Abstract
Portal hypertensive biliopathy (PHB) is characterized by anatomical and functional abnormalities of the intrahepatic, extrahepatic and pancreatic ducts, in patients with portal hypertension associated to extrahepatic portal vein obstruction and less frequently to cirrhosis. These morphological changes, consisting in dilatation and stenosis of the biliary tree, are due to extensive venous collaterals occurring in an attempt to decompress the portal venous blockage. It is usually asymptomatic until it progresses to more advanced stages with cholestasis, jaundice, biliary sludge, gallstones, cholangitis and finally biliary cirrhosis. Imaging modalities of the biliary tree such as Doppler ultrasound, computed tomography, magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography are essential to establish the diagnosis and the need of therapeutical interventions. Once the diagnosis is established, treatment with ursodesoxycholic acid seems to be beneficial. Decompression of the biliary tree to dilate, remove stones or implant biliary prosthesis by endoscopic or surgical procedures (hepato-yeyunostomy) usually resolves the cholestatic picture and prevents septic complications. The ideal treatment is the decompression of the portal system, with transjugular intrahepatic porto-systemic shunt or a surgical porto-systemic shunt. Unfortunately, few patients will be candidates for these procedures due to the extension of the thrombotic process. The purpose of this paper is to report the first 3 cases of PHB seen in a Colombian center and to review the literature.
Keywords: Bile duct diseases, Biliary obstruction, Banti’s syndrome, Cholestasis in children, Portal vein obstruction, Interventional endoscopy
INTRODUCTION
Portal hypertensive biliopathy (PHB) is defined as the set of anatomical and functional alterations of the intra-and extrahepatic bile ducts in patients with portal hypertension due to extrahepatic portal vein obstruction (EHPVO). These changes include dilatation and stenosis of the bile ducts, common hepatic duct, gallbladder and intrahepatic ducts and they are due to extrinsic compression of these pathways by paracholecystic and paracholedochal venous plexuses that expand and compress in an attempt to decompress the venous blockage generated by the portal vein thrombosis[1].
Initially the process is silent and without any specific symptoms. However, as it progresses to more advanced stages, the patient presents with cholestasis, jaundice, biliary sludge, gallstones and finally secondary biliary cirrhosis. Some researchers have reported the presence of these symptoms in 70%-100% in patients from India with EHPVO[2].
It is a relatively new disease, and according to Löhr et al[3] early associations between jaundice and EHPVO were reported in 1944 by Fraser et al, in 1965 by Gibson et al[4], and it was finally Dhiman et al[5], who in 1999 proposed the term “portal hypertensive biliopathy”; as a newly described disease it is expected to be underdiagnosed. The incidence of PHB in patients with EHPVO (81%-100%)[6] is much higher than in patients with liver cirrhosis (0%-33%) or idiopathic portal hypertension (9%-40%)[1]. To our knowledge, there have been no cases of PHB reported in Colombia.
The aim of this paper is to present the first three cases documented in Colombia and to review the literature on the subject.
CASE REPORT
Case 1
This male patient first consulted at 7 years of age for upper gastrointestinal bleeding due to esophageal varices secondary to portal vein thrombosis. Endoscopic sclerotherapy and band ligation were performed on several occasions and treatment with oral propranolol was started. Liver function tests were normal and diagnostic imaging [Doppler ultrasound, computed tomography (CT)] showed cavernomatous degeneration of the portal vein and collateral circulation through spontaneous splenorenal shunts. A percutaneous liver biopsy showed non-cirrhotic liver with minimal nonspecific changes and portal fibrosis. Over the next 10 years he had 3 episodes of variceal bleeding controlled by endoscopic ligation.
At the age of 21 (14 years later), he was readmitted to the hospital due to diffuse abdominal pain, cholestatic liver test pattern and diagnostic images compatible with thrombosis and cavernomatous degeneration of the portal vein, associated with spleno-mesenteric thrombosis and diffuse intrahepatic dilatation of the biliary tree (Figure 1). A magnetic resonance cholangiopancreatography (MRCP) confirmed the findings and showed extrinsic compression of the bile duct by venous collaterals and distal common bile duct stenosis not passable endoscopically (Figures 2 and 3). All laboratory tests to rule out other liver diseases were negative including viral and autoimmune serologies, tumor markers and testing for procoagulant processes.
Figure 1.
