Abstract
Although innate immune responses are necessary for the initiation of acquired immune responses and the subsequent successful elimination of pathogens, excessive responses occasionally result in lethal endotoxic shock accompanied by a cytokine storm. B and T lymphocyte attenuator (BTLA), a coinhibitory receptor with similarities to cytotoxic T-lymphocyte antigen (CTLA)-4 and programmed death (PD)-1, is expressed in not only B and T cells but also dendritic cells (DCs) and macrophages (Mϕs). Recently, several studies have reported that BTLA-deficient (BTLA−/−) mice show enhanced pathogen clearance compared with WT mice in early phase of infections. However, the roles of BTLA expressed on innate cells in overwhelming and uncontrolled immune responses remain unclear. Here, we found that BTLA−/− mice were highly susceptible to LPS-induced endotoxic shock. LPS-induced TNF-α and IL-12 production in DCs and Mϕs was significantly enhanced in BTLA−/− mice. BTLA−/− DCs also produced high levels of TNF-α on stimulation with Pam3CSK4 but not poly(I:C) or CpG, suggesting that BTLA functions as an inhibitory molecule on Toll-like receptor signaling at cell surface but not endosome. Moreover, BTLA−/− DCs showed enhanced MyD88- and toll/IL-1R domain-containing adaptor inducing IFN (TRIF)-dependent signaling on LPS stimulation, which is associated with impaired accumulation of Src homology 2-containing protein tyrosine phosphatase in lipid rafts. Finally, we found that an agonistic anti-BTLA antibody rescued mice from LPS-induced endotoxic shock, even if the antibody was given to mice that had developed a sign of endotoxic shock. These results suggest that BTLA directly inhibits LPS responses in DCs and Mϕs and that agonistic agents for BTLA might have therapeutic potential for LPS-induced endotoxic shock.
Septic shock is a life-threatening disease, which is caused by bacterial infection, especially with Gram-negative bacteria (1, 2). Toll-like receptor 4 (TLR4), one of representative pattern recognition receptors, recognizes LPS from Gram-negative bacteria and transduces signals in innate cells, such as macrophages (Mϕs) and dendritic cells (DCs), for the production of proinflammatory cytokines and chemokines (2–4). These innate responses are necessary for the initiation of acquired immune responses and subsequent successful elimination of bacteria. However, excessive innate immune responses occasionally result in a cytokine storm that is a potentially fatal immune reaction consisting of a positive feedback loop between highly elevated levels of various cytokines and immune cells, which leads to lethal endotoxic shock within a few days (1, 3, 5–8). However, lethal endotoxic shock is difficult to control by inhibitors for a particular cytokine (2, 7), and thus, novel therapeutic strategies for lethal endotoxic shock are desired.
B and T lymphocyte attenuator (BTLA; CD272) is the third inhibitory coreceptor, which has been identified as an inhibitory coreceptor expressed on CD4+ T cells and B cells with similarities to CTLA-4 and PD-1 (9). Thereafter, accumulating evidence has revealed that BTLA is expressed on not only CD4+ T and B cells but also a wide range of hematopoietic cells, including CD8+ T cells, natural killer T cells, natural killer cells, Mϕs, and DCs at various levels (10). The ligand for BTLA is the TNF receptor family member Herpesvirus entry mediator (HVEM), which is broadly expressed on hematopoietic cells, including T cells, Mϕs, and DCs (10). Ligation of BTLA by HVEM induces the recruitment of SHP-1/SHP-2 and then attenuates cell activation (9–11). Analyses of BTLA-deficient (BTLA−/−) mice have revealed that BTLA plays inhibitory roles in a variety of disease models, including experimental autoimmune encephalomyelitis (9), partially MHC-mismatched cardiac allograft (12), experimental colitis (13), and experimental hepatitis (14). We have also shown that the deficiency of BTLA spontaneously causes the breakdown of self-tolerance, resulting in the development of an autoimmune hepatitis-like disease and lymphocytic infiltration in multiple organs in aged mice (15). However, the administration of an agonistic anti-BLTA antibody has been shown to prevent graft-versus-host disease (16) and hapten-induced contact hypersensitivity (17). These results suggest that BTLA plays an important role in the homeostasis of acquired immune responses.
In addition to the role of BTLA in acquired immune responses, recent studies have shown that BTLA also plays a role in immune responses against infectious pathogens. Sun et al. (18) have shown that BTLA−/− mice exhibit significantly higher bacterial clearance compared with WT mice in the early phase of bacterial infection. Shubin et al. (19) have also shown that BTLA−/− mice exhibited a higher rate of survival and protection from cecal ligation and puncture. Moreover, Adler et al. (20) have shown that BTLA−/− mice exhibit strongly enhanced parasite clearance and that the increased clearance is seen before the onset of acquired immune responses. These findings suggest that BTLA is involved in the clearance of pathogens in the early phase of immune responses and that BTLA expressed on innate cells might be involved in the process. However, the role of BTLA in overwhelming and uncontrolled immune responses that lead to endotoixic shock remains unclear.
In this study, we examined the role of BTLA in innate immune responses and found that BTLA signaling inhibited LPS-induced endotoxic shock and proinflammatory cytokine production from innate cells. We also found that BTLA signaling inhibited both LPS-induced MyD88- and TRIF-dependent pathways in DCs, possibly by inducing the recruitment of SHP-2 into lipid rafts. We also showed that an agonistic anti-BTLA antibody had therapeutic potential for endotoxic shock. Our results highlight the importance of BTLA in innate immune responses.
Results
BTLA-Deficient Mice Are Highly Susceptible to LPS-Induced Endotoxic Shock.
