Abstract
Maternal stress is a key risk factor for neurodevelopmental disorders, including schizophrenia and autism, which often exhibit a sex bias in rates of presentation, age of onset, and symptom severity. The placenta is an endocrine tissue that functions as an important mediator in responding to perturbations in the intrauterine environment and is accessible for diagnostic purposes, potentially providing biomarkers predictive of disease. Therefore, we have used a genome-wide array approach to screen placental expression across pregnancy for gene candidates that are sex-biased and stress-responsive in mice and translate to human tissue. We identifed O-linked-N-acetylglucosamine (O-GlcNAc) transferase (OGT), an X-linked gene important in regulating proteins involved in chromatin remodeling, as fitting these criteria. Levels of both OGT and its biochemical mark, O-GlcNAcylation, were significantly lower in males and further reduced by prenatal stress. Examination of human placental tissue found similar patterns related to X chromosome dosage. As a demonstration of the importance of placental OGT in neurodevelopment, we found that hypothalamic gene expression and the broad epigenetic microRNA environment in the neonatal brain of placental-specific hemizygous OGT mice was substantially altered. These studies identified OGT as a promising placental biomarker of maternal stress exposure that may relate to sex-biased outcomes in neurodevelopment.
Keywords: O-glycosylation, extra-embryonic tissue, neuropsychiatric disorders
Maternal stress has been identified as a key risk factor for neurodevelopmental disorders, including schizophrenia and autism, which often exhibit a sex bias in rates of presentation, age of onset, and symptom severity (1–4). Specifically, males exposed to stress during the first trimester have an increased risk for schizophrenia, suggesting early pregnancy may be a sensitive window of developmental vulnerability (5). Such epidemiological findings also support a sex specificity of effects during a period of rapid fetal and placental development. Further, sex differences in gene expression in the placenta may represent unique modes of increased disease risk or resilience (4, 6).
Alterations in prenatal programming associated with neurodevelopmental disorders likely involve complex interactions between the maternal environment, the placenta, and factors of the developing fetus, including sex (4, 6). In eutharian mammals, including humans and mice, the placenta serves to mediate communication between the maternal and fetal compartments, delivering nutrients and oxygen and protecting the developing fetus from environmental insults (7). Although derived from both maternal and fetal contributions, the majority of the placenta develops out of the trophoblastic lineage of fetal origin, yielding a predominantly XX or XY placenta, from which sex differences in response to otherwise similar intrauterine perturbations may emerge (8, 9).
In our mouse model of early prenatal stress (EPS), we have previously established that male offspring exposed to maternal stress during early gestation exhibit endophenotypes associated with neurodevelopmental disease, including stress dysregulation and cognitive deficits that persist into the second generation, confirming early pregnancy as a point of increased susceptibility to the effects of maternal stress experience (5, 10–12). Dynamic growth and programming of the placenta occurs during this early period, and therefore alterations in its function resulting from maternal stress exposure could ultimately affect neurodevelopment (13–16). Therefore, we have used a genomic and proteomics approach to identify potential placental biomarkers predictive of prenatal stress exposure. Our proposed criteria for biomarkers assumed that candidate genes would (i) show a significant sex difference in expression, (ii) be significantly altered in our EPS model, and (iii) be similarly regulated in human placental tissue. These criteria were established based upon the hypothesis that a sex bias in disease risk stems from basal sex differences in abilities to adapt or respond to a perturbed environment.
Results
Effects of Offspring Sex and EPS on Placental Gene Expression.