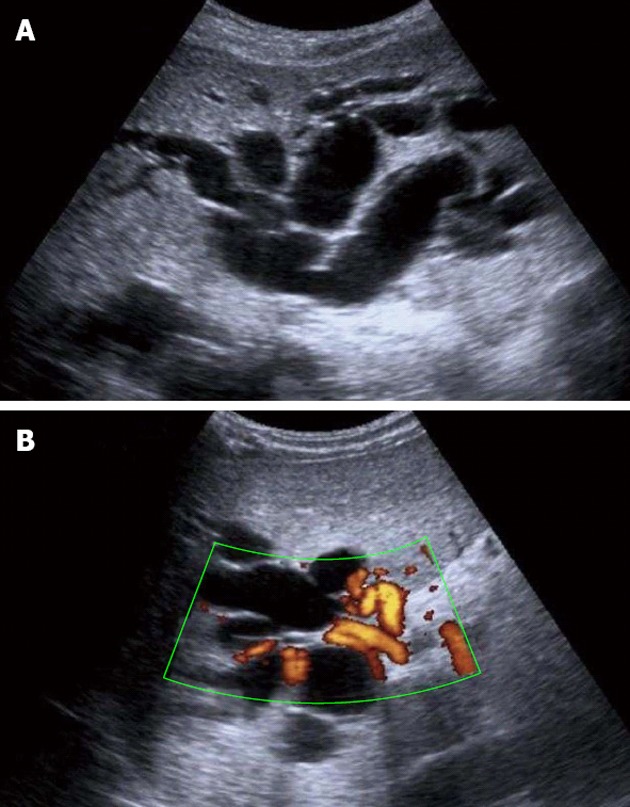
Ultrasound examination. A: Ultrasound examination showing dilatation of the intrahepatic bile ducts; B: Doppler ultrasound examination demonstrating cavernomatous degeneration of the portal vein with hilar collateral circulation.
Figure 2.
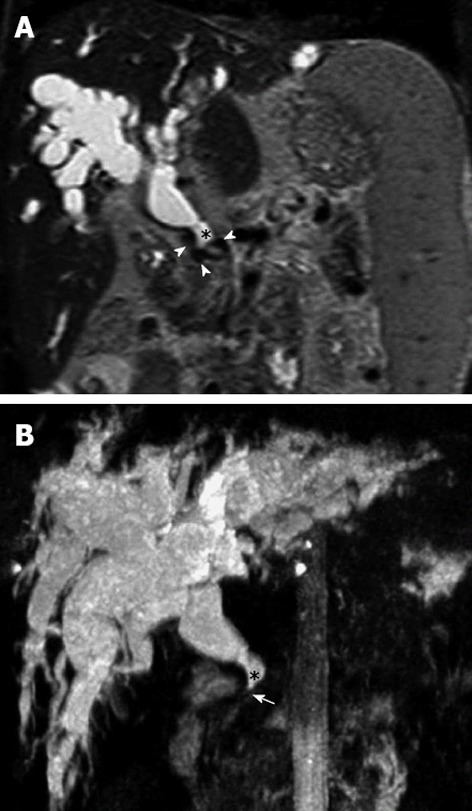
Magnetic resonance cholangiopancreatography. A: Coronal T2 weight Magnetic resonance indicates marked dilatation of the intra-and extra-hepatic bile duct (*), with presence of vascular structures with void flow that exert extrinsic compression (arrowheads), corresponding to the cavernoma. B: Coronal maximum intensity reconstruction shows the severity of the dilatation of the intra- and extra-hepatic bile duct (*) with notable narrowing of the distal common bile duct (arrow).
Figure 3.
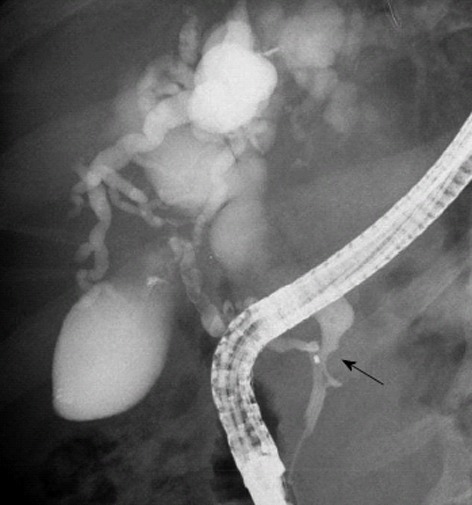
Pancreatic endoscopic retrograde cholangiopancreatography image indicates the site of stenosis of the bile duct (arrow) and a marked dilation of the biliary radicals.
With this information a diagnosis of PHB was established and treatment with ursodeoxycholic acid and low-molecular-weight heparin was started. He was discharged after establishing that he was not a candidate for a portal-systemic shunt or liver transplantation and improvement in his cholestatic pattern was confirmed (Table 1).
Table 1.
Liver function tests
| Liver function test | Case 1 | Case 2 | Case 3 | |||
| At diagnosis | Follow up | At diagnosis | Follow up | At diagnosis | Follow up | |
| Alkaline phosphatase (U/L) | 424 | 347 | 437 | 207 | 936 | 311 |
| GGT (U/L) | 131 | 128 | 201 | 99 | 241 | 111 |
| Total bilirubin (mg/dL) | 16.1 | 5.9 | 6.62 | 3.05 | 17.7 | 9.28 |
| Direct bilirubin (mg/dL) | 12.8 | 4.9 | 4.61 | 1.99 | 13.5 | 7.7 |
| ALT (U/L) | 114 | 63 | 125 | 68 | 235 | 24 |
| AST (U/L) | 159 | 83 | 151 | 59 | 131 | 33 |
| INR | 1.17 | 1.06 | 1.66 | |||
| Albumin (g/dL) | 2.6 | 2.8 | 3.68 | 4.13 | 1.5 | 2.4 |
GGT: γ glutamyl transferase; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio. Normal values: Alkaline phosphatase 35-104 U/L; GGT 0-38 U/L; Total bilirubin 0.3-1.2 mg/dL; Direct bilirubin 0-0.3 mg/dL; ALT 10-49 U/L; AST 0-34 U/L; Albumin 3.4-5.0 g/dL.
