (82). Parenchymal arterioles depolarize and constrict to a greater extent than pial arteries at lower pressure (23, 51, 88). These observations are consistent with (i) the predominance of Ca2+ waves in PA VSMCs and (ii) the lack of effect of RyR and BK channel blockers on PA diameter. A likely explanation for the inability of Ca2+ waves to activate BK channels is that Ca2+ waves do not deliver sufficient Ca2+ to activate BK channels (95). In contrast, Ca2+ sparks deliver micromolar Ca2+ to closely apposed BK channels to cause substantial channel activation.
3.2 Local Ca2+ release initiates Ca2+ waves
The ability of Ca2+ sparks to initiate Ca2+ waves which travel through the cytoplasm following a CICR mechanism was first described in cardiac myocytes (20, 21) and is still an area of active investigation (18). Similarly, Ca2+ spark-initiated Ca2+ waves have been observed in several vascular beds including the hepatic portal vein (4, 48), small mesenteric arteries (99), and retinal arterioles (116). The prevalent model explaining the propagation of the initial Ca2+ release to the entire cell is that a local increase in Ca2+ activates neighboring RyRs because resting open state probability (Po) of RyRs is high (Figure 1) (10, 13, 16). When resting Po is low, local RyR Ca2+ release (e.g. a spark) is not sufficient to activate nearby RyRs to cause a Ca2+ wave (Figure 1) (10, 13). It is also possible that ambient InsP3 levels are elevated in PAs, and that a combination of InsP3Rs and RyRs in non-junctional SR elements is causing Ca2+ waves (47).
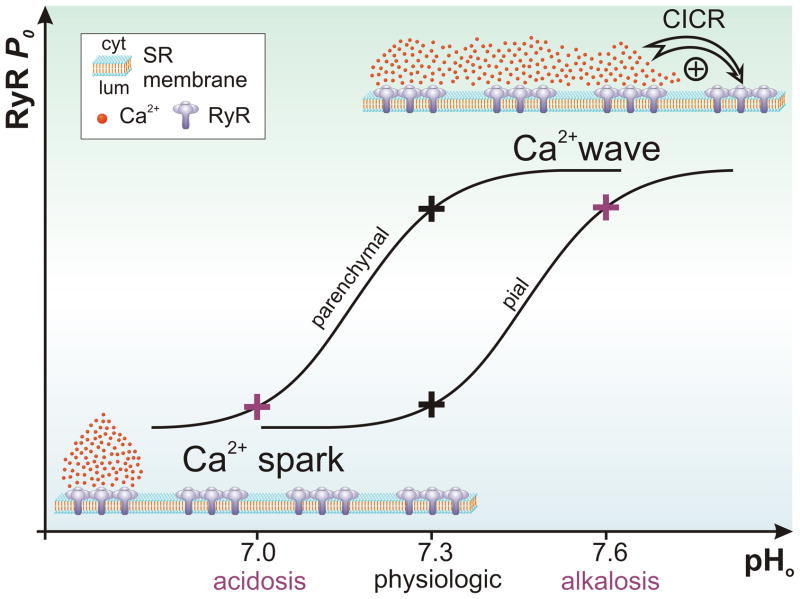
Figure 1. Effect of pH on RyR-mediated Ca2+ signals in smooth muscle cells of pressurized pial arteries and parenchymal arterioles.
By modulating RyR excitability, protons can influence the shape of the Ca2+ signals in cerebrovascular smooth muscle cells. At the physiologic pH of cerebrospinal fluid (pH=7.3) myocytes in large (pial) arteries display spatially limited and brief Ca2+ release events (sparks). When pH increases (alkalosis), RyR open probability (Po) increases as well and the Ca2+ ions released by a cluster of RyRs activate neighboring clusters, forming a Ca2+ wave by propagation of the signal. In contrast, parenchymal arterioles display Ca2+ waves under normal conditions. When pH decreases (acidosis), RyR Po is low and this spatially restricts the Ca2+ release (Ca2+ spark). This reshaping of Ca2+ signals in the brain microcirculation plays a fundamental role during the vasodilation induced by acidosis.
