Abstract
Purpose
We hypothesized that the addition of gefitinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, to docetaxel would enhance therapeutic efficacy in squamous cell carcinoma of the head and neck (SCCHN).
Patients and Methods
Patients with recurrent or metastatic SCCHN with Eastern Cooperative Oncology Group (ECOG) performance status of 2, or patients with ECOG performance status of 0 to 2 but were previously treated with chemotherapy, were randomly assigned to receive weekly docetaxel plus either placebo (arm A) or gefitinib 250 mg/d, orally (arm B) until disease progression. At the time of progression, patients in the placebo arm could receive single-agent gefitinib. EGFR, c-MET, and KRAS mutations and polymorphisms in drug metabolizing enzymes and transporters were evaluated by pyrosequencing.
Results
Two hundred seventy patients were enrolled before the study was closed early at interim analysis (arm A, n = 136; arm B, n = 134). Median overall survival was 6.0 months in arm A versus 7.3 months in arm B (hazard ratio, 0.93; 95% CI, 0.72 to 1.21; P = .60). An unplanned subset analysis showed that gefitinib improved survival in patients younger than 65 years (median 7.6 v 5.2 months; P = .04). Also, there was a trend for improved survival in patients with c-MET wild-type (5.7 v 3.6 months; P = .09) regardless of treatment. Grade 3/4 toxicities were comparable between the two arms except that grade 3/4 diarrhea was more common with docetaxel/gefitinib. Of 18 eligible patients who received gefitinib after disease progression in arm A, one patient had a partial response.
Conclusion
The addition of gefitinib to docetaxel was well tolerated but did not improve outcomes in poor prognosis but otherwise unselected patients with SCCHN.
INTRODUCTION
Approximately 52,000 new instances of head and neck cancer are diagnosed annually in the United States.1 Although locally advanced squamous cell carcinoma of the head and neck (SCCHN) is potentially curable with combined-modality therapy, recurrent or metastatic (R/M) disease carries a poor prognosis. Patients with disease progression after first-line therapy for R/M SCCHN or early recurrence after potentially curative chemoradiotherapy have a particularly poor outcome. Performance status (PS) is a strong predictor of survival in SCCHN.2 There are limited data on therapeutic outcomes in patients with compromised PS.3 A number of single agents have activity in previously treated patients with R/M SCCHN, including the taxanes and methotrexate, however, there is no standard treatment. Weekly docetaxel was active in a phase II trial in the first-line treatment of R/M SCCHN with a reported a response rate of 42% and median overall survival (OS) of 11.3 months.4 A phase II randomized study of weekly docetaxel versus methotrexate showed higher response rates for docetaxel but comparable survival rates.5
Epidermal growth factor receptor (EGFR) inhibitors have antitumor activity and tolerable toxicity profiles in SCCHN. Cetuximab, a monoclonal antibody against EGFR, has demonstrated efficacy in the management of SCCHN.6 A randomized Eastern Cooperative Oncology Group (ECOG) study (E5397) in R/M SCCHN showed that adding cetuximab to cisplatin improves objective response rate but not overall survival.7 In contrast, a larger phase III trial conducted by Vermorken et al8 showed that adding cetuximab to platinum/fluorouracil prolongs survival in first-line treatment of R/M SCCHN.
Gefitinib, an oral quinazoline, is a highly selective EGFR-tyrosine kinase inhibitor (TKI). Its common adverse effects included rash, diarrhea, and elevated transaminases. Gefitinib resulted in single-agent response rates in phase II trials in R/M SCCHN of 1% to 11%.9–11 A phase III trial showed that gefitinib at doses of 250 mg or 500 mg was not superior to methotrexate.3 EGFR-TKIs can potentiate the effect of chemotherapy in a manner that may be tumor type– and schedule-dependent. The combination of docetaxel with gefitinib is supported by preclinical observations in SCCHN models. Simultaneous administration or sequencing gefitinib after chemotherapy was optimal in the laboratory.12–14
Clinical data with docetaxel plus gefitinib have been reported in many cancers, including phase II data with cisplatin/docetaxel plus gefitinib in SCCHN.15 The combination of erlotinib and docetaxel resulted in significant toxicities in a phase I trial in patients with SCCHN necessitating reduction of the erlotinib dose to 50 mg daily.16 This prompted us to study gefitinib as the EGFR-TKI of choice. Our hypothesis was that the addition of gefitinib to docetaxel will be synergistic and improve the outcome of previously treated and/or compromised performance status patients with recurrent or metastatic SCCHN.
PATIENTS AND METHODS
Patient Selection
Eligible patients were at least 18 years old with R/M SCCHN considered incurable with locoregional therapies; adequate hematologic and liver function test parameters; and measurable or nonmeasurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST)17; PS 2, if previously untreated (including prior chemotherapy as part of potentially curative therapy > 6 months); or PS 0 to 2, if previously treated for R/M disease or prior chemotherapy as part of potentially curative therapy within 6 months of study enrollment. Any number of prior regimens was permitted except prior treatment with an EGFR inhibitor or docetaxel. Prior paclitaxel was allowed, if disease did not progress while receiving paclitaxel. Patients with peripheral neuropathy of grade 2 or worse, unstable comorbid disease, or hypercalcemia were excluded. Female patients of childbearing potential could not be pregnant or breastfeeding. Patients with major tumor-related hemorrhagic events in the previous 3 months, on therapeutic anticoagulation, or with tumors that invaded major vessels were also excluded. All patients signed informed consent and the protocol was approved by the respective institutional review boards.
Treatment Plan
Docetaxel was administered as a 60-minute infusion at a dose of 35 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Placebo (arm A) or gefitinib (Iressa, AstraZeneca, Wilmington, DE; arm B) at a dose of 250 mg (one tablet) was administered orally each day starting on day 1 and continuing for days 1 to 28 of each cycle. Premedication with dexamethasone was given for a total of three doses: 4 mg orally 12 hours before docetaxel, 4 mg intravenously or orally 30 to 60 minutes before docetaxel, and 4 mg orally 12 hours after docetaxel. Docetaxel plus placebo/gefitinib treatment continued until disease progression. Patients assigned to arm A had the option of unblinding at disease progression and registering (step 2) to receive single-agent gefitinib 250 mg once daily until disease progression. This option was eliminated in September 2007. Docetaxel and gefitinib dose modifications were applied for hematologic and nonhematologic toxicities according to protocol-specified criteria. Gefitinib and matching placebo were provided by AstraZeneca (Wilmington, DE) and distributed by the Pharmaceutical Management Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
Patient Assessments and Monitoring
Patients were evaluated by computed tomography of the chest and abdomen and computed tomography or magnetic resonance imaging of the neck at baseline, within 4 weeks of registration, and after every 2 cycles (8 weeks). Bone scan was performed at baseline and then as clinically indicated. Objective response was evaluated using RECIST version 1.0.17 Complete blood counts were obtained on days 1 and 8 and serum chemistry tests were administered on day 1 of each cycle. All toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0.