Case 2
A 20-year-old male presented with a history of gastrointestinal bleeding on several occasions, and esophageal varices secondary to portal hypertension, noncirrhotic, massive splenomegaly, hypersplenism and intermittent abdominal pain. Past medical history included umbilical vein catheterization in the neonatal period secondary to indirect hyperbilirubinemia due to ABO/Rh incompatibility. Esophagogastric devascularization, splenectomy, vagotomy and pyloroplasty were performed. His chief complaints were abdominal pain, jaundice with slight tinge in the sclera. Liver function tests were performed, CT of the abdomen showed dilatation of the intrahepatic bile duct (Figure 4), endoscopic retrograde cholangiopancreatography (ERCP) showed extrinsic compression of the distal common bile duct. In August 2011, he required hospitalization following another episode of jaundice, MRCP showed dilatation of the intrahepatic and extrahepatic bile ducts and cavernomatous degeneration of the portal vein (Figure 5). Due to extensive vascular thrombosis of the portal venous system, a liver transplant was not feasible and the risk of hepatoyeyunostomy was unacceptably high. Ursodeoxycholic acid treatment was started with apparent benefit (Table 1). Etiological tests to rule out other causes of liver disease were negative including viral, autoimmunity, tumor markers and procoagulant processes.
Figure 4.
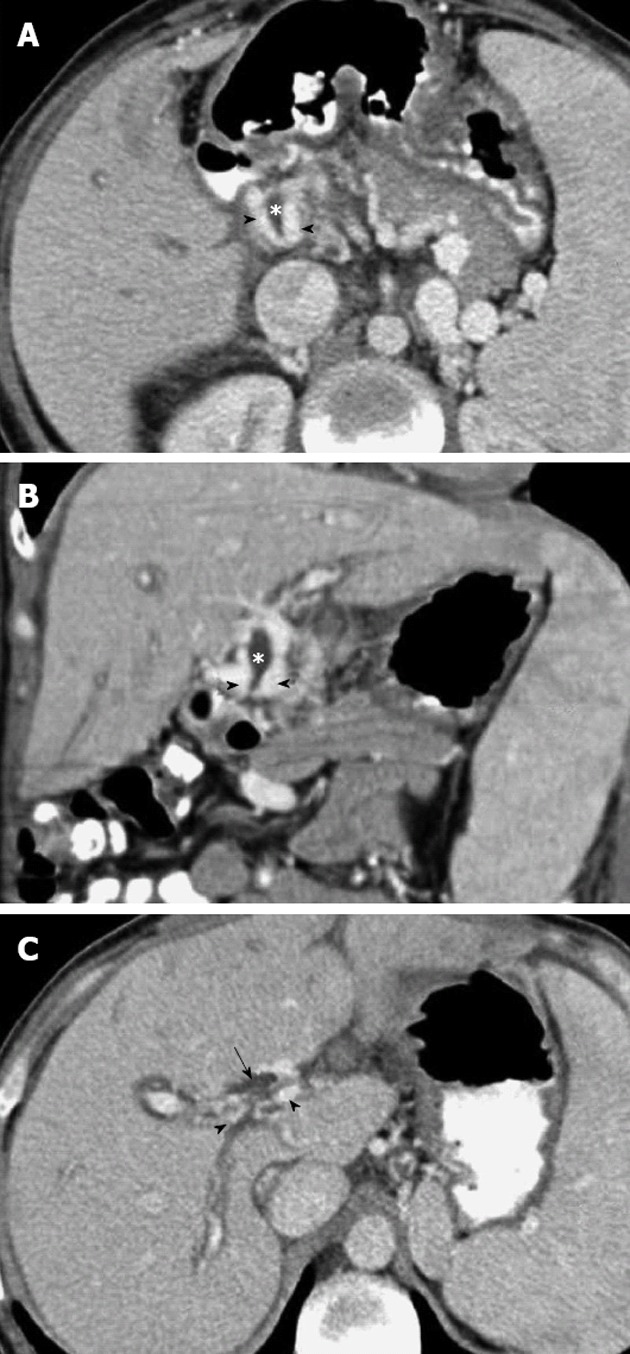
Portal phase abdominal computed tomography. A, B: Axial (A) and coronal planes (B) show multiple dilated collateral representing the paracholedochal plexus (arrowheads) with reduced diameter of the bile duct (*), secondary to extrinsic compression by the cavernoma; C: The superior axial image shows dilatation of the bile ducts (arrow).
Figure 5.

Magnetic resonance cholangiopancreatography. Coronal maximum intensity reconstruction identifies irregularities and extrinsic compression of the distal common bile duct (arrowheads).