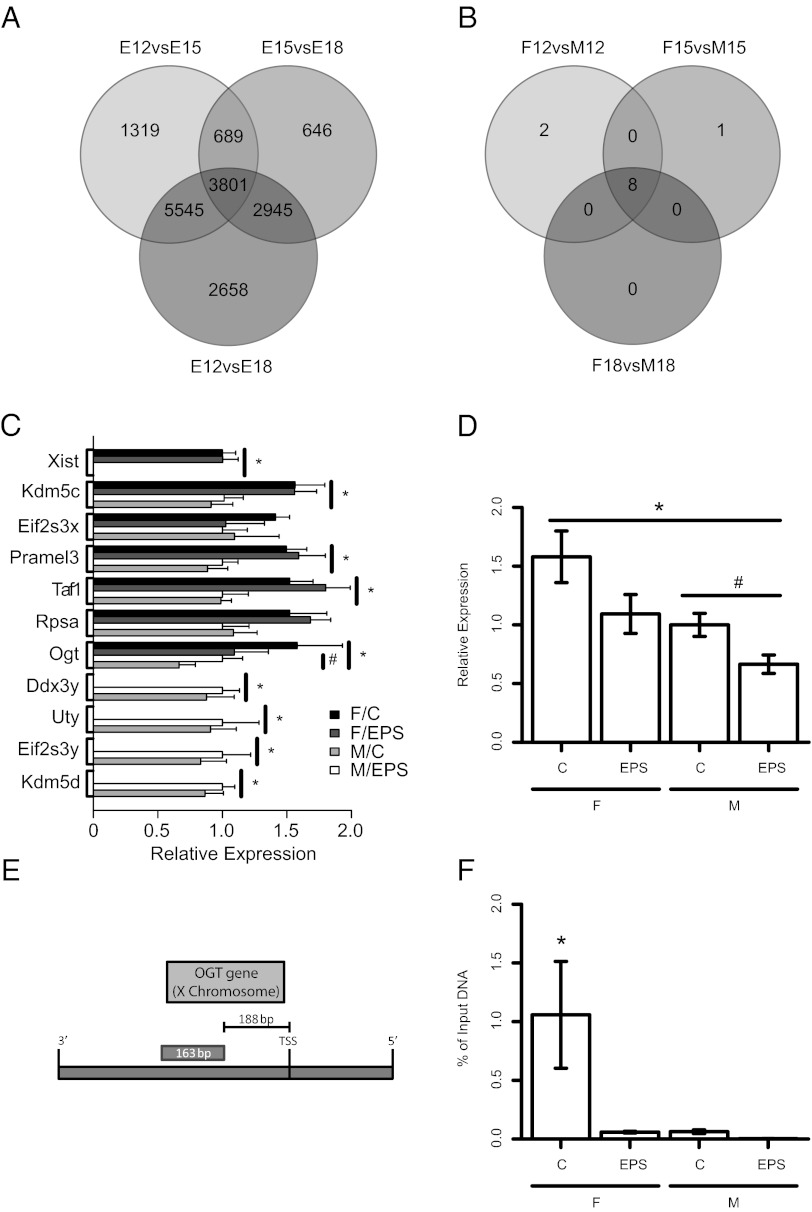
To identify placental genes with sex differences in patterns of expression consistent across gestation [embryonic day (E) 12.5, E15.5, and E18.5], we used a genome-wide microarray analysis. These gestational time points were selected because they reflect maturing stages of fetal development, representing likely important changes in placental function and interactions with the maternal environment (17). Therefore, we hypothesized that identification of genes with sex differences in expression consistent across this period would indicate important candidates potentially responsible for sex differences in programming outcomes in response to a perturbed maternal environment. Differential expression analyses [false discovery rate (FDR) < 0.05] revealed dynamic developmental changes in gene expression patterns, with 2,658 placental genes changing expression from E12.5 to E18.5. Surprisingly, sex differences were only found for 11 X- and Y-linked genes, of which 4 are X and Y paralogs (Fig. 1 A and B and Table S1). Eight of these genes exhibited significant sex differences in patterns of expression across pregnancy (Table S1). As expected, X-linked genes were higher in females, and Y-linked genes were higher in males.
Fig. 1.
Identification of OGT as a potential biomarker of maternal stress. (A) Venn diagram of developmental differential expression analyses of mouse placental genes via Affymetrix GeneChip Mouse Gene 1.0 ST microarray. The labels of each circle refer to specific embryonic time point comparisons represented: E12vsE15 are the comparisons between E12.5 (n = 6) and E15.5 (n = 6), E15vsE18 are the comparisons between E15.5 and E18.5 (n = 6), and E12vsE18 are the comparisons between E12.5 and E18.5. The numbers within the diagram represent the number of genes characterized as having differential expression between these groups, independent of sex, using a false discovery rate of 0.05. (B) Venn diagram of sex differences in mRNA expression levels analyses of mouse placental genes via microarray. Circle labels refer to the sex comparisons made during each developmental time point. The numbers are derived as in A. (C) Genes identified as having sex differences in expression examined in our EPS model. C, control; F, female; M, male (n = 7); EPS, early prenatal stress (n = 8). Data for Xist were normalized to the female control levels; data for all other traits were normalized to male control levels. Bars are the maximum likelihood estimate for each group ± the 95% confidence interval for that estimate. Symbols (* for sex and # for EPS) indicate a main effect with a confidence interval that does not bound zero as determined by the linear model (y ∼ Sex + EPS + Sex*EPS). (D) OGT was identified as fitting our biomarker criteria for having both sex differences in expression and responding to EPS. Symbols (* for sex and # for EPS) indicate a main effect with a confidence interval that does not bound zero as determined by the linear model (y ∼ Sex + EPS + Sex*EPS). Normalization is as in C. (E) Diagram of the promoter region of OGT used for ChIP analysis for presence at H3K4me3. (F) ChIP analysis of the OGT promoter demonstrating the expression changes in OGT are associated with the presence or absence of the transcriptional activator mark, H3K4me3. Data are representative of the amount of DNA (normalized to the amount of input or nonimmunoprecipitated DNA) from the promoter region of OGT associated with the H3K4me3 chromatin mark. Bars are the maximum likelihood estimate for each group ± the 95% confidence interval for that estimate. Asterisk indicates a measurable difference between female control and all other groups as determined by nonoverlapping confidence intervals for these estimates.