Genotyping and Mutation Analyses
Genotyping studies were performed to identify biomarkers that would correlate with treatment. Genomic DNA was extracted from whole blood or paraffin-embedded tumor blocks for analyses of single nucleotide polymorphisms (SNPs) or mutations, respectively, using standard extraction procedures. SNPs in CYP3A4, CYP3A5, ABCB1, EGFR Q787, and ABCG2 genes, as well as mutations in EGFR exons 18 to 2118–21; c-MET exons 2, 14, 15, and the tyrosine kinase domain22–26; and KRAS exons 12 and 1327–29 were analyzed by pyrosequencing. EGFRvIII was analyzed by polymerase chain reaction–amplifying-specific regions of the EGFR gene and visualizing samples on a 2% agarose-ethidium bromide gel for the absence or presence of mutant EGFR.19
Statistical Design and Analysis
This was a double blind, placebo-controlled phase III randomized trial. Patients were randomly assigned equally to docetaxel/placebo (arm A) and docetaxel/gefitinib (arm B). Randomization was done using permuted blocks within strata, with dynamic balancing within main institutions and their affiliate networks, with stratification by prior chemotherapy status (treated/untreated), PS (0, 1, or 2), weight loss in the last 6 months (< 5% v ≥ 5%), and prior cetuximab (yes v no). The study was designed to detect an improvement in median OS from 6 months in the control arm to 8.4 months in the experimental arm. A total accrual of 314 eligible patients and total information of 286 deaths were needed to attain 80% power with 2.5% type I error, using a one-sided log-rank test. To allow for up to 5% of the patients to be ineligible, a total of 330 patients were to be accrued. The trial was monitored according to principles of group-sequential methods using a one-sided O'Brien-Fleming30 upper boundary. Interim analyses were scheduled beginning at 25% of full information, then semiannually with stopping rules in favor of the null and alternative hypotheses based on repeated CI31 on the hazard ratio (HR), using the O'Brien-Fleming boundary. In November 2008, the ECOG Data Monitoring Committee recommended study closure to accrual because it was unlikely that the primary end point would be reached.
The analysis of efficacy outcomes excluded ineligible patients, whereas the toxicity summary included all patients who received treatment. OS was defined as the time from registration to death from any cause or censored at the time of last contact. Time-to-progression (TTP) was defined as time from registration to evidence of disease progression or censored at the last disease evaluation. Categorical data were summarized by frequency and percentage. Exact binomial confidence intervals were estimated for response rates.32 Wilcoxon rank sum and Fisher's exact tests were used to compare continuous and categoric variables, respectively, between groups. The survival data were analyzed using the Kaplan-Meier33 method and the significance was tested by log-rank tests. Cox's proportional hazards models34 were used to estimate HR and evaluate interaction effects. All P values are two-sided. A level of 5% was considered statistically significant. SNPs were investigated in blood samples and efficacy was compared by genotype (variant v nonvariant, including wild-type and heterozygote) for each polymorphism. Mutations were examined in tumor samples and efficacy was compared by mutation status (wild-type v mutation, including heterozygote and variant). Because the analysis of correlatives was exploratory, no statistical adjustment was performed for multiple comparisons.
RESULTS
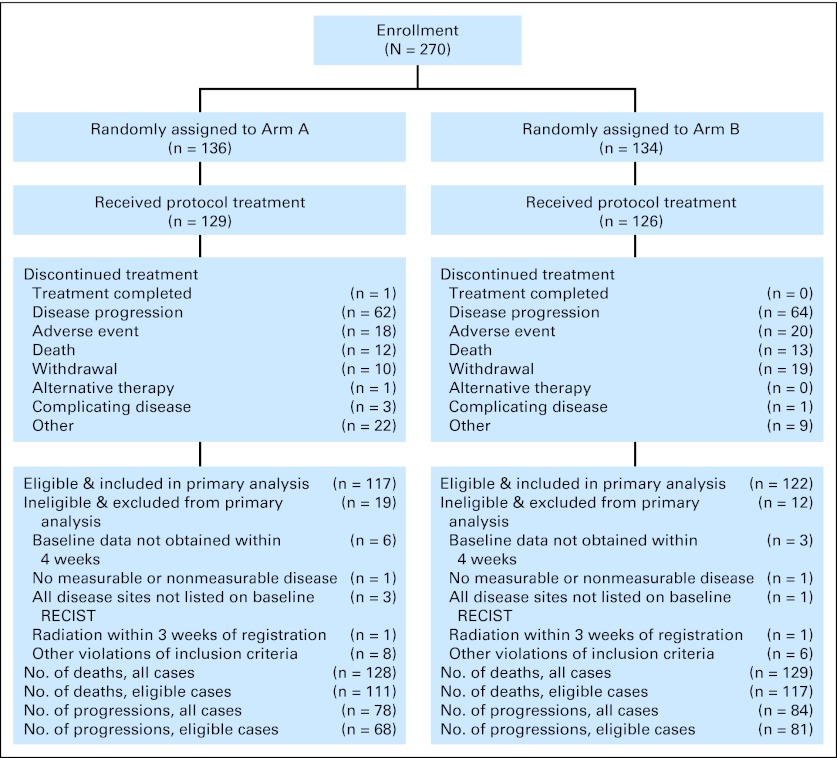
From August 2004 to November 2008, a total of 270 patients (136 in arm A; 134 in arm B) were enrolled onto the study, of whom 239 were eligible (117 in arm A; 122 in arm B; Fig 1). Fifteen patients (seven in arm A; eight in arm B) never started their assigned treatment.
Fig 1.
CONSORT diagram representing enrollment and outcomes of patients in the docetaxel/placebo (arm A) or docetaxel/gefitinib (arm B) treatment group. RECIST, Response Evaluation Criteria in Solid Tumors.
Twenty-four patients initially assigned to docetaxel plus placebo were registered to step 2 following disease progression; of those patients, 22 were eligible and four patients never started gefitinib. A total of six patients received cetuximab after study treatment completion and before documented disease progression per study criteria (four patients in arm A; two in arm B).
Patient Characteristics and Treatment Delivery
Table 1 provides baseline patient demographics and disease characteristics for eligible patients (N = 239). Except for the prior biologic/targeted therapy status, there were no statistically significant imbalances between the two arms. The median number of treatment cycles received was two (range, 0 to 10) and two (range, 0 to 18) for arms A and B, respectively. Appendix Table A1 (online-only) presents the reasons for treatment discontinuation, the most common of which was progressive disease. Although a similar number of gefitinib cycles was administered to younger and elderly patients (median, two), a higher proportion of the elderly required gefitinib dose interruptions in arm B (72% v 41%; P < .001) but not placebo in arm A (47% v 46%; P = 1.00). No significant association between docetaxel dose modifications and treatment arm was observed for either younger patients or older patients.
