Abstract
Cognitive impairment (CI) and behavioral disturbances can be the earliest symptoms of Parkinson’s disease (PD), ultimately afflict the vast majority of PD patients, and increase caregiver burden. Our two Morris K. Udall Centers of Excellence for Parkinson’s Disease Research were supported by the National Institute of Neurological Disorders and Stroke (NINDS) to recommend a comprehensive yet practical approach to cognitive and behavioral assessment to fuel collaborative research. We recommend a step-wise approach with two levels of standardized evaluation to establish a common battery, as well as an alternative testing recommendation for severely impaired subjects, and review supplemental tests that may be useful in specific research settings. Our flexible approach may be applied to studies with varying emphasis on cognition and behavior, does not place undue burden on participants or resources, and has a high degree of compatibility with existing test batteries to promote collaboration.
INTRODUCTION
Cognitive and behavioral impairments are key features of Alzheimer’s diseases, as recently highlighted by the National Alzheimer’s Project Act [1] and the Alzheimer’s Disease Research Summit 2012: Path to Treatment and Prevention [2], and are increasingly recognized in other types of neurodegenerative disease. Indeed, non-motor symptoms are prevalent in Parkinson’s disease (PD) and contribute substantially to its morbidity and mortality. Up to 80% of patients with PD will eventually develop dementia, and cognitive impairment (CI) in the absence of dementia can be the earliest symptom of PD [3–5]. Similarly, behavioral complications–including psychosis, apathy, depression, and anxiety–commonly are associated with PD [6]. Together, cognitive and behavioral disturbances in PD account for increasing disability and caregiver burden as the disease progresses [7–9].
Given the range of deleterious effects from cognitive and behavioral impairments and increased emphasis on the neuropsychiatric features of PD, there is a compelling need for a standard approach to neuropsychiatric assessment in collaborative PD research. However, the lack of a common core assessment battery, such as that used in Alzheimer’s Disease Centers (ADCs)[10], limits comparisons across studies. In addition, some instruments traditionally used in PD are insensitive to the earliest cognitive changes. For example, the Mini-Mental State Examination (MMSE), which has been widely used in dementia evaluation, may not be the optimal tool for assessment of general cognition in PD [11]. Although a number of different cognitive batteries have been used among large clinical investigations of PD (Table 1), a broad consensus has not emerged for the use of any particular set of tests [10, 12–15].
Table 1.
Comparison of Penn-UW Battery with Major Studies of Parkinson’s Disease or National Programs
| TEST | DATATOP | PD-DOC | PPMI | NINDS CDE | NACC UDS | Penn-UW | |||
|---|---|---|---|---|---|---|---|---|---|
| Level I | Level II | ||||||||
| GENERAL COGNITION | Clinical Dementia Rating | X | |||||||
| Mattis Dementia Rating Scale-2 | X | ||||||||
| Mini–Mental State Exam | X | X | X | X | |||||
| Montréal Cognitive Assessment | X | X | X | ||||||
| PREMORBID FUNCTION | Shipley Institute of Living Scale-2, Vocabulary | X | |||||||
| DOMAIN-SPECIFIC COGNITION | Frontal-Executive | Digit Span | X | X | |||||
| Letter-Number Sequencing | X | X | X | ||||||
| Odd Man Out | X | ||||||||
| Symbol-Digit Modalities | X | X | |||||||
| Digit symbol–Coding | X | X | |||||||
| Trail Making | X | X | |||||||
| Memory | Hopkins Verbal Learning Test– Revised | X | X | X | |||||
| Logical Memory | X | ||||||||
| Selective Reminding Test | X | ||||||||
| Visual Spatial | Clock Drawing Test | X | X | X | |||||
| Judgment of Line Orientation | X | X | X | ||||||
| New Dot | X | ||||||||
| Language | Phonemic Verbal Fluency | X | X | X | |||||
| Semantic Verbal Fluency | X | X | X | X | X | ||||
| Short Boston Naming | X | X | X | ||||||
| BEHAVIOR/AFFECT | Geriatric Depression Scale, 15-Item | X | X | X | X | ||||
| Hamilton Depression Scale | X | ||||||||
| MDS-UPDRS, Part I | X | ||||||||
| Neuropsychiatric Inventory | X | X | X | ||||||
| QUIP (Weintraub et al) | X | ||||||||
| State-Trait Anxiety Inventory | X | ||||||||
Abbreviations. DATATOP: Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonsism; PD-DOC: Parkinson’s Disease Data Organizing Center; PPMI: Parkinson’s Progression Markers Initiative; NINDS-CDE: National Institute of Neurological Disorders and Stroke Common Data Elements; NACC UDS: National Alzheimer’s Coordinating Center Uniform Data Set. QUIP: Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease.
The University of Pennsylvania and the University of Washington Udall Centers both have a primary focus on cognitive and behavioral aspects of this disease, yet have been limited in our ability to collaborate more closely with each other, as well as other groups with a similar research focus, because of incompatibilities in cognitive and behavioral assessments [16]. The NINDS recently sponsored our efforts to develop a cross-institutional collaborative approach to the assessment of cognition and behavior in research subjects with PD.
PENN-UW CONSENSUS PROCESS
A working group comprising scientists and clinicians at our Udall Centers was convened and met on a regular basis by teleconference. A review of neuropsychological batteries previously used in studies of cognition in PD or national programs (Table 1) was undertaken. We considered each test’s psychometric properties, research and clinical utility, inclusion in previous and ongoing batteries, measurement of important aspects of CI in PD, and testing burden on both patients and research staff with the goal of assembling a relatively comprehensive but efficient approach to cognitive and behavioral testing for varying research contexts.
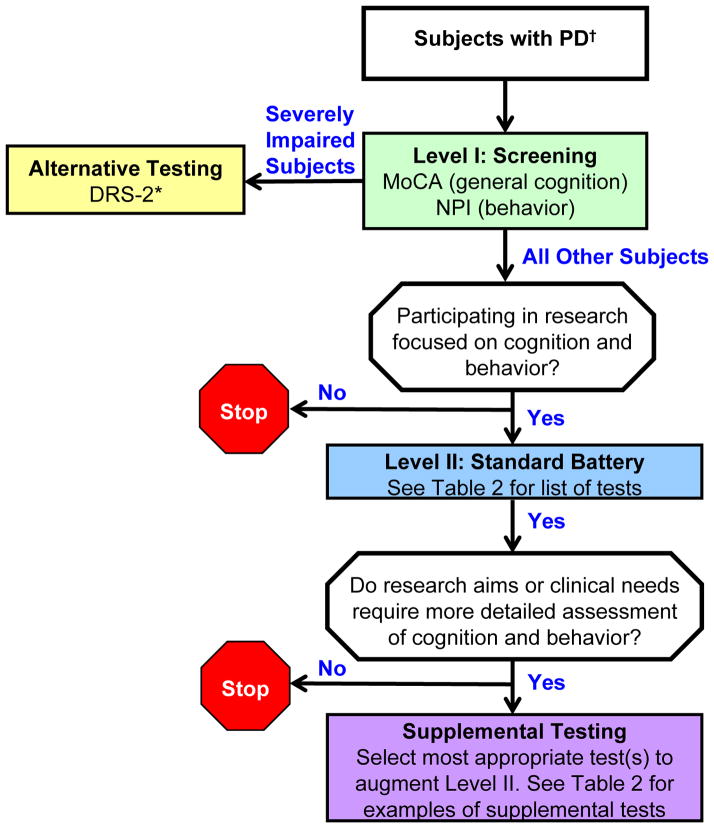
The resulting recommendations follow a step-wise approach with two Levels, as well as Supplemental Testing that may be applicable for more detailed testing in particular research settings (Figure and Table 2); we also include an Alternative Testing recommendation for severely impaired subjects. Level I contains two brief screening tests of global cognition and behavior recommended for all subjects with PD. Level II is a battery of domain-specific cognitive tests and secondary assessment of behavior and psychiatric functioning, as they may impact cognition, and is recommended for PD subjects participating in research focused on cognition and behavior. We also stress the value of study-specific supplemental testing and include multiple examples that may have value in PD research. These recommendations were submitted for comments from a range of stakeholders including Udall Center personnel, representatives of the NINDS CDE process, and experts in PD and cognition. Their feedback was incorporated into the final recommendations.
Figure. Penn-UW recommendations for cognitive and behavioral testing of PD subjects.
Our recommended strategy for assessing cognition and behavior are shown. Refer to Table 2 for the test battery recommended for Level II and for examples of additional tests to consider for Supplemental Testing. Abbreviations. MoCA: Montréal Cognitive Assessment. NPI: Neuropsychiatric Inventory. DRS-2: Mattis Dementia Rating Scale-2.
†Meet United Kingdom Parkinson’s Disease Society Brain Bank (UKPDSBB) clinical diagnostic criteria [30].
*While patients may not be able to complete the DRS-2, there is the capacity to examine subscale performances in the sections that they are able to complete.
Table 2.