To determine any effects of EPS on sexually dimorphic genes, messenger RNA (mRNA) was compared between male and female control or EPS placentas (Fig. 1C). One gene, O-linked-N-acetylglucosamine transferase (OGT), an X-linked gene with lower baseline expression in male placentas, also had a reduction of expression with the administration of EPS (Fig. 1D and Dataset S1). It should be noted that EPS had no effect on litter size or sex ratios in this study (Dataset S1), as was previously reported (10).
Characterization of Placental OGT Protein, Enzymatic Activity, and Chromatin State at the Ogt Locus.
To determine any chromatin regulation associated with the locus, we measured histone H3 trimethyl Lys4 (H3K4me3), a permissive chromatin mark, at the promoter region (Fig. 1E). Consistent with the basal sex differences and EPS pattern of expression found for OGT, we detected a decreased association of this locus with H3K4me3 in both male and in EPS placentas by chromatin immunoprecipitation (ChIP) (Fig. 1F and Dataset S1).
Changes in OGT protein levels corresponded with those found for mRNA, with less protein in males compared with females, and less protein in male EPS placentas compared with control males (Fig. 2 A and B and Dataset S1). We compared total levels of O-GlcNAc modified proteins as a biochemical readout of OGT enzymatic activity. We observed robust decreases of this mark in male placentas compared with females (Fig. 2C and Dataset S1) but no overall difference in O-GlcNAcylation between male control or EPS placentas (Dataset S1). Despite no overall difference, two bands at 28 and 37 kDa, which were visibly different between male control and EPS placentas, were excised for proteomic analysis (Fig. 2D). These proteins were identified as annexin A1 (ANXA1) and peroxiredoxin 1 (PRDX1) (25 and 21, respectively, unique peptide sequences identified with >95% probability for these proteins; Fig. 2 E and F and Table S2). Total protein levels of both ANXA1 and PRDX1 were not affected by EPS (Fig. S1 A and B and Dataset S1). There were no global sex differences in levels of total serine or threonine phosphorylation, demonstrating that all posttranslational modifications were not affected in the same manner as O-GlcNAcylation (Fig. S2 A and B and Dataset S1).
Fig. 2.
Biochemical assessment of OGT and O-GlcNAcylation in mouse and human placentas. (A) Representative Western blot images of OGT levels from male and female mouse placentas. Histogram is the maximum likelihood estimate for the normalized optical densities of each group (n = 7) ± the 95% confidence interval for that estimate. Asterisk indicates measurable difference between groups as determined by nonoverlapping confidence intervals for the estimates. (B) Representative Western blot images of OGT levels from control (n = 7) and EPS (n = 8) male mouse placentas. Histogram was derived and annotated as in A. (C) Representative Western blot images of total O-GlcNAcylated proteins from male and female mouse placentas. Histogram was derived and annotated as in A (n = 7). (D) Representative Western blot images of total O-GlcNAcylated proteins from male control (n = 7) and EPS (n = 8) placentas. The image is annotated to highlight the bands visibly identified with differential O-GlcNAcylation between treatment groups excised for proteomic analyses. (E) Amino acid sequence coverage of LC-MS/MS where the band at 28 kDA was identified as peroxiredoxin-1 (PRDX1) and the band at 37 kDA was identified as annexin A1 (ANXA1) via LC-MS/MS with greater than 99.9% certainty. (F) Representative spectrum image of peptide fragments used to identify both ANXA1 and PRDX1. (G) Expression of OGT by RT-PCR in human placental tissue associated with male births. Data were normalized to XY levels. Bars are the maximum likelihood estimate for each group (n = 4) ± the 95% confidence interval for that estimate. XX, maternal; XY, fetal. (H) Representative Western blot images of total O-GlcNAcylated proteins from both XX and XY placental contributions from human placentas. Histogram was derived as in A (n = 4). Asterisk indicates a measurable difference between groups with different X-chromosome complement as determined by nonoverlapping confidence intervals for each estimate.
Effect of X Chromosome Complement on Human Placental OGT mRNA and Protein.