Case 3
A 30-year-old male with a past medical history of several episodes of upper gastrointestinal bleeding that started in 2005. Cavernomatous degeneration of the portal vein, non-cirrhotic portal hypertension with complications of hypersplenism and esophagogastric varices were documented. All etiological laboratory tests to rule out other liver diseases were negative, including viral and autoimmune serologies, tumor markers and procoagulant processes. Liver biopsy was reported as normal. In 2006, a meso-caval shunt was performed which, in the immediate postoperative period, showed signs of dysfunction and occlusion.
The case was presented to the liver transplant committee and it concluded that, given the patient’s history and the characteristics of bleeding, esophagogastric devascularization surgery was performed, as there was no other possibility of a surgical shunt or transjugular intrahepatic porto-systemic shunts (TIPS). In August 2011, he presented to the emergency room with abdominal pain over 2 mo associated with jaundice and cholestasis. A hepatobiliary scan (up to 6 h) indicated signs of cholestasis without passage of tracer into the intestine or bile duct. The inability to perform an ERCP due to complete biliary obstruction resulted in him undergoing a hepato-yeyunostomy with an adequate postoperative course (Table 1).
DISCUSSION
Literature review and as subtitle definition
Portal hypertensive biliopathy is defined as changes in the biliary tract in patients with portal hypertension due to EHPVO[7]. These include stenosis and dilatation of the intrahepatic and extrahepatic bile ducts and secondary varicose veins surrounding the common bile duct and the gallbladder wall. When these changes evolve, the individual presents with cholestasis and jaundice, and choledocholithiasis is a common sequela[2,8]. In general, this disease has been reported in 70%-100% of patients with extrahepatic obstruction of the portal vein[8]. It is much less common in cirrhotic portal hypertension, and it is speculated that the reason is that in cirrhotic patients blocking of the portal circulation occurs at the level of hepatic sinusoid, giving origin to collateral circulation far from the vein complexes around the extrahepatic bile ducts.
Prevalence
Liver cirrhosis is the most common cause of portal hypertension in the Western world and a rare cause of PHB. However, non-cirrhotic portal hypertension constitutes 40% of all cases of portal hypertension in developing countries[9]. Other causes in non-cirrhotic patients include: non-cirrhotic portal fibrosis, schistosomiasis, EHPVO, the idiopathic Budd Chiari syndrome, congenital hepatic fibrosis, nodular regenerative hyperplasia and sinusoidal obstruction syndrome (veno-occlusive disease of the liver). Although there are no population studies and its frequency is much higher in India, South Korea and Turkey[10,11], PHB is an increasingly important entity that seems to impact the natural history of EHPVO. We found proof of this in a literature review we carried out in PubMed using the term “portal biliopathy” in which we found 70 English-language publications worldwide, most of them consisting of reports of a few patients and review articles with 9 publications reporting more than 10 cases[10-18]. We found a total of 223 cases most in Asian countries and to a lesser extent in Europe and North America. In South America, PHB has been reported in Mexico, Chile and Brazil[19-21]. To the best of our knowledge, no cases have ever been reported in Colombia.
PVT presents frequently during childhood and adolescence[22], but PHB usually presents later in life.
Pathophysiology
It is well known that, to maintain hepatic blood flow, the development of multiple collateral veins occurs in response to obstruction of the extrahepatic portal vein. One study showed that the time between complete acute thrombosis and the formation of the cavernoma is 6 wk[23]. These collateral veins, called portal cavernoma, form a dense vascular pattern and fibrous stroma in the peripancreatic region along the portal vein occluded, and provide an alternative route around the thrombosed segment of the portal vein[4]. In normal conditions, the venous drainage of the bile duct is divided into two special plexuses. The first is formed by the pericholedochal Saint’s venous plexus which extends as a fine grid around the bile duct and main hepatic ducts. The second is formed by the pericholedochal Petren’s venous plexus that is parallel to the bile duct and is connected to the gastric vein, the pancreatic-duodenal and portal vein. Its conversion into collateral veins causing pressure and bulging of the thin and flexible bile duct walls is called portal biliopathy[24,25]. In addition to the hypothesis of compression of the varices around the bile duct, there is an ischemic hypothesis that implies that the vascularization of the bile ducts is compromised, leading to scarring of the lining of the ducts, resulting in biliary strictures and cholangiectasias[2].
Differential diagnosis
The differential diagnosis is extensive and includes: primary and secondary sclerosing cholangitis, gallstone disease, ischemic cholangiopathy, acute and chronic rejection, primary biliary cirrhosis, cystic fibrosis and autoimmune pancreatitis. In tropical areas, parasitic diseases that compromise the bile duct should be considered[26].
Symptomatology
Although morphological changes have been reported in 80%-100% of patients with EHPVO[1,6], the majority remains asymptomatic for many years and is rare in children. Several investigators have reported frequencies of 5%-17%[1,4,6,27], depending on the duration and frequency of clinical follow-up especially in the adult population. The most common clinical presentation is recurrent abdominal pain, fever, jaundice and cholangitis with partial or, in some cases complete, biliary obstruction[14]. Alkaline phosphatase is elevated in 80% of cases[28], aminotransferases are normal until advanced stages of disease, and coagulation tests, and albumin levels may be abnormal in cases of complete obstruction with secondary biliary cirrhosis.