The fundamental role played by the RyR Po in shaping intracellular Ca2+ signals is particularly well illustrated in the observations reported by Heppner et al. in VSMCs of pial arteries (53). These investigators found that a small increase in pH (7.4 to 7.5) increases Ca2+ spark frequency. However, further increases in pH beyond 7.6, shifted Ca2+ signaling from stationary sparks to Ca2+ waves (Figure 1) (53). The simplest explanation for this phenomenon is that a decrease in intracellular proton (H+) levels increased RyR Po, and therefore the likelihood of Ca2+ waves. However, the induced Ca2+ waves contributed little to the observed vasoconstriction (53).
3.3 Acidosis converts Ca2+ waves to sparks to activate BK channels
Because smooth muscle cells in PAs exhibit mostly Ca2+ waves at physiological pH, we hypothesized that acidic pH might dilate PAs by reshaping the predominant intracellular Ca2+ signal from Ca2+ waves to Ca2+ sparks, which would activate BK channels to cause membrane potential hyperpolarization and vasodilation (Figure 1). Using pharmacological and genetic approaches, we found that this mechanism accounts for 60% of the vasodilation observed by acidifying the external pH from 7.4 to 7.0 (29). The concept that acidosis, over a narrow and physiologically relevant pH range, can induce vasodilation principally by remodeling Ca2+ signals is entirely novel, and as described below, may not be limited to the intracerebral microcirculation.
The reshaping of the Ca2+ signaling in VSMCs of PAs by protons likely occurs because changes in extracellular pH (pHo) lead to changes in intracellular pH (pHi). The relationship between pHo and pHi has been established in VSMCs from many tissues including the brain (3, 6, 92, 113). This relationship appears to be linear with a ratio ΔpHi/ΔpHo of about 0.73 (7). Moreover, both normocapnic and hypercapnic acidosis induce a RyR-BK channel component of acidic pH-induced dilation. In a 1989 study, Toda et al. investigated the relaxation of dog cerebral artery strips in response to acidosis induced by hypercapnia or manipulation of the bicarbonate concentration (114). These investigators showed that the increase in CO2 from 5% to 15%, as in our study (29), has a vasorelaxant effect mediated by decreasing pH rather than acting through direct effects of CO2 in the bathing media. This finding is consistent with our observations concerning the similarity between normocapnic and hypercapnic acidosis effects on Ca2+ signaling in PAs. Additionally, a reduction in pHo lowers cytoplasmic Ca2+ (8) which would decrease RyR activity. Interestingly, Heppner et al. showed that inhibition of Ca2+ influx through VDCC did not prevent the induction or the persistence of alkaline pH-induced Ca2+ waves in rat cerebral pial arteries (53). Thus the reshaping of RyR-dependent Ca2+ signaling by pHo appears to be due primarily to the direct regulation of RyR P0. The simplest interpretation of the observed remodeling of Ca2+ signals at pH 7.0 is that protons lower RyR P0 and then prevent local Ca2+ release to activate neighboring RyRs (Figure 1) and Ca2+ release remains limited to single RyR (Ca2+ quark) or clusters of RyRs (Ca2+ sparks). However we have not directly assessed this mechanism and more complex models can be considered. Intracellular acidification in combination with the subsequent reduction of intracellular Ca2+ could also decrease the Po of both RyRs and InsP3Rs, and thereby terminate Ca2+ waves. The subsequent reduced leak of Ca2+ from the SR would then elevate SR luminal [Ca2+], and thereby may enhance local spark probability at the junctional SR elements which abut the plasma membrane with BK channels (131). Indeed it is well demonstrated and recognized that an increase in the SR Ca2+ load activates RyRs (35) and increases Ca2+ spark frequency along with spontaneous transient outward currents occurrence (131). Unfortunately the influence of SR Ca2+ load on InsP3Rs is less clear and experimental evidence is scarce. A luminal Ca2+ binding site has been described (104) and Bezprozvanny & Ehrlich showed that InsP3R activity is inhibited when luminal Ca2+ concentration is raised from 3 μM to 10 mM (14), whereas Bottman and coworkers reported a potentiation of the InsP3-induced Ca2+ release by luminal Ca2+ (17). These issues remain to be resolved and are important topics for future investigation.
