Abstract
Recognition of viral double-stranded RNA by Toll-like receptor 3 (TLR3) triggers activation of the transcription factors NF-κB and interferon regulated factor 3, leading to induction of type I interferons and proinflammatory cytokines. TIR-domain–containing adapter-inducing interferon-β (TRIF) is an adapter protein required for TLR3-mediated signaling. Here we identified the E3 ubiquitin ligase WW domain-containing protein 2 (WWP2) as a TRIF-associated protein by biochemical purification. WWP2 mediated K48-linked ubiquitination and degradation of TRIF upon TLR3 activation. Overexpression of WWP2 inhibited TLR3-mediated NF-κB and interferon regulated factor 3 activation, whereas knockdown of WWP2 had opposite effects. We generated Wwp2-deficient mice to further investigate the roles of Wwp2 in innate immune responses. Consistently, production of IFN-β, CCL5, TNFα, and IL-6 in response to the TLR3 ligand poly(I:C) was elevated in Wwp2−/− macrophages and Wwp2-deficient mice exhibited increased susceptibility to poly(I:C)-induced death than the control littermates. Our findings suggest that WWP2 negatively regulates TLR3-mediated innate immune and inflammatory responses by targeting TRIF for ubiquitination and degradation.
Toll-like receptors (TLRs) are evolutionarily conserved pattern recognition receptors (PRRs) that are critically involved in host defense from plants to humans. So far, 13 TLRs (named TLR1 to TLR13) have been identified in humans and mice, each of which recognizes a distinct set of pathogen-associated molecular patterns (PAMPs) (1). TLRs contain an extracellular domain consisting of leucine rich repeats which is responsible for PAMP recognition, a transmembrane domain, and a conserved cytoplasmic toll/IL-1 receptor (TIR) domain which is able to mediate homotypic protein–protein interactions (2). The TIR domains of TLRs are responsible for their homo- or hetero-dimerization, and upon ligand stimulation, they also act as platforms to recruit downstream TIR domain-containing adaptor proteins and signaling molecules, leading to the activation of transcription factors such as NF-κB and interferon regulated factor 3 (IRF3) (1, 3, 4). These transcription factors act alone or in collaboration to induce transcription of proinflammatory cytokines and/or type I interferons (IFNs).
Among the TLRs, TLR3 has been reported to recognize viral dsRNA as well as its analog poly(I:C). Recognition of these ligands by TLR3 activates signaling pathways leading to the activation of NF-κB and IRF3 and subsequent production of type I IFNs and proinflammatory cytokines (5). TLR3-mediated signaling critically depends on the TIR domain-containing adapter TIR-domain–containing adapter-inducing interferon-β (TRIF; also called TICAM-1) (6). It has been shown that Trif−/− lung fibroblasts are defective in poly(I:C)-induced activation of NF-κB and IRF3 as well as production of type I IFNs, demonstrating that TRIF is indispensable for TLR3-mediated signaling (7). In addition, the TLR4 ligand LPS has been shown to signal through TRIF-dependent and independent [myeloid differentiation factor 88 (MyD88)-dependent] pathways (7, 8). In Trif−/− macrophages, although LPS-induced expression of proinflammatory cytokines such as Tnf, Cxcl1, and Cxcl2 is normal, LPS-induced expression of Ccl5/Rantes and Ifnb is abolished, indicating that LPS-induced activation of IRF3 and subsequent expression of type I IFNs and IFN-inducible genes are dependent on TRIF (8, 9).
TRIF contains an N-terminal proline-rich region, a middle TIR domain and a C-terminal receptor-interacting protein (RIP) homotypic interaction motif (RHIM). The N-terminal domain of TRIF is responsible for its association with TBK1, which is a downstream kinase required for TRIF-mediated IRF3 activation (10–12). The N-terminal domain of TRIF also contains a consensus TRAF-binding motif (250-PEEMSW-255) that is required for the recruitment of TRAF6 for NF-κB activation (13). The C-terminal RHIM motif of TRIF can recruit RIP1 via RHIM homotypic interaction, which may account for the NF-κB activation in TRAF6-deficient macrophages (14, 15). Moreover, TRIF also mediates apoptosis through the RIP–FADD–caspase-8 pathway (12). These studies suggest that TRIF plays divergent roles in TLR3-mediated signaling. However, the mechanisms by which TRIF are regulated remain unclear.
In this study, we identified a HECT-domain containing E3 ubiquitin ligase WW domain-containing protein 2 [WWP2, also called atrophin-1 interacting protein 2 (AIP2)] as a TRIF-associated protein by biochemical purification experiments. WWP2 was associated with TRIF upon TLR3 activation and targeted TRIF for K48-linked ubiquitination and degradation. Knockdown of WWP2 resulted in compromised TRIF ubiquitination, elevated TRIF protein level and enhanced expression of IFNΒ after TLR3 activation. Furthermore, gene knockout of Wwp2 in mice resulted in increased expression of proinflammatory cytokines and type I IFNs in macrophages and susceptibility to poly(I:C)-induced death in vivo. These results reveal a previously uncharacterized mechanism by which TLR3-mediated innate immune and inflammatory responses are regulated at the TRIF adapter level.