Diagnostic imaging
Typical indications of cavernomatous degeneration of the portal vein are visible by ultrasound: a decrease in its diameter, increased echogenicity of the tissue in the hilum, associated with multiple anechoic tubular structures, corresponding to distended paracholedochal veins, and it is technically difficult to identify the common bile duct. The indentations visible by ultrasound on the common bile duct are secondary to extrinsic compression by the enlarged paracholedochal venous plexus; these are larger and are connected with the gastric vein, pancreaticoduodenal and portal vein, while the irregularities caused by dilation of pericholedochal varices may not be observed by ultrasound, since their size is less than 1 mm[29]. Gallbladder varices, present in between 30%-55% of cases[29,30], are visualized as anechoic tubular structures of 1-5 mm in diameter in the external refractive surface of the gallbladder, outside the muscular layer. These varicose veins correlate with ERCP findings[29].
Evaluation of the extrahepatic bile duct by endoscopic Doppler ultrasound, shows compression of the biliary tree by the collateral circulation in patients with cavernomatous degeneration of the portal vein. This allows the exclusion of other causes such as stones, biliary sludge and tumors are not vizualised in other imaging modalities[30,31].
Multi-detector CT (MDCT), using narrower collimation and lower acquisition time, gives high quality images for visualizing the collateral circulation, the product of portal vein obstruction[32]. MDCT angiography techniques and post-processing, clearly demarcate the signs of cavernous transformation of portal vein, the compressive effect of collateral circulation around the bile duct, and gallbladder varices[15,29]. It has been claimed that 3D portography using MDCT has an accuracy similar to conventional portal angiography in demonstrating the characteristics of porto-systemic collaterals[33].
MRCP is currently the noninvasive diagnostic modality of choice, allowing an adequate characterization of the intra-and extrahepatic bile duct and with a capacity similar to ERCP for visualization of changes in the bile duct[34]. Condat et al[35] studied 25 patients with cavernous transformation of portal vein by crisis resource management (CRM), excluding those with malignancies and/or cirrhosis. Stenosis of the common bile ducts was seen in 16 cases while 5 had intra- and extrahepatic bile duct abnormalities, most consisting of short length stenosis (13 of 21) associated with retrograde dilatation in 16 cases. Using portography sequences with gadolinium, it was demonstrated in all cases that bile duct alterations corresponded with the mass effect of the cavernoma. Thus, CRM sequences with contrast portography are superior to CRM alone for detecting alterations of the intrahepatic bile duct and common bile duct stones and for recognizing and differentiating them from collateral circulation[35].
ERCP features described most often in the literature, include biliary segments with narrowing of variable length and degree, indentations and irregularity of the contours of the bile duct and the presence of angles, ectasia and calculi. An absence of branching can be seen in the intrahepatic bile ducts and in some dilated ducts[35]. Cholangiographic features are not specific and similar to other entities, explaining the previous name of “pseudo-cholangiocarcinoma”[36] and “pseudo-sclerosing cholangitis”[6]. Patients with extrahepatic occlusion of the portal vein have abnormal ERCP in 81% to 100% of cases, with involvement of the extrahepatic bile duct in 60%-97%, right hepatic duct in 40% to 56% and left hepatic duct in 55% to 100%[35]. One study identified alterations in the intra-and extrahepatic bile duct in 85% (17 of 20) of patients with extrahepatic obstruction of the portal vein, whereas cirrhosis without extrahepatic portal obstruction were found only in 27% (3 of 11) of cases[30]. Chandra et al[2] have proposed a morphological classification based on the topography of the cholangiography findings (Table 2), although its usefulness for management remains to be demonstrated.
Table 2.
Portal hypertensive biliopathy: Morphological classification[2]
| Type | Findings |
| I | Involvement of extrahepatic bile duct |
| II | Involvement of intrahepatic bile ducts only |
| IIIa | Involvement of extrahepatic bile duct and unilateral intrahepatic bile duct |
| IIIb | Involvement of extrahepatic bile duct and bilateral intrahepatic ducts |
Some reports on patients with cavernous transformation of the portal vein, especially those with extension to the superior mesenteric or splenic vein, have shown pancreatic head enlargement and images of a pseudo-mass at this location, with heterogeneous signal intensity in the simple phase in relation to the cavernoma, constituting intra and/or peripancreatic collateral circulation. Dynamic sequences with contrast allow an adequate assessment of intrapancreatic cavernoma, differentiating it from neoplastic lesions. In contrast to the high frequency of alterations in the biliary tract, altered pancreatic ducts have been reported in only a minority of cases[37]. Furthermore, a greater number of cases of extrahepatic portal biliopathy have been reported in cirrhotic patients with hypercoagulable states when the portal thrombosis extends to the superior mesenteric vein[18].