Table 1.
Patient Characteristics, Disease Status, and Prior Treatment (N = 239)
| Characteristic | Treatment Arm |
Total |
P* | ||||
|---|---|---|---|---|---|---|---|
| Placebo Arm |
Gefitinib Arm |
||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Age, years | .57 | ||||||
| Median | 61.4 | 60.8 | |||||
| Range | 28.0-86.5 | 41.6-84.4 | |||||
| < 65 | 72 | 62 | 84 | 69 | 156 | 65 | .28 |
| ≥ 65 | 45 | 38 | 38 | 31 | 83 | 35 | |
| Sex | .75 | ||||||
| Male | 92 | 79 | 98 | 80 | 190 | 79 | |
| Female | 25 | 21 | 24 | 20 | 49 | 21 | |
| Race | .59 | ||||||
| White | 101 | 86 | 102 | 84 | 203 | 85 | |
| Nonwhite | 16 | 14 | 20 | 16 | 36 | 15 | |
| Performance status | .72 | ||||||
| 0 | 15 | 13 | 12 | 10 | 27 | 11 | |
| 1 | 31 | 26 | 31 | 25 | 62 | 26 | |
| 2 | 71 | 61 | 79 | 65 | 150 | 63 | |
| Weight loss in previous 6 months | .82 | ||||||
| < 5% of body weight | 64 | 55 | 71 | 58 | 135 | 57 | |
| 5-10% of body weight | 24 | 21 | 24 | 20 | 48 | 20 | |
| 10 to < 20% of body weight | 19 | 16 | 15 | 12 | 34 | 14 | |
| ≥ 20% of body weight | 10 | 9 | 12 | 10 | 22 | 9 | |
| Smoking history | .19 | ||||||
| Never smoked | 13 | 12 | 7 | 6 | 20 | 9 | |
| Pipe or cigar smoker only | 1 | 1 | 2 | 2 | 3 | 1 | |
| Cigarette smoker, pack-years | |||||||
| < 20 | 17 | 15 | 10 | 8 | 27 | 11 | |
| 20-40 | 35 | 31 | 46 | 38 | 81 | 34 | |
| > 40 | 47 | 42 | 56 | 46 | 103 | 43 | |
| Unknown | 4 | 1 | 5 | ||||
| Average alcohol consumption | .89 | ||||||
| < 1 drink/month | 6 | 19 | 4 | 14 | 10 | 4 | |
| 1-5 drinks/month | 5 | 16 | 8 | 28 | 13 | 5 | |
| 1-10 drinks/week | 10 | 32 | 8 | 28 | 18 | 7 | |
| 11-30 drinks/week | 7 | 23 | 6 | 21 | 13 | 5 | |
| > 30 drinks/week | 3 | 10 | 3 | 10 | 6 | 2 | |
| Unknown | 5 | 5 | 10 | ||||
| Histologic grade | .90 | ||||||
| Well differentiated | 10 | 9 | 14 | 11 | 24 | 10 | |
| Moderately differentiated | 59 | 50 | 56 | 46 | 115 | 48 | |
| Poorly differentiated | 33 | 28 | 37 | 30 | 70 | 29 | |
| Undifferentiated | 2 | 2 | 3 | 2 | 5 | 2 | |
| Grade cannot be assessed | 13 | 11 | 12 | 10 | 25 | 11 | |
| Disease status at study entry | .53 | ||||||
| Eradicated, no recurrence | 32 | 28 | 37 | 31 | 69 | 29 | |
| Eradicated, recurred locally | 58 | 51 | 49 | 42 | 107 | 45 | |
| Residual after prior therapy | 21 | 18 | 27 | 23 | 48 | 20 | |
| Untreated | 3 | 3 | 5 | 4 | 8 | 3 | |
| Unknown | 3 | 4 | 7 | ||||
| Overall disease status | .82 | ||||||
| Locally or locoregionally recurrent/persistent only | 39 | 33 | 44 | 36 | 83 | 35 | |
| Distant metastases only | 29 | 25 | 32 | 26 | 61 | 25 | |
| Both | 49 | 42 | 46 | 38 | 95 | 40 | |
| Regional lymph node status | .15 | ||||||
| Unknown | 6 | 11 | 17 | ||||
| Never involved | 17 | 15 | 19 | 17 | 36 | 15 | |
| Never involved but removed | 1 | 1 | 5 | 5 | 6 | 3 | |
| Involved nodes, eradicated | 49 | 44 | 32 | 29 | 81 | 34 | |
| Involved nodes eradicated, new involvement | 22 | 20 | 26 | 23 | 48 | 20 | |
| Involved nodes, not treated | 13 | 12 | 14 | 13 | 27 | 11 | |
| Other | 9 | 8 | 15 | 14 | 24 | 10 | |
| Primary site | .40 | ||||||
| Oral cavity | 30 | 26 | 23 | 19 | 53 | 22 | |
| Oropharynx | 36 | 31 | 42 | 34 | 78 | 33 | |
| Larynx | 28 | 24 | 33 | 27 | 61 | 26 | |
| More than one | 4 | 3 | 9 | 7 | 13 | 5 | |
| Other† | 19 | 16 | 15 | 12 | 34 | 14 | |
| Prior chemotherapy | .38 | ||||||
| No | 33 | 28 | 28 | 23 | 61 | 26 | |
| Yes | 84 | 72 | 94 | 77 | 178 | 74 | |
| Prior radiotherapy | .47 | ||||||
| No | 20 | 17 | 16 | 13 | 36 | 15 | |
| Yes | 97 | 83 | 106 | 87 | 203 | 85 | |
| Prior surgery | .24 | ||||||
| No | 41 | 35 | 52 | 43 | 93 | 39 | |
| Yes | 76 | 65 | 70 | 57 | 146 | 61 | |
| Prior biologic/targeted therapy | .03 | ||||||
| No | 111 | 96 | 122 | 100 | 233 | 98 | |
| Yes | 5 | 4 | 0 | 0 | 5 | 2 | |
| Metastatic site involvement | |||||||
| Lung | .70 | ||||||
| Unknown | 2 | 1 | 3 | ||||
| Not involved | 50 | 43 | 56 | 46 | 106 | 44 | |
| Involved | 65 | 57 | 65 | 54 | 130 | 54 | |
| Liver | .78 | ||||||
| Unknown | 4 | 5 | 9 | ||||
| Not involved | 106 | 95 | 109 | 93 | 215 | 90 | |
| Involved | 6 | 5 | 8 | 7 | 14 | 6 | |
| Bone | .16 | ||||||
| Unknown | 0 | 1 | 1 | ||||
| Not involved | 94 | 80 | 106 | 88 | 200 | 84 | |
| Involved | 23 | 20 | 15 | 12 | 38 | 15 | |
P value calculation excludes unknown values.