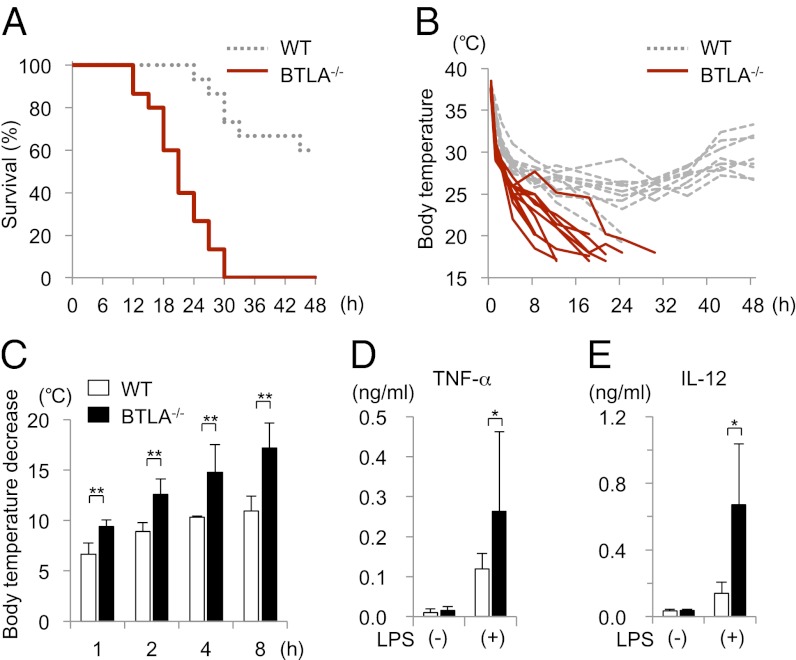
To determine whether BTLA plays a role in the regulation of innate immune responses, we first investigated the role of BTLA in a murine model of endotoxic shock by using BTLA−/− mice. BTLA−/− mice and littermate WT control mice on a C57BL/6 background were injected i.p. with LPS (500 μg/body), and their survival, rectal temperature and cytokine levels in sera were monitored. As shown in Fig. 1A, all BTLA−/− mice died within 30 h after LPS injection, whereas over one-half of WT mice survived at 48 h after LPS injection (n = 15 for each genotype, log rank test, P < 0.0001). Body temperature was significantly decreased in BTLA−/− mice compared with WT mice at 1, 2, 4, and 8 h after LPS injection (Fig. 1 B and C). The levels of TNF-α and IL-12, key cytokines causing endotoxic shock (1, 21), were significantly elevated in sera in BTLA−/− mice compared with sera in WT mice (n = 5 each, P < 0.05) (Fig. 1 D and E). These results indicate that BTLA suppresses cytokine production and endotoxic shock on LPS stimulation.
Fig. 1.
BTLA−/− mice are highly susceptible to LPS-induced endotoxic shock. BTLA−/− and littermate WT mice (8 wk) were injected i.p. with LPS (500 μg/body). (A) Survival of the mice was analyzed by Kaplan–Meier survival analysis. n = 15 for each genotype, log rank test, P < 0.0001. (B and C) Body temperature (B) and body temperature decrease from the baseline (C) are shown. Data are mean ± SD, n = 10 for each group. **Significantly different from the mean value of WT mice, P < 0.01. (D and E) Sera were collected from the mice at 1 and 4 h after the LPS injection, and the levels of TNF-α (1 h) and IL-12 (4 h) were determined by ELISA. Data are mean ± SD, n = 5. *Significantly different from the mean value of WT mice, P < 0.05.
BTLA Suppresses LPS-Induced Cytokine Production in Mϕs and DCs.
It has been shown that BTLA is expressed not only on T and B cells but also DCs and Mϕs, although the expression levels of BTLA on innate cells, including Mϕs, vary depending on the strain of mice (9, 22). To address the mechanisms underlying the enhanced susceptibility of BTLA−/− mice to LPS-induced endotoxic shock, we first examined the development of Mϕs and DCs in BTLA−/− mice. We found that the numbers of Mϕs (F4/80+ CD11b+ cells) and DCs (CD11c+ MHC II+ cells) in spleen in BTLA−/− mice were similar to the number in WT mice (Fig. S1A), although the frequency of CD4− CD8− CD11c+ DCs in spleen was slightly increased in BTLA−/− mice (Fig. S1B). In addition, no significant difference was found in the numbers of bone marrow-derived Mϕs (BMMϕs) and bone marrow-derived DCs (BMDCs), which were obtained from the culture of bone marrow cells in BTLA−/− and WT mice (Fig. S1A). These results suggest that BTLA is not essential for the development of DCs and Mϕs.
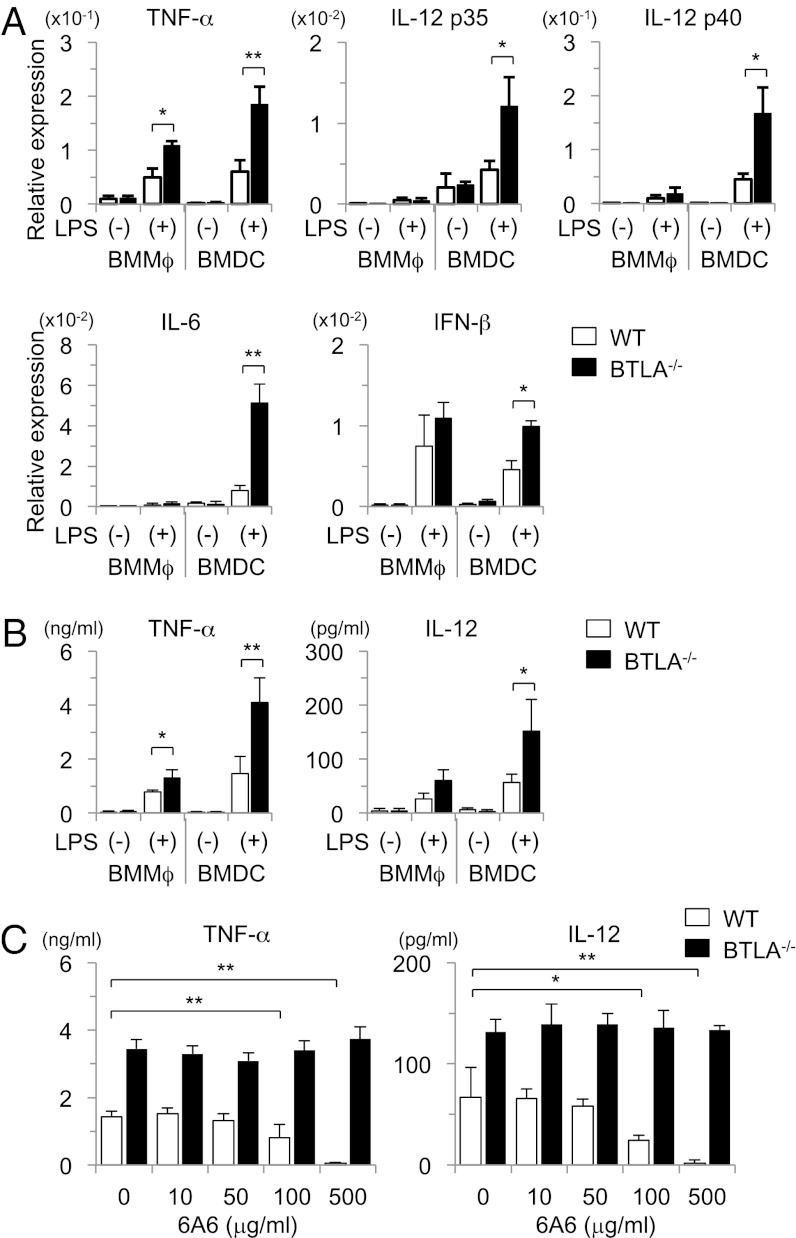
To determine whether BTLA is involved in the regulation of proinflammatory cytokine production in DCs and Mϕs, we next examined LPS-induced cytokine production of BMMϕs and BMDCs in BTLA−/− and WT mice. As shown in Fig. 2A, BTLA−/− BMDCs expressed higher levels of TNF-α, IL-6, IL-12, and IFN-β mRNA than WT BMDCs (Fig. 2A). In addition, BTLA−/− BMDCs produced larger amounts of TNF-α and IL-12 than WT BMDCs (Fig. 2B). However, the induction of these cytokines in BMMϕs was weaker compared with BMDCs, and the difference between BTLA−/− BMMϕs and WT BMMϕs in the expression levels of these cytokines was less obvious (Fig. 2 A and B). These results suggest that BTLA expressed on DCs is involved in the suppression of LPS-induced cytokine production.