Histopathological changes
The portal vein is replaced by an extensive vascular network in a stromal support with multiple anastomoses that eventually achieve the passage of blood to the liver. This vascular structure called a “cavernoma” extends along the entire porta and in some cases to the intrahepatic portal branches[38]. The liver has a generally smooth or finely granular aspect with some fibrous septa that project into the parenchyma, a consequence of thrombosis of intrahepatic portal vessels[39]. Nodular regenerative hyperplasia has been reported, especially in cases of EHPVO associated with human immunodeficiency virus[40]. It is exceptional to find cirrhotic changes and liver function tests are normal in most cases. In clinically manifest biliopathy the spectrum of changes ranges from intrahepatic cholestasis, ductal proliferation and acute cholangitis. In cases of complete biliary obstruction secondary biliary cirrhosis usually occurs with time.
Treatment
In asymptomatic patients with EHPVO, specific treatment is not recommended to improve bile flow. However, the identification of early bile duct morphological alterations by MRCP, makes it possible to design a management program and to intervene early when symptoms begin. The use of therapeutic doses of ursodeoxycholic acid (10-15 mg/kg per day) is the first choice. This should always be associated with a detailed examination of the bile duct by ERCP, CRM and intervention if necessary to dilate, remove stones or implant prosthesis (14). Clearly there is a risk of injuring the dilated venous complex, causing hemobilia and worsening cholestasis[41,42]. The ideal treatment for EHPVO associated with biliopathy is to decompress the portal system with a surgical porto-systemic shunt or interventional radiology with a TIPS. There is regression of ectopic varices and improvement of cholestasis in most patients, although a proportion of them continue with significant changes in bile ducts. Unfortunately, in most cases this treatment is not possible due to the extension of thrombosis to most vascular territories that drain into the portal vein.
In a recent study, Agarwal et al[10] reported on 39 patients who underwent porto-systemic shunt (34) and 2 bilio-digestive anastomosis, finding improvement of biliary symptoms in more than 60% of cases and an acceptable complications rate. However, the vast surgical experience of this group is not easy to reproduce in other centers. Liver transplantation is not generally indicated although there are some reports of liver and intestinal transplants when any of the above mentioned procedures have not been successful[43]. When the patient does not benefit from medical or endoscopic treatment and a porto-systemic shunt is not possible, hepaticojejunostomy performed without decompressing the portal system is a high risk option because multiple venous collaterals can generate uncontrollable bleeding.
In conclusion, portal hypertensive biliopathy is a newly recognized complication that occurs primarily in patients with EHPVO and less frequently in cases of cirrhosis. Modern imaging technology allows identification of morphological abnormalities even before symptoms occur. The most appropriate treatment sequence is to start ursodeoxycholic acid administration, combinatined with instrumentation of the bile duct when necessary. It is imperative to decompress the portal system with a porto-systemic shunt or TIPS when anatomy permits, and is not advisable to perform bilio-digestive shunts without previous decompression of the portal vein because of the high risk of bleeding. In cases of procoagulant phenomena associated with EHPVO, anticoagulation with low molecular weight heparins can lead to recanalization of the portal vein with significant clinical improvement.
Footnotes
P- Reviewer Maruyama H S- Editor Li JY L- Editor Hughes D E- Editor Li JY
References
- 1.Khuroo MS, Yattoo GN, Zargar SA, Javid G, Dar MY, Khan BA, Boda MI. Biliary abnormalities associated with extrahepatic portal venous obstruction. Hepatology. 1993;17:807–813. [PubMed] [Google Scholar]
- 2.Chandra R, Kapoor D, Tharakan A, Chaudhary A, Sarin SK. Portal biliopathy. J Gastroenterol Hepatol. 2001;16:1086–1092. doi: 10.1046/j.1440-1746.2001.02562.x. [DOI] [PubMed] [Google Scholar]
- 3.Löhr JM, Kuchenreuter S, Grebmeier H, Hahn EG, Fleig WE. Compression of the common bile duct due to portal-vein thrombosis in polycythemia vera. Hepatology. 1993;17:586–592. doi: 10.1002/hep.1840170410. [DOI] [PubMed] [Google Scholar]