Results
Identification of WWP2 as a TRIF-Associated Protein.
Previous studies have shown that TRIF is a critical adapter protein in TLR3-mediated NF-κB and IRF3 activation pathways (16). To unambiguously identify potential new proteins associated with TRIF, we performed tandem affinity purification (TAP) assays with full-length TRIF as bait. An expression plasmid for TRIF tagged with streptavidin binding peptide (SBP) and calmodulin binding peptide (CBP) was transfected into 293 cells, and TRIF-associated proteins were purified by the mammalian TAP system. The eluted proteins were identified by a shotgun mass spectrometry analysis. By comparing with other nonrelated purifications with the same method, we identified WWP2 as a candidate protein associated with TRIF. WWP2 is an E3 ubiquitin ligase containing a C-terminal HECT domain, four WW repeats, and an N-terminal C2 domain that may be important for its subcellular localization (17). It has been reported that WWP2 is involved in inhibition of activation-induced T-cell death by ubiquitinating EGR2 (18) as well as in early mammalian development by targeting the octamer-binding transcription factor 4 (Oct-4) (19). In addition, recent studies have suggested that WWP2 can facilitate the budding process of retrovirus (20). Whether and how WWP2 is involved in TRIF-mediated signaling is unknown.
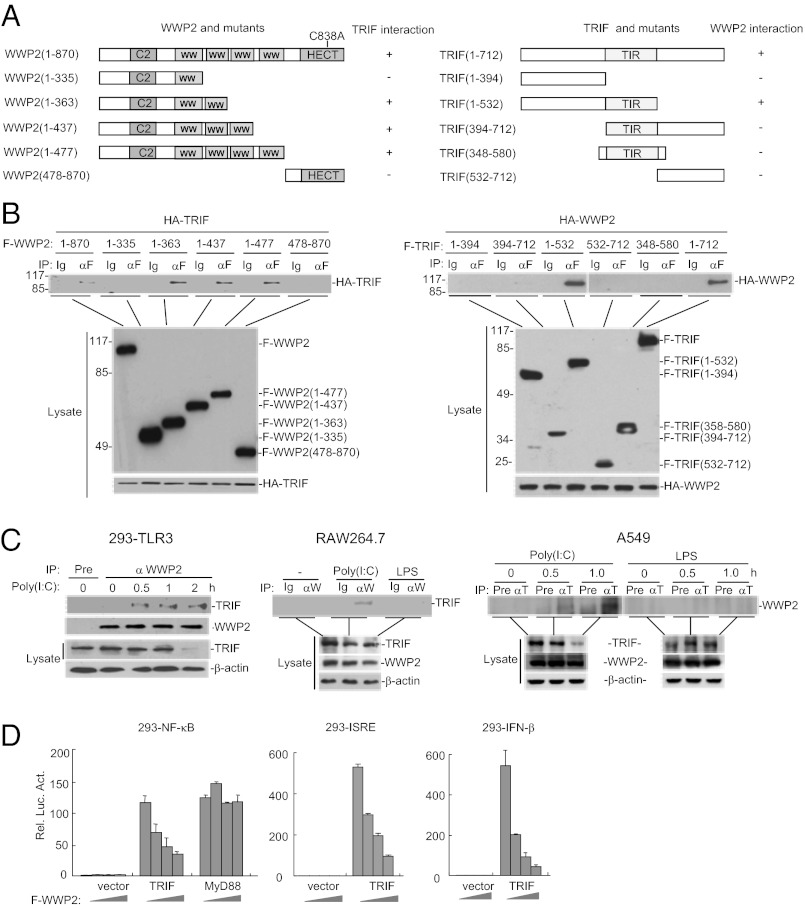
To confirm the association between WWP2 and TRIF, we performed transient transfection and coimmunoprecipitation experiments in 293 cells. The results indicated that WWP2 interacted with TRIF in mammalian overexpression system (Fig. 1 A and B). Domain mapping experiments indicated that the second WW domain of WWP2 and the N-terminal and TIR domains of TRIF are important for their interaction (Fig. 1 A and B).
Fig. 1.