3.4 RyRs and neurovascular coupling
Parenchymal arterioles play a critical role during NVC (34). These arterioles dilate in response to neuronal activation, which results in a local increase in blood flow (58, 67, 96). Activation of BK channels by Ca2+ sparks through RyR is a powerful vasodilator mechanism and can be activated in PAs by acidification (29). However, the origin and nature of Ca2+ signals that occur in parenchymal VSMCs during NVC remain to be identified. Interestingly, in brain slices, VSMCs of PAs exhibit synchronous Ca2+ oscillations (36, 37) which are rapidly suppressed during NVC simulated through neuronal depolarization by electric field stimulation. This observation suggests that Ca2+ signals in these arterioles are influenced by neural stimulation via vasoactive substances released from astrocytes and neurons. In newborn piglets, glutamate increases Ca2+ spark frequency and decreases cytoplasmic Ca2+ concentration in the VSMCs of brain slice arterioles (126). This effect is thought to be mediated by carbon monoxide (CO) derived from astrocytic heme oxygenase metabolism (70, 125, 126). However, CO has been described as a tonic vasoconstrictor, preventing H2S-induced vasodilation in cerebellar slices from neonatal mice (80) and CO failed to dilate isolated pial arteries from adult rats and mice (2). Therefore, the involvement of CO during NVC in the mature brain remains to be established. Others have suggested that epoxyeicosatrienoic acids (EETs) produced by cytochrome P450 epoxygenase may mediate NVC (72, 76, 93, 103). The mode of action of EETs in NVC has not been fully resolved, but these compounds induce BK channel-mediated hyperpolarization of VSMCs (42), and increase Ca2+ spark frequency in pial arteries (31). Consequently, EETs are potential modulators of RyR activity during NVC. As in the case of acidosis, Ca2+ spark-driven BK currents can be activated in PAs and can induce large, sustained and reversible vasodilation (29) (Figure 1). Then Ca2+ spark-driven activation of BK channels may also play a role in the vasodilator effects of substances such as CO and EETs, whereby VSMC relaxation occurs.
In their classic study, Roy and Sherrington suggested a possible role for H+ during NVC (102). Protons produced by neuronal metabolism could cause local acidification and then vasodilation. While this hypothesis has been challenged, (58, 74) local release of protons from the astrocytic endfoot onto the smooth muscle could produce dilation. Indeed, alkalinization of astrocytic cell body during cortical stimulation (22) or metabotropic glutamate receptor activation (1) has been reported, which would be consistent with the release of H+. However, using the fluorescent pH indicator SNARFR-5, we did not observe changes in endfoot pH in brain slices following electrical field stimulation (Dabertrand and Nelson, unpublished observations). It is more likely that vasodilation induced by a decrease in pH within the brain may occur during pathologic conditions.
In normal conditions, appropriate pH levels are maintained by the lungs and kidneys. The CO2 resulting from metabolism is expired by the lungs, but if it is retained or rapidly eliminated, it causes respiratory acidosis or respiratory alkalosis, respectively, The kidney, as a regulatory organ, adjusts acid and base excretion and dysregulation in this balance leads to metabolic acidosis or metabolic alkalosis. Nevertheless, acidification of nervous tissue can be triggered rapidly with reduced respiratory rate, during CO2 inhalation (132) or during cerebral lactic acidosis related to ischemia (101) and hypoxia (71). Indeed, without oxygen, the brain switches to anaerobic glycolysis, leading to accumulation of lactic acid. Therefore, during ischemia, tissue pH falls by as much as 1 unit (84, 105) and this acidification is thought to play a role in the injury caused, for example, by ischemic stroke (81, 107, 128). Consequently moderate hyperventilation of patients with cerebral ischemia has been considered in the past to counteract the acidification. However this also increases vascular tone and therefore globally decreases CBF, which worsens the ischemia. (100). Consequently, blockade of downstream targets activated by lactic acidosis appears currently more promising clinically (81). Nevertheless, in the case of cerebral hypoxia or ischemia, acidosis-related vasodilation of PAs might represent a mechanismto maintain perfusion by promoting collateral blood flow.