To determine the translational potential of our findings, OGT levels were evaluated in human term placenta. Biopsies were obtained from male placentas, enriched for fetal (XY) or maternal (XX) contributions to assess X-chromosomal complement effects. OGT was highly expressed in human placenta from both maternal and fetal contributions. Similar to our findings in mice, gene expression in XY samples was measurably lower for OGT compared with XX samples (Fig. 2G and Dataset S1). Biochemical analysis of O-GlcNAc modified proteins followed this same pattern (Fig. 2H and Dataset S1).
Reduced Placental OGT Results in Broad Neurodevelopmental Changes.
To determine the potential programming effects that reduced placental OGT would impose on the developing brain, we used a placental-specific Cre-recombinase–expressing mouse to conditionally target the Ogt gene (18). Using this genetic model, female mice had reduced placental OGT expression but no difference in the liver (Dataset S1). We examined broad patterns of gene expression in the neonatal hypothalamus, because we were most interested in changes that would potentially affect neuroendocrine systems. In addition, we compared neonatal whole-brain microRNA patterns as a marker of the potential differences in the epigenetic environment in the developing brain. Because OGT is X-linked, wild-type and hemizygous females were compared because our goal was to examine a model of controlled reduced OGT, and not a knockout of this gene, which would be the result in males. We detected robust changes in hypothalamic gene regulation, with 370 genes showing significant differences (FDR < 0.05; Fig. 3A and Dataset S2). Further, functional annotation clustering of these affected genes revealed a significant enrichment for pathways involved in energy utilization, protein regulation, and synapse formation, processes important in neurodevelopment (Fig. 3A) (19, 20). Similar to the extensive gene expression changes we detected in the hypothalamus, we also found robust differences in the brain microRNA environment, where hemizygous placental OGT expression shifted the pattern of 250 of the most abundant brain microRNAs to be distinct from that of wild-type females by hierarchical clustering analyses (Fig. 3B).
Fig. 3.
Placental specific reduction of OGT broadly affects early neurodevelopment. (A) Heat map of PN2 hypothalamic genes with significantly different levels of expression between XOgt/ XWT females, with or without placental Cre expression [P.Cre+ (n = 4) or P.Cre– (n = 5), respectively], resulting in a 50% reduction in OGT expression in the P.Cre+ females (Fig. S3). Genes that were either significantly up-regulated (n = 218) or significantly down-regulated (n = 161) with reduced placental OGT, as determined by a FDR of 0.01, were grouped into functional annotation categories using the DAVID functional annotation clustering tool. (B) Heat map of whole-brain microRNA expression levels from the same animals in A. Hierarchical clustering discriminates all but one sample between the two groups [P.Cre+/− (n = 5) vs. P.Cre−/− (n = 4)], suggesting broad epigenetic changes in the PN2 brain.
Discussion
An increased risk for neurodevelopmental disorders such as schizophrenia has been associated with fetal antecedents including prenatal stress. Because such diseases often present with a large sex bias in rates of diagnosis, age of onset, and symptom severity, we used a genomics approach to identify potential placental candidate genes predictive of prenatal stress exposure from male and female placental tissue taken at E12.5, E15.5, and E18.5 (1–4). From this screen, OGT was identified as an important placental effector of programmatic changes in neurodevelopment, providing a potential mechanism for neurodevelopmental changes resulting from maternal stress in early pregnancy (EPS). The criteria used in this assessment included that the candidate gene must (i) show a significant sex difference in expression across pregnancy, (ii) be significantly altered in our EPS model, and (iii) be similarly regulated in human placental tissue. The hypothesis in these studies centered on a potential threshold effect whereby basal sex differences in placental gene expression may provide a sex bias for risk or resiliency in response to prenatal insults.
OGT is a key cellular regulator through the unique posttranslational modification it places on serine and threonine residues of both nuclear and cytosolic proteins. More specifically, OGT plays an important role in programmatic chromatin remodeling by O-glycosylation (O-GlcNAcylation) of protein targets, including RNA polymerase II, histone deacetylases, and histone 2B (21). Because O-GlcNAcylation competes with serine/threonine phosphorylation, it serves a critical function in the regulation of enzymatic activity important in somatic cell function and embryo viability (22–25). Additionally, OGT has been characterized as a cellular nutrient sensor (26) and therefore may play an important role in the protective effects of the placenta on the developing brain from insults such as maternal food deprivation (7). We hypothesized that levels of this important enzyme below a critical point following maternal stress could significantly affect specific aspects of placental function. Therefore, because male placentas showed a lower baseline expression of OGT, a stress-mediated further reduction may specifically place male fetuses at a disadvantage in being able to adapt to a changing environment, and at an increased risk for long-term effects on neurodevelopment. Additionally, OGT has been characterized as a cellular nutrient sensor (26).