Penn-UW Recommended Screening and Standard Battery with Examples of Supplemental Tests
| LEVEL I: SCREENING | ||||||
|---|---|---|---|---|---|---|
| General Cognition | Premorbid | Frontal- Executive | Memory | Visual Spatial | Language | Behavior |
| Montréal Cognitive Assessment (MoCA) | Neuropsychiatric Inventory (NPI) | |||||
| LEVEL II: STANDARD BATTERY | ||||||
| General Cognition | Premorbid | Frontal-Executive | Memory | Visual Spatial | Language | Behavior |
| Shipley Institute of Living Scale-2, Vocabulary | Trail Making Test | Hopkins Verbal Learning Test-Revised (HVLT-R) | Judgment of Line Orientation (JoLO) | Short Boston Naming Test | Geriatric Depression Scale-15 item (GDS-15) | |
| Digit Symbol-Coding1 | Clock Drawing2 | Phonemic verbal fluency (FAS) | ||||
| Letter-Number Sequencing1 | Semantic verbal fluency (Animals) | |||||
| EXAMPLES of SUPPLEMENTAL TESTS | ||||||
| General Cognition | Premorbid | Frontal-Executive | Memory | Visual Spatial | Language | Behavior |
| Mattis Dementia Rating Scale-2 (DRS-2)* | Target Cancellation | Logical Memory3 | Rey Complex Figure Test | Narrative Writing Sample6 | Hamilton Depression Scale (HAM-D) | |
| Mini Mental State Exam (MMSE) | Symbol Search1 | California Verbal Learning Test (CVLT) | Block Design1 | Semantic verbal fluency (fruits, vegetables, super-market items) | Beck Depression Inventory-II (BDI-II) | |
| AD Assessment Scale, Cognitive (ADAS-Cog) | Stroop (Golden version) | Rey Auditory Verbal Learning Test (RAVLT) | Hooper Visual Organization Test | MDS-sponsored revision of Unified PD Rating Scale (MDS-UPDRS), part 1 | ||
| Scales of Outcomes of PD Cognition (SCOPA-cog) | Digit Span1 | Visual Reproduction3 | Complex Figure and Line Orientation4 | Starkstein Apathy Scale | ||
| PD Cognitive Rating Scale (PD-CRS) | Tower of London | Benton Visual Retention Test (BVRT) | Constructional Praxis5 | |||
| Wisconsin Card Sort Test | Recognition Memory Test (RMT) | Hospital Anxiety and Depression Scale (HADS) | ||||
| Similarites1 | ||||||
From Wechsler Adult Intelligence Scale-III.
From Wechsler Memory Scale-III.
Extracted from MoCA; additional scoring required.
From Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
From Consortium to Establish a Registry for AD (CERAD) battery.
From Boston Diagnostic Aphasia Examination (BDAE).
Also recommended for severely impaired individuals; see Figure legend.
We deliberately selected assessments from other published batteries to maximize common assessments for subjects with neurodegenerative diseases. The NINDS has recently published the CDE for PD and included a recommended list of cognitive and behavioral measures [14]. The cognitive measures from the CDE focused on general cognition, and as such, are not intended to focus on domain-specific assessments. Our current proposal is designed to complement and build upon the efforts of the CDE by adding a focus on domain-specific cognitive assessments in Level II. The Montréal Cognitive Assessment (MoCA) [17], recommended for Level I screening, was included among the recommended scales of the CDE, and five of the remaining ten general cognitive scales are also included in our list of supplemental tests, thus permitting sites historically using these alternate global assessments to continue to use those measures. For behavioral evaluation, all of our recommended or supplemental tests are included in the CDE for psychiatric symptoms. Importantly, our approach is compatible with the cognitive domains and behaviors recommended by the Movement Disorders Society’s diagnostic criteria for dementia associated with PD [18, 19]. Our recommendations also contain many of the same tests in the battery from the recently-closed PD-DOC and the Parkinson’s Progression Markers Initiative [14, 15]. Similarly, there is broad compatibility between the approach recommended here and the Uniform Data Set used by the ADCs [10]. In contrast to the diagnostic guidelines set forth by the Movement Disorers Society Task Force recommendations, we sought to restrict our Level II assessment to a battery that could be completed in its entirety without undue burden in a research setting. Future efforts should emphasize harmonization among researchers interested in cognition and behavior in neurodegenerative disorders. Our step-wise approach with study-specific supplementation is sufficiently flexible to accommodate such collaborations. Ultimately, our proposal is intended as a first step toward a process of broader consensus.
RECOMMENDED TESTING
Level I. Screening
Level I is intended for global assessment of cognition and behavior with minimal burden on subjects or Center resources, and is recommended for all PD subjects, even for studies without a focus on cognition or behavior because of the pervasive nature of these deficits in patients with PD. For this level, we recommend the MoCA (cognition) and the Neuropsychiatric Inventory (NPI) [20] (behavior). The MoCA is a 30-point screening instrument originally designed to detect mild CI. It possesses adequate psychometric properties for the detection of CI and measures a broad spectrum of cognitive abilities that are relevant to PD. Several studies have shown that the MoCA demonstrates improved sensitivity over other general screening measures in PD patients [11, 17, 21–23] and the Parkinson Study Group Cognitive/Psychiatric Working Group has recommended the MoCA for use in PD populations over multiple alternate screening tools [24]. We note, however, that although the MoCA has good psychometric properties, this instrument alone is not sufficient to diagnose CI or dementia in patients with PD [25]. The NPI is a rater-administered, informant-based neuropsychiatric inventory that surveys a range of behavioral disturbances and is widely used by the PD research community. The NPI has been used in epidemiologic and treatment studies in both demented and non-demented patients with PD [6], although it has never been validated for any individual psychiatric disorder in PD. These measures are brief, can be administered by a range of study personnel, and require minimal effort from patients, caregivers, and investigators However, they are not comprehensive instruments, and we encourage the use of more domain-specific measures (described in Level II) for centers with a specific focus on cognition. See Appendix for detailed characteristics of these tests.
Level II. Standard Battery
The Level II tests were chosen as the recommended standard battery for all PD subjects who are enrolled in cognitive or behavioral research protocols at our Centers. Level II testing may also be valuable for subjects who score below 26 on the MoCA, even if enrolled in a study that is not focused on cognition or behavior, in order to define better their cognitive profile. We stress that the choice of tests in Level II reflects a balance between comprehensiveness and efficiency, informed by our experience, as we strive to develop an acceptable common battery to fuel collaboration.
The nine recommended cognitive tests in Level II (Table 2) build on Level I by adding assessment of premorbid abilities and four basic cognitive domains: frontal-executive skills, memory, visual spatial abilities, and language. This battery forms the foundation for broad neuropsychological characterization. Given the high frequency of depression in PD [26], we also recommend additional characterization of mood symptoms with the Geriatric Depression Scale, 15-item version (GDS-15) [26, 27]. See Appendix for detailed characteristics of these tests.
Supplemental Testing
The testing proposed in Levels I and II establishes a step-wise common battery but clearly is not exhaustive and cannot meet the needs of all studies focused on cognition and behavior in PD. For this reason we underscore the importance of study-specific supplemental testing that may be used when research aims or clinical needs require more detailed assessment of cognitive and behavioral functions. Indeed, the supplemental tests listed in Table 2 are examples of some tests that can address more detailed cognitive features outside the scope of the Level II standard battery. However, we do not recommend that supplemental tests be administered routinely unless they for a study-specific research need, nor do we recommend that these tests be administered in their entirety or as a stand-alone battery. See Appendix for more information.
Alternative testing for severely impaired subjects
Alternative testing is recommended for subjects who, according to the best clinical judgment of the study investigators, are not capable of completing Level II testing. As a group, these individuals are difficult to evaluate, largely due to floor effects on cognitive testing. In addition to the MoCA and the NPI, we recommend the Mattis Dementia Rating Scale-2 (DRS-2) [28] as an appropriate tool to rate the level of impairment across domains in participants with substantial impairments. Some severely impaired subjects will not be able even to complete the DRS-2; however, there is the capacity to examine subscale performance in the sections that an individual is able to complete. We considered recommending additional tests designed specifically for severely impaired subjects, but rejected this approach because outcomes on these tests are difficult to compare with results from less impaired subjects.
DISCUSSION
The primary motivation for this common approach is to harmonize cognitive and behavioral characterization of subjects with PD across the clinical research programs of our two Udall Centers and with our colleagues at other institutions to thereby promote data sharing and propel collaborative research.
Despite the advantages of promoting cross-site collaborative research, a common cognitive and behavioral approach also has limitations. On the one hand, it may require more time than some researchers think necessary to address specific research questions. Conversely, it may not cover all the specific functions of interest for some research groups. Furthermore, a common battery may exclude or place at a lower priority certain instruments favored by some researchers. We have attempted to minimize these limitations with our step-wise approach that has brief global assessments in Level I, focuses on a limited number of domain-specific assessments in Level II, and is expandable through study-specific supplemental testing.
Due to the motor impairment in PD, there is appropriate concern about the utilization of paper-and-pencil tasks that include motor and/or timed components. In the current approach, we attempted to minimize the use of such tests; however, certain tasks (including Trail Making) are validated and frequently used in this population. Nevertheless, for domains in which paper-and-pencil tasks are utilized, we also included tasks without a motor component. In addition, tasks such as Trail Making can be evaluated with regard to motor speed (Trails A) versus complex divided attention (Trails B), and scores can be adjusted accordingly. In addition, motor symptoms can be taken into account both at the level of clinical assessment and at the point of statistical analyses. Finally, depending upon the goals of the study or extent of motor impairment, inclusion of supplemental tests that have a non-motor component (e.g., Stroop) may be appropriate and useful in helping to determine whether motor or CIs are more prominent.
In conclusion, we recommend a step-wise approach to the cognitive and behavioral characterization of PD subjects to fuel collaborative research. Our approach allows an appropriately broad and harmonized assessment of cognition and behavior that is not overly burdensome to participants, and can be tailored to specific research interests. This approach already is promoting greater collaboration between our Udall Centers and our colleagues at other sites. Finally, our proposed battery was design to be compatible with other national research programs focused on neurodegenerative diseases and cognition, and potentially may be extended to clinical and health effectiveness research into brain aging, as proposed by the National Alzheimer’s Project Act [1] and the Alzheimer’s Disease Research Summit 2012: Path to Treatment and Prevention [2].
Supplementary Material
Appendix: Detailed Review Of Assessments
LEVEL I
General Cognitive Screening
Montréal Cognitive Assessment (MoCA)
The MoCA is a 30-point cognitive screening instrument, originally designed to detect mild cognitive impairments. This test briefly assesses orientation, attention and concentration, memory, language, abstract verbal reasoning, and visual spatial skills. The MoCA contains items that are similar to several of the tests described in the Level II battery; however, MoCA items are less demanding and form the basis for a general assessment, but are inadequate to evaluate domain-specific functioning.
Test characteristics
The MoCA has good test-retest and inter-rater reliability, as well as good construct validity when correlated with a complete neuropsychological battery in PD patients [1]. The test protocol, administration instructions, validation data, and references are readily accessible on the website http://www.mocatest.org/, and are available for clinical and non-profit research use without cost.
Administration time
5–10 minutes
Alternate forms
No
Normative data
Validation data and recommended cutoff scores are published on the MoCA website.