Fig. 2.
BTLA−/− DCs produce large amounts of proinflammatory cytokines on LPS stimulation. (A and B) BMMϕs and BMDCs from BTLA−/− and WT mice were stimulated with LPS (100 ng/mL). (A) Four hours later, mRNA levels of TNF-α, IL-12 p35, IL-12 p40, IL-6, and IFN-β were measured by quantitative PCR. Data are mean ± SD, n = 4. *P < 0.05, **P < 0.01. (B) Twelve hours later, the levels of TNF-α and IL-12 in the supernatants were measured by ELISA. Data are mean ± SD, n = 4. *P < 0.05, **P < 0.01. (C) BMDCs were stimulated with LPS (100 ng/mL) in the presence or absence of an agonistic anti-BTLA antibody (6A6), and the levels of TNF-α and IL-12 in the supernatants were measured by ELISA at 12 h after the stimulation. Data are mean ± SD, n = 4. *P < 0.05, **P < 0.01.
It has been shown that HVEM, which is a ligand of BTLA, also interacts with other molecules, such as LIGHT, CD160, and glycoprotein D (10, 23). Therefore, it is possible that BTLA deficiency may affect the function of DCs through the enhancement of HVEM-mediated signaling rather than the lack of BTLA signaling in DCs. To determine whether the enhanced cytokine production in BTLA−/− BMDCs is caused by the absence of inhibitory signals from BTLA, we next examined the effect of 6A6, an agonistic antibody against BTLA (16, 24), on LPS-induced cytokine production of BMDCs. As shown in Fig. 2C, 6A6 suppressed TNF-α and IL-12 production from WT BMDCs but not BTLA−/− BMDCs in a dose-dependent manner. These results suggest that signals through BTLA expressed on DCs inhibit LPS-induced cytokine production.
BTLA-Deficient DCs Produce High Levels of TNF-α on Stimulation with LPS and Pam3CSK4 but Not poly(I:C) or CpG.
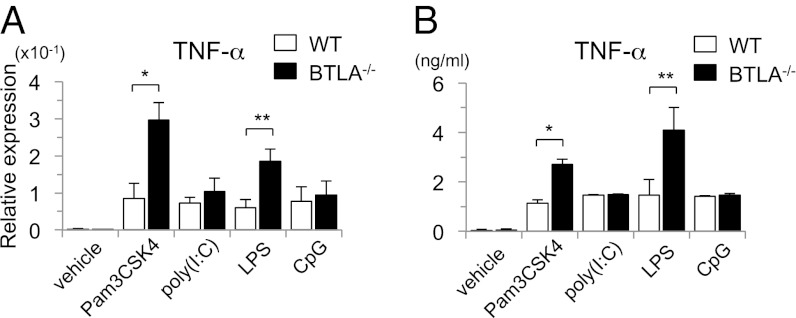
To determine whether the inhibitory function of BTLA is specific for LPS-TLR4 signaling, we next examined the effect of other TLR ligands, such as Pam3CSK4 (TLR2/1 ligand), poly(I:C) (TLR3 ligand), and CpG oligodeoxynucleotides (TLR9 ligand), on cytokine production in WT BMDCs and BTLA−/− BMDCs. Analogous to stimulation with LPS, the induction of TNF-α mRNA (Fig. 3A) as well as the levels of TNF-α in the supernatants (Fig. 3B) were significantly enhanced in BTLA−/− BMDCs compared with WT BMDCs on stimulation with Pam3CSK4. However, the production of TNF-α was not enhanced in BTLA−/− BMDCs on the stimulation with poly(I:C) or CpG at both mRNA and protein levels (Fig. 3). These results suggest that BTLA functions as an inhibitory molecule on TLR signaling at cell surface, where TLR4 and TLR2/1 are located, but not endosome, where TLR3 and TLR9 are located.
Fig. 3.
BTLA−/− DCs produce large amounts of TNF-α on stimulation with LPS and Pam3CSK4 but not poly(I:C) or CpG. BTLA−/− BMDCs and WT BMDCs were stimulated with LPS (100 ng/mL), Pam3CSK4 (200 ng/mL), poly(I:C) (10 μg/mL), and CpG (10 μg/mL). (A) Four hours later, the expression levels of TNF-α mRNA were measured by quantitative PCR. Data are mean ± SD, n = 4. *P < 0.05, **P < 0.01. (B) Twelve hours later, the levels of TNF-α in the supernatants were measured by ELISA. Data are mean ± SD, n = 4. *P < 0.05, **P < 0.01.
BTLA Inhibits both MyD88- and TRIF-Dependent Pathways of TLR4 Signaling.