Lip and oral cavity, nasopharynx, hypopharynx, salivary glands, paranasal sinuses, no primary identified, other.
Overall Survival and Time-to-Progression
Seven patients (3%) were alive at the time of the analysis (arm A, four patients; arm B, three), with a median follow-up time of 35 months (range, 24 to 54 months). For all patients, median OS was 6.8 months (95% CI, 5.72 to 7.52 months). The median OS was 6.0 months (95% CI, 4.93 to 7.43) and 7.3 months (95% CI, 5.75 to 8.44) in arms A and B, respectively (HR, 0.93; 95% CI, 0.72 to 1.21; P = .60). Median TTP was 2.1 months for arm A and 3.5 months for arm B (HR, 0.81; 95% CI, 0.58 to 1.11; P = .19). Figure 2 shows the Kaplan-Meier curves for OS and TTP. The median OS and TTP for the 22 patients who registered for step 2, calculated from the time of cross-over registration, were 6.3 and 2.6 months, respectively.
Fig 2.
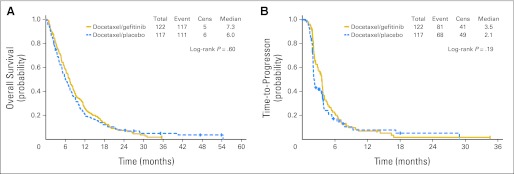
Kaplan-Meier estimates of (A) overall survival by treatment arm (n = 239) and (B) time-to-progression by treatment arm (n = 239). Cens, censored.
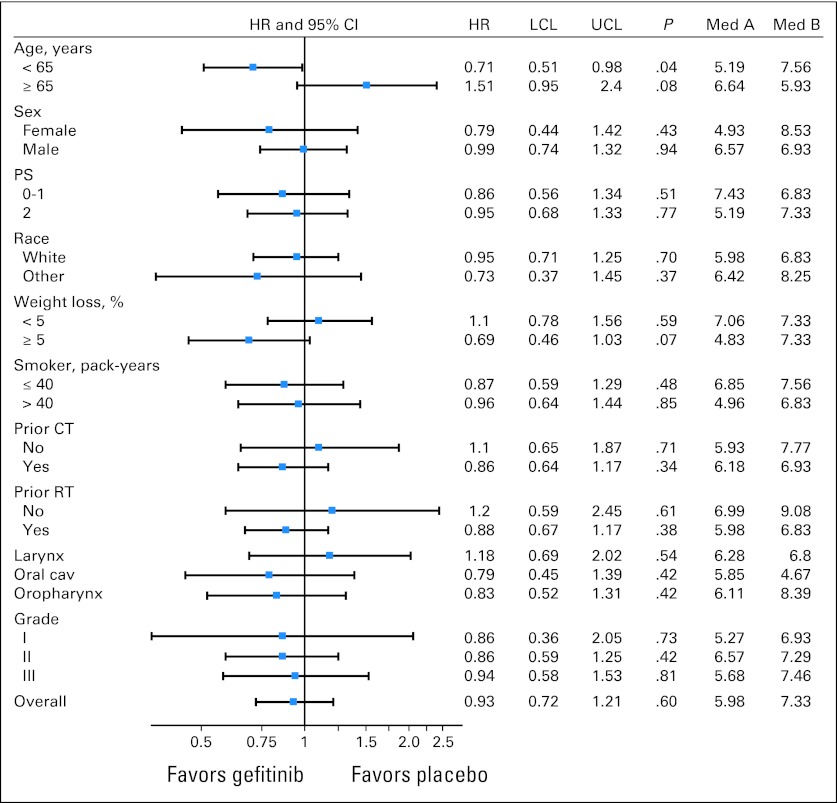
In an unplanned subgroup analysis, we found that patients younger than 65 years derived survival benefit from combination therapy (median OS, 7.6 months with docetaxel/gefitinib v 5.2 months with docetaxel/placebo; P = .04) but patients 65 years or older did not (median OS, 5.9 months with docetaxel/gefitinib v 6.6 months with docetaxel/placebo; P = .08; Fig 3 shows forest plot of HRs of OS and Fig 4 shows survival curves). The Cox proportional hazards regression analysis showed that the interaction effect by age and treatment arm was highly significant (P = .007). There was also improvement in TTP with the addition of gefitinib in younger patients (median, 3.6 v 2.0 months; P = .01) but not in patients ≥ 65 years (median, 3.4 v 3.7 months; P = .58).
Fig 3.
Forest plot representing hazard ratios (HRs) with 95% CIs of overall survival in patient subgroups. Cav, cavity; CT, chemotherapy; LCL, lower confidence limit; Med A, median overall survival in arm A; Med B, median overall survival in arm B; PS, performance status; RT, radiotherapy; UCL, upper confidence limit.
Fig 4.
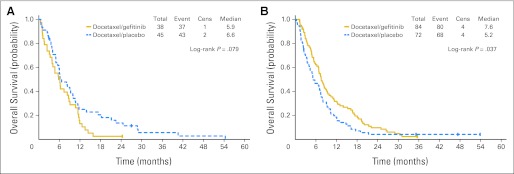
Kaplan-Meier estimates of overall survival by treatment arm for (A) patients ≥ 65 years (n = 83) and (B) patients younger than 65 years (n = 156). Cens, censored.
Response
The distribution of response in arm A was: two patients with complete responses, three patients with partial responses, 28 patients with stable disease, 48 patients with progressive disease, and 36 patients unevaluable. In arm B, there were two patients with complete responses, 10 patients with partial responses, 41 patients with stable disease, 43 patients with progressive disease, 26 patients who were unevaluable. In evaluable patients, the overall response rate (ORR) was 6.2% (95% CI, 2.01% to 13.82%) and 12.5% (95% CI, 6.61% to 20.84%), in arms A and B, respectively, a difference that was not statistically significant (P = .13). Of 16 evaluable patients registered to step 2, one had an objective response and six had stable disease as best response.
Toxicity
Toxicities were assessed in a total of 253 patients (129 in arm A; 124 in arm B) in step 1 of the study (Table 2). The incidence of grade 3/4 toxicities was comparable between the two arms, except for a higher incidence of diarrhea with gefitinib (13% v 2%; P < .001). Two treatment-related lethal toxicities occurred in patients in arm A (lung infection and pulmonary hemorrhage) and six in patients in arm B (pneumonitis, septicemia, hypotension, and three sudden deaths). No statistically significant difference in grade 3 to 5 toxicities was noted between younger (< 65 years) and older patients (≥ 65 years) in either arm, except for a higher rate of grade 3 to 5 infection on the gefitinib arm (8% in older v 0% in younger patients; P = .03).