Inclusion in major PD-related studies
Parkinson’s Progression Markers Initiative (PPMI), National Institute of Neurological Disorders and Stroke Common Data Elements (NINDS-CDE).
Utility for PD
Several studies have shown that the MoCA demonstrates improved sensitivity over other general screening measures in PD patients [2–6], and the Parkinson Study Group Cognitive/Psychiatric Working Group has recommended the MoCA for use in PD populations over multiple alternate screening tools [7]. It should be noted, however, that although the MoCA has good psychometric properties, this instrument alone is not considered sufficient to diagnose cognitive impairment or dementia in patients with PD [8].
Behavior
Neuropsychiatric Inventory (NPI)
The NPI is a rater-administered, informant-based neuropsychiatric inventory that broadly assesses ten neuropsychiatric domains: delusions, hallucinations, dysphoria, agitation/aggression, apathy, euphoria, anxiety, disinhibition, irritability/lability, and aberrant motor behavior [9]. Each domain is rated for severity (3-point scale) and frequency (4-point scale) of symptoms, with a single score that considers both aspects.
Test characteristics
Good content and concurrent validity, test-retest reliability, inter-rater reliability, and internal consistency have been reported [9]. Characteristics are further described on the NPI website (http://npitest.net/about-npi.html).
Administration time
5–20 minutes, depending upon the extent of the neuropsychiatric symptoms reported by the caregiver.
Alternate forms
Although the NPI is typically administered by a clinician to an informant, there also is a self-completed version of the NPI called the NPI-Q, (also informant-based) [10], as well as a version designed for administration by nursing home personnel (NPI-NH) [11].
Normative data
Normative data do not appear to be available at this time.
Inclusion in major PD-related studies
Parkinson’s Disease Data Organizing Center (PD-DOC), National Alzheimer’s Coordinating Center Uniform Data Set (NACC UDS).
Utility for PD
The NPI has been widely used in epidemiologic and treatment studies in both demented and non-demented patients with PD [12], although it has never been validated for any individual psychiatric disorder in PD.
LEVEL II
Premorbid Abilities
Inclusion of a measure that provides an estimate of premorbid cognitive abilities facilitates evaluation of changes associated with neurodegenerative disease [13–15]. Research participants vary in their innate abilities and lifetime experiences; comparison of individual test results with an individual’s likely peak cognitive abilities should provide a better estimate of decline from previous functioning. For example, when an individual is highly educated, an average performance on a given test may represent a decline from prior abilities. Conversely, a person with lower lifetime educational opportunities may appear to be impaired on cognitive testing in the absence of a true decline. In particular, assessment of subtle cognitive impairments requires increased sensitivity to individual changes in cognition.
Shipley Institute of Living Scale-2, Vocabulary
The Shipley Institute of Living Scale-2, Vocabulary [16] is a 40-item test of vocabulary knowledge in which a target word is presented along with four possible synonyms. Because vocabulary knowledge tends to be relatively stable into the eighth decade of life [17], it can serve as a surrogate for premorbid cognitive abilities [14,15].
Test characteristics
The Shipley has good test-retest reliability and internal consistency, and concurrent validity has been demonstrated consistently by moderate to high correlations with both intelligence batteries and achievement tests [16].
Administration time
Typically 5–10 minutes; however, this measure is self-paced and can take longer.
Alternate forms
No; however, for the purposes of obtaining a premorbid estimate of intellectual functioning, this measure can be administered at baseline only for longitudinal studies.
Normative data
Age-based normative data are available in the Shipley-2 manual [16].
Inclusion in major PD-related studies
None
Utility for PD
Although the Shipley has not been specifically validated for use in PD populations, vocabulary scores have been widely administered to older adults as a measure of crystallized ability [18]. The Shipley is considerably briefer than full intelligence batteries and is self-administered; therefore, it is generally well-tolerated in aging and brain-injured populations [19].
Frontal-Executive
Attention, concentration, and working memory are supported primarily by the frontal lobes and frontal pathways [20–22]. Simple attention does not frequently appear to be compromised pervasively in patients with PD; however, more complex aspects of attention and working memory, including divided attention, planning, response inhibition, working memory, mental flexibility, and abstract reasoning commonly are impaired [23–26]. For basic assessment of patients with PD, we recommend measures that entail both motor and auditory complex sequencing, working memory, and processing speed. If additional focus on higher executive functions, such as abstract reasoning, is desired, supplemental executive tests (described later) may be added.
The Trail Making Test–Parts A and B
The Trail Making Test [20–22] is a commonly used neuropsychological instrument with demonstrated clinical and research utility that measures attentional speed, sequencing, visual search, and mental flexibility [27]. Part A assesses simple graphomotor sequencing of numbered circles. Part B, a test of divided attention, assesses complex graphomotor sequencing of alternating numbers and letters.
Test characteristics
The Trail Making Test is established as a highly sensitive test for brain damage [22]. Trails B is correlated with other tests of executive function [22] and has been associated with activation of the left dorsolateral prefrontal cortex, prefrontal gyrus, cingulate gyrus, and medial frontal gyrus [28]. High inter-rater reliability is reported [22], as well as relative insensitivity to practice effects [27].
Administration time
4–12 minutes
Alternate forms
No
Normative data
The Trailmaking Test has been the focus of many normative studies [21,22]. We recommend use of the Mayo Older Adult Normative Studies (MOANS) [29,39], which are psychometrically sound and facilitate comparison across a number of neuropsychological instruments.
Inclusion in major PD-related studies
NACC UDS
Utility for PD
In patients with PD, few studies report impairments on Trails A, whereas Trails B is typically impaired [24–26, 31]. McDowd and colleagues recently observed that non-demented patients with PD performed better than patients with Alzheimer’s disease, but worse than healthy older adults, on both Trails A and B [32]. Because this test has a strong motor and timed component, evaluation of Trails B alone may not provide an accurate portrayal of visuospatial working memory in patients with PD. Rather, subtracting Trails A from Trails B or statistically controlling for motor impairment may provide a better indication of complex visual attention.
Digit Symbol-Coding
Digit Symbol-Coding (from the Wechsler Adult Intelligence Scale®, 3rd edition [WAIS®–III] [33])a is a measure of graphomotor working memory and processing speed. For this test, subjects are shown a key that consists of unique digit-symbol pairs and then complete a matrix consisting of numbered boxes paired with empty boxes in which they are asked to write the corresponding symbol.
Test characteristics
High test-retest and inter-rater reliability, as well as good construct validity, have been reported for Digit Symbol-Coding [34].
Administration time
3 minutes
Alternate forms
No.
Normative data
The WAIS-III Manual provides age-adjusted normative data.
Inclusion in major PD-related studies
PD-DOC, PPMI
Utility in PD
Non-demented patients with PD have been shown to perform better than patients with AD but worse than healthy older adults on this task [32]. Significant associations between performance on Digit Symbol and CSF levels of Brain-Derived Neurotrophic Factor (BDNF), Aβ42 and Aβ42/total tau (t-tau) have been observed in non-demented patients with PD [35]. A variation of this test has been used to investigate the effects of therapeutic interventions [36], predict driving abilities [37], and characterize the effects of depression on bradyphrenia [38] in patients with PD.
Letter-Number Sequencing
Letter-Number Sequencing (LNS; from theWAIS®–III and the Wechsler Memory Scale®—Third Edition [WMS®–III]) is a measure of auditory working memory and processing speed. For this test, the subject hears a combination of single-digit numbers and letters and is asked to repeat the numbers in ascending order followed by the letters in alphabetical order.
Test characteristics
No significant practice effects are reported with LNS in middle- to older-aged adults, suggesting that this measure can be readministered effectively in a longitudinal sample [39]. Good inter-rater reliability, as well as good construct validity, have also been reported for LNS [34].
Administration time
5 minutes
Alternate forms
No
Normative data
The WAIS-III Manual provides age-adjusted normative data.
Inclusion in major PD-related studies
PD-DOC, PPMI
Utility in PD
Functional magnetic residence imaging (fMRI) suggests that LNS is sensitive to age-related changes in cognition. Emery and colleagues [40] recently reported that older adults demonstrated more extensive activation in the bilateral prefrontal cortical working memory network than younger adults when completing this task, relative to a memory maintenance task. Although this test has not been used extensively in the investigation of PD, our clinical experience suggests that LNS likely has great potential with this population, and psychometric studies suggest that it is sensitive to the effects of the catechol-O-methyltransferase (COMT) valine-158, methionine polymorphism (val(158)met) and tolcapone [41].
Memory
PD-associated memory performance deficits have been attributed largely to dysfunction of the fronto-striatal pathways resulting from characteristic dopamine loss in the basal ganglia [42–45]. Conventionally, it was postulated that the resulting dysexecutive syndrome primarily interfered with the ability to recall information on demand (free recall), while generally sparing recognition ability [44, 46, 47]. More recently, however, disruptions in both encoding [42,48] and recognition [47, 49–51] have been demonstrated in PD patients with and without dementia. Patterns of responding (impaired organizational strategies and positive response bias) again suggest that these deficits are primarily related to frontal-executive dysfunction rather than memory loss mediated by the temporal lobes [47, 49, 52]. However, PD has also been associated with temporal and parietal hypoperfusion and atrophy [53–59], and some PDD patients also have neuropathologic changes consistent with AD [31, 60, 61]. As a result, some individuals with PD may present with AD-like memory impairments that are independent of executive dysfunction [48]. In addition, memory performance pattern may be dependent upon initial motor symptom presentation and/or early laterality of motor symptoms [62, 63]. Given the scope of memory problems potentially associated with PD, utilization of a task that provides clues as to the origin of the memory dysfunction (encoding, retention, retrieval) is essential for PD-related cognitive research.
Hopkins Verbal Learning Test-Revised
The Hopkins Verbal Learning Test-Revised (HVLT-R) [64] is a 12-item list-learning task that permits examination of learning, free recall, and recognition. Because the words are selected from three semantic categories, higher-order organizational strategies that require effective executive functioning can be evaluated. In addition, learning slope and cumulative word learning can be assessed by comparing scores across the three learning trials. The initial encoding trials are followed by free recall delay and recognition trials, which help to clarify whether the memory problems are associated with encoding or retrieval processes.