Levels of OGT mRNA, protein, and O-GlcNAc were all related to X-chromosome complement, suggesting this gene likely escapes X inactivation in the placenta. These levels were all further reduced in our mouse model of EPS. Factors modulating OGT expression and translation have not been well characterized. However, biallelic expression in female embryonic stem cells at varying stages of differentiation has been reported (27, 28). As further evidence for regulation of OGT expression at a transcriptional level, ChIP was conducted at the Ogt locus with H3K4me3, a permissive chromatin mark indicative of transcriptional activation. A pattern of association with this activational mark was found similar to that detected for OGT mRNA and protein levels, where there was a reduction for males and EPS groups compared with control females. Interestingly, the histone demethylases, lysine-specific demethylase 5C (KDM5C) and lysine-specific demethylase 5D (KDM5D), which demethylate H3K4me3, were genes also identified as having a sex-dependent expression pattern in our placental array. Taken together, these data support additional modes of sex-dependent epigenetic regulation whereby a potential imbalance in chromatin regulation may affect placental function.
In addition to OGT identification in this genomic screen as an important candidate placental biomarker, additional genes of interest were also noted. Overall, there were dynamic changes in gene expression patterns and within unique gene sets over the three gestational time points examined. Despite the dynamic nature of this tissue, only a small subset of genes demonstrated sex differences in patterns of expression, all of which were sex chromosome-linked: Ogt; X-inactive specific transcript (Xist); Kdm5c; eukaryotic translation initiation factor 2, subunit 3, structural gene X-linked (Eif2s3x); preferentially expressed antigen in melanoma-like 3 (Pramel3); TATA box binding protein-associated factor 1 (Taf1); ribosomal protein SA (Rpsa); DEAD box polypeptide 3, Y-linked (Ddx3y); ubiquitously transcribed tetratricopeptide repeat containing, Y-linked (Uty); eukaryotic translation initiation factor 2, subunit 3, structural gene Y-linked (Eif2s3y); and Kdm5d. These genes have functions involved in protein translation, histone methylation, and protein glycosylation (Table S1). Although these genes showed significant sex differences, no further effect of EPS was detected. However, the sex differences in their patterns of expression suggest that they may play an important role in sex differences in placental function that could also contribute to a sex bias in neurodevelopmental disease. Further, the surprisingly limited number of genes exhibiting sex differences in expression in the placenta across pregnancy using our strict statistical requirements highlights the unique control of gene expression in the placenta and the potential impact perturbations during gestation may have on sex biases in programming outcomes in the developing fetus.
In the biochemical assessment of OGT activity, O-GlcNAc levels were significantly reduced in male placentas, fitting with their decreased OGT mRNA and protein. In response to EPS, we noted two distinct bands in male placental samples that were apparently reduced. These proteins were identified as ANXA1 and PRDX1 by proteomics analyses. Both proteins are known targets of OGT; however, the exact site of O-glycosylation has not been determined (29). ANXA1 is an important modulator of cellular inflammatory signaling, and PRDX1 is an intracellular peroxidase that functions as a chaperone with clients including nuclear factor-kappa β (30–34). These are two specific examples of mechanisms by which EPS may produce sex differences in placental function that could affect brain development trajectories.
To determine the translational potential of our findings, OGT levels and biochemistry were evaluated in human term placental tissue. Biopsies obtained from male placentas enriched for fetal (XY) or maternal (XX) contributions to assess X-chromosomal complement found that OGT was highly expressed and, similar to our findings in mice, XY samples were significantly lower for OGT compared with XX samples. Biochemical analysis of O-GlcNAcylated proteins followed this same pattern (Fig. 2H). Human placental tissue was obtained from deidentified cesarean-section deliveries, and thus the pregnancy experience and exposure to various stressors or other fetal antecedents could not be determined. As such, to avoid likely confounding of these uncontrollable variables, only X-chromosomal dosage effects on OGT expression were evaluated (i.e., from male births). Together, these data support OGT as meeting all three of our criteria as a predictive placental biomarker.