Table 2.
Treatment-Related Adverse Events
| Adverse Event | Gefitinib Arm (n = 124) |
Placebo Arm (n = 129) |
||||
|---|---|---|---|---|---|---|
| Grade 3 (%) | Grade 4 (%) | Grade 5 (%) | Grade 3 (%) | Grade 4 (%) | Grade 5 (%) | |
| Allergic reaction | 1 | 2 | ||||
| Hemoglobin | 4 | |||||
| Leukopenia | 3 | 2 | 2 | 2 | ||
| Lymphopenia | 2 | 1 | 5 | 2 | ||
| Neutropenia | 2 | 1 | 1 | 2 | ||
| Thrombocytopenia | 1 | |||||
| Atrial fibrillation | 1 | 1 | ||||
| Ventricular flutter | 1 | |||||
| Hypotension | 2 | 1 | 2 | 1 | ||
| Left ventricular diastolic dysfunction | 1 | |||||
| Fatigue | 11 | 13 | 3 | |||
| Insomnia | 1 | |||||
| Weight loss | 1 | 2 | ||||
| International normalized ratio | 1 | |||||
| Partial thromboplastin time | 1 | |||||
| Nail changes | 1 | 1 | ||||
| Pruritus/itching | 1 | |||||
| Rash/desquamation | 2 | |||||
| Hand-foot reaction | 1 | |||||
| Death/sudden death | 2 | |||||
| Anorexia | 5 | 2 | ||||
| Dehydration | 6 | 1 | 5 | |||
| Diarrhea without prior colostomy | 11 | 2 | 2 | |||
| Dysphagia | 2 | 1 | ||||
| Fistula, colon/cecum/appendix | 1 | |||||
| Oral mucositis by examination | 2 | |||||
| Oral mucositis by symptoms | 2 | 2 | ||||
| Nausea | 6 | 3 | 1 | |||
| Perforation, duodenum | 1 | |||||
| Vomiting | 2 | 1 | 3 | |||
| Abdomen, hemorrhage NOS | 1 | |||||
| Esophagus, hemorrhage | 1 | |||||
| Oral cavity, hemorrhage | 1 | |||||
| Bronchus, hemorrhage | 1 | |||||
| Colitis, infectious | 1 | |||||
| Febrile neutropenia | 1 | |||||
| Infection with neutropenia | ||||||
| Grade 3-4 | ||||||
| Abdomen NOS | 1 | |||||
| Bladder | 1 | |||||
| Lung | 1 | |||||
| Blood | 1 | |||||
| Grade 0-2 | ||||||
| Abdomen | 1 | |||||
| Catheter | 2 | |||||
| Colon | 1 | |||||
| Lung | 3 | 1 | 5 | 1 | 1 | |
| Neck | 1 | 1 | ||||
| Skin | 1 | 3 | ||||
| Urinary tract | 1 | |||||
| Wound | 1 | |||||
| Blood | 2 | 1 | ||||
| Infection with unknown neutrophils, lung | 1 | |||||
| Infection with unknown neutrophils, skin | 1 | 1 | ||||
| Opportunistic infection with lymphopenia | 1 | |||||
| Infection, other | 1 | |||||
| Edema | ||||||
| Head and neck | 2 | 1 | ||||
| Limb | 1 | |||||
| Hypoalbuminemia | 1 | |||||
| Alkaline phosphatase | 1 | |||||
| ALT | 1 | |||||
| Creatinine | 1 | |||||
| Hyperglycemia | 2 | 1 | ||||
| Hypophosphatemia | 1 | |||||
| Hypokalemia | 1 | |||||
| Hyponatremia | 1 | 2 | 1 | |||
| Non-neuropathic lower extremity muscle weakness | 1 | 1 | ||||
| Non-neuropathic generalized weakness | 1 | 5 | ||||
| Trismus | 1 | |||||
| Neuropathy, motor | 1 | |||||
| Neuropathy, sensory | 3 | |||||
| Syncope | 2 | |||||
| Abdomen, pain | 1 | |||||
| Chest pain NOS | 1 | |||||
| Head/headache | 1 | |||||
| Adult respiratory distress syndrome | 1 | 1 | ||||
| Bronchospasm, wheezing | 1 | |||||
| Cough | 1 | |||||
| Dyspnea | 3 | 1 | 4 | 1 | ||
| Hypoxia | 1 | 1 | 1 | |||
| Pleural effusion, nonmalignant | 2 | 1 | ||||
| Pneumonitis/pulmonary infiltrates | 1 | 1 | 2 | |||
| Renal failure | 1 | |||||
| Thrombosis/embolism | 1 | |||||
| Vessel injury, carotid | 1 | |||||
| Worst degree | 37 | 6 | 5 | 36 | 12 | 2 |
NOTE. Grade 3, severe; grade 4, life threatening; grade 5, lethal.
Abbreviation: NOS, not otherwise specified.
Of 19 patients assessed for toxicity in step 2, two experienced grade 3 toxicities (fatigue and dysphagia, respectively). No grade 4 or 5 toxicities were reported.
Correlative Studies
SNP analysis was performed on germline DNA samples and mutation analysis was performed on somatic DNA samples from tumor tissue. Among the 239 eligible patients, 89 blood samples and 69 tumor samples were available for analysis (Table 3 and Appendix Tables 2-4). No significant difference was found in any patient characteristic between patients who had blood or tumor sample analyzed and those who did not. No association was found between SNP genotypes and toxicity or efficacy. Two EGFR mutations were detected, one EGFRvIII and one EGFR A767T (both in arm B). The patient with an EGFRvIII mutation had an objective response, whereas two of 35 patients with wild-type EGFR achieved an objective response (100% v 6%; P = .08) in arm B and had an OS of 19.6 months versus 5.7 months in patients with wild type. The patient with the EGFR A767T mutation was not evaluable for response. Regardless of treatment, the presence of c-MET mutations tended to predict decreased OS. In 10 patients with c-MET mutations, the median OS was 3.6 months (95% CI, 1.1 to 8.6) versus a median OS of 5.7 months (95% CI, 3.5 to 8.3; P = .09) in 41 patients with wild-type c-MET. TTP was also decreased, although not significantly (median TTP, 2.1 v 2.9 months; P = .07), in patients with c-MET mutations. There were no KRAS mutations identified.
Table 3.