WWP2 interacts with TRIF. (A) A schematic presentation of full-length WWP2, TRIF and their mutants. (B) Domain mapping of TRIF and WWP2 interaction. The 293 cells (2 × 106) were transfected with the indicated expression plasmids (5 μg each). Coimmunoprecipitation were performed with anti-Flag (αF) or control mouse lgG (Ig). (Upper) The immunoprecipitates were analyzed by immunoblot with anti-HA. (Lower) Expression of the transfected proteins were analyzed by immunoblots with anti-Flag or anti-HA. (C) Endogenous association of WWP2 with TRIF. The 293-TLR3 (5 × 107), RAW264.7 (1 × 108), or A549 (1 × 108) cells were left untreated or treated with poly(I:C) (50 μg/mL) or LPS (50 ng/mL) for the indicated times. Cell lysates were immunoprecipitated, and immunoprecipitates were analyzed by immunoblots with the indicated antibodies. For experiments with 293-TLR3 cells, coimmunoprecipitation was performed with mouse preimmune serum (Pre) or mouse anti-WWP2 serum. The blots were detected by rabbit anti-TRIF or rabbit anti-WWP2. For experiments with RAW264.7 cells, coimmunoprecipitation was performed with rabbit IgG control (Ig) or rabbit anti-WWP2 (αW). The blots were detected by mouse anti-TRIF. For experiments with A549 cells, coimmunoprecipitation was performed with mouse preimmune serum (Pre) or mouse anti-TRIF (αT) serum. The blots were detected by rabbit anti-WWP2. (Lower) The expression levels of the proteins were examined by immunoblots with the indicated antibodies. (D) WWP2 inhibits TRIF- but not MyD88-mediated signaling. The 293 cells (1 × 105) were transfected with TRIF or MyD88 expression plasmid (0.2 μg each), increased amounts of WWP2 expression plasmid and the indicated reporter plasmid (0.1 μg). Reporter assays were performed 20 h after transfection.
We next determined whether endogenous WWP2 could associate with TRIF. Endogenous coimmunoprecipitation experiments indicated that WWP2 was not associated with TRIF under physiological conditions. However, their association was readily detected following poly(I:C) stimulation of 293 cells stably expressing TLR3 (293-TLR3) (Fig. 1C). Poly(I:C) stimulation also induced endogenous association between WWP2 and TRIF in macrophage cells RAW264.7 and epithelial cells A549 (Fig. 1C), which have previously been shown to express TLR3 (21). In similar experiments, LPS stimulation did not induce the interaction between WWP2 and TRIF in RAW264.7 and A549 cells (Fig. 1C). These results suggest that poly(I:C) binding to TLR3 induces the association between WWP2 and TRIF.
Because WWP2 is associated with TRIF after TLR3 activation, we determined the effects of WWP2 on TRIF-mediated signaling. In reporter assays, WWP2 inhibited TRIF-mediated activation of NF-κB, interferon stimulated response element (ISRE), and the IFN-β promoter in a dose-dependent manner. In the same experiments, WWP2 did not inhibit MyD88-mediated NF-κB activation (Fig. 1D). These results suggest that WWP2 specifically inhibits TRIF-mediated signaling.
WWP2 Targets TRIF for Ubiquitination and Degradation.
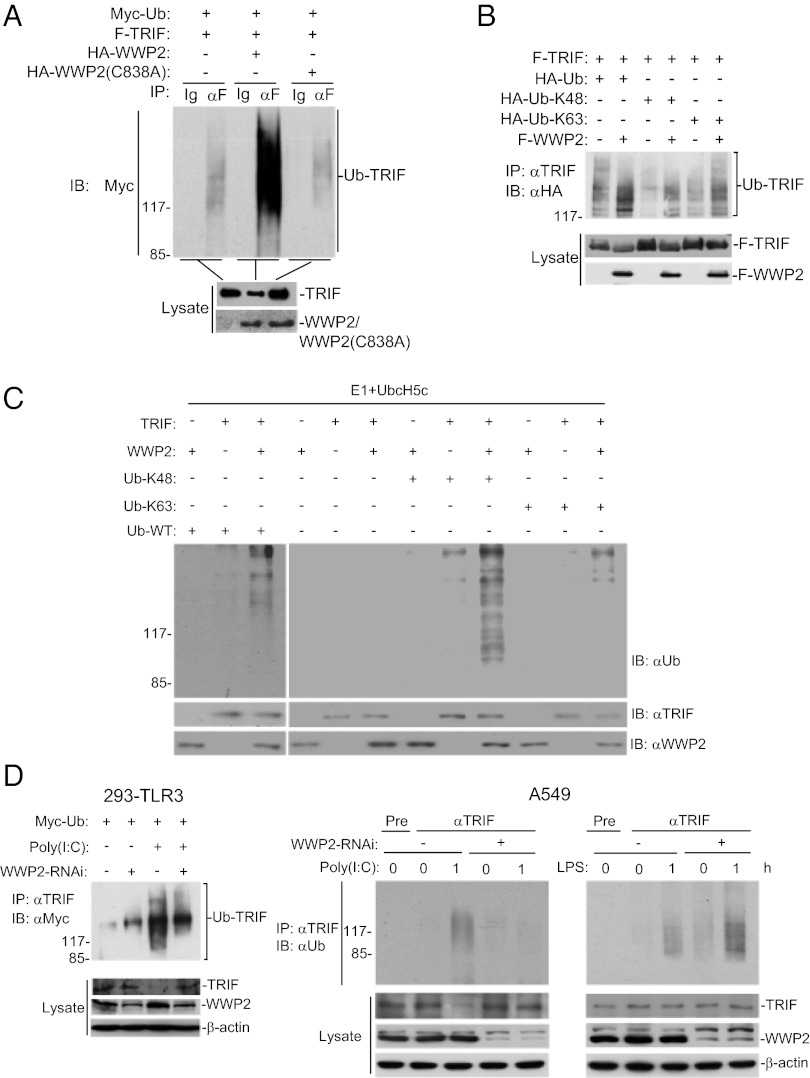