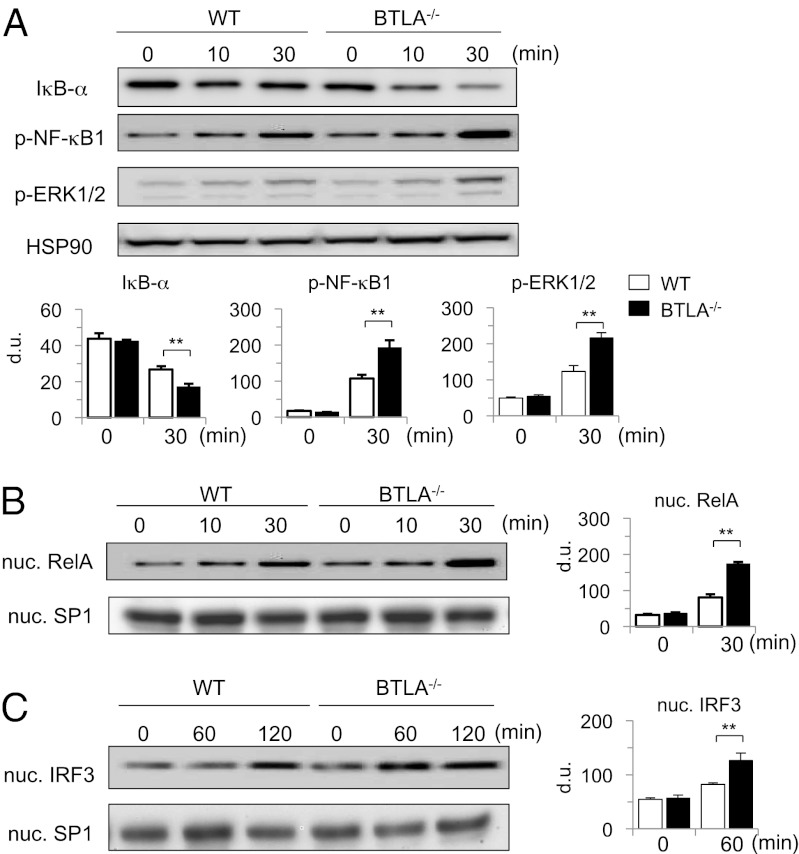
TLR4 signaling activates diverse transcription factors and induces proinflammatory cytokine expression through MyD88- or TRIF-dependent pathways (4). MyD88-dependent pathway activates NF-κB and ERK/MAPK pathways, whereas TRIF-dependent pathway activates interferon regulatory factor (IRF)-3–dependent pathways, although there is partial cross-talk between MyD88- and TRIF-dependent pathways (4). To determine the mechanisms underlying BTLA-mediated inhibition of LPS-induced TNF-α expression in DCs, we examined the activation of these signaling pathways in LPS-stimulated BTLA−/− BMDCs and WT BMDCs. LPS stimulation strongly induced IκB-α degradation, phosphorylation of NF-κB1 and ERK1/2 in the cytoplasm, and nuclear translocation of RelA in BTLA−/− BMDCs compared with those changes in WT BMDCs (Fig. 4 A and B). In addition, LPS-induced nuclear translocation of IRF-3 was significantly enhanced in BTLA−/− BMDCs compared with WT BMDCs (Fig. 4C). These results suggest that BTLA inhibits both MyD88- and TRIF-dependent pathways of TLR4 signaling.
Fig. 4.
LPS-induced activation of NF-κB, MAPK, and IRF-3 pathways is enhanced in BTLA−/− DCs. (A and B) BTLA−/− BMDCs and WT BMDCs were stimulated with LPS (100 ng/mL) for indicated time periods. (A) Whole-cell lysates were subjected to immunoblotting with antibodies against IκB-α, p-NF-κB1, p-ERK1/2, and HSP90α/β (as a control). (B) Nuclear extracts were subjected to immunoblotting with antibodies against RelA and SP1 (as a control). Shown are representative blots and densitometric analyses of relative intensity of three independent experiments. (C) BTLA−/− BMDCs and WT BMDCs were stimulated with LPS (1 μg/mL) for indicated time periods. Nuclear extracts were subjected to immunoblotting with antibodies against IRF-3 and SP1. Shown are representative blots and densitometric analyses of relative intensity of three independent experiments. ** P < 0.01.
BTLA Inhibits TLR4 Signaling Through SHP-2 Activation.
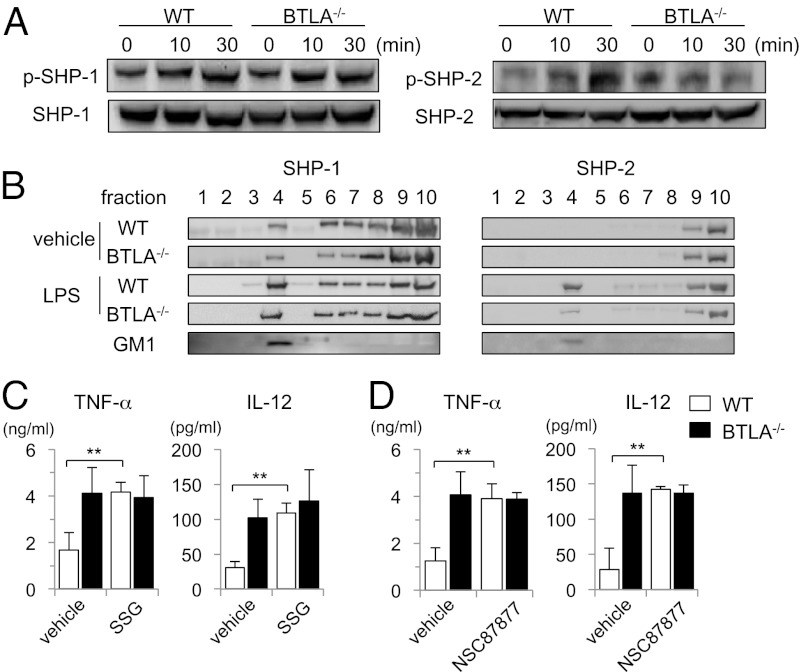
Given that BTLA inhibits a wide range of TLR4 signaling, we next examined proximal events of TLR4 signaling. We first examined the expression levels of TLR4 itself in BTLA−/− BMDCs and WT BMDCs and found that BTLA−/− BMDCs expressed TLR4 at the similar levels to WT BMDCs with or without LPS stimulation (Fig. S2). It has been reported that BTLA has two immunoreceptor tyrosine-based inhibitory motifs in the cytoplasmic region and interacts directly with SHP-1 and SHP-2 (SHP-1/2) in T cells (9, 11). It has also been reported that SHP-1/2 regulates TLR4 signaling and cytokine production in several cell types (25–28). To determine whether BTLA regulates TLR4 signaling through the activation of SHP-1/2 in DCs, we first examined SHP-1/2 phosphorylation in BTLA−/− BMDCs and WT BMDCs on LPS stimulation. As shown in Fig. 5A, SHP-2 phosphorylation was significantly increased by LPS stimulation in WT BMDCs but not BTLA−/− BMDCs. However, SHP-1 phosphorylation was similarly induced in BTLA−/− BMDCs and WT BMDCs on LPS stimulation (Fig. 5A).
Fig. 5.