Efficacy by the Presence of c-MET Tumor Mutation
| cMET Mutation by Arm | Objective Response |
P | Overall Survival |
P | Time to Progression |
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No |
Yes |
||||||||||
| No. of Patients | % | No. of Patients | % | Median | 95% CI | Median | 95% CI | ||||
| Docetaxel (n = 23) | |||||||||||
| No | 19 | 95 | 1 | 5 | 1.00 | 5.4 | 2.0 to 14.7 | — | 3.2 | 1.4 to 4.8 | — |
| Yes | 3 | 100 | 0 | 0 | 2.4 | 1.6 to 3.5 | 1.5 | 0.8 to 2.2 | |||
| Gefitinib (n = 28) | |||||||||||
| No | 20 | 95 | 1 | 5 | 1.00 | 5.9 | 3.1 to 10.3 | .44 | 2.4 | 1.5 to 6.1 | .19 |
| Yes | 7 | 100 | 0 | 0 | 5.7 | 1.1 to 9.1 | 2.1 | 1.6 to 3.8 | |||
| Both (n = 51) | |||||||||||
| No | 39 | 95 | 2 | 5 | 1.00 | 5.7 | 3.5 to 8.3 | .09 | 2.9 | 1.8 to 3.7 | .07 |
| Yes | 10 | 100 | 0 | 0 | 3.6 | 1.1 to 8.6 | 2.1 | 0.8 to 2.3 | |||
NOTE. If tumor mutation was observed on cMET V1110I (n = 2), H1112Y (n = 5), H112RL (n = 0), T1010I (n = 1), R988C (n = 1), V1333I (n = 1), or any cMET exon 14 del (n = 2), then cMET mutation was coded as “Yes.” If wild type was observed in all these biomarkers, then cMET mutation status was coded as “No.” Otherwise, the status was coded as missing and excluded from data analysis.
DISCUSSION
This trial was one of the first phase III, placebo-controlled trials in poor PS and/or heavily pretreated patients with R/M SCCHN. For this patient population, we did not demonstrate a survival benefit by adding gefitinib to docetaxel. The median overall survival with or without gefitinib was 7.3 months versus 6.0 months, respectively, a difference that did not reach statistical significance. Moreover, there was no significant difference in TTP between the two arms (median TTP was 2.1 months for arm A and 3.5 months for arm B). The addition of gefitinib to docetaxel resulted in a higher rate of grade 3 or 4 diarrhea but other toxicities were comparable between the two arms.
Unplanned subgroup analysis showed that patients younger than 65 years derived survival benefit with the addition of gefitinib to docetaxel (median, 7.6 v 5.2 months), but not patients ≥ 65 years. There were no significant differences in grade 3 to 5 toxicities between younger and elderly patients except a higher incidence of infections with docetaxel and gefitinib in the elderly. However, the elderly required more dose interruptions of gefitinib dosing, which may explain the differential survival outcome by age. It has been previously reported that elderly patients with R/M SCCHN treated with chemotherapy have increased toxicities when compared with younger patients, even though survival outcomes appear comparable.35,36 With advancing age, there are biologic changes and a higher incidence of comorbidities that may predispose the elderly to increased risks from chemotherapy.37 Our observation of a potential survival benefit with the addition of gefitinib to docetaxel in younger but not older patients may warrant further validation in clinical studies.
A prior ECOG trial showed poor survival results in previously treated patients with R/M SCCHN dosed with irinotecan and docetaxel resulting in an ORR of 3% and median OS of 5 months.38 The results seen with single-agent docetaxel in the control arm of the current trial were as expected. Although cross-over to single-agent gefitinib was initially allowed in this trial, only 18 eligible patients received it. This is unlikely to have had any impact on the survival results of our study.
Two phase II trials explored the addition of an EGFR-TKI to cisplatin and docetaxel in patients with R/M SCCHN.15,39 Kim et al39 reported an ORR of 66% in the first 37 patients treated with cisplatin/docetaxel plus erlotinib, whereas Belon et al15 reported an ORR of 50% in 24 patients treated with cisplatin/docetaxel plus gefitinib. The combination of an EGFR-TKI (erlotinib) and chemotherapy resulted in survival benefit, albeit marginal, in advanced pancreatic cancer40 but not in advanced non–small-cell lung cancer.41–44 Whether the lack of efficacy in combined EGFR-TKI and chemotherapy in lung cancer can be attributed to a sequence-dependent effect is the subject of ongoing research.45
Our analysis of correlative biomarkers in a rather small fraction of available patient samples indicate that c-MET mutations are possible prognostic markers for survival and disease progression but do not predict outcomes after EGFR inhibitor therapy, which is consistent with other reports that suggest the c-MET amplification does not predict response to EGFR inhibitors and that c-MET is a negative prognostic marker.46–48 The single patient found to have a tumor with an EGFRvIII mutation responded to docetaxel/gefitinib in our study. Although mutations in EGFR are rare in SCCHN,49 the potential benefit with EFGR-TKI treatment for these patients may warrant further study.
In conclusion, the addition of gefitinib to docetaxel was well tolerated but it did not enhance therapeutic efficacy across all patients in this clinical setting. The outcome of patients with SCCHN with previously treated disease or performance status of 2 remains poor and the study of other novel agents in this setting should continue.
Supplementary Material
Appendix
Table A1.
Reasons Eligible Patients Withdrew From Treatment (Step 1)
| Reason | Placebo (n = 110) |
Gefitinib (n = 117) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Treatment completed per protocol criteria | 1 | 1 | 0 | 0 |
| Disease progression during active treatment | 55 | 50 | 62 | 53 |
| Adverse events/adverse effects/complications | 14 | 13 | 19 | 16 |
| Death on study | 11 | 10 | 12 | 10 |
| Withdrawal or refusal after beginning protocol therapy | 10 | 9 | 15 | 13 |
| Alternative therapy | 0 | 0 | 0 | 0 |
| Other complicating disease | 3 | 3 | 1 | 1 |
| Other | 16 | 15 | 8 | 7 |
Table A2.
Frequency and Percentage by Polymorphism and Genotype (SNP analysis)
| Genotype | No. of Patients | % | ||
| ABCB1_3435 | 19 | 21 | ||
| CC | ||||
| CT | 52 | 58 | ||
| TT* | 18 | 20 | ||
| ABCB1_2677 | 3 | — | ||
| Unknown | ||||
| GG | 25 | 2 | ||
| GT | 44 | 51 | ||
| TT* | 16 | 19 | ||
| AT* | 1 | 1 | ||
| ABCB1_1236 | 4 | — | ||
| Unknown | ||||
| CC | 22 | 2 | ||
| CT | 46 | 54 | ||
| TT* | 17 | 20 | ||
| CYP3A4 | 73 | 82 | ||
| AA | ||||
| AG | 15 | 17 | ||
| GG* | 1 | 1 | ||
| CYP3A5 | 2 | 2 | ||
| AA | ||||
| AG | 19 | 21 | ||
| GG* | 68 | 76 | ||
| ABCG2_C421A | 13 | 15 | ||
| AC | ||||
| CC* | 76 | 85 | ||
| ABCG2_C1143T | 1 | — | ||
| Unknown | ||||
| CC | 53 | 60 | ||
| CT | 29 | 33 | ||
| TT* | 6 | 7 | ||
| EGFR_Q787Q | 2 | — | ||
| Unknown | ||||
| AA | 23 | 27 | ||
| AG | 42 | 48 | ||
| GG* | 22 | 25 |
Abbreviation: SNP, single nucleotide polymorphisms.