To examine a more direct link between reduced placental OGT and potential programming changes in the developing brain, we used a placental-specific Cre-recombinase–expressing mouse to conditionally target the Ogt gene. We examined broad patterns of gene expression in the neonatal hypothalamus, because we were interested in programming changes that would potentially affect neuroendocrine systems based on our EPS model, by Affymetrix microarray (12, 35). In addition, we compared neonatal whole-brain microRNA patterns by ABI Taqman array as a marker of the potential differences in the epigenetic environment in the developing brain, and based on our previous findings for dynamic microRNA changes in our EPS model (35). Because OGT is X-linked, wild-type and hemizygous females were compared to examine a model of controlled reduced OGT, and not a knockout of this gene, which would be the result in males. Importantly, gene expression changes as a result of reduced placental OGT in this context are representative of the impact this biomarker has on neurodevelopment irrespective of chromosomal sex and were not directly compared with the effects of EPS on early brain development. We detected profound changes in hypothalamic gene regulation, with over 375 genes showing significant differences in expression patterns in the hypothalamus from placental hemizygous Ogt mice compared with controls. Further, functional annotation clustering of these affected genes revealed a significant enrichment for pathways involved in energy utilization, protein regulation, and synapse formation, all processes important in neurodevelopment (19, 20). Similar to the extensive gene expression changes we detected in the hypothalamus, we also found robust differences in the brain microRNA environment, where hemizygous placental OGT expression shifted the pattern of 250 of the most abundant brain microRNAs to be distinct from that of control animals by hierarchical clustering analyses. These results support our previous studies showing similar broad changes produced by EPS in the postnatal brain microRNA environment indicative of epigenetic programming involvement in shaping plasticity during neonatal brain development (35). These data substantiate the importance of regulation of placental OGT levels and provide a potential link with profound changes in gene transcription in neurodevelopment with a candidate biomarker.
The endocrine placenta is poised to be a key mediating tissue in responding to a dynamic and changing maternal environment. Sex differences in how this tissue responds to the same maternal milieu likely contribute to the altered susceptibility of male and female fetuses to long-term programming outcomes (4). Our studies identified OGT, an intracellular glycotransferase important in regulating key chromatin programming events, as a potential placental biomarker that was sex-biased in its expression, responsive to EPS, and similar in X-linked expression pattern in human tissue. Because the placenta is readily accessible, these studies have high translational potential and may be useful in predicting gestational stress exposure.
Methods
Animals.
Male C57BL/6J and female 129S1/SvImJ mice were obtained from Jackson Laboratories and subsequently used as breeding stock to produce C57BL/6J:129S1/SvImJ hybrids (F1 hybrids). F1 hybrid breeding pairs (n = 33) were checked daily at 7:00 AM for copulation plugs. Noon on the day that the plug was observed was considered to be embryonic day 0.5 (E0.5). F1 hybrids were used for the placental microarray and the prenatal stress experiments. For the placental-specific reduction of OGT, double heterozygous [B6.129-Ogttm1Gwh/J (XOgt/ XWT); B6-CYP19-Cre (placental-Cre recombinase heterozygote, P.Cre+/−)] females (n = 17) were bred to hemizygous B6.129-Ogttm1Gwh/J (XOgt/ Y)/ heterozygous (P.Cre+/−) males (n = 14), resulting in offspring representing all potential genotypes. To identify the effects of a placental-specific reduction of OGT, female XOgt/ XWT P.Cre+/− (P.Cre+/−, n = 10) were compared with XOgt/ XWT P.Cre−/− (P.Cre+/−, n = 10) for placental specificity validation and brain analyses. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Early Prenatal Stress.
Administration of chronic variable stress was performed as previously described (12). Dams were randomly assigned to treatment groups to receive stress during days 1–7 of gestation (EPS; n = 8) or to a control (n = 7) nonstressed group. Pregnant mice assigned to the EPS group experienced each of the following stressors on a different day of the EPS period: 60 min (beginning at 1:00 PM) of fox odor exposure (1:5,000 2,4,5-trimethylthiazole; Acros Organics), 15 min of restraint (beginning at 1:00 PM) in a modified 50-mL conical tube, 36 h of constant light, novel noise (White Noise/Nature Sound-Sleep Machine; Brookstone) overnight, three cage changes (at ∼9:00 AM, 12:00 PM, and 3:00 PM) throughout the light cycle, saturated bedding (700 mL, 23 °C water) overnight, and novel object (eight marbles of similar shape and color) exposure overnight. These stressors were selected to be nonhabituating and did not induce pain or directly influence maternal food intake, weight gain, or behavior (10).
Mouse Tissue Dissection.
For the placental microarray experiment mouse placentas and embryonic somatic tissue were dissected at E12.5, E15.5, and E18.5 (n =6 at each gestational time point). DNA was extracted from the embryonic somatic tissue, and the sex of the embryo was determined using methods described elsewhere (36). One male and one female placenta from each litter were used for microarray analysis; these placentas were quartered and put in 500 μL of RNAlater and stored at −80C until processed as below. For the prenatal stress experiment, E12.5 male and female placentas from control (n = 7) and EPS (n = 8) litters were used for gene expression, ChIP, and protein analyses; these placentas were bisected via transverse sectioning and one half was placed in 500 μL of RNAlater and stored at −80C (for gene expression analyses) and one half was flash-frozen in liquid nitrogen and stored at −80C (for ChIP and Western blot analyses).