Conclusion
Since the discovery of Ca2+ sparks in smooth muscle in 1995 (85), many roles of RyRs in vascular function have come into focus. Ca2+ release through RyRs contributes to Ca2+ waves in VSMCs of pressurized arterioles, but the role of Ca2+ waves and their contribution to parenchymal arteriolar tone remains obscure. It is also possible that Ca2+ waves modulate the activity of Ca2+-dependent transcription factors such as Nuclear factor of activated T-cells (NFAT) (45), and thereby exert transcriptional control. It is, however, clear that at physiological external pH, Ca2+ waves do not activate BK channels in VSMCs of pressurized parenchymal arterioles. Acidification leads to a loss of Ca2+ waves and the appearance of Ca2+ sparks causing profound, rapid and reversible vasodilation. Over a narrow and physiologically relevant pH range, the majority of the proton-induced vasodilation depends on Ca2+ spark-driven activation of BK channels (29). These results suggest that the role of the RyRs in vascular smooth muscle is context-dependent. The lack of Ca2+ sparks and absence of BK channel-mediated hyperpolarization to oppose pressure-induced constriction at physiological pH can explain why PAs are more depolarized and constricted at lower pressures, an appropriate physiological response for arterioles that experience lower pressures. Acidification-induced Ca2+ spark engagement of BK channels provides a novel mechanism to enhance cerebral blood flow, and may play an important role in pathological responses in the brain. Therefore, elucidating the regulatory mechanisms of RyRs is crucial for understanding the control of cerebral blood flow.
Acknowledgments
Sources of support that require acknowledgement:
This work was supported by National Institutes of Health grants R37DK053832, RO1HL44455, RO1HL58231, and PO1HL095488, the Fondation Leducq for the Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain, the Totman Trust for Medical Research, and a postdoctoral fellowship from the American Heart Association (09POST2290090) to Fabrice Dabertrand.
The authors would like to thank Dr Kathryn M. Dunn for her insightful comments on the manuscript.
Abbreviations used
- [Ca2+]i
intracellular calcium concentration
- BK
large conductance Ca2+-activated potassium channel
- CBF
cerebral blood flow
- CICR
Ca2+-induced Ca2+ release
- CO
carbon monoxide
- CO2
carbon dioxide
- E-C
excitation-contraction
- EET
epoxyeicosatrienoic acid
- H+
proton
- InsP3
inositol 1,4,5-trisphosphate
- InsP3R
inositol 1,4,5-trisphosphate receptor
- K+
potassium
- NFAT
nuclear factor of activated T-cells
- PA
parenchymal arteriole
- pHi
intracellular pH
- pHo
extracellular pH
- Po
open state probability
- RyR
ryanodine receptor
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
- VDCC
voltage-dependent Ca2+ channel
- VSMC
vascular smooth muscle cell
References
- 1.Amos BJ, Mathie A, Richards CD. Activation of group I metabotropic glutamate receptors elicits pH changes in cultured rat cortical glia and neurons. Neuroscience. 1998;86:1109–1120. doi: 10.1016/s0306-4522(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 2.Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol Heart Circ Physiol. 2006;291:H223–230. doi: 10.1152/ajpheart.00058.2006. [DOI] [PubMed] [Google Scholar]
- 3.Apkon M, Boron WF. Extracellular and intracellular alkalinization and the constriction of rat cerebral arterioles. J Physiol. 1995;484 ( Pt 3):743–753. doi: 10.1113/jphysiol.1995.sp020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnaudeau S, Boittin FX, Macrez N, Lavie JL, Mironneau C, Mironneau J. L-type and Ca2+ release channel-dependent hierarchical Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1997;22:399–411. doi: 10.1016/s0143-4160(97)90024-5. [DOI] [PubMed] [Google Scholar]
- 5.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin C, Dilly K, Eisner D, Wray S. Simultaneous measurement of intracellular pH, calcium, and tension in rat mesenteric vessels: effects of extracellular pH. Biochem Biophys Res Commun. 1996;222:537–540. doi: 10.1006/bbrc.1996.0779. [DOI] [PubMed] [Google Scholar]
- 7.Austin C, Wray S. Changes of intracellular pH in rat mesenteric vascular smooth muscle with high-K+ depolarization. J Physiol. 1993;469:1–10. doi: 10.1113/jphysiol.1993.sp019800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin C, Wray S. Interactions between Ca(2+) and H(+) and functional consequences in vascular smooth muscle. Circ Res. 2000;86:355–363. doi: 10.1161/01.res.86.3.355. [DOI] [PubMed] [Google Scholar]