Refers to variant.
Table A3.
Frequency and Percentage by EGFR Biomarker and Genotype/Mutation
| Genotype | No. of Patients | % |
| EGFR_CA_SSR1_CA repeats | 4 | — |
| Unknown | ||
| Mutant (n < 16) | 14 | 22 |
| WT | 51 | 78 |
| EGFR_vIII | 3 | — |
| Unknown | ||
| Mutant | 1 | 2 |
| WT | 65 | 98 |
| EGFR_L858R | 4 | — |
| Unknown | ||
| TT* | 65 | 100 |
| EGFR_del_746_759 | 4 | — |
| Unknown | ||
| WT | 65 | 100 |
| EGFR_E749K | 4 | — |
| Unknown | ||
| GG* | 65 | 100 |
| EGFR_T790M | 69 | 100 |
| CC | ||
| EGFR_L861Q | 3 | — |
| Unknown | ||
| TT* | 66 | 100 |
| EGFR_E762G | 1 | — |
| Unknown | ||
| AA* | 68 | 100 |
| EGFR_A767T | 4 | — |
| Unknown | ||
| GA | 1 | 2 |
| GG* | 64 | 98 |
Abbreviations: EGFR, epidermal growth factor receptor; WT, wild type.
Refers to WT.
Table A4.
Frequency and Percentage by c-MET Mutation and Genotype
| Genotype | No. of Patients | % |
| cMET_Y1230C | ||
| Unknown | 6 | — |
| GG* | 63 | 100 |
| cMET_Y1230D | ||
| Unknown | 6 | — |
| GG* | 63 | 100 |
| cMET_Y1235D | ||
| Unknown | 6 | — |
| TT* | 63 | 100 |
| cMET_V1110I | ||
| Unknown | 14 | — |
| GA | 2 | 4 |
| GG* | 53 | 96 |
| cMET_H1112Y | ||
| Unknown | 7 | — |
| CC* | 57 | 92 |
| CT | 3 | 5 |
| TT | 2 | 3 |
| cMET_H1112RL | ||
| Unknown | 6 | — |
| AA* | 63 | 100 |
| cMET_M1149T | ||
| Unknown | 3 | — |
| TT* | 66 | 100 |
| cMET_V1206L | ||
| Unknown | 3 | — |
| TT* | 66 | 100 |
| cMET_V1238I | ||
| Unknown | 3 | — |
| GG* | 66 | 100 |
| cMET_D1246NH | ||
| Unknown | 4 | — |
| AA* | 65 | 100 |
| cMET_Y1248HD | ||
| Unknown | 2 | — |
| TT* | 67 | 100 |
| cMET_Y1248C | ||
| Unknown | 3 | — |
| AA* | 66 | 100 |
| cMET_M1268T | ||
| Unknown | 3 | — |
| TT* | 66 | 100 |
| cMET_T1010I | ||
| Unknown | 12 | — |
| CC* | 56 | 98 |
| TT | 1 | 2 |
| cMET_R988C | ||
| Unknown | 12 | — |
| CC* | 56 | 98 |
| TT | 1 | 2 |
| cMET_E168D | ||
| Unknown | 3 | — |
| GG* | 66 | 100 |
| cMET_T230M | ||
| Unknown | 3 | — |
| CC | 66 | 100 |
| cMET_T1275I | ||
| Unknown | 6 | — |
| CC | 63 | 100 |
| cMET_V1333I | ||
| Unknown | 3 | — |
| GA | 1 | 2 |
| GG | 65 | 98 |
| cMET_Exon14_del* | ||
| cMET_ex14_1GAT | ||
| WT | 69 | 100 |
| cMET_lnt13ex14_del_n16_3078 | ||
| Mutant | 2 | 3 |
| WT | 67 | 97 |
| cMET_ln13_del_n26_n48 | ||
| WT | 69 | 100 |
| cMET_ln13_del_n8_n18 | ||
| Mutant | 1 | 1 |
| WT | 68 | 99 |
| cMET_ln13_del_n27_n6 | ||
| WT | 69 | 100 |
| cMET_ex14_del_3195_p7 | ||
| WT | 69 | 100 |
Abbreviation: WT, wild type.
Refers to WT.
Footnotes
Written on behalf of the Eastern Cooperative Oncology Group.
Supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA39229, and CA16116 from the National Cancer Institute, National Institutes of Health, US Department of Health and Human Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00088907.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Barbara Burtness, AstraZeneca (C), Boehringer Ingelheim (C), Genentech (C), sanofi-aventis (C) Stock Ownership: None Honoraria: Athanassios Argiris, AstraZeneca Research Funding: Athanassios Argiris, AstraZeneca; Jill M. Kolesar, AstraZeneca; Barbara Burtness, Genentech, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Athanassios Argiris, Musie Ghebremichael, Jill Gilbert, Barbara Burtness, Arlene A. Forastiere
Administrative support: Athanassios Argiris, Arlene A. Forastiere
Provision of study materials or patients: Athanassios Argiris
Collection and assembly of data: Athanassios Argiris, Musie Ghebremichael, Kamakshi Sachidanandam, Jill M. Kolesar
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–2229. doi: 10.1002/cncr.20640. [DOI] [PubMed] [Google Scholar]
- 3.Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27:1864–1871. doi: 10.1200/JCO.2008.17.0530. [DOI] [PubMed] [Google Scholar]
- 4.Hitt R, Amador ML, Quintela-Fandino M, et al. Weekly docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Cancer. 2006;106:106–111. doi: 10.1002/cncr.21579. [DOI] [PubMed] [Google Scholar]
- 5.Guardiola E, Peyrade F, Chaigneau L, et al. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer. 2004;40:2071–2076. doi: 10.1016/j.ejca.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 7.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EE, Kane MA, List MA, et al. Phase II trial of gefitinib 250 mg daily in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2005;11:8418–8424. doi: 10.1158/1078-0432.CCR-05-1247. [DOI] [PubMed] [Google Scholar]
- 10.Cohen EE, Rosen F, Stadler WM, et al. Phase II trial of ZD1839 in recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2003;21:1980–1987. doi: 10.1200/JCO.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Kirby AM, A'Hern RP, D'Ambrosio C, et al. Gefitinib (ZD1839, Iressa) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94:631–636. doi: 10.1038/sj.bjc.6602999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe MS, Chen Z, Klass CM, et al. Enhancement of docetaxel-induced cytotoxicity by blocking epidermal growth factor receptor and cyclooxygenase-2 pathways in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:3015–3023. doi: 10.1158/1078-0432.CCR-06-2959. [DOI] [PubMed] [Google Scholar]
- 13.Klass CM, Choe MS, Hurwitz SJ, et al. Sequence dependence of cell growth inhibition by EGFR-tyrosine kinase inhibitor ZD1839, docetaxel, and cisplatin in head and neck cancer. Head Neck. 2009;31:1263–1273. doi: 10.1002/hed.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saigal B, Glisson BS, Johnson FM. Dose-dependent and sequence-dependent cytotoxicity of erlotinib and docetaxel in head and neck squamous cell carcinoma. Anticancer Drugs. 2008;19:465–475. doi: 10.1097/CAD.0b013e3282fc46c4. [DOI] [PubMed] [Google Scholar]
- 15.Belon J, Irigoyen A, Rodriguez I, et al. Preliminary results of a phase II study to evaluate gefitinib combined with docetaxel and cisplatin in patients with recurrent and/or metastatic squamous-cell carcinoma of the head and neck. J Clin Oncol. 2005;23(suppl):295s. abstr 5563. [Google Scholar]