Test characteristics
Good alternate forms and test-retest reliability have been established [64–66]. The HVLT-R also has good convergent validity with similar measures, including the California Verbal Learning Test (CVLT) [67, 68], yet the shorter administration time increases the likelihood that it will be well-tolerated by patients. It is thus frequently used in elderly populations [68].
Administration time
7–10 minutes, excluding a 20–25 minute delay.
Alternate forms
Six parallel forms are available.
Normative data
Normative studies have been completed in older adults [64, 69, 70].
Inclusion in major PD-related studies
PD-DOC, PPMI
Utility in PD
List-learning test performance correlates with motor symptoms, processing speed, independent activities of daily living, mood, and executive functions in PD [71–74], and is a strong predictor of functional outcome in older adults [75]. Specifically, the HVLT-R discriminates between PD and AD [76], and can be used to identify subsets of PD patients with different memory profiles [48].
Visual Spatial
Visuospatial dysfunction, which may be among the first cognitive changes noticed in PD [59, 77], is predictive of both cognitive decline and eventual dementia [78, 79]. The neuroanatomic substrate of defective visuospatial processing in PD is not well understood, but is hypothesized to result from asymmetric dopamine loss in the right basal ganglia [80], deficits in the dorsolateral prefrontal pathways with resulting structural and functional changes in the parietal lobes [81, 82], disruptions in the fronto-striatal loop [83], or other neuropathologic changes in the temporal, parietal, and/or occipital lobes [84]. Spatial processing and concept formation are difficult to assess in patients with PD, however, given the motor component often required to complete spatial tasks. In addition, impaired executive processes may contribute to visuomotor dysfunction and make it difficult to assess pure spatial performance [85, 86]. In order to address both of these issues, we include two visuospatial measures with varying levels of motor and frontal-executive demand.
Judgment of Line Orientation
The Judgment of Line Orientation (JoLO) [87] is considered to be a more “pure” visual-perceptual task that has a minimal motor component and does not require substantial higher-order organizational abilities [88]. The test asks the subject to match pairs of angled lines to a display array of lines.
Test characteristics
Venderploeg et al. [89] found the 15-item short form method (which involves doubling the short form score) to be a well-tolerated, valid, and reliable method of test administration. High test-retest reliability and an absence of practice effects in both PD patients and controls have been demonstrated [88]. Good construct validity has also been established with the JoLO [90]; performance on this task correlates with tests that require visual perception and spatial updating [91].
Administration time
5–10 minutes
Alternate forms
Yes. To reduce subject fatigue, we recommend using a 15-item short form, in which either the odd or even numbers are given; these can be administered as parallel forms [89].
Normative data
MOANS age-based normative data are available and recommended to facilitate comparison across tests [92].
Inclusion in major PD-related studies
PD-DOC, PPMI
Utility in PD
Both global score and detailed error classification [93] can be useful in discriminating between patients with PD, AD, and normal controls. For example, individuals with PD may demonstrate specific error profiles (e.g., fewer “simple” errors and more complex intraquadratic errors), relative to controls and patients with AD [88, 94].
Clock Drawing Test.b
The Clock Drawing Test (CDT) [22] is a brief, well-tolerated measure that is used to assess visuospatial and executive difficulties in which participants are asked to draw a clock to command [95, 96]. We recommend extracting the CDT from the MoCA for this task.
Test characteristics
The CDT has good interrater and test-retest reliability, as well as good concurrent validity with the MMSE and executive function measures [96–99].
Administration time
Given as part of the MoCA (see above).
Alternate forms
No
Normative data
Limited age-based normative data are available for the CDT using the 10-point scoring system [100]; however, cut-off scores rather than means are frequently used [22].
Inclusion in major PD-related studies
PPMI, NINDS-CDE (as part of the MoCA)
Utility for PD
Individuals with PD may be more likely to perform poorly on complex visuo-construction tasks that require executive organizational skills. The CDT appears to be sensitive to cognitive changes associated with PD and can discriminate between MCI due to AD and PD [101]. It has also been shown to be sensitive to changes associated with treatment in PD medication trials [102, 103]. Scoring required for the MoCA is relatively simple; however, other more detailed scoring techniques exist and may be helpful in further discriminating PD from controls and other neurodegenerative diseases (e.g., patients with PD are more likely to make stimulus-bound errors than patients with AD) [97]. We thus recommend that the ten-point scoring system be used for patients with PD [98, 99].
Language
Although language dysfunction is not typically identified as a core clinical feature of PD, deficits in both verbal fluency and naming have been reported even early in the disease process [104–107]. Language deficits may be a consequence of the dysexecutive syndrome commonly associated with PD [108–111] or secondary to impaired speech production resulting from motor dysfunction and bradykinesia [112]. Others have suggested a specific disruption of the semantic networks that leads to language impairment [107]. Thus, pure language impairment may be present in PD (although to a lesser degree than other deficits); alternatively, compromised language performance may reflect deficits in other cognitive domains [26]. In either case, neuropsychological characterization of PD would be incomplete without language assessment. For this reason, we recommend assessing both confrontation naming (a more “pure” test of anomia) and verbal fluency (which has a strong executive component).
Short Boston Naming Test
One of the most commonly used visual confrontation naming measures is the Boston Naming Test (BNT) [113], in which the subject is shown a picture of an object and is asked to provide the name of that object. We are recommending the use of an abbreviated form of the BNT (Short BNT) to ameliorate frustration and fatigue often observed in patients with lower levels of education, lower intellectual levels, or severe cognitive impairments [114, 115].
Test characteristics
The BNT has sound psychometric properties, including adequate test-retest reliability [116] and concurrent validity [117] as well as clinical utility in discriminating different forms of dementia [118]. The current version of the BNT contains a 15-item form validated by Mack and colleagues [119, 120].
Administration time
5 minutes
Alternate Forms
Normative data
Multiple normative studies are available for verbal fluency measures [21, 22].
Administration time
5 minutes
Inclusion in major PD-related studies
PD-DOC, NACC UDS
Utility in PD
There is evidence that naming is impaired in patients with PD both with and without dementia [59, 106]; however, nonspecific effects may contribute to anomia, which is common to many neurodegenerative diseases and traumatic brain injury [20, 121].
Verbal fluency skills depend heavily on retrieval strategies (e.g., clustering words by sound or meaning and switching to a new clustering strategy when the current strategy is exhausted), knowledge of semantic relationships, and long-term memory for words [32]. They have been used to assess semantic processing, word knowledge, and executive functions [32]. The overall pattern of test results (as well as the relative impairment on semantic vs. phonemic fluency) determines whether impaired verbal fluency represents a primary language or executive deficit. Verbal fluency tasks have been used in the study of PD to assess the therapeutic effects of cognitive training, rasagiline [122], duodenal levodopa administration, and deep brain stimulation [123], as well as the correlates of gender, laterality of motor impairment, and freezing of gait [124].
Phonemic verbal fluency
We recommend FAS [20–22] as the standard phonemic verbal fluency task. Respondents are instructed to freely generate as many words as possible in 60 seconds that begin with a specified letter.
Test characteristics
Phonemic verbal fluency tests have been shown to exhibit adequate internal consistency and test-retest reliability in healthy adults [125] and good inter-rater and test-retest reliability in patients with multiple sclerosis [126]. It is likely that phonemic verbal fluency performance is affected by age and education but not by gender [20].
Administration time
4 minutes
Alternate forms
Other letter combinations (e.g., CFL) have been validated; however, raw scores on these different forms may not be equivalent. We note that Ruff et al. suggest that z-scores and percentiles may be equivalent for the FAS and CFL versions of phonemic verbal fluency [125].
Normative data
Many normative studies are available for verbal fluency measures [21, 22].
Inclusion in major PD-related studies
PD-DOC, Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonsism (DATATOP).
Utility in PD
Phonemic verbal fluency activates the left inferior and middle frontal cortices, anterior cingulate cortex, putamen, thalamus and cerebellum [127]. Interestingly, right cerebellar lesions impair phonemic verbal fluency and, to a lesser extent, semantic verbal fluency, corroborating a role for lateralized posterior involvement in verbal fluency [128]. Patients having undergone pallidotomy performed worse on phonemic verbal fluency relative to baseline evaluation, especially if lesions were mostly in the internal pallidal segment rather than the external pallidal segment or internal capsule [122].
Semantic verbal fluency
We recommend using the category of “animals” as the standard semantic verbal fluency task. Respondents are asked to produce as many animal names as possible in 1 minute.
Test characteristics
Semantic verbal fluency test-retest reliability is adequate, and performance appears to be influenced by age, education, and IQ but not by gender [20, 129]. Performance on semantic verbal fluency appears to be sensitive to the presence of dementia [20].
Administration time
2 minutes
Alternate forms
Other semantic categories have been validated; however, these are not considered to be equivalent measures.
Normative data
Many normative studies are available for verbal fluency measures [21, 22].
Inclusion in major PD-related studies
PD-DOC, PPMI, DATATOP, NACC UDS
Utility in PD
There are conflicting reports concerning the relative impairment of semantic and phonemic fluency in PD patients as compared to controls and other dementia types [130]; however, semantic verbal fluency may be more strongly associated with dementia in PD patients [131]. COMT val(158)met status appears to modulate left inferior frontal gyrus activation during a semantic verbal fluency task [132].
Behavioral
Given the high frequency of depression in PD [133], we recommend that the Level II evaluation include a measure specifically to assess mood in addition to the NPI.
Geriatric Depression Scale –15 item (GDS-15)
The GDS-15 is a 15-item, self-administered instrument that is widely used in depression, including in patients with PD [134, 135]. Each item is scored as a 0 or 1, with higher scores indicating increasing depression severity. The GDS-15 does not include a suicide item, but is brief and easy for patients to use, as it utilizes a “yes/no” format. The GDS has fewer physical symptoms than most depression rating scales, so it is less subject to symptom overlap in PD patients.
Test characteristics
The GDS-15 has good sensitivity and specificity for detecting depression in older adults, and correlates highly with scores on other depression measures [136]. Moderate internal consistency retest reliability has been reported [137].