- 9.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499 ( Pt 2):291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008;586:5047–5061. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 14.Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 16.Bootman MD, Berridge MJ. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 17.Bootman MD, Missiaen L, Parys JB, De Smedt H, Casteels R. Control of inositol 1,4,5-trisphosphate-induced Ca2+ release by cytosolic Ca2+ Biochem J. 1995;306 ( Pt 2):445–451. doi: 10.1042/bj3060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bovo E, Mazurek SR, Blatter LA, Zima AV. Regulation of sarcoplasmic reticulum Ca(2)(+) leak by cytosolic Ca(2)(+) in rabbit ventricular myocytes. J Physiol. 2011;589:6039–6050. doi: 10.1113/jphysiol.2011.214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandt NR, Caswell AH, Brandt T, Brew K, Mellgren RL. Mapping of the calpain proteolysis products of the junctional foot protein of the skeletal muscle triad junction. J Membr Biol. 1992;127:35–47. doi: 10.1007/BF00232756. [DOI] [PubMed] [Google Scholar]
- 20.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 21.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 22.Chesler M, Kraig RP. Intracellular pH of astrocytes increases rapidly with cortical stimulation. Am J Physiol. 1987;253:R666–670. doi: 10.1152/ajpregu.1987.253.4.R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol. 2004;44:1–8. doi: 10.1097/00005344-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabertrand F, Fritz N, Mironneau J, Macrez N, Morel JL. Role of RYR3 splice variants in calcium signaling in mouse nonpregnant and pregnant myometrium. Am J Physiol Cell Physiol. 2007;293:C848–854. doi: 10.1152/ajpcell.00069.2007. [DOI] [PubMed] [Google Scholar]
- 26.Dabertrand F, Mironneau J, Henaff M, Macrez N, Morel JL. Comparison between gentamycin and exon skipping treatments to restore ryanodine receptor subtype 2 functions in mdx mouse duodenum myocytes. Eur J Pharmacol. 2010;628:36–41. doi: 10.1016/j.ejphar.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Dabertrand F, Mironneau J, Macrez N, Morel JL. Full length ryanodine receptor subtype 3 encodes spontaneous calcium oscillations in native duodenal smooth muscle cells. Cell Calcium. 2008;44:180–189. doi: 10.1016/j.ceca.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Dabertrand F, Morel JL, Sorrentino V, Mironneau J, Mironneau C, Macrez N. Modulation of calcium signalling by dominant negative splice variant of ryanodine receptor subtype 3 in native smooth muscle cells. Cell Calcium. 2006;40:11–21. doi: 10.1016/j.ceca.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabertrand F, Porte Y, Macrez N, Morel JL. Spaceflight regulates ryanodine receptor subtype 1 in portal vein myocytes in the opposite way of hypertension. J Appl Physiol. 2012;112:471–480. doi: 10.1152/japplphysiol.00733.2011. [DOI] [PubMed] [Google Scholar]
- 31.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 32.Edwards RM, Stack EJ, Trizna W. Calcitonin gene-related peptide stimulates adenylate cyclase and relaxes intracerebral arterioles. J Pharmacol Exp Ther. 1991;257:1020–1024. [PubMed] [Google Scholar]
- 33.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 36.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 37.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 38.Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5- trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 39.Fleming BP, Barron KW, Howes TW, Smith JK. Response of the microcirculation in rat cremaster muscle to peripheral and central sympathetic stimulation. Circ Res. 1987;61:II26–31. [PubMed] [Google Scholar]
- 40.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franzini-Armstrong C. STUDIES OF THE TRIAD : I. Structure of the Junction in Frog Twitch Fibers. J Cell Biol. 1970;47:488–499. doi: 10.1083/jcb.47.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 43.Giannini G, Sorrentino V. Molecular structure and tissue distribution of ryanodine receptors calcium channels. Med Res Rev. 1995;15:313–323. doi: 10.1002/med.2610150405. [DOI] [PubMed] [Google Scholar]