LPS-induced accumulation of SHP-2 in lipid rafts is impaired in BTLA−/− DCs. (A) BTLA−/− BMDCs and WT BMDCs were stimulated with LPS (100 ng/mL) for indicated time periods. Whole-cell lysates were subjected to immunoblotting with antibodies against phosphorylated SHP-1 (p-SHP-1), SHP-1, phosphorylated SHP-2 (p-SHP-2), and SHP-2. Shown are representative blots of three independent experiments. (B) BTLA−/− BMDCs and WT BMDCs were stimulated with LPS (1 μg/mL) for 30 min. Cell lysates were fractionated by sucrose density gradient centrifugation, and immunoblotting with anti-SHP-1 antibody and anti-SHP-2 antibody was performed. Lipid raft fraction containing glycosphingolipid GM1 was labeled with HRP-conjugated cholera toxin B subunit. (C and D) BTLA−/− BMDCs and WT BMDCs were treated with SSG (C), NSC87877 (D), or vehicle for 30 min and then stimulated with LPS (100 μg/mL) for 12 h. The levels of TNF-α and IL-12 in the supernatants were measured by ELISA. Data are mean ± SD, n = 4. **P < 0.01.
Recently, we reported that BTLA accumulates in lipid rafts after T cell receptor stimulation in T-cell lines (29). TLR4 has also been reported to recruit into lipid rafts after LPS stimulation (30). We, therefore, examined subcellular distribution of SHP1/2 in BTLA−/− BMDCs and WT BMDCs on LPS stimulation. As shown in Fig. 5B, SHP-1 was present in lipid raft fraction (Fig. 5B, lane 4), which was defined by the presence of ganglioside GM1 before LPS stimulation and accumulated in lipid rafts with LPS stimulation in both BTLA−/− BMDCs and WT BMDCs. By contrast, SHP-2 was absent in lipid rafts before LPS stimulation and accumulated in lipid rafts on LPS stimulation in WT BMDCs but not BTLA−/− BMDCs (Fig. 5B). These results suggest that BTLA is required for the accumulation of SHP-2 in lipid rafts on LPS stimulation.
To address the functional roles of SHP-1/2 in BTLA-mediated suppression of TLR4 signaling, we examined the effect of two SHP-1/2 inhibitors, sodium stibogluconate (SSG) and NSC87877, on LPS-induced cytokine production in BTLA−/− BMDCs and WT BMDCs. LPS-induced TNF-α and IL-12 production was significantly enhanced in SSG-treated WT BMDCs compared with vehicle-treated WT BMDCs and reached levels similar to the levels in LPS-stimulated BTLA−/− BMDCs (Fig. 5C). However, SSG did not enhance TNF-α and IL-12 production in LPS-stimulated BTLA−/− BMDCs (Fig. 5C). Similar results were obtained if LPS-stimulated WT BMDCs were treated with NSC87877 (Fig. 5D). Taken together, these results suggest that SHP-1/2, especially SHP-2, is involved in BTLA-mediated suppression of TLR4 signaling.
Agonistic Anti-BTLA Antibody Rescues Mice from LPS-Induced Endotoxic Shock.
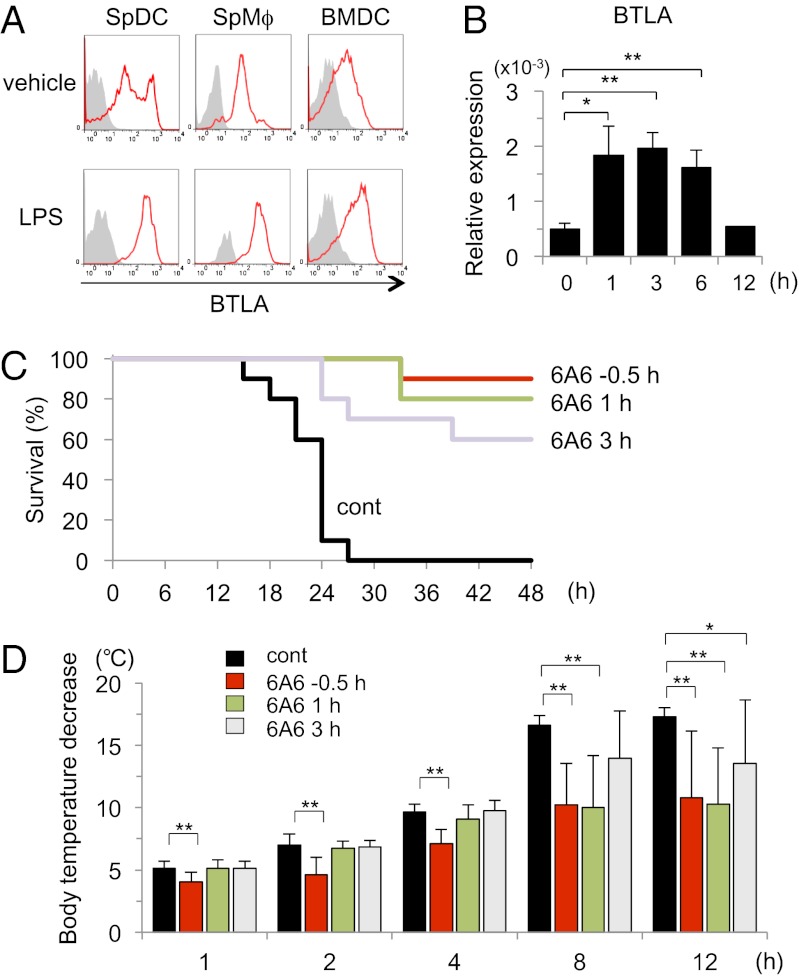
To determine whether agonistic agents for BTLA might have therapeutic potential for ongoing LPS-induced endotoxic shock, we first examined whether the addition of the agonistic anti-BTLA antibody (6A6) inhibited LPS-induced cytokine production from WT BMDCs, even if 6A6 was added to the culture after LPS stimulation. The production of TNF-α and IL-12 from WT BMDCs was significantly reduced by 6A6, even when 6A6 was added at 3 h after LPS stimulation (Fig. S3). Consistent with the observation, we found that LPS induced BTLA expression in innate cells, including splenic DCs, splenic Mϕs, and BMDCs (Fig. 6A). The induction of BTLA expression by LPS stimulation was confirmed at transcription levels in BMDCs (Fig. 6B). These results suggest that, in addition to BTLA expressed on DCs at steady state, newly expressed BTLA may also be involved in the inhibition of LPS-induced cytokine production in DCs.
Fig. 6.