- 4.Gibson JB, Johnston GW, Fulton TT, Rodgers HW. Extrahepatic portal-venous obstruction. Br J Surg. 1965;52:129–139. doi: 10.1002/bjs.1800520211. [DOI] [PubMed] [Google Scholar]
- 5.Dhiman RK, Puri P, Chawla Y, Minz M, Bapuraj JR, Gupta S, Nagi B, Suri S. Biliary changes in extrahepatic portal venous obstruction: compression by collaterals or ischemic? Gastrointest Endosc. 1999;50:646–652. doi: 10.1016/s0016-5107(99)80013-3. [DOI] [PubMed] [Google Scholar]
- 6.Dilawari JB, Chawla YK. Pseudosclerosing cholangitis in extrahepatic portal venous obstruction. Gut. 1992;33:272–276. doi: 10.1136/gut.33.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarin SK, Sollano JD, Chawla YK, Amarapurkar D, Hamid S, Hashizume M, Jafri W, Kumar A, Kudo M, Lesmana LA, et al. Consensus on extra-hepatic portal vein obstruction. Liver Int. 2006;26:512–519. doi: 10.1111/j.1478-3231.2006.01269.x. [DOI] [PubMed] [Google Scholar]
- 8.Sarin SK, Agarwal SR. Extrahepatic portal vein obstruction. Semin Liver Dis. 2002;22:43–58. doi: 10.1055/s-2002-23206. [DOI] [PubMed] [Google Scholar]
- 9.Anand CS, Tandon BN, Nundy S. The causes, management and outcome of upper gastrointestinal haemorrhage in an Indian hospital. Br J Surg. 1983;70:209–211. doi: 10.1002/bjs.1800700407. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal AK, Sharma D, Singh S, Agarwal S, Girish SP. Portal biliopathy: a study of 39 surgically treated patients. HPB (Oxford) 2011;13:33–39. doi: 10.1111/j.1477-2574.2010.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin SM, Kim S, Lee JW, Kim CW, Lee TH, Lee SH, Kim GH. Biliary abnormalities associated with portal biliopathy: evaluation on MR cholangiography. AJR Am J Roentgenol. 2007;188:W341–W347. doi: 10.2214/AJR.05.1649. [DOI] [PubMed] [Google Scholar]
- 12.Chevallier P, Denys A, Novellas S, Schmidt S, Schnyder P, Bruneton JN. Magnetic resonance cholangiography features of biliary abnormalities due to cavernous transformation of the portal vein. Clin Imaging. 2006;30:190–194. doi: 10.1016/j.clinimag.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 13.Ertuğrul I, Köklü S, Başar O, Yüksel O, Uçar E, Coban S, Ibiş M, Arhan M, Odemiş B, Saşmaz N. Thrombosis of the portal venous system: a prospective study. J Clin Gastroenterol. 2008;42:835–838. doi: 10.1097/MCG.0b013e318046eadc. [DOI] [PubMed] [Google Scholar]
- 14.Oo YH, Olliff S, Haydon G, Thorburn D. Symptomatic portal biliopathy: a single centre experience from the UK. Eur J Gastroenterol Hepatol. 2009;21:206–213. doi: 10.1097/MEG.0b013e3283060ee8. [DOI] [PubMed] [Google Scholar]
- 15.Ozkavukcu E, Erden A, Erden I. Imaging features of portal biliopathy: frequency of involvement patterns with emphasis on MRCP. Eur J Radiol. 2009;71:129–134. doi: 10.1016/j.ejrad.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Belhadjbrik N, Kchaou-Ouakaa A, Kharrat J, Elloumi H, Gargouri D, Kochlef A, Ghorbel A. [Biliary abnormalities associated to portal Cavernoma. About 17 cases] Tunis Med. 2006;84:291–295. [PubMed] [Google Scholar]
- 17.Vibert E, Azoulay D, Aloia T, Pascal G, Veilhan LA, Adam R, Samuel D, Castaing D. Therapeutic strategies in symptomatic portal biliopathy. Ann Surg. 2007;246:97–104. doi: 10.1097/SLA.0b013e318070cada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walser EM, Runyan BR, Heckman MG, Bridges MD, Willingham DL, Paz-Fumagalli R, Nguyen JH. Extrahepatic portal biliopathy: proposed etiology on the basis of anatomic and clinical features. Radiology. 2011;258:146–153. doi: 10.1148/radiol.10090923. [DOI] [PubMed] [Google Scholar]
- 19.Mercado-Díaz MA, Hinojosa CA, Chan C, Anthon FJ, Podgaetz E, Orozco H. [Portal biliopathy] Rev Gastroenterol Mex. 2004;69:37–41. [PubMed] [Google Scholar]
- 20.Guerrero Hernández I, Weimersheimer Sandoval M, López Méndez E, Hernández Calleros J, Tapia AR, Uribe M. Biliary stricture caused by portal biliopathy: case report and literature review. Ann Hepatol. 2005;4:286–288. [PubMed] [Google Scholar]
- 21.Besa C, Cruz JP, Huete A, Cruz F. Portal biliopathy: a multitechnique imaging approach. Abdom Imaging. 2012;37:83–90. doi: 10.1007/s00261-011-9765-2. [DOI] [PubMed] [Google Scholar]
- 22.Schettino GC, Fagundes ED, Roquete ML, Ferreira AR, Penna FJ. Portal vein thrombosis in children and adolescents. J Pediatr (Rio J) 2006;82:171–178. doi: 10.2223/JPED.1484. [DOI] [PubMed] [Google Scholar]
- 23.De Gaetano AM, Lafortune M, Patriquin H, De Franco A, Aubin B, Paradis K. Cavernous transformation of the portal vein: patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol. 1995;165:1151–1155. doi: 10.2214/ajr.165.5.7572494. [DOI] [PubMed] [Google Scholar]