For the placental-specific reduction of OGT experiment, two cohorts of animals were used: (i) an embryonic cohort (n = 4 P.Cre+/− and P.Cre−/−) to validate the specificity of OGT reduction to the placenta and (ii) a cohort (n = 4 P.Cre+/−; n = 5 P.Cre−/−) killed on postnatal day 2 (PN2) for brain analyses. For the validation, placentas and embryonic somatic tissue were dissected at E12.5. DNA was extracted as above; placentas and liver tissue were flash-frozen in liquid nitrogen and stored at −80C. For the brain analyses, pups were killed at PN2 and brains were dissected and flash-frozen in liquid nitrogen and stored at −80C. Tissue punches (300 μm) of the hypothalamus [∼3.0–3.3 mm posterior in the anterior–posterior plane and 1.5 mm ventral in the dorsal–ventral plane, structures corresponding to the paraventricular area in a developmental mouse brain atlas (37)] were collected using a 1.0-mm circular punch (Ted Pella) and then used for microarray analysis; the remaining brain tissue was used for microRNA analysis.
Human Tissue Samples.
Placental tissues associated with deidentified male fetuses (n = 4) were quartered by cutting in the transverse section through the location of the emergence of the umbilical cord in two directions. Biopsies (∼5 mg) were obtained from both the fetal and maternal contributions to the placenta from three locations, 5 cm from the umbilical cord. Biopsies were flash-frozen in liquid nitrogen and stored at −80 C until further analysis. Human tissue collection protocols were reviewed and approved by the Institutional Board of the University of Pennsylvania.
Brain microRNA Environment.
Total RNA was extracted from brains [P.Cre+/− (n = 4) and P.Cre−/− (n = 5)] using TRIzol reagent (Invitrogen). One microgram of total RNA was reverse-transcribed to cDNA using Megaplex RT pool A primers and Multiscribe reverse transcriptase (Applied Biosystems). Expression levels of 245 microRNAs were determined using the Taqman Array MicroRNA card A Array (Applied Biosystems). Analysis was performed using the comparative cycle threshold (Ct) method. For each sample, the average of the Ct values of small nucleolar RNA (sno)135 and sno202 was used as an endogenous loading control. Expression levels of each sample were normalized to the average expression level of P.Cre−/− females. Uninformed hierarchical clustering using Pearson correlations was used to discriminate differential microRNA expression between individuals (42).
Statistical Analyses.
Analyses were performed using R (version 2.14.2) and the packages arm, (43), bbmle (44), and limma (39, 45) to fit the gene expression and optical density data to linear models, and estimates for main and interaction effects were determined from these models. For all analyses, maximum likelihood estimates were calculated and 95% confidence intervals were constructed for regression coefficients as the reported statistical values. We used this statistical approach over the more traditional null hypothesis significance testing to provide the magnitude for any observed experimental effect, to provide a measure of dispersion around this effect size to allow assessment of the predictive value of our models, and to relate any statistical conclusions to the experimental hypothesis (46). A confidence interval for an estimate that did not bound zero was considered to be significant; comparisons among experimental groups were made using confidence interval evaluation, and those that did not overlap were considered to be significantly different. Database for Annotation, Visualization and Integrated Discovery (DAVID) functional annotation clustering (47) was used for the PN2 hypothalamic analysis, and MeV v4.8 was used for the PN2 microRNA analysis.
Supplementary Material
Acknowledgments
We thank C. Taylor for technical assistance and animal care and G. Leone at The Ohio State University for the generous donation of Cyp19-Cre mice. This work is supported by National Institutes of Health Grants MH099910, MH091258, and MH087597.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300065110/-/DCSupplemental.