- 44.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem. 2002;277:37756–37764. doi: 10.1074/jbc.M203596200. [DOI] [PubMed] [Google Scholar]
- 46.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C279–288. doi: 10.1152/ajpcell.00550.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP(3) receptors as a factor shaping spontaneous Ca(2+)-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordienko DV, Greenwood IA, Bolton TB. Direct visualization of sarcoplasmic reticulum regions discharging Ca(2+)sparks in vascular myocytes. Cell Calcium. 2001;29:13–28. doi: 10.1054/ceca.2000.0180. [DOI] [PubMed] [Google Scholar]
- 49.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton SL. Ryanodine receptors. Cell Calcium. 2005;38:253–260. doi: 10.1016/j.ceca.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 51.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harder DR. A cellular mechanism for myogenic regulation of cat cerebral arteries. Ann Biomed Eng. 1985;13:335–339. doi: 10.1007/BF02584252. [DOI] [PubMed] [Google Scholar]
- 53.Heppner TJ, Bonev AD, Santana LF, Nelson MT. Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurized cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;283:H2169–2176. doi: 10.1152/ajpheart.00603.2002. [DOI] [PubMed] [Google Scholar]
- 54.Hill-Eubanks DC, Werner ME, Heppner TJ, Nelson MT. Calcium signaling in smooth muscle. Cold Spring Harb Perspect Biol. 2011;3:a004549. doi: 10.1101/cshperspect.a004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirsch S, Reichold J, Schneider M, Szekely G, Weber B. Topology and hemodynamics of the cortical cerebrovascular system. J Cereb Blood Flow Metab. 2012;32:952–967. doi: 10.1038/jcbfm.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horiuchi T, Dietrich HH, Hongo K, Dacey RG., Jr Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33:2692–2699. doi: 10.1161/01.str.0000034791.52151.6b. [DOI] [PubMed] [Google Scholar]
- 57.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 58.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 59.Itoh Y, Suzuki N. Control of brain capillary blood flow. J Cereb Blood Flow Metab. 2012;32:1167–1176. doi: 10.1038/jcbfm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278:C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- 61.Kamishima T, McCarron JG. Regulation of the cytosolic Ca2+ concentration by Ca2+ stores in single smooth muscle cells from rat cerebral arteries. J Physiol. 1997;501 ( Pt 3):497–508. doi: 10.1111/j.1469-7793.1997.497bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 ( Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508 ( Pt 1):211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci U S A. 2012;109:E1387–1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koide M, Nystoriak MA, Krishnamoorthy G, O'Connor KP, Bonev AD, Nelson MT, Wellman GC. Reduced Ca2+ spark activity after subarachnoid hemorrhage disables BK channel control of cerebral artery tone. J Cereb Blood Flow Metab. 2011;31:3–16. doi: 10.1038/jcbfm.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol. 2010;2:a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 68.Ledbetter MW, Preiner JK, Louis CF, Mickelson JR. Tissue distribution of ryanodine receptor isoforms and alleles determined by reverse transcription polymerase chain reaction. J Biol Chem. 1994;269:31544–31551. [PubMed] [Google Scholar]
- 69.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leffler CW, Parfenova H, Jaggar JH. Carbon monoxide as an endogenous vascular modulator. Am J Physiol Heart Circ Physiol. 2011;301:H1–H11. doi: 10.1152/ajpheart.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 72.Liu X, Li C, Falck JR, Roman RJ, Harder DR, Koehler RC. Interaction of nitric oxide, 20-HETE, and EETs during functional hyperemia in whisker barrel cortex. Am J Physiol Heart Circ Physiol. 2008;295:H619–631. doi: 10.1152/ajpheart.01211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res. 2001;89:1051–1057. doi: 10.1161/hh2301.100250. [DOI] [PubMed] [Google Scholar]