An agonistic anti-BTLA antibody rescues mice from LPS-induced endotoxic shock. (A) Splenocytes were stimulated with LPS (100 ng/mL) for 12 h, and the expression levels of BTLA on DCs (CD11c+ MHC II+ cells) and Mϕs (F4/80+ CD11b+ cells) were analyzed by flow cytometry. BMDCs were stimulated with LPS (100 ng/mL) for 12 h, and the expression levels of BTLA were analyzed by flow cytometry. Shown are representative histograms of BTLA staining with control staining (gray histograms) of three independent experiments. (B) BMDCs were stimulated with LPS (100 ng/mL) for the indicated time period, and mRNA levels of BTLA were measured by quantitative PCR. Data are mean ± SD, n = 4. *P < 0.05, **P < 0.01. (C and D) C57BL/6 mice were injected i.p. with LPS (750 μg/body). Where indicated, 6A6 (400 μg/mouse) was administered to the mice at −0.5, +1, and +3 h from the timing of LPS administration. (C) Survival of the mice was analyzed by Kaplan–Meier survival analysis. n = 10 mice for each group; control vs. −0.5 h (P < 0.0001), control vs. 1 h (P < 0.0001), and control vs. 3 h (P < 0.0005; log rank test). (D) Body temperature was evaluated at 0, 1, 2, 4, 8, and 12 h after LPS injection. Body temperature decreases from the baseline are shown. Data are mean ± SD, n = 10 for each group. *P < 0.05, **P < 0.01.
We finally examined therapeutic potential of 6A6 on endotoxic shock by lethal doses of LPS (750 μg/body). As shown in Fig. 6C, when 6A6 was administered at 30 min before the LPS administration, 6A6 almost completely inhibited LPS-induced lethal endotoxic shock in C57BL/6 mice. Importantly, even if 6A6 was administered to the mice at 3 h after the LPS stimulation (when the mice had developed a sign of endotoxic shock), 6A6 rescued over one-half of mice from the lethal endotoxic shock (Fig. 6C). Body temperature decrease was also significantly inhibited by 6A6, even when 6A6 was administered to the mice at 3 h after the LPS administration (Fig. 6D). These results suggest that agonistic agents for BTLA might have therapeutic potential for LPS-induced endotoxic shock.
Discussion
In this study, we showed that BTLA inhibits LPS-induced endotoxic shock (Fig. 1) by suppressing proinflammatory cytokine production from DCs and Mϕs (Figs. 1 and 2). We also showed that BTLA inhibits both MyD88- and TRIF-dependent pathways on LPS stimulation in DCs (Fig. 4), presumably by inducing the recruitment of SHP-2 into lipid rafts (Fig. 5). Finally, we showed that an agonistic anti-BTLA antibody reduces mortality of LPS-induced endotoxic shock (Fig. 6). These results suggest that agonistic agents for BTLA may have therapeutic potential against endotoxic shock.
We show that BTLA inhibits TLR4 signaling in DCs. We found that cytokine responses against LPS were significantly enhanced in BTLA−/− DCs (Fig. 2). This finding is consistent with a previous study showing that BTLA−/− splenocytes produce large amounts of proinflammatory cytokines in response to heat-killed Listeria monocytogenes (18). In addition, we found that cytokine responses against Pam3CSK4, a ligand for TLR2/1, were significantly enhanced in BTLA−/− DCs compared with WT DCs (Fig. 3), indicating that the inhibitory effect of BTLA on TLR signaling is not restricted to TLR4 signaling. However, we found that cytokine responses against ligands for TLR3 [poly(I:C)] and TLR9 (CpG) were normal in BTLA−/− DCs (Fig. 3). Because TLR4 and TLR2/1 are located at cell surface but TLR3 and TLR9 are located at endosome (4), it is suggested that BTLA inhibits the signaling of TLRs that are located at cell surface but not at endosome.
HVEM, a ligand of BTLA, is broadly expressed on hematopoietic cells, including DCs and Mϕs (10). A number of studies using BTLA−/− mice and HVEM−/− mice indicates that the interaction between BTLA and HVEM causes bidirectional signaling that balances the immune responses. However, because HVEM has other ligands, including LIGHT, that may influence immune responses, data obtained from the analysis of BTLA−/− mice should be interpreted carefully. It is possible that the absence of BTLA results in increased interaction between LIGHT and HVEM, which in turn, may induce proinflammatory signals through the activation of NF-κB (10). However, we showed here that the administration of the agonistic anti-BTLA antibody 6A6 inhibited LPS-induced cytokine production from WT DCs but not BTLA−/− DCs (Fig. 2C). We also showed that the administration of 6A6 inhibited LPS-induced endotoxic shock in WT mice (Fig. 6). These results indicate that BTLA signaling in DCs is involved in the suppression of TLR4 signaling.
Accumulating evidence indicates that TLR signaling pathways are regulated by TIR domain-containing adaptors, such as MyD88, toll/interleukine-1 receptor (TIRAP), toll/IL-1 receptor domain-containing adaptor protein (TRIF), and TRIF-related adaptor molecule (TRAM) (4). The different uses of these adaptors provide specificity of individual TLR-mediated signaling pathways (4). For instance, TLR2, TLR7, and TLR9 transduce their signal through MyD88-dependent pathways, which results in the activation of the NF-κB pathway, whereas TLR3 transduces its signal through MyD88-independent/TRIF-dependent pathways, which results in the activation of IRF-3 (4). However, TLR4 uses both MyD88- and TRIF-dependent pathways (4). We showed that not only TLR4-mediated degradation of IκB-α and nuclear translocation of RelA but also TLR4-mediated nuclear translocation of IRF-3 was enhanced in BTLA−/− BMDCs compared with WT BMDCs (Fig. 4). These findings suggest that the MyD88-dependent pathways as well as the TRIF-dependent pathways were suppressed by BTLA in DCs.
Ligation of BTLA by HVEM induces tyrosine phosphorylation of ITIM and subsequent association of SHP-1/SHP-2 (10). Recently, we have shown that BTLA accumulates in lipid rafts in activated T cells (29). We showed here that LPS stimulation induced the recruitment of SHP-2 into lipid rafts and induced phosphorylation of SHP-2 in WT BMDCs but not BTLA−/− BMDCs (Fig. 5 A and B). We also found that SHP-2 inhibitor enhanced LPS-mediated cytokine production in WT BMDCs but not BTLA−/− BMDCs (Fig. 5 C and D). In this regard, An et al. (25) have shown that SHP-2 suppresses TLR-triggered TRIF-dependent pathways by binding to and inhibiting activation of tank-binding kinase 1. More recently, Xu et al. (28) reported that SHP-2 suppresses MyD88-dependent pathways by interacting with TNF receptor-associated factor (TRAF)6 and controlling its ubiquitination. Taken together, these results suggest that BTLA is required for the accumulation of SHP-2 in lipid rafts on LPS stimulation and that the activated SHP-2 inhibits LPS-triggered MyD88- and TRIF-dependent pathways.