Alternate forms
No
Normative data
Normative data do not appear to be available at this time.
Inclusion in major PD-related studies
PD-DOC, PPMI, NACC UDS
Utility for PD
The GDS also has good sensitivity and predictive value in PD [133]. It performs well in patients with mild-moderate cognitive impairment and is entirely in the public domain. A cutoff score of 5 has been recommended in PD to indicate clinically significant depressive symptoms [138, 139].
SUPPLEMENTAL TESTS
Additional measures may be appropriate for more detailed evaluation of specific cognitive and behavioral domains in patients with PD. These measures may be added to the core Level II battery as required for study aims and/or more detailed clinical assessments. Given that these are supplemental measures to be added at the discretion of the clinician and/or study site, we have not described these measures in the same detail as the tests above. The reader interested in descriptions and test characteristics is referred to MD Lezak, DB Howieson, and DW Loring, Neuropsychological Assessment (Fourth Edition), New York: Oxford University Press, 2004 [20], and to E Strauss, EMS Sherman, and O Spreen, A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary (Third Edition), New York: Oxford University Press, 2006) [22].
General cognitive function
Included in the Supplemental tests are a number of measures of general cognitive abilities that may be administered in addition to the MoCA due to their frequency of use in both older and PD populations: the Mattis Dementia Rating Scale-2 (DRS-2) [140], the Mini-Mental State Exam (MMSE) [141], Alzheimer’s Disease Assessment Scale-Cognitive (ADAS-Cog) [142], Scales of Outcomes of Parkinson’s Disease–Cognition (SCOPA-cog) [143], and the Parkinson’s Disease Cognitive Rating Scale (PD-CRS) [144]. However, these measures may not be as sensitive to PD-related changes in global functioning as the MoCA, and thus are not recommended as substitutes [2, 7].
Frontal-Executive
Given the frequency of dysfunction reported in PD patients in the areas of attention, concentration, working memory, and executive abilities, more comprehensive exploration of these cognitive domains may be a primary focus of PD-related neuropsychological research. Thus, the inclusion of additional measures to determine complex scanning and visual tracking and processing speed (Target Cancellation [20], Symbol Search [WAIS-III]), sustained and selective attention (Stroop test [22]), working memory/mental control (Digit Span [WAIS-III]), planning and complex executive functions (Tower of London [145], Wisconsin Card Sorting Test [146]), or verbal reasoning and concept formation abilities (Similarities [WAIS-III]) may be appropriate.
Memory
More detailed memory assessment may be undertaken when research aims call for comparison of list-learning performance and story recall (Logical Memory [WMS-III]), examination of proactive and retroactive interference effects (California Verbal Learning Test-II [CVLT-II] [147] or Rey Auditory Verbal Learning Test [RAVLT] [148]), performance following semantic cues (CVLT-II), visual memory (Visual Reproduction [WMS-III], Benton Visual Retention Test [BVRT] [149]), and comparison of right- vs. left-sided recognition performance (Recognition Memory Test [150]).
Visual Spatial
If more in-depth assessment of visuospatial functioning is desired, the Rey Complex Figure Test [151] requires a higher level of visual organization (as well as memory and executive components), Block Design (WAIS-III) may provide more information concerning conceptual-spatial processing, and the Hooper Visual Organization Test [152] offers a measure of visual integration that separates the perceptual spatial component from motor abilities. For dementia-specific research, the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) [153] construction tasks and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [154] complex figure and line orientation subtests may be appropriate, particularly for more impaired groups.
Language
For additional language measures, the Narrative Writing Sample (“cookie theft” from the Boston Diagnostic Aphasia Examination) [155] can provide insight into changes in semantic processing, perceptual abilities, and motor problems (micrographia). Alternate semantic fluency tasks, including fruits and vegetables and/or supermarket items, may provide supplementary information concerning semantic strategies.
Behavior
Depending upon study goals, more in-depth assessment of mood and behavior also may be warranted. The Hamilton Depression Scale (HAM-D) [156], Beck Depression Inventory-II (BDI-II) [157], Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part I [158], Starkstein Apathy Scale [159], or the Hospital Anxiety and Depression Scale (HADS) [160] may be added to the Level II behavior assessments for this purpose.
QUALITY CONTROL
For all levels of assessment, appropriate measures to ensure quality control are strongly recommended. While the MoCA may be administered by a range of study personnel, domain-specific cognitive tests should be administered by trained psychometrists with regular performance review and ongoing auditing of assessment procedures. In addition to quality standards for test administration procedures, careful control of testing conditions should be undertaken. For example, given the potential for fluctuations in cognition in participants with Lewy Body disease, documentation of the current cognitive state of the participant should be made at each study visit. Further, although testing participants with PD while on medication is preferred, thorough documentation of medication dose, timing, and those not on medication should be undertaken. For longitudinal studies, careful review of prior test conditions (on/off medication, cognitive fluctuation status) will permit reassessment under similar conditions.
REFERENCES
- 1.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23(7):1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 2.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 3.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 5.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57(2):304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23(2):297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 7.Chou KL, Amick MM, Brandt J, et al. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord. 2010;25(15):2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasten M, Bruggemann N, Schmidt A, Klein C. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2010;75(5):478. doi: 10.1212/WNL.0b013e3181e7948a. author reply 478–479. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 10.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Wood S, Cummings JL, Hsu MA, et al. The use of the neuropsychiatric inventory in nursing home residents. Characterization and measurement. Am J Geriatr Psychiatry. 2000;8(1):75–83. doi: 10.1097/00019442-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78(1):36–42. doi: 10.1136/jnnp.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.APA. Guidelines for the evaluation of dementia and age-related cognitive decline. Washington, DC: American Psychological Association; 1997. [Google Scholar]
- 14.Harnish MJ, Beatty WW, Nixon SJ, Parsons OA. Performance by normal subjects on the Shipley Institute of Living Scale. J Clin Psychol. 1994;50(6):881–883. doi: 10.1002/1097-4679(199411)50:6<881::aid-jclp2270500611>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Matthews TD, Lassiter K, Habedank H. Validity of two brief measures: the General Abilities Measure for Adults and the Shiple Institute of Living Scale. Percept Mot Skills. 2001;92(3 Pt 1):881–887. doi: 10.2466/pms.2001.92.3.881. [DOI] [PubMed] [Google Scholar]
- 16.Shipley WC, Gruber CP, Martin TA, Klein AM. Shipley-2 Manual. Los Angeles, CA: Western Psychological Services; 2009. [Google Scholar]
- 17.Singer T, Verhaeghen P, Ghisletta P, Lindenberger U, Baltes PB. The fate of cognition in very old age: six-year longitudinal findings in the Berlin Aging Study (BASE) Psychol Aging. 2003;18(2):318–331. doi: 10.1037/0882-7974.18.2.318. [DOI] [PubMed] [Google Scholar]
- 18.Verhaeghen P. Aging and vocabulary scores: a meta-analysis. Psychol Aging. 2003;18(2):332–339. doi: 10.1037/0882-7974.18.2.332. [DOI] [PubMed] [Google Scholar]
- 19.Yuspeh RL, Vanderploeg RD, Kershaw DA. Normative data on a measure of estimated premorbid abilities as part of a dementia evaluation. Appl Neuropsychol. 1998;5(3):149–153. doi: 10.1207/s15324826an0503_6. [DOI] [PubMed] [Google Scholar]
- 20.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 21.Mitrushina MN. Handbook of normative data for neuropsychological assessment. 2. New York: Oxford University Press; 2005. [Google Scholar]
- 22.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administration, norms, and commentary. 3. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 23.Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130(Pt 11):2887–2897. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. Cognitive changes in Parkinson’s disease patients with visual hallucinations. Dement Geriatr Cogn Disord. 2007;23(5):281–288. doi: 10.1159/000100850. [DOI] [PubMed] [Google Scholar]
- 25.Troster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev. 2008;18(1):103–119. doi: 10.1007/s11065-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 26.Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson’s disease. Brain Pathol. 2010;20(3):640–645. doi: 10.1111/j.1750-3639.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc. 2006;1(5):2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 28.Zakzanis KK, Mraz R, Graham SJ. An fMRI study of the Trail Making Test. Neuropsychologia. 2005;43(13):1878–1886. doi: 10.1016/j.neuropsychologia.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Lucas JA, Ivnik RJ, Smith GE, et al. Mayo’s Older African Americans Normative Studies: norms for Boston Naming Test, Controlled Oral Word Association, Category Fluency, Animal Naming, Token Test, Wrat-3 Reading, Trail Making Test, Stroop Test, and Judgment of Line Orientation. Clin Neuropsychol. 2005;19(2):243–269. doi: 10.1080/13854040590945337. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clin Neuropsychol. 2005;19(3–4):329–377. doi: 10.1080/13854040590945210. [DOI] [PubMed] [Google Scholar]