- 74.Magnotta VA, Heo HY, Dlouhy BJ, Dahdaleh NS, Follmer RL, Thedens DR, Welsh MJ, Wemmie JA. Detecting activity-evoked pH changes in human brain. Proc Natl Acad Sci U S A. 2012;109:8270–8273. doi: 10.1073/pnas.1205902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meininger GA, Davis MJ. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992;263:H647–659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- 76.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monkawa T, Miyawaki A, Sugiyama T, Yoneshima H, Yamamoto-Hino M, Furuichi T, Saruta T, Hasegawa M, Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J Biol Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- 78.Morel JL, Dabertrand F, Fritz N, Henaff M, Mironneau J, Macrez N. The decrease of expression of ryanodine receptor sub-type 2 is reversed by gentamycin sulphate in vascular myocytes from mdx mice. J Cell Mol Med. 2009;13:3122–3130. doi: 10.1111/j.1582-4934.2009.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Morel JL, Fritz N, Lavie JL, Mironneau J. Crucial role of type 2 inositol 1,4,5- trisphosphate receptors for acetylcholine-induced Ca2+ oscillations in vascular myocytes. Arterioscler Thromb Vasc Biol. 2003;23:1567–1575. doi: 10.1161/01.ATV.0000089013.82552.5D. [DOI] [PubMed] [Google Scholar]
- 80.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, Suematsu M. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc Natl Acad Sci U S A. 2012;109:1293–1298. doi: 10.1073/pnas.1119658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, Cole WC, Jones PP, Chen SR, Welsh DG. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol. 2010;588:3983–4005. doi: 10.1113/jphysiol.2010.193300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Narayanan D, Adebiyi A, Jaggar JH. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol. 2012;302:H2190–2210. doi: 10.1152/ajpheart.01146.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260:R581–588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 86.Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- 87.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogawa Y, Kurebayashi N, Murayama T. Putative roles of type 3 ryanodine receptor isoforms (RyR3) Trends Cardiovasc Med. 2000;10:65–70. doi: 10.1016/s1050-1738(00)00050-5. [DOI] [PubMed] [Google Scholar]
- 90.Osol G, Halpern W. Myogenic properties of cerebral blood vessels from normotensive and hypertensive rats. Am J Physiol. 1985;249:H914–921. doi: 10.1152/ajpheart.1985.249.5.H914. [DOI] [PubMed] [Google Scholar]
- 91.Paterno R, Faraci FM, Heistad DD. Role of Ca(2+)-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke. 1996;27:1603–1607. doi: 10.1161/01.str.27.9.1603. discussion 1607–1608. [DOI] [PubMed] [Google Scholar]
- 92.Peng HL, Jensen PE, Nilsson H, Aalkjaer C. Effect of acidosis on tension and [Ca2+]i in rat cerebral arteries: is there a role for membrane potential? Am J Physiol. 1998;274:H655–662. doi: 10.1152/ajpheart.1998.274.2.H655. [DOI] [PubMed] [Google Scholar]
- 93.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 94.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 96.Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 98.Priori SG, Napolitano C. Cardiac and skeletal muscle disorders caused by mutations in the intracellular Ca2+ release channels. J Clin Invest. 2005;115:2033–2038. doi: 10.1172/JCI25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pucovsky V, Gordienko DV, Bolton TB. Effect of nitric oxide donors and noradrenaline on Ca2+ release sites and global intracellular Ca2+ in myocytes from guinea-pig small mesenteric arteries. J Physiol. 2002;539:25–39. doi: 10.1113/jphysiol.2001.012978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raichle ME, Plum F. Hyperventilation and cerebral blood flow. Stroke. 1972;3:566–575. doi: 10.1161/01.str.3.5.566. [DOI] [PubMed] [Google Scholar]
- 101.Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85–158. 117. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–125. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sienaert I, De Smedt H, Parys JB, Missiaen L, Vanlingen S, Sipma H, Casteels R. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1996;271:27005–27012. doi: 10.1074/jbc.271.43.27005. [DOI] [PubMed] [Google Scholar]
- 105.Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J Neurosurg. 1992;77:169–184. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- 106.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Simon RP. Acidotoxicity trumps excitotoxicity in ischemic brain. Arch Neurol. 2006;63:1368–1371. doi: 10.1001/archneur.63.10.1368. [DOI] [PubMed] [Google Scholar]