References
- 1.(2012) Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2008. MMWR CDC Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 2.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9(12):911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale TL, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howerton CL, Bale TL. Prenatal programing: At the intersection of maternal stress and immune activation. Horm Behav. 2012;62(3):237–242. doi: 10.1016/j.yhbeh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khashan AS, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65(2):146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 6.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14(4):348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 7.Broad KD, Keverne EB. Placental protection of the fetal brain during short-term food deprivation. Proc Natl Acad Sci USA. 2011;108(37):15237–15241. doi: 10.1073/pnas.1106022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossant J, Cross JC. Placental development: Lessons from mouse mutants. Nat Rev Genet. 2001;2(7):538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld CS. Effects of maternal diet and exposure to bisphenol A on sexually dimorphic responses in conceptuses and offspring. Reprod Domest Anim. 2012;47(Suppl 4):23–30. doi: 10.1111/j.1439-0531.2012.02051.x. [DOI] [PubMed] [Google Scholar]
- 10.Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav. 2006;88(4-5):605–614. doi: 10.1016/j.physbeh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91(1):55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE. 2012;7(3):e33794. doi: 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell KJ, et al. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. 2012;37(6):818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Pena CJ, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11 beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyrwoll CS, Kerrigan D, Holmes MC, Seckl JR, Drake AJ. Altered placental methyl donor transport in the dexamethasone programmed rat. Placenta. 2012;33(3):220–223. doi: 10.1016/j.placenta.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Keverne EB. Epigenetically regulated imprinted genes and foetal programming. Neurotox Res. 2010;18(3-4):386–392. doi: 10.1007/s12640-010-9169-z. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel PL, Leone G. Expression of Cre recombinase in early diploid trophoblast cells of the mouse placenta. Genesis. 2007;45(3):129–134. doi: 10.1002/dvg.20276. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HWM, Rutten BPF. An environmental analysis of genes associated with schizophrenia: Hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry. 2012;17(12):1194–1205. doi: 10.1038/mp.2011.183. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, et al. Schizophrenia gene networks and pathways and their applications for novel candidate gene selection. PLoS ONE. 2010;5(6):e11351. doi: 10.1371/journal.pone.0011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujiki R, et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480(7378):557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafi R, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97(11):5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24(4):1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachara NE, et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279(29):30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 25.Comer FI, Hart GW. O-Glycosylation of nuclear and cytosolic proteins. Dynamic interplay between O-GlcNAc and O-phosphate. J Biol Chem. 2000;275(38):29179–29182. doi: 10.1074/jbc.R000010200. [DOI] [PubMed] [Google Scholar]
- 26.Lazarus MB, Nam YS, Jiang JY, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469(7331):564–567. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin H, et al. Dosage compensation in the mouse balances up-regulation and silencing of X-linked genes. PLoS Biol. 2007;5(12):e326. doi: 10.1371/journal.pbio.0050326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao J, et al. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci USA. 2010;107(12):5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Torii M, Liu H, Hart GW, Hu Z-Z. dbOGAP – An integrated bioinformatics resource for protein O-GlcNAcylation. BMC Bioinformatics. 2011;12(1):91. doi: 10.1186/1471-2105-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XM, He SH, Sun JM, Delcuve GP, Davie JR. Selective association of peroxiredoxin 1 with genomic DNA and COX-2 upstream promoter elements in estrogen receptor negative breast cancer cells. Mol Biol Cell. 2010;21(17):2987–2995. doi: 10.1091/mbc.E10-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann CA, Cao JX, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8(24):4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chhipa RR, Lee KS, Onate S, Wu Y, Ip C. Prx1 enhances androgen receptor function in prostate cancer cells by increasing receptor affinity to dihydrotestosterone. Mol Cancer Res. 2009;7(9):1543–1552. doi: 10.1158/1541-7786.MCR-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flower RJ, Blackwell GJ. The importance of phospholipase-A2 in prostaglandin biosynthesis. Biochem Pharmacol. 1976;25(3):285–291. doi: 10.1016/0006-2952(76)90216-1. [DOI] [PubMed] [Google Scholar]
- 34.Parente L, Solito E. Annexin 1: more than an anti-phospholipase protein. Inflamm Res. 2004;53(4):125–132. doi: 10.1007/s00011-003-1235-z. [DOI] [PubMed] [Google Scholar]
- 35.Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J Neurosci. 2011;31(33):11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clapcote SJ, Roder JC. Simplex PCR assay for sex determination in mice. Biotechniques. 2005;38(5):702. doi: 10.2144/05385BM05. 706, 704, 706. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Halliday H, Watson C, Koutcherov Y, Wang H. Atlas of the Developing Mouse Brain at E17.5, P0, and P6. London: Academic Press; 2007. [Google Scholar]
- 38.Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 39.Smyth GK. Limma: Linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry RA, Huber W, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
- 40.Irizarry RA, et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. JR Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 42.Berry MPR, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelman A, Hill J. 2006. Data Analysis Using Regression and Multilevel/Hierarchical Models (Cambridge Univ Press, Cambridge, UK)
- 44.Bolker B. 2010. bbmle: Tools for general maximum likelihood estimation. (The Comprehensive R Archive Network, Vienna)
- 45.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(1):e3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 46.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- 47.Sherman BT, et al. DAVID Knowledgebase: A gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinformatics. 2007;8:426. doi: 10.1186/1471-2105-8-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.