- 31.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson’s disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81(10):1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 32.McDowd J, Hoffman L, Rozek E, et al. Understanding verbal fluency in healthy aging, Alzheimer’s disease, and Parkinson’s disease. Neuropsychology. 2011;25(2):210–225. doi: 10.1037/a0021531. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. WAiS-III® Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation Harcourt Brace & Company; 1997. [Google Scholar]
- 34.Wechsler D. WAIS-III® Wechsler Adult Intelligence Scale - Third Edition WMS-III® Wechsler Memory Scale - Third Edition Technical Manual. San Antonio, Tx: The Psychological Corporation® Harcourt Brace & Company; 1997. [Google Scholar]
- 35.Leverenz JB, Watson GS, Shofer J, Zabetian CP, Zhang J, Montine TJ. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(1):61–64. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt FA, Farlow MR, Meng X, Tekin S, Olin JT. Efficacy of rivastigmine on executive function in patients with Parkinson’s disease dementia. CNS Neurosci Ther. 2010;16(6):330–336. doi: 10.1111/j.1755-5949.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Worringham CJ, Wood JM, Kerr GK, Silburn PA. Predictors of driving assessment outcome in Parkinson’s disease. Mov Disord. 2006;21(2):230–235. doi: 10.1002/mds.20709. [DOI] [PubMed] [Google Scholar]
- 38.Rogers D, Lees AJ, Smith E, Trimble M, Stern GM. Bradyphrenia in Parkinson’s disease and psychomotor retardation in depressive illness. An experimental study Brain. 1987;110(Pt 3):761–776. doi: 10.1093/brain/110.3.761. [DOI] [PubMed] [Google Scholar]
- 39.Lemay S, Bedard MA, Rouleau I, Tremblay PL. Practice effect and test-retest reliability of attentional and executive tests in middle-aged to elderly subjects. Clin Neuropsychol. 2004;18(2):284–302. doi: 10.1080/13854040490501718. [DOI] [PubMed] [Google Scholar]
- 40.Emery L, Heaven TJ, Paxton JL, Braver TS. Age-related changes in neural activity during performance matched working memory manipulation. Neuroimage. 2008;42(4):1577–1586. doi: 10.1016/j.neuroimage.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biol Psychiatry. 2009;66(11):997–1004. doi: 10.1016/j.biopsych.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Bronnick K, Alves G, Aarsland D, Tysnes OB, Larsen JP. Verbal memory in drug-naive, newly diagnosed Parkinson’s disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25(1):114–124. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- 43.Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC. Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain. 1998;121(Pt 5):949–965. doi: 10.1093/brain/121.5.949. [DOI] [PubMed] [Google Scholar]
- 44.Stefanova ED, Kostic VS, Ziropadja LJ, Ocic GG, Markovic M. Declarative memory in early Parkinson’s disease: serial position learning effects. J Clin Exp Neuropsychol. 2001;23(5):581–591. doi: 10.1076/jcen.23.5.581.1239. [DOI] [PubMed] [Google Scholar]
- 45.Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- 46.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244(1):2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 47.Higginson CI, Wheelock VL, Carroll KE, Sigvardt KA. Recognition memory in Parkinson’s disease with and without dementia: evidence inconsistent with the retrieval deficit hypothesis. J Clin Exp Neuropsychol. 2005;27(4):516–528. doi: 10.1080/13803390490515469. [DOI] [PubMed] [Google Scholar]
- 48.Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17(4):195–200. [PubMed] [Google Scholar]
- 49.Higginson CI, King DS, Levine D, Wheelock VL, Khamphay NO, Sigvardt KA. The relationship between executive function and verbal memory in Parkinson’s disease. Brain Cogn. 2003;52(3):343–352. doi: 10.1016/s0278-2626(03)00180-5. [DOI] [PubMed] [Google Scholar]
- 50.Whittington CJ, Podd J, Kan MM. Recognition memory impairment in Parkinson’s disease: power and meta-analyses. Neuropsychology. 2000;14(2):233–246. doi: 10.1037//0894-4105.14.2.233. [DOI] [PubMed] [Google Scholar]
- 51.Whittington CJ, Podd J, Stewart-Williams S. Memory deficits in Parkinson’s disease. J Clin Exp Neuropsychol. 2006;28(5):738–754. doi: 10.1080/13803390590954236. [DOI] [PubMed] [Google Scholar]
- 52.O’Brien TJ, Wadley V, Nicholas AP, Stover NP, Watts R, Griffith HR. The contribution of executive control on verbal-learning impairment in patients with Parkinson’s disease with dementia and Alzheimer’s disease. Arch Clin Neuropsychol. 2009;24(3):237–244. doi: 10.1093/arclin/acp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borghammer P, Chakravarty M, Jonsdottir KY, et al. Cortical hypometabolism and hypoperfusion in Parkinson’s disease is extensive: probably even at early disease stages. Brain Struct Funct. 2010;214(4):303–317. doi: 10.1007/s00429-010-0246-0. [DOI] [PubMed] [Google Scholar]
- 54.Bruck A, Kurki T, Kaasinen V, Vahlberg T, Rinne JO. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson’s disease is related to cognitive impairment. J Neurol Neurosurg Psychiatry. 2004;75(10):1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu MT, Taylor-Robinson SD, Chaudhuri KR, et al. Cortical dysfunction in non-demented Parkinson’s disease patients: a combined (31)P-MRS and (18)FDG-PET study. Brain. 2000;123(Pt 2):340–352. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- 56.Ibarretxe-Bilbao N, Tolosa E, Junque C, Marti MJ. MRI and cognitive impairment in Parkinson’s disease. Mov Disord. 2009;24(Suppl 2):S748–753. doi: 10.1002/mds.22670. [DOI] [PubMed] [Google Scholar]
- 57.Jokinen P, Bruck A, Aalto S, Forsback S, Parkkola R, Rinne JO. Impaired cognitive performance in Parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat Disord. 2009;15(2):88–93. doi: 10.1016/j.parkreldis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Nobili F, Abbruzzese G, Morbelli S, et al. Amnestic mild cognitive impairment in Parkinson’s disease: a brain perfusion SPECT study. Mov Disord. 2009;24(3):414–421. doi: 10.1002/mds.22381. [DOI] [PubMed] [Google Scholar]
- 59.Song IU, Kim JS, Jeong DS, Song HJ, Lee KS. Early neuropsychological detection and the characteristics of Parkinson’s disease associated with mild dementia. Parkinsonism Relat Disord. 2008;14(7):558–562. doi: 10.1016/j.parkreldis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Brown DF, Dababo MA, Bigio EH, et al. Neuropathologic evidence that the Lewy body variant of Alzheimer disease represents coexistence of Alzheimer disease and idiopathic Parkinson disease. J Neuropathol Exp Neurol. 1998;57(1):39–46. doi: 10.1097/00005072-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 61.Compta Y, Marti MJ, Ibarretxe-Bilbao N, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord. 2009;24(15):2203–2210. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 62.Foster PS, Drago V, Crucian GP, et al. Verbal and visuospatial memory in lateral onset Parkinson disease: time is of the essence. Cogn Behav Neurol. 2010;23(1):19–25. doi: 10.1097/WNN.0b013e3181c20de7. [DOI] [PubMed] [Google Scholar]
- 63.Lyros E, Messinis L, Papathanasopoulos P. Does motor subtype influence neurocognitive performance in Parkinson’s disease without dementia? Eur J Neurol. 2008;15(3):262–267. doi: 10.1111/j.1468-1331.2007.02046.x. [DOI] [PubMed] [Google Scholar]
- 64.Benedict RHB, Schretlen D, Groninger L, Brandt J. The Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and inter-rater reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 65.Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. J Clin Exp Neuropsychol. 1998;20(3):339–352. doi: 10.1076/jcen.20.3.339.822. [DOI] [PubMed] [Google Scholar]
- 66.Rasmussen DX, Bylsma FW, Brandt J. Stability of performance on the Hopkins Verbal Learning Test. Archives of Clinical Neuropsychology. 1994;10:21–26. [PubMed] [Google Scholar]
- 67.Lacritz LH, Cullum CM. The Hopkins Verbal Learning Test and CVLT: a preliminary comparison. Arch Clin Neuropsychol. 1998;13(7):623–628. [PubMed] [Google Scholar]
- 68.Shapiro AM, Benedict RH, Schretlen D, Brandt J. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. doi: 10.1076/clin.13.3.348.1749. [DOI] [PubMed] [Google Scholar]
- 69.Friedman MA, Schinka JA, Mortimer JA, Graves AB. Hopkins Verbal Learning Test-Revised: norms for elderly African Americans. Clin Neuropsychol. 2002;16(3):356–372. doi: 10.1076/clin.16.3.356.13857. [DOI] [PubMed] [Google Scholar]
- 70.Vanderploeg RD, Schinka JA, Jones T, Small BJ, Graves AB, Mortimer JA. Elderly norms for the Hopkins Verbal Learning Test-Revised. Clin Neuropsychol. 2000;14(3):318–324. doi: 10.1076/1385-4046(200008)14:3;1-P;FT318. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez HH, See RH, Gary MF, et al. Depressive symptoms in Parkinson disease correlate with impaired global and specific cognitive performance. J Geriatr Psychiatry Neurol. 2009;22(4):223–227. doi: 10.1177/0891988709335792. [DOI] [PubMed] [Google Scholar]
- 72.Grace J, Amick MM, D’Abreu A, Festa EK, Heindel WC, Ott BR. Neuropsychological deficits associated with driving performance in Parkinson’s and Alzheimer’s disease. J Int Neuropsychol Soc. 2005;11(6):766–775. doi: 10.1017/S1355617705050848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabbagh MN, Lahti T, Connor DJ, et al. Functional ability correlates with cognitive impairment in Parkinson’s disease and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(5):327–334. doi: 10.1159/000108340. [DOI] [PubMed] [Google Scholar]
- 74.Zahodne LB, Bowers D, Price CC, et al. The Case for Testing Memory With Both Stories and Word Lists Prior to DBS Surgery for Parkinson’s Disease. Clin Neuropsychol. 2011;25(3):348–358. doi: 10.1080/13854046.2011.562869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gross AL, Rebok GW, Unverzagt FW, Willis SL, Brandt J. Word list memory predicts everyday function and problem-solving in the elderly: results from the ACTIVE cognitive intervention trial. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18(2):129–146. doi: 10.1080/13825585.2010.516814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aretouli E, Brandt J. Episodic memory in dementia: Characteristics of new learning that differentiate Alzheimer’s, Huntington’s, and Parkinson’s diseases. Arch Clin Neuropsychol. 2010;25(5):396–409. doi: 10.1093/arclin/acq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson’s disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16(3):177–180. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson DK, Galvin JE. Longitudinal changes in cognition in Parkinson’s disease with and without dementia. Dement Geriatr Cogn Disord. 2011;31(2):98–108. doi: 10.1159/000323570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stepkina DA, Zakharov VV, Yakhno NN. Cognitive impairments in progression of Parkinson’s disease. Neurosci Behav Physiol. 2010;40(1):61–67. doi: 10.1007/s11055-009-9223-6. [DOI] [PubMed] [Google Scholar]