- 24.Hunt AH. Compression of the common bile-duct by an enlarging collateral vein in a case of portal hypertension. Br J Surg. 1965;52:636–637. doi: 10.1002/bjs.1800520820. [DOI] [PubMed] [Google Scholar]
- 25.Saint JH. The epicholedochal venous plexus and its importance as a means of identifying the common duct during operations on the extrahepatic biliary tract. Br J Surg. 1961;48:489–498. doi: 10.1002/bjs.18004821104. [DOI] [PubMed] [Google Scholar]
- 26.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44:1063–1074. doi: 10.1002/hep.21405. [DOI] [PubMed] [Google Scholar]
- 27.Webb LJ, Sherlock S. The aetiology, presentation and natural history of extra-hepatic portal venous obstruction. Q J Med. 1979;48:627–639. [PubMed] [Google Scholar]
- 28.Malkan GH, Bhatia SJ, Bashir K, Khemani R, Abraham P, Gandhi MS, Radhakrishnan R. Cholangiopathy associated with portal hypertension: diagnostic evaluation and clinical implications. Gastrointest Endosc. 1999;49:344–348. doi: 10.1016/s0016-5107(99)70011-8. [DOI] [PubMed] [Google Scholar]
- 29.Chawla Y, Dilawari JB, Katariya S. Gallbladder varices in portal vein thrombosis. AJR Am J Roentgenol. 1994;162:643–645. doi: 10.2214/ajr.162.3.8109513. [DOI] [PubMed] [Google Scholar]
- 30.Palazzo L, Hochain P, Helmer C, Cuillerier E, Landi B, Roseau G, Cugnenc PH, Barbier JP, Cellier C. Biliary varices on endoscopic ultrasonography: clinical presentation and outcome. Endoscopy. 2000;32:520–524. doi: 10.1055/s-2000-9009. [DOI] [PubMed] [Google Scholar]
- 31.Takamatsu M, Furutake M, Hisa T, Ueda M. Obstructive jaundice caused by a portal cavernoma. Jpn J Radiol. 2010;28:754–758. doi: 10.1007/s11604-010-0480-7. [DOI] [PubMed] [Google Scholar]
- 32.Kang HK, Jeong YY, Choi JH, Choi S, Chung TW, Seo JJ, Kim JK, Yoon W, Park JG. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics. 2002;22:1053–1061. doi: 10.1148/radiographics.22.5.g02se011053. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal A, Jain M. Multidetector CT portal venography in evaluation of portosystemic collateral vessels. J Med Imaging Radiat Oncol. 2008;52:4–9. doi: 10.1111/j.1440-1673.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- 34.Dhiman RK, Behera A, Chawla YK, Dilawari JB, Suri S. Portal hypertensive biliopathy. Gut. 2007;56:1001–1008. doi: 10.1136/gut.2006.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Condat B, Vilgrain V, Asselah T, O’Toole D, Rufat P, Zappa M, Moreau R, Valla D. Portal cavernoma-associated cholangiopathy: a clinical and MR cholangiography coupled with MR portography imaging study. Hepatology. 2003;37:1302–1308. doi: 10.1053/jhep.2003.50232. [DOI] [PubMed] [Google Scholar]
- 36.Bayraktar Y, Balkanci F, Kayhan B, Ozenç A, Arslan S, Telatar H. Bile duct varices or “pseudo-cholangiocarcinoma sign” in portal hypertension due to cavernous transformation of the portal vein. Am J Gastroenterol. 1992;87:1801–1806. [PubMed] [Google Scholar]
- 37.Vilgrain V, Condat B, O’Toole D, Plessier A, Ruszniewski P, Valla DC. Pancreatic portal cavernoma in patients with cavernous transformation of the portal vein: MR findings. Eur Radiol. 2009;19:2608–2613. doi: 10.1007/s00330-009-1459-6. [DOI] [PubMed] [Google Scholar]
- 38.Arora R, Mohanty M, Nundy S, Nayak NC. Phlebothrombosis as a common pathogenic denominator in noncirrhotic portal fibrosis & amp; extrahepatic portal splenic venous obstruction. Indian J Med Res. 1984;79:392–403. [PubMed] [Google Scholar]
- 39.Sengupta KP, Mallik KC, Maddrey WC, Biswas SK, Pal NC, Das MM, Basu AK. Liver changes in extrahepatic portal venous obstruction. Indian J Med Res. 1968;56:1643–1650. [PubMed] [Google Scholar]
- 40.Crum-Cianflone NF, Weekes J, Bavaro M. Review: thromboses among HIV-infected patients during the highly active antiretroviral therapy era. AIDS Patient Care STDS. 2008;22:771–778. doi: 10.1089/apc.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tighe M, Jacobson I. Bleeding from bile duct varices: an unexpected hazard during therapeutic ERCP. Gastrointest Endosc. 1996;43:250–252. doi: 10.1016/s0016-5107(96)70327-9. [DOI] [PubMed] [Google Scholar]
- 42.Mutignani M, Shah SK, Bruni A, Perri V, Costamagna G. Endoscopic treatment of extrahepatic bile duct strictures in patients with portal biliopathy carries a high risk of haemobilia: report of 3 cases. Dig Liver Dis. 2002;34:587–591. doi: 10.1016/s1590-8658(02)80093-7. [DOI] [PubMed] [Google Scholar]
- 43.Filipponi F, Urbani L, Catalano G, Iaria G, Biancofiore G, Cioni R, Campatelli A, Mosca F, Campani D, Romagnoli P. Portal biliopathy treated by liver transplantation. Transplantation. 2004;77:326–327. doi: 10.1097/01.TP.0000101795.29250.10. [DOI] [PubMed] [Google Scholar]