Because WWP2 is an E3 ubiquitin ligase, and our earlier results indicated that TRIF was down-regulated following TLR3 activation and TRIF-mediated signaling was inhibited by WWP2, we reasoned that WWP2 may mediate TRIF ubiquitination and degradation. In mammalian overexpression system, WWP2 but not its enzymatic inactive mutant WWP2(C838A) markedly increased the ubiquitination and degradation of TRIF (Fig. 2A). Using K48-only or K63-only ubiquitin mutant, we found that WWP2 enhanced K48-linked ubiquitination of TRIF, whereas it had minimal effect on K63-linked ubiquitination of TRIF (Fig. 2B). In vitro ubiquitination assays further confirmed that WWP2 could directly ubiquitinate TRIF (Fig. 2C). In these in vitro experiments, WWP2 dramatically increased K48-linked ubiquitination of TRIF but had little effect on K63-linked ubiquitination of TRIF (Fig. 2C). These results suggest that WWP2 preferably mediates K48-linked ubiquitination of TRIF.
Fig. 2.
WWP2 targets TRIF for K48-linked ubiquitination and degradation. (A) Overexpression of wild-type but not mutant WWP2 promotes ubiquitination of TRIF. The 293 cells (1 × 107) were transfected with the indicated plasmids. Twenty hours after transfection, cell lysates were immunoprecipitated with anti-Flag (αF) or control mouse lgG (Ig). The immunoprecipitates were analyzed by immunoblot with anti-Myc (Upper). The expression levels of the proteins were examined by immunoblots with the indicated antibodies (Lower). (B) WWP2 markedly enhances K48-linked ubiquitination of TRIF. The 293 cells (2 × 106) were transfected with the indicated plasmids. Twenty hours after transfection, cell lysates were immunoprecipitated with anti-TRIF. The immunoprecipitates were re-extracted in lysis buffer containing 1% SDS and denatured by heating for 5 min at 95 °C. Supernatants were diluted with lysis buffer until the concentration of SDS was decresed to 0.1%, followed by reimmunoprecipitation with anti-TRIF and then analyzed by immunoblot with anti-HA (Upper). The expression levels of the proteins were examined by immunoblots with the indicated antibodies (Lower). (C) WWP2 mediates K48-linked ubiquitination of TRIF in vitro. TRIF, WWP2, ubiquitin, and ubiquitin mutants were translated in vitro, and E1 and UbcH5c were added for ubiquitination assays. (Upper) Ubiquitin-conjugated TRIF was detected by immunoblot with anti-ubiquitin antibody. (Lower) The levels of input proteins were examined by immunoblots with the indicated antibodies. (D) Effects of WWP2-RNAi on poly(I:C)-induced ubiquitination and degradation of endogenous TRIF. The 293-TLR3 (5 × 107) or A549 (5 × 107) cells were transfected with a control or WWP2-RNAi plasmid. The cells were left untreated or treated with poly(I:C) (50 μg/mL) or LPS (100 ng/mL) for 1 h. The cell lysates were immunoprecipitated with mouse preimmune serum control or mouse anti-TRIF serum, and the immunoprecipitates were analyzed by immunoblot with anti-Myc (Upper Left) or anti-ubiquitin (Upper Right). (Lower) The expression levels of the related proteins were examined by immunoblots with the indicated antibodies.
We next determined whether WWP2 would regulate ubiquitination and degradation of TRIF in untransfected cells. As shown in Fig. 2D, in both 293-TLR3 and A549 cells, knockdown of WWP2 had minimal effects on basal ubiquitination and protein level of TRIF. However, knockdown of WWP2 inhibited poly(I:C)-induced ubiquitination and degradation of TRIF (Fig. 2D). In contrast, knockdown of WWP2 did not inhibit LPS-induced ubiquitination of TRIF in A549 cells (Fig. 2D). These results suggest that WWP2 mediates ubiquitination and degradation of TRIF after TLR3 activation in untransfected cells.