- 80.Schendan HE, Amick MM, Cronin-Golomb A. Role of a lateralized parietal-basal ganglia circuit in hierarchical pattern perception: evidence from Parkinson’s disease. Behav Neurosci. 2009;123(1):125–136. doi: 10.1037/a0013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cronin-Golomb A, Braun AE. Visuospatial dysfunction and problem solving in Parkinson’s disease. Neuropsychology. 1997;11(1):44–52. doi: 10.1037//0894-4105.11.1.44. [DOI] [PubMed] [Google Scholar]
- 82.Firbank MJ, Colloby SJ, Burn DJ, McKeith IG, O’Brien JT. Regional cerebral blood flow in Parkinson’s disease with and without dementia. Neuroimage. 2003;20(2):1309–1319. doi: 10.1016/S1053-8119(03)00364-1. [DOI] [PubMed] [Google Scholar]
- 83.Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson’s disease. Neuropsychologia. 2006;44(3):339–349. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson’s disease. Mov Disord. 2009;24(8):1193–1199. doi: 10.1002/mds.22560. [DOI] [PubMed] [Google Scholar]
- 85.Inzelberg R, Schechtman E, Hocherman S. Visuo-motor coordination deficits and motor impairments in Parkinson’s disease. PLoS One. 2008;3(11):e3663. doi: 10.1371/journal.pone.0003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D. Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J Int Neuropsychol Soc. 2010;16(2):268–277. doi: 10.1017/S1355617709991299. [DOI] [PubMed] [Google Scholar]
- 87.Benton AL, Sivan AB, Hamsher K, Varney N, Spreen O. Contributions to Neuropsychological Assessment-A Clinical Manual. Lutz, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- 88.Montse A, Pere V, Carme J, Francesc V, Eduardo T. Visuospatial deficits in Parkinson’s disease assessed by judgment of line orientation test: error analyses and practice effects. J Clin Exp Neuropsychol. 2001;23(5):592–598. doi: 10.1076/jcen.23.5.592.1248. [DOI] [PubMed] [Google Scholar]
- 89.Venderploeg RD, LaLone LV, Greblo P, Schinka JA. Odd-even short forms of the Judgment of Line Orientation Test. Appl Neuropsychol. 1997;4(4):244–246. doi: 10.1207/s15324826an0404_6. [DOI] [PubMed] [Google Scholar]
- 90.Tranel D, Vianna E, Manzel K, Damasio H, Grabowski T. Neuroanatomical correlates of the Benton Facial Recognition Test and Judgment of Line Orientation Test. J Clin Exp Neuropsychol. 2009;31(2):219–233. doi: 10.1080/13803390802317542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montgomery P, Silverstein P, Wichmann R, Fleischaker K, Andberg M. Spatial updating in Parkinson’s disease. Brain Cogn. 1993;23(2):113–126. doi: 10.1006/brcg.1993.1050. [DOI] [PubMed] [Google Scholar]
- 92.Steinberg BA, Bieliauskas LA, Smith GE, Langellotti C, Ivnik RJ. Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Boston Naming Test, the MAE Token Test, and the Judgment of Line Orientation Test. Clin Neuropsychol. 2005;19(3–4):280–328. doi: 10.1080/13854040590945229. [DOI] [PubMed] [Google Scholar]
- 93.Ska B, Poissant A, Joanette Y. Line orientation judgment in normal elderly and subjects with dementia of Alzheimer’s type. J Clin Exp Neuropsychol. 1990;12(5):695–702. doi: 10.1080/01688639008401012. [DOI] [PubMed] [Google Scholar]
- 94.Finton MJ, Lucas JA, Graff-Radford NR, Uitti RJ. Analysis of visuospatial errors in patients with Alzheimer’s disease or Parkinson’s disease. J Clin Exp Neuropsychol. 1998;20(2):186–193. doi: 10.1076/jcen.20.2.186.1167. [DOI] [PubMed] [Google Scholar]
- 95.Freedman M, Leach L, Kaplan E, Wincour G, Shulman K, Delis DC. Clock Drawing: A Neuropsychological Analysis. New York: Oxford Univeristy Press; 1994. [Google Scholar]
- 96.Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15(6):548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 97.Lee AY, Kim JS, Choi BH, Sohn EH. Characteristics of clock drawing test (CDT) errors by the dementia type: quantitative and qualitative analyses. Arch Gerontol Geriatr. 2009;48(1):58–60. doi: 10.1016/j.archger.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 98.Sunderland T, Hill JL, Mellow AM, et al. Clock drawing in Alzheimer’s disease. A novel measure of dementia severity. J Am Geriatr Soc. 1989;37(8):725–729. doi: 10.1111/j.1532-5415.1989.tb02233.x. [DOI] [PubMed] [Google Scholar]
- 99.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc. 1989;37(8):730–734. doi: 10.1111/j.1532-5415.1989.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 100.Hubbard EJ, Santini V, Blankevoort CG, et al. Clock drawing performance in cognitively normal elderly. Arch Clin Neuropsychol. 2008;23(3):295–327. doi: 10.1016/j.acn.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saka E, Elibol B. Enhanced cued recall and clock drawing test performances differ in Parkinson’s and Alzheimer’s disease-related cognitive dysfunction. Parkinsonism Relat Disord. 2009;15(9):688–691. doi: 10.1016/j.parkreldis.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Litvinenko IV, Odinak MM, Mogil’naya VI, Emelin AY. Efficacy and safety of galantamine (reminyl) for dementia in patients with Parkinson’s disease (an open controlled trial) Neurosci Behav Physiol. 2008;38(9):937–945. doi: 10.1007/s11055-008-9077-3. [DOI] [PubMed] [Google Scholar]
- 103.Litvinenko IV, Odinak MM, Mogil’naya VI, Perstnev SV. Use of memantine (akatinol) for the correction of cognitive impairments in Parkinson’s disease complicated by dementia. Neurosci Behav Physiol. 2010;40(2):149–155. doi: 10.1007/s11055-009-9244-1. [DOI] [PubMed] [Google Scholar]
- 104.Beatty WW, Monson N. Lexical processing in Parkinson’s disease and multiple sclerosis. J Geriatr Psychiatry Neurol. 1989;2(3):145–152. doi: 10.1177/089198878900200305. [DOI] [PubMed] [Google Scholar]
- 105.Beatty WW, Monson N, Goodkin DE. Access to semantic memory in Parkinson’s disease and multiple sclerosis. J Geriatr Psychiatry Neurol. 1989;2(3):153–162. doi: 10.1177/089198878900200306. [DOI] [PubMed] [Google Scholar]
- 106.Cotelli M, Borroni B, Manenti R, et al. Action and object naming in Parkinson’s disease without dementia. Eur J Neurol. 2007;14(6):632–637. doi: 10.1111/j.1468-1331.2007.01797.x. [DOI] [PubMed] [Google Scholar]
- 107.Piatt AL, Fields JA, Paolo AM, Koller WC, Troster AI. Lexical, semantic, and action verbal fluency in Parkinson’s disease with and without dementia. J Clin Exp Neuropsychol. 1999;21(4):435–443. doi: 10.1076/jcen.21.4.435.885. [DOI] [PubMed] [Google Scholar]
- 108.Grossman M, Kalmanson J, Bernhardt N, Morris J, Stern MB, Hurtig HI. Cognitive resource limitations during sentence comprehension in Parkinson’s disease. Brain Lang. 2000;73(1):1–16. doi: 10.1006/brln.2000.2290. [DOI] [PubMed] [Google Scholar]
- 109.Grossman M, Lee C, Morris J, Stern MB, Hurtig HI. Assessing resource demands during sentence processing in Parkinson’s disease. Brain Lang. 2002;80(3):603–616. doi: 10.1006/brln.2001.2630. [DOI] [PubMed] [Google Scholar]
- 110.Grossman M, Zurif E, Lee C, et al. Information processing speed and sentence comprehension in Parkinson’s disease. Neuropsychology. 2002;16(2):174–181. doi: 10.1037//0894-4105.16.2.174. [DOI] [PubMed] [Google Scholar]
- 111.Hochstadt J, Nakano H, Lieberman P, Friedman J. The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain Lang. 2006;97(3):243–257. doi: 10.1016/j.bandl.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 112.Cummings JL, Darkins A, Mendez M, Hill MA, Benson DF. Alzheimer’s disease and Parkinson’s disease: comparison of speech and language alterations. Neurology. 1988;38(5):680–684. doi: 10.1212/wnl.38.5.680. [DOI] [PubMed] [Google Scholar]
- 113.Kaplan E, Goodglass HSW. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 114.Calero MD, Arnedo ML, Navarro E, Ruiz-Pedrosa M, Carnero C. Usefulness of a 15-item version of the Boston Naming Test in neuropsychological assessment of loweducational elders with dementia. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):P187–191. doi: 10.1093/geronb/57.2.p187. [DOI] [PubMed] [Google Scholar]
- 115.Jefferson AL, Wong S, Gracer TS, Ozonoff A, Green RC, Stern RA. Geriatric performance on an abbreviated version of the Boston naming test. Appl Neuropsychol. 2007;14(3):215–223. doi: 10.1080/09084280701509166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Flanagan JL, Jackson ST. Test-retest reliability of three aphasia tests: performance of non-brain-damaged older adults. J Commun Disord. 1997;30(1):33–42. doi: 10.1016/s0021-9924(96)00039-1. quiz 42–33. [DOI] [PubMed] [Google Scholar]
- 117.Axelrod BN, Ricker JH, Cherry SA. Concurrent validity of the MAE visual naming test. Arch Clin Neuropsychol. 1994;9(4):317–321. [PubMed] [Google Scholar]
- 118.Diehl J, Monsch AU, Aebi C, et al. Frontotemporal dementia, semantic dementia, and Alzheimer’s disease: the contribution of standard neuropsychological tests to differential diagnosis. J Geriatr Psychiatry Neurol. 2005;18(1):39–44. doi: 10.1177/0891988704272309. [DOI] [PubMed] [Google Scholar]
- 119.Graves RE, Bezeau SC, Fogarty J, Blair R. Boston naming test short forms: a comparison of previous forms with new item response theory based forms. J Clin Exp Neuropsychol. 2004;26(7):891–902. doi: 10.1080/13803390490510716. [DOI] [PubMed] [Google Scholar]
- 120.Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer’s disease. J Gerontol. 1992;47(3):P154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- 121.Albert ML, Spiro A, 3rd, Sayers KJ, et al. Effects of health status on word finding in aging. J Am Geriatr Soc. 2009;57(12):2300–2305. doi: 10.1111/j.1532-5415.2009.02559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.York MK, Levin HS, Grossman RG, Lai EC, Krauss JK. Clustering and switching in phonemic fluency following pallidotomy for the treatment of Parkinson’s disease. J Clin Exp Neuropsychol. 2003;25(1):110–121. doi: 10.1076/jcen.25.1.110.13626. [DOI] [PubMed] [Google Scholar]
- 123.Merola A, Zibetti M, Angrisano S, Rizzi L, Lanotte M, Lopiano L. Comparison of subthalamic nucleus deep brain stimulation and Duodopa in the treatment of advanced Parkinson’s disease. Mov Disord. 2011;26(4):664–670. doi: 10.1002/mds.23524. [DOI] [PubMed] [Google Scholar]
- 124.Miller IN, Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord. 2010;25(16):2695–2703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 126.Vlaar AM, Wade DT. Verbal fluency assessment of patients with multiple sclerosis: test-retest and inter-observer reliability. Clin Rehabil. 2003;17(7):756–764. doi: 10.1191/0269215503cr674oa. [DOI] [PubMed] [Google Scholar]