- 16.Kraut EH, Rhoades C, Zhang Y, et al. Phase I and pharmacokinetic study of erlotinib (OSI-774) in combination with docetaxel in squamous cell carcinoma of the head and neck (SSCHN) Cancer Chemother Pharmacol. 2011;67:579–586. doi: 10.1007/s00280-010-1332-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Hama T, Yuza Y, Saito Y, et al. Prognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinoma. Oncologist. 2009;14:900–908. doi: 10.1634/theoncologist.2009-0058. [DOI] [PubMed] [Google Scholar]
- 19.Ramalingam SS, Lee JW, Belani CP, et al. Cetuximab for the treatment of advanced bronchioloalveolar carcinoma (BAC): An Eastern Cooperative Oncology Group phase II study (ECOG 1504) J Clin Oncol. 2011;29:1709–1714. doi: 10.1200/JCO.2010.33.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takano T, Ohe Y, Sakamoto H, et al. Epidermal growth factor receptor gene mutations and increased copy numbers predict gefitinib sensitivity in patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:6829–6837. doi: 10.1200/JCO.2005.01.0793. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Nagahara H, Mimori K, et al. Mutations of epidermal growth factor receptor in colon cancer indicate susceptibility or resistance to gefitinib. Oncol Rep. 2008;19:1541–1544. [PubMed] [Google Scholar]
- 22.Di Renzo MF, Olivero M, Martone T, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- 23.Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66:283–289. doi: 10.1158/0008-5472.CAN-05-2749. [DOI] [PubMed] [Google Scholar]
- 24.Onozato R, Kosaka T, Kuwano H, et al. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4:5–11. doi: 10.1097/JTO.0b013e3181913e0e. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt L, Junker K, Nakaigawa N, et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene. 1999;18:2343–2350. doi: 10.1038/sj.onc.1202547. [DOI] [PubMed] [Google Scholar]
- 26.Seiwert TY, Jagadeeswaran R, Faoro L, et al. The MET receptor tyrosine kinase is a potential novel therapeutic target for head and neck squamous cell carcinoma. Cancer Res. 2009;69:3021–3031. doi: 10.1158/0008-5472.CAN-08-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogino S, Meyerhardt JA, Irahara N, et al. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 2009;15:7322–7329. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber A, Langhanki L, Sommerer F, et al. Mutations of the BRAF gene in squamous cell carcinoma of the head and neck. Oncogene. 2003;22:4757–4759. doi: 10.1038/sj.onc.1206705. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 31.Jennison C, Turnbull BW. Statistical approaches to interim monitoring of medical trials: A review and commentary. Statistical Science. 1990;5:299–317. [Google Scholar]
- 32.Cox DR, Snell EJ. ed 2. London, United Kingdom: Chapman and Hall; 1989. Analysis of binary data. [Google Scholar]
- 33.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assn. 1958;53:457–481. [Google Scholar]
- 34.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 35.Argiris A, Li Y, Murphy BA, et al. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol. 2004;22:262–268. doi: 10.1200/JCO.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimmick GG, Fleming R, Muss HB, et al. Cancer chemotherapy in older adults: A tolerability perspective. Drugs Aging. 1997;10:34–49. doi: 10.2165/00002512-199710010-00004. [DOI] [PubMed] [Google Scholar]
- 38.Argiris A, Buchanan A, Brockstein B, et al. Docetaxel and irinotecan in recurrent or metastatic head and neck cancer: A phase 2 trial of the Eastern Cooperative Oncology Group. Cancer. 2009;115:4504–4513. doi: 10.1002/cncr.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim ES, Kies MS, Glisson BS, et al. Phase II study of combination cisplatin, docetaxel and erlotinib in patients with metastatic/recurrent head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2006;24(suppl):285s. abstr 5521. [Google Scholar]
- 40.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 41.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: A phase III trial—INTACT 2. J Clin Oncol. 2004;22:785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 43.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 44.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 45.Davies AM, Ho C, Beckett L, et al. Intermittent erlotinib in combination with pemetrexed: Phase I schedules designed to achieve pharmacodynamic separation. J Thorac Oncol. 2009;4:862–868. doi: 10.1097/JTO.0b013e3181a94b08. [DOI] [PubMed] [Google Scholar]
- 46.Chau NG, Perez-Ordonez B, Zhang K, et al. The association between EGFR variant III, HPV, p16, c-MET, EGFR gene copy number and response to EGFR inhibitors in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:11. doi: 10.1186/1758-3284-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim EH, Zhang SL, Li JL, et al. Using whole genome amplification (WGA) of low-volume biopsies to assess the prognostic role of EGFR, KRAS, p53, and CMET mutations in advanced-stage non-small cell lung cancer (NSCLC) J Thorac Oncol. 2009;4:12–21. doi: 10.1097/JTO.0b013e3181913e28. [DOI] [PubMed] [Google Scholar]
- 48.Ludovini V, Bianconi F, Pistola L, et al. Optimization of patient selection for EGFR-TKIs in advanced non-small cell lung cancer by combined analysis of KRAS, PIK3CA, MET, and non-sensitizing EGFR mutations. Cancer Chemother Pharmacol. 2012;69:1289–1299. doi: 10.1007/s00280-012-1829-7. [DOI] [PubMed] [Google Scholar]
- 49.McIntyre JB, Bose P, Klimowicz AC, et al. Specific and sensitive hydrolysis probe-based real-time PCR detection of epidermal growth factor receptor variant III in oral squamous cell carcinoma. PLoS One. 2012;7:e31723. doi: 10.1371/journal.pone.0031723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.