Overexpression of WWP2 Inhibits TLR3-Mediated Signaling.
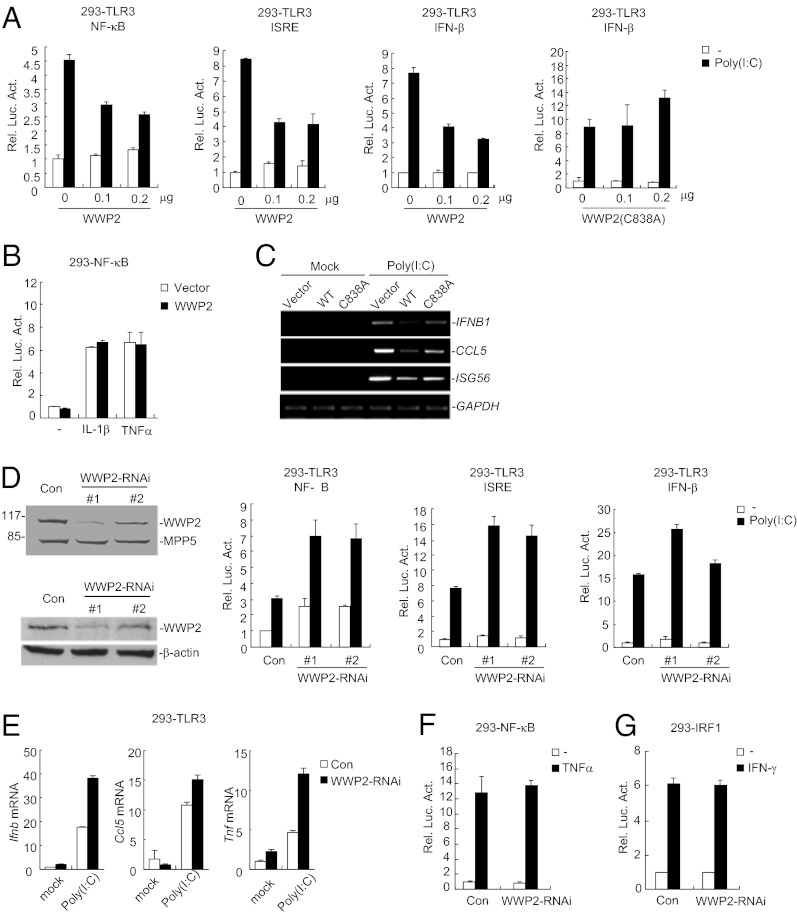
Because WWP2 interacted with TRIF following poly(I:C) stimulation in 293-TLR3 cells, we determined whether WWP2 was involved in TLR3-mediated signaling. As shown in Fig. 3A, overexpression of WWP2 but not its enzymatic inactive mutant WWP2(C838A) inhibited poly(I:C)-induced activation of NF-κB, ISRE, and the IFN-β promoter in 293-TLR3 cells in reporter assays. In similar experiments, WWP2 did not inhibit IL-1β- or TNFα-induced NF-κB activation (Fig. 3B). Results from RT-PCR analysis indicated that overexpression of WWP2 but not WWP2(C838A) inhibited poly(I:C)-induced transcription of endogenous IFNB1, CCL5, and ISG15 genes in 293-TLR3 cells (Fig. 3C). These data suggest that WWP2 inhibits TLR3-mediated signaling and such an inhibitory function requires its E3 ubiquitin ligase activity.
Fig. 3.