In contrast, we found that LPS-mediated phosphorylation of SHP-1 (Fig. 5A) and its accumulation in lipid rafts (Fig. 5B) were not affected by the absence of BTLA in DCs. The precise mechanisms underlying the difference are currently unclear. However, because several molecules, including dendritic cell-derived immunoglobulin receptor (31), immunoglobulin-like transcript (32), and dendritic cell immunoreceptor (33), have been reported to be expressed in DCs and associated with SHP-1, we assume that these molecules other than BTLA might be sufficient for the phosphorylation of SHP-1 and its accumulation in lipid rafts in DCs.
We showed that BTLA−/− mice are highly susceptible to LPS-induced endotoxic shock (Fig. 1), suggesting that BTLA prevents excessive innate immune responses that occasionally result in lethal shock. We also showed that the administration of the agonistic anti-BTLA antibody 6A6 rescues WT mice from LPS-induced endotoxic shock even if 6A6 is administered 3 h after LPS injection (Fig. 6). In addition, we found that LPS signal induces the expression of BTLA in innate cells (Fig. 6). These results suggest that BTLA functions as a negative feedback regulator of TLR4 signaling in innate cells.
Because BTLA−/− mice have been reported to exhibit significantly higher pathogen clearance compared with WT mice (18–20), the administration of 6A6 is of potential concern for the suppression of pathogen clearance. However, it has been reported that 6A6 does not perturb protective immunity against pathogens (16, 24). Taken together with our finding that the administration of 6A6 rescues mice from endotoxic shock, even if 6A6 is administered to the mice that have developed a sign of endotoxic shock, agonistic agents of BTLA signaling may be applicable for the prevention of septic shock in the early phase of severe bacterial infection.
In conclusion, our study shows that BTLA suppresses LPS-induced endotoxic shock, presumably by suppressing cytokine production from LPS-stimulated DCs and Mϕs. Although additional studies are required for understanding the molecular mechanisms of the findings, our results should highlight the therapeutic potential of agonistic agents for BTLA in the prevention of pathological conditions in which excessive activation of innate immune cells is involved.
Materials and Methods
Mice.
BTLA−/− mice and littermate WT mice on a C57BL/6 background (9) were bred and housed in the animal facility at Chiba University. All mice were housed in microisolator cages under specific pathogen-free conditions. The Chiba University Animal Care and Use Committee approved all procedures used in this study.
Cell Culture.
For the preparation of BMMϕs, BM cells (2 × 106 cells) were cultured in complete RPMI medium 1640 (supplemented with 10% (vol/vol) FCS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 μM 2-ME) in the presence of macrophage colony-stimulating factor (10 ng/mL; R&D Systems) for 5 d. For the preparation of BMDCs, BM cells were cultured in complete RPMI medium 1640 containing recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (20 ng/mL; Peprotech); 3 d later, an equal volume of fresh medium containing GM-CSF (20 ng/mL) was added to the culture. At days 6 and 8 of the culture, one-half of the medium was replaced with fresh medium containing GM-CSF. Nonadherent cells were harvested as BMDCs at day 10.
BMMϕs or BMDCs were stimulated with LPS (100 ng/mL or 1 μg/mL, Escherichia coli 0127:B8; Sigma-Aldrich), Pam3CSK4 (200 ng/mL; InvivoGen), CpG (10 μg/mL, ODN 1585; InvivoGen), or poly(I:C) (10 μg/mL; InvivoGen). In some experiments, cells were treated with an agonistic anti-BTLA antibody (clone: 6A6) (16, 22). In other experiments, cells were pretreated with SSG (#567565; EMD Chemicals) or NSC87877 (#565851; Merck) at 30 min before LPS stimulation.
Quantitative Real-Time PCR Analysis.
Total cellular RNA was extracted with the PureLink RNA Mini Kit (Invitrogen). Reverse transcription was performed using an iScript cDNA Synthesis Kit (BioRad Laboratories). Quantitative PCR was performed with an ABI PRISM 7300 Sequence Detection System (Applied Biosystems). The levels of TNF-α, IL-6, IL-12a (IL-12 p35), IL-12b (IL-12 p40), IFN-β, and BTLA mRNA were normalized to the levels of β-actin mRNA. The sequences of PCR primers are shown in SI Text.
Flow Cytometric Analysis.
Cells were incubated with anti-CD16/32 mAb (BD Biosciences) to block Fc-mediated binding, stained with indicated antibodies, and analyzed on a FACSCalibur (BD Biosciences) using CellQuestPro software (BD Biosciences) and FlowJo software (Tree Star). FITC-conjugated anti-CD11c (HL3), PE-conjugated anti-CD8α (Ly-2), PE-conjugated anti-CD11b (M1/70), and allophycocyanin-conjugated anti-B220 (RA3-6B2) were purchased from BD Biosciences. PE-conjugated anti-CD11c (N418), PerCP/Cy5.5-conjugated CD11b (M1/70), allophycocyanin-conjugated CD4 (RM4-5), and Alexa Fluor 488-conjugated anti–I-A/I-E (M5/114.15.2) were purchased from BioLegend. FITC-conjugated F4/80 (BM8), PE-conjugated anti-PDCA1 (eBio927), PE-conjugated anti-BTLA (6F7), and Alexa Fluor 647-conjugated anti-BTLA (8F4) were purchased from eBioscience. PE-conjugated anti-TLR4 (MTS510) was purchased from Santa Cruz Biotechnology.
Cytokine Assay.
The amounts of TNF-α and IL-12 p70 in sera and culture supernatants were measured by ELISA kits (BD Biosciences) according to the manufacturer’s protocols.
Immunoblotting.
Whole-cell lysates and nuclear extracts were prepared as described elsewhere (34). Immunoblotting was performed as described previously (35). The following antibodies were purchased from Cell Signaling Technology: anti–IκB-α (#9242), antiphospho–NF-κB1 (Ser933, clone: 18E6), antiphospho-pERK1/2 (Thr202/Tyr204, clone: D13.14.E), anti-RelA (clone: C22B4), anti–IRF-3 (clone: D83B9), and antiphospho–SHP-2 (Tyr542, #3751). Antibodies against HSP90α/β (#sc-7947), SP1 (#sc-59), SHP-1 (sc-287), and SHP-2 (sc-280) were purchased from Santa Cruz Biotechnology. Antibody against phospho–SHP-1 (Tyr-536) was purchased from Abcom.