- 108.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of cerebral arterioles in response to activation of adenylate cyclase is dependent on activation of Ca(2+)-dependent K+ channels. Circ Res. 1995;76:1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- 110.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte- mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 111.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 112.Tasker PN, Taylor CW, Nixon GF. Expression and distribution of InsP(3) receptor subtypes in proliferating vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;273:907–912. doi: 10.1006/bbrc.2000.3036. [DOI] [PubMed] [Google Scholar]
- 113.Tian R, Vogel P, Lassen NA, Mulvany MJ, Andreasen F, Aalkjaer C. Role of extracellular and intracellular acidosis for hypercapnia-induced inhibition of tension of isolated rat cerebral arteries. Circ Res. 1995;76:269–275. doi: 10.1161/01.res.76.2.269. [DOI] [PubMed] [Google Scholar]
- 114.Toda N, Hatano Y, Mori K. Mechanisms underlying response to hypercapnia and bicarbonate of isolated dog cerebral arteries. Am J Physiol. 1989;257:H141–146. doi: 10.1152/ajpheart.1989.257.1.H141. [DOI] [PubMed] [Google Scholar]
- 115.Tu H, Wang Z, Bezprozvanny I. Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region. Biophys J. 2005;88:1056–1069. doi: 10.1529/biophysj.104.049601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tumelty J, Scholfield N, Stewart M, Curtis T, McGeown G. Ca2+-sparks constitute elementary building blocks for global Ca2+-signals in myocytes of retinal arterioles. Cell Calcium. 2007;41:451–466. doi: 10.1016/j.ceca.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tunwell RE, Wickenden C, Bertrand BM, Shevchenko VI, Walsh MB, Allen PD, Lai FA. The human cardiac muscle ryanodine receptor-calcium release channel: identification, primary structure and topological analysis. Biochem J. 1996;318 ( Pt 2):477–487. doi: 10.1042/bj3180477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vaithianathan T, Narayanan D, Asuncion-Chin MT, Jeyakumar LH, Liu J, Fleischer S, Jaggar JH, Dopico AM. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. Am J Physiol Cell Physiol. 2010;299:C264–278. doi: 10.1152/ajpcell.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vinall PE, Simeone FA. Cerebral autoregulation: an in vitro study. Stroke. 1981;12:640–642. doi: 10.1161/01.str.12.5.640. [DOI] [PubMed] [Google Scholar]
- 120.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 121.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol. 2012;590:1849–1869. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H1616–1630. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev. 2010;90:113–178. doi: 10.1152/physrev.00018.2008. [DOI] [PubMed] [Google Scholar]
- 124.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res. 2008;102:1118–1126. doi: 10.1161/CIRCRESAHA.108.173948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xi Q, Tcheranova D, Basuroy S, Parfenova H, Jaggar JH, Leffler CW. Glutamate-induced calcium signals stimulate CO production in piglet astrocytes. Am J Physiol Heart Circ Physiol. 2011;301:H428–433. doi: 10.1152/ajpheart.01277.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xi Q, Umstot E, Zhao G, Narayanan D, Leffler CW, Jaggar JH. Glutamate regulates Ca2+ signals in smooth muscle cells of newborn piglet brain slice arterioles through astrocyte-and heme oxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2010;298:H562–569. doi: 10.1152/ajpheart.00823.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiao B, Masumiya H, Jiang D, Wang R, Sei Y, Zhang L, Murayama T, Ogawa Y, Lai FA, Wagenknecht T, Chen SR. Isoform-dependent formation of heteromeric Ca2+ release channels (ryanodine receptors) J Biol Chem. 2002;277:41778–41785. doi: 10.1074/jbc.M208210200. [DOI] [PubMed] [Google Scholar]
- 128.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 129.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao G, Neeb ZP, Leo MD, Pachuau J, Adebiyi A, Ouyang K, Chen J, Jaggar JH. Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells. J Gen Physiol. 2010;136:283–291. doi: 10.1085/jgp.201010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol. 1999;113:215–228. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ziemann AE, Allen JE, Dahdaleh NS, Drebot, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 134.Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, Meissner G, MacLennan DH. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]