WWP2 regulates TLR3-mediated signaling pathways. (A) WWP2 inhibits poly(I:C)-induced signaling in 293-TLR3 cells. The cells (1 × 105) were transfected with the indicated luciferase reporter plasmid (0.1 μg) and increased amounts of empty control vector (empty bars) or WWP2 expression plasmid (filled bars). Twenty hours after transfection, the cells were treated with poly(I:C) (50 μg/mL) or left untreated for 8 h before luciferase assays were performed. (B) WWP2 has no marked effects on TNFα- and IL-1β- induced NF-κB activation. The 293 cells (1 × 105) were transfected with the indicated luciferase reporter plasmids (0.1 μg) and empty control vector (empty bars) or WWP2 expression plasmid (filled bars). Twenty hours after transfection, cells were treated with TNFα (20 ng/mL) and IL-1β (20 ng/mL) or left untreated for 8 h before luciferase assays were performed. (C) WWP2 inhibits poly(I:C)-induced transcription of endogenous IFNB1, CCL5, and ISG15 genes. The 293-TLR3 cells (2 × 105) were transfected with the indicated expression plasmids (1 μg each) for 20 h. The cells were treated with poly(I:C) or left untreated for the indicated times before RT-PCR for the indicated genes were performed. (D) Effects of WWP2-RNAi plasmids on expression of WWP2 and on poly(I:C)-induced signaling. For the blots, the 293 cells (2 × 105) were transfected with control or WWP2-RNAi plasmid (1 μg each) for 24 h. Cell lysates were analyzed by immunoblots with the indicated antibodies. For the reporter assays, 293-TLR3 cells (1 × 105) were transfected with the indicated luciferase reporter (0.1 μg) and RNAi (0.5 μg each) plasmids. Twenty-four hours after transfection, cells were left untreated or treated with poly(I:C) (50 μg/mL) for 8 h before luciferase assays were performed. (E) Effects of WWP2-RNAi on poly(I:C)-induced transcription of downstream genes. The 293-TLR3 cells (2 × 105) were transfected with a control or WWP2-RNAi plasmid (1 μg each). Twenty hours after transfection, cells were treated with poly(I:C) or left untreated for 1 h before qRT-PCR was performed. (F) Effects of WWP2-RNAi on TNFα-induced NF-κB activation. The experiments were similarly performed as in A except that TNFα (20 ng/mL) was used for stimulation. (G) Effects of WWP2-RNAi on IFN-γ-induced activation of the IRF1 promoter. The 293 cells (1 × 105) were transfected with IRF1 promoter reporter (0.1 μg) and WWP2-RNAi (0.5 μg each) plasmids. Twenty-four hours after transfection, cells were left untreated or treated with IFN-γ (100 ng/mL; filled bars) or left untreated (open bars) for 6 h before reporter assays were performed.
Knockdown of WWP2 Potentiates TLR3-Mediated Signaling.
Because overexpression of WWP2 inhibits TLR3-mediated signaling, we next determined the roles of endogenous WWP2 in TLR3-mediated signaling by RNAi-mediated knockdown experiments. As shown in Fig. 3D, knockdown of WWP2 markedly potentiated poly(I:C)-induced activation of NF-κB, ISRE, and the IFN-β promoter in reporter assays. Consistently, knockdown of WWP2 also potentiated poly(I:C)-induced expression of IFNB1, CCL5, and TNFα genes in 293-TLR3 cells (Fig. 3E). In similar experiments, however, knockdown of WWP2 had no marked effect on TNF-induced NF-κB activation or IFN-γ–induced IRF1 activation (Fig. 3 F and G). These results demonstrate that WWP2 is a negative regulator of TLR3-mediated signaling pathways.
Wwp2 Negatively Regulates TLR3-Mediated Induction of Cytokines in Macrophages and Mice.
Because human WWP2 and its mouse ortholog share 96% sequence identity at the amino acid level, we hypothesize that they share similar functions. To further elucidate the physiological role of WWP2 in TLR-mediated innate immune responses, we generated Wwp2-deficient mice by standard conditional gene knockout technology (Fig. S1A). The heterozygosity and nullizygosity were verified by PCR and immunoblot analysis (Fig. S1 B and C). The Wwp2−/− mice were born at the normal Mendelian ratio and grew healthily. The total cell numbers of thymocytes, splenocytes, and cells in peripheral lymph nodes were comparable between wild-type and Wwp2−/− mice. The compositions of CD4+, CD8+, or CD4+CD8+ double positive thymocytes, and T cells and B cells in spleen were comparable between wild-type and Wwp2−/− mice (Fig. S1 D–G). In addition, Wwp2-deficiency did not affect the composition of naïve (CD62L+) and memory (CD44+) T cells in spleen and peripheral lymph nodes (Fig. S1G), indicating that WWP2 was dispensable for animal or immune cell development.
To determine whether mouse Wwp2 is involved in innate immune response, we collected bone marrow-derived macrophages (BMDMs) from wild-type and Wwp2-deficient mice and stimulated these cells with poly(I:C), LPS (ligand for TLR4), R848 (ligand for TLR7 and TLR8), PGN (ligand for TLR2), and Sendai virus (SeV) (stimulating RIG-I-like receptors). The results showed that poly(I:C)- and LPS-induced transcription of Ifnb1 and Ccl5 was significantly increased in Wwp2−/− BMDMs in comparison with their wild-type or heterozygous counterparts (Fig. 4A). Interestingly, although both poly(I:C)- and LPS-induced transcription of Tnf and Il6 genes was enhanced in Wwp2−/− BMDMs compared with their wild-type or heterozygous counterparts (Fig. 4A), secreted TNFα and IL-6 cytokines were increased only in poly(I:C)- but not LPS-induced Wwp2−/− BMDMs (Fig. 4B). In these experiments, the induction of Ifnb1 and Ccl5/Rantes mRNA or TNFα and IL-6 cytokines by R848, PGN, or SeV was not discernable among wild-type, heterozygous, and Wwp2−/− BMDMs (Fig. 4 A and B). These results suggest that deficiency of Wwp2 specifically promotes TLR3-mediated induction of Ifnb1 and its downstream gene Ccl5 as well as the proinflammatory cytokines TNFα and IL-6.