Isolation of Lipid Rafts by Sucrose Density Gradient Centrifugation.
BMDCs (1 × 108 cells) were harvested, washed one time in cold PBS, stimulated with LPS (1 μg/mL) for 30 min, and lysed in 0.5 mL ice-cold Mes-buffered saline (25 mM Mes, 150 mM NaCl, 1% Triton X-100, 5 mM Na3VO4, 5 mM EDTA, 1 mM PMSF, and protease inhibitor mixture; Sigma Chemical). Sucrose density gradient centrifugation was performed as described previously (36).
LPS-Induced Endotoxin Shock.
BTLA−/− and WT mice (8 wk) on a C57BL/6 background were injected i.p. with LPS (500 or 750 μg/body). In some experiments, an agonistic anti-BTLA antibody 6A6 (400 μg/body) was injected i.p. at indicated time points. Survival of the mice was monitored every 3 h for 48 h. Body temperature of the mice was measured at indicated time points using a rectal thermometer. For serum cytokine assay, sera were collected at 1 and 4 h after the LPS injection.
Statistical Analysis.
Data are summarized as mean ± SD. The statistical analysis of the results was performed by unpaired t test. P values < 0.05 were considered significant. The statistical analysis of survival was performed by log rank test.
Supplementary Material
Acknowledgments
We thank Professor K. Murphy for BTLA−/− mice and Ms. J. Iwata for excellent technical assistance. This work was supported in part by Grants-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, the Japanese Government and the Global Center of Excellence Program (Global Center for Education and Research in Immune System Regulation and Treatment), Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222093110/-/DCSupplemental.
References
- 1.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9(5):517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 2.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munford RS. Severe sepsis and septic shock: The role of gram-negative bacteremia. Annu Rev Pathol. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 5.Karima R, Matsumoto S, Higashi H, Matsushima K. The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today. 1999;5(3):123–132. doi: 10.1016/s1357-4310(98)01430-0. [DOI] [PubMed] [Google Scholar]
- 6.Ulloa L, Tracey KJ. The “cytokine profile”: A code for sepsis. Trends Mol Med. 2005;11(2):56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y, et al. MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology. 2011;115(3):555–567. doi: 10.1097/ALN.0b013e31822a22f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4(7):670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 10.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 11.Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312(4):1236–1243. doi: 10.1016/j.bbrc.2003.11.070. [DOI] [PubMed] [Google Scholar]
- 12.Tao R, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol. 2005;175(9):5774–5782. doi: 10.4049/jimmunol.175.9.5774. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg MW, et al. A crucial role for HVEM and BTLA in preventing intestinal inflammation. J Exp Med. 2008;205(6):1463–1476. doi: 10.1084/jem.20071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata A, et al. Protective roles of B and T lymphocyte attenuator in NKT cell-mediated experimental hepatitis. J Immunol. 2010;184(1):127–133. doi: 10.4049/jimmunol.0900389. [DOI] [PubMed] [Google Scholar]
- 15.Oya Y, et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis Rheum. 2008;58(8):2498–2510. doi: 10.1002/art.23674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albring JC, et al. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. J Exp Med. 2010;207(12):2551–2559. doi: 10.1084/jem.20102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagomi D, et al. Therapeutic potential of B and T lymphocyte attenuator expressed on CD8(+) T cells for contact hypersensitivity. J Invest Dermatol. 2013;133(3):702–711. doi: 10.1038/jid.2012.396. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, et al. B and T lymphocyte attenuator tempers early infection immunity. J Immunol. 2009;183(3):1946–1951. doi: 10.4049/jimmunol.0801866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shubin NJ, et al. BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol. 2012;92(3):593–603. doi: 10.1189/jlb.1211641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adler G, et al. B and T lymphocyte attenuator restricts the protective immune response against experimental malaria. J Immunol. 2011;187(10):5310–5319. doi: 10.4049/jimmunol.1101456. [DOI] [PubMed] [Google Scholar]
- 21.Ozmen L, et al. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180(3):907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurchla MA, et al. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174(6):3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 23.Cheung TC, et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci USA. 2009;106(15):6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lepenies B, et al. Ligation of B and T lymphocyte attenuator prevents the genesis of experimental cerebral malaria. J Immunol. 2007;179(6):4093–4100. doi: 10.4049/jimmunol.179.6.4093. [DOI] [PubMed] [Google Scholar]
- 25.An H, et al. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity. 2006;25(6):919–928. doi: 10.1016/j.immuni.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 26.An H, et al. Phosphatase SHP-1 promotes TLR- and RIG-I-activated production of type I interferon by inhibiting the kinase IRAK1. Nat Immunol. 2008;9(5):542–550. doi: 10.1038/ni.1604. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran IR, et al. The phosphatase SRC homology region 2 domain-containing phosphatase-1 is an intrinsic central regulator of dendritic cell function. J Immunol. 2011;186(7):3934–3945. doi: 10.4049/jimmunol.1001675. [DOI] [PubMed] [Google Scholar]
- 28.Xu S, et al. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat Immunol. 2012;13(6):551–559. doi: 10.1038/ni.2283. [DOI] [PubMed] [Google Scholar]
- 29.Owada T, et al. Activation-induced accumulation of B and T lymphocyte attenuator at the immunological synapse in CD4+ T cells. J Leukoc Biol. 2010;87(3):425–432. doi: 10.1189/jlb.0309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115(Pt 12):2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- 31.Shi L, et al. DIgR2, dendritic cell-derived immunoglobulin receptor 2, is one representative of a family of IgSF inhibitory receptors and mediates negative regulation of dendritic cell-initiated antigen-specific T-cell responses. Blood. 2006;108(8):2678–2686. doi: 10.1182/blood-2006-04-015404. [DOI] [PubMed] [Google Scholar]
- 32.Liang S, et al. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6—STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105(24):8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanazawa N, Tashiro K, Miyachi Y. Signaling and immune regulatory role of the dendritic cell immunoreceptor (DCIR) family lectins: DCIR, DCAR, dectin-2 and BDCA-2. Immunobiology. 2004;209(1–2):179–190. doi: 10.1016/j.imbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, et al. Proteolytic processing of Stat6 signaling in mast cells as a negative regulatory mechanism. J Exp Med. 2002;196(1):27–38. doi: 10.1084/jem.20011682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maezawa Y, et al. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J Immunol. 2006;176(2):1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: Its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9(2):239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.