- 127.Senhorini MC, Cerqueira CT, Schaufelberger MS, et al. Brain activity patterns during phonological verbal fluency performance with varying levels of difficulty: A functional magnetic resonance imaging study in Portuguese-speaking healthy individuals. J Clin Exp Neuropsychol. 2011:1–10. doi: 10.1080/13803395.2011.561299. [DOI] [PubMed] [Google Scholar]
- 128.Schweizer TA, Alexander MP, Susan Gillingham BA, Cusimano M, Stuss DT. Lateralized cerebellar contributions to word generation: a phonemic and semantic fluency study. Behav Neurol. 2010;23(1–2):31–37. doi: 10.3233/BEN-2010-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: normal performance, validity and test-retest reliability. Br J Clin Psychol. 2000;39(Pt 2):181–191. doi: 10.1348/014466500163202. [DOI] [PubMed] [Google Scholar]
- 130.Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2004;10(4):608–622. doi: 10.1017/S1355617704104141. [DOI] [PubMed] [Google Scholar]
- 131.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 132.Krug A, Markov V, Sheldrick A, et al. The effect of the COMT val(158)met polymorphism on neural correlates of semantic verbal fluency. Eur Arch Psychiatry Clin Neurosci. 2009;259(8):459–465. doi: 10.1007/s00406-009-0010-8. [DOI] [PubMed] [Google Scholar]
- 133.Thompson AW, Liu H, Hays RD, et al. Diagnostic accuracy and agreement across three depression assessment measures for Parkinson’s disease. Parkinsonism Relat Disord. 2011;17(1):40–45. doi: 10.1016/j.parkreldis.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravina B, Elm J, Camicioli R, et al. The course of depressive symptoms in early Parkinson’s disease. Mov Disord. 2009;24(9):1306–1311. doi: 10.1002/mds.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ravina B, Camicioli R, Como PG, et al. The impact of depressive symptoms in early Parkinson disease. Neurology. 2007;69(4):342–347. doi: 10.1212/01.wnl.0000268695.63392.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14(10):858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 137.Friedman B, Heisel MJ, Delavan RL. Psychometric properties of the 15-item geriatric depression scale in functionally impaired, cognitively intact, community-dwelling elderly primary care patients. J Am Geriatr Soc. 2005;53(9):1570–1576. doi: 10.1111/j.1532-5415.2005.53461.x. [DOI] [PubMed] [Google Scholar]
- 138.Weintraub D, Oehlberg KA, Katz IR, Stern MB. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14(2):169–175. doi: 10.1097/01.JGP.0000192488.66049.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weintraub D, Xie S, Karlawish J, Siderowf A. Differences in depression symptoms in patients with Alzheimer’s and Parkinson’s diseases: evidence from the 15-item Geriatric Depression Scale (GDS-15) Int J Geriatr Psychiatry. 2007;22(10):1025–1030. doi: 10.1002/gps.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mattis S. Dementia Rating Scale: Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 141.Folstein MF, Folstein SE, McHugh PR. Mini Mental State: A Practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 142.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 143.Mannus J, Visser M, Verwey NA, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61:1222–1228. doi: 10.1212/01.wnl.0000091864.39702.1c. [DOI] [PubMed] [Google Scholar]
- 144.Pagonabarroga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A. Parkinson’s Disease-Cognitive Rating Scale: A new cognitive scale specific for Parkinson’s disease. Movement Disorders. 2008;23:998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- 145.Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 146.Heaton RK, Chelune GJ, Talley JL, Kay GC, Curtis G. Wisconsin Card Sorting Test Manual. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 147.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. 2. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 148.Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 149.Benton AL. Revised Visual Retention Test. 4. New York, NY: Psychological Corporation; 1974. [Google Scholar]
- 150.Warrington EK. Recognition Memory Test: Manual. Berkshire, UK: NFER-Nelson; 1984. [Google Scholar]
- 151.Rey A, Osterreith PA. Translations of excerpts from Andre Rey’s psychological examination of traumatic encephalopathy and P.A. Osterreith’s The Complex Figure Copy Test. The Clinical Neuropsychologist. 1993;10:375–381. [Google Scholar]
- 152.Hooper HE. Use of the Hooper Visual Organization Test in the differentiation of organic brain pathology from formal, psychoneurotic, and schizophrenic reactions. American Psychologist. 1952;7:350. [Google Scholar]
- 153.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24(4):641–652. [PubMed] [Google Scholar]
- 154.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 155.Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. San Antonio, TX: Pearson; 2000. [Google Scholar]
- 156.Hamilton M. Rating depressive patients. Journal of Clinical Psychiatry. 1980;(41):21–24. [PubMed]
- 157.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX: Pearson; 1996. [Google Scholar]
- 158.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 159.Starkstein SE, MAyberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neuroscience. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- 160.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Footnotes
The Wechsler Adult Intelligence Scale and the Wechsler Memory Scale are currently in their 4th Edition (published 2008 and 2009, respectively). Given that the WAIS-III and WMS-III have been in use since 1998, use of these scales may facilitate consistency across sites.
Many neuropsychological tests measure multiple cognitive domains. For example, the CDT measures both spatial functioning and executive skills, while verbal fluency (particularly phonemic fluency) can reflect language ability and/or executive functions. We thus recommend evaluating performance across tasks in relation to performance on other tasks to determine whether individual performance is related to primary deficits in one or more areas of cognition.
Financial disclosure and acknowledgments: NINDS (NS062684, NS053488) and the Nancy and Buster Alvord Endowment. We thank Dr. Kathy Montine for editorial assistance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khachaturian ZS, Khachaturian AS, Thies W. The draft “National Plan” to address Alzheimer’s disease - National Alzheimer’s Project Act (NAPA) Alzheimers Dement. 2012;8:234–236. doi: 10.1016/j.jalz.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease Research Summit 2012: Path to Treatment and Prevention. http://www.nia.nih.gov/newsroom/alzheimers-disease-research-summit-2012-recommendations.
- 3.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Bronnick K, Fladby T. Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:371–378. doi: 10.1007/s11910-011-0203-1. [DOI] [PubMed] [Google Scholar]
- 5.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Bronnick K, Ehrt U, De Deyn PP, Tekin S, Emre M, Cummings JL. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78:36–42. doi: 10.1136/jnnp.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14:866–874. [PubMed] [Google Scholar]
- 8.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48:938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 9.Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 10.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 11.Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, Weintraub D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurlan R, Murphy D. Parkinson’s disease data and organizing center. Movement disorders: official journal of the Movement Disorder Society. 2007;22:904–905. doi: 10.1002/mds.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73:1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Neurological Disorders and Stroke. Common Data Elements, Parkinson’s Disease. 2012 doi: 10.1177/1740774512438980. http://www.commondataelements.ninds.nih.gov/PD.aspx. [DOI] [PMC free article] [PubMed]
- 15.Michael J Fox Foundation. Parkinson’s Progression Marker Initiative. 2011 http://www.ppmi-info.org/study-design/research-documents-and-sops/
- 16.Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, Srulijes K, Trojanowski JQ, Lee VM, Siderowf AD, Hurtig H, Litvan I, Schiess MC, Peskind ER, Masuda M, Hasegawa M, Lin X, Pan C, Galasko D, et al. Phosphorylated alpha-synuclein in Parkinson’s disease. Sci Transl Med. 2012;4:121ra120. doi: 10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 18.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 19.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 20.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 21.Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, Porter RJ, Watts R, Anderson TJ. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 22.Nazem S, Siderowf AD, Duda JE, Have TT, Colcher A, Horn SS, Moberg PJ, Wilkinson JR, Hurtig HI, Stern MB, Weintraub D. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RM, Wadia P, Miyasaki J, Duff-Canning S, Lang AE, Marras C. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 24.Chou KL, Amick MM, Brandt J, Camicioli R, Frei K, Gitelman D, Goldman J, Growdon J, Hurtig HI, Levin B, Litvan I, Marsh L, Simuni T, Troster AI, Uc EY. A recommended scale for cognitive screening in clinical trials of Parkinson’s disease. Mov Disord. 2010;25:2501–2507. doi: 10.1002/mds.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasten M, Bruggemann N, Schmidt A, Klein C. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2010;75:478. doi: 10.1212/WNL.0b013e3181e7948a. [DOI] [PubMed] [Google Scholar]
- 26.Thompson AW, Liu H, Hays RD, Katon WJ, Rausch R, Diaz N, Jacob EL, Vassar SD, Vickrey BG. Diagnostic accuracy and agreement across three depression assessment measures for Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:40–45. doi: 10.1016/j.parkreldis.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheikh JI, Yesavage J. Clinical Gerontology: A Guide to Assessment and Intervention. Haworth Press; New York: 1986. Geriatric Depression Scale (GDS): Recent Evidence and Development of a Shorter Version; pp. 165–173. [Google Scholar]
- 28.Mattis S. Dementia Rating Scale: Manual. Odessa, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 29.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.