Fig. 4.
Effects of Wwp2-deficiency on expression of various inflammatory and innate immune genes in macrophages. Wwp2+/+, Wwp2+/−, or Wwp2−/− BMDMs were treated with poly(I:C) (20 μg/mL), LPS (25 ng/mL), PGN (50 μg/mL), and R848 (40 nM) or infected with SeV for 3 h before qRT-PCR was performed (A), or the cells were treated for 18 h and then culture supernatants were collected for measurement of TNFα and IL-6 by ELISA (B). **P < 0.01; *P < 0.05 (t test).
Wwp2−/− Mice Were More Susceptible to Poly(I:C)-Induced Death.
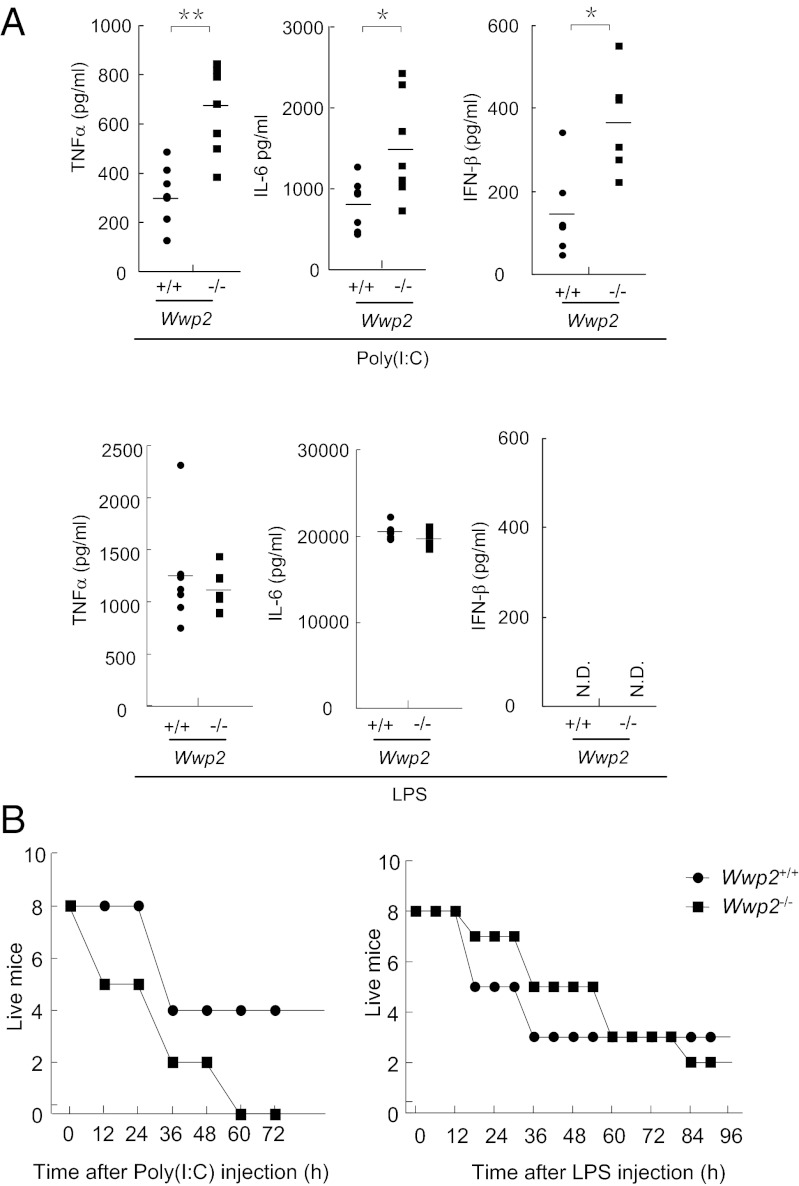
To examine whether Wwp2 regulates TLR3-mediated innate immune response in vivo, we monitored poly(I:C)-induced production of type I IFNs, proinflammatory cytokines, as well as poly(I:C)-induced inflammatory death of wild-type and Wwp2-deficient mice. Age- and sex-matched wild-type and Wwp2−/− mice were injected with poly(I:C) or LPS through i.p. route. The induced cytokines in the serum were measured 2 h after injection, and the survived animals were recorded every 12 h. As shown in Fig. 5A, poly(I:C)-induced production of TNFα, IL-6, and IFN-β was significantly enhanced in the sera from Wwp2−/− mice compared with that in their wild-type counterparts. In contrast, LPS-induced production of TNFα or IL-6 in the sera was not markedly increased in Wwp2−/− mice (Fig. 5A).
Fig. 5.
Wwp2−/− mice are more susceptible to poly(I:C)- but not LPS-induced death. (A) Serum cytokine concentrations in Wwp2+/+ and Wwp2−/− mice (n = 6 or 7) injected intraperitoneally with poly(I:C) plus D-galactosamine or LPS for 2 h. **P < 0.01; *P < 0.05 (t test). (B) Sex- and age-matched Wwp2+/+ and Wwp2−/− mice (n = 8) were injected intraperitoneally with poly(I:C) plus D-galactosamine or LPS, and their survival was monitored every 12 h for 4 d.
In consistent with the results of cytokine production in the sera, Wwp2−/− mice showed an early death onset (12 h v.s. 36 h) and a higher percentage of lethality (100% v.s. 50%) within 60 h in comparison with their wild-type counterparts after injection of poly(I:C) plus D-galactosamine (Fig. 5B). In contrast, wild-type and Wwp2−/− mice responded similarly to LPS-induced death (Fig. 5B). These results suggest that Wwp2 negatively regulated TLR3-mediated cytokine production, inflammatory and innate immune responses in vivo.
Discussion
The adapter protein TRIF plays a critical role in TLR3-mediated signaling, which activates the transcription factors NF-κB and IRF3 and leads to induction of proinflammatory cytokines and type I IFNs. However, little is known about how TRIF is regulated, particularly, at a posttranscriptional level. In this study, we performed biochemical purification experiments and identified WWP2 as a TRIF-associated protein. Although WWP2 constitutively interacted with TRIF in mammalian overexpression system, endogenous WWP2 only interacted with TRIF following poly(I:C) stimulation. Overexpression of WWP2 inhibited poly(I:C)-triggered activation of NF-κB and IRF3, whereas knockdown of WWP2 had opposite effects, suggesting that WWP2 negatively regulates TLR3-mediated signaling in mammalian cells. In addition, Wwp2−/− BMDMs and mice produced higher levels of proinflammatory cytokines and type I IFNs after poly(I:C) treatment and Wwp2-deficient mice were more susceptible to poly(I:C)-induced death. Therefore, our data demonstrate that WWP2 regulates TLR3-mediated inflammatory and innate immune responses both in vitro and in vivo.
WWP2 is an E3 ubiquitin ligase. Our results suggest that WWP2 mediates K48-linked ubiquitination and degradation of TRIF following TLR3 activation, which might be responsible for its negative regulation of TLR3-mediated signaling. It has been reported that the TIR-containing protein SARM specifically regulates TRIF-mediated signal transduction by interacting with TRIF, but the underlying molecular mechanism is still unclear (22). Interestingly, overexpression of WWP2 inhibited TRIF- but not MyD88-mediated NF-κB activation. Consistently, Wwp2-deficiency increased TLR3-, but not TLR2- or TLR7/8-mediated production of the proinflammatory cytokines TNFα and IL-6 in macrophages. Moreover, Wwp2-deficiency did not affect SeV-induced expression of IFN-β, TNFα, and IL-6 in mouse macrophages, suggesting that WWP2 does not regulate innate immune response mediated by RIG-I-like receptors in these cells.
Previous studies have demonstrated that TRIF is also involved in TLR4-mediated signaling (8). However, TRIF plays distinct roles in TLR3- and TLR4-mediated signaling pathways. TRIF is an essential adapter in TLR3-mediated signaling because all TLR3-mediated innate immune responses were abolished in Trif−/− mice (7). In contrast, TLR4 uses both MyD88 and TRIF for signaling. These two adapter molecules play both redundant and distinct roles in LPS-induced gene expression. It is demonstrated that MyD88 is primarily involved in TLR4-mediated activation of NF-κB and transcription of numerous proinflammatory cytokines such as TNFα and IL-6 (9). Consistently, Myd88−/− mice are highly resistant to LPS-induced death (23). On the other hand, TRIF is mostly required for TLR4-mediated expression of type I IFNs and their downstream genes. Interestingly, our studies suggest that Wwp2 deficiency increased poly(I:C)-induced production of proinflammatory cytokines TNFα and IL-6 in the serum, as well as sensitized mice to poly(I:C)-induced systematic inflammation and death. However, Wwp2 deficiency did not elevate LPS-induced production of TNFα and IL-6 in the serum, nor had any marked effects on LPS-induced death. These observations are consistent with the previous studies showing that TRIF is required for all TLR3-mediated responses but dispensable for TLR4-induced and MyD88-mediated production of certain proinflammatory cytokines such as TNFα and IL-6. Taken together, our findings suggest that WWP2 specifically targets TRIF and plays a critical regulatory role in TLR3-mediated inflammatory and innate immune responses.
In this study, we found that Wwp2 deficiency up-regulated mRNA levels of Ifnb, Ccl5, Tnfa, and cretion following LPS stimulation in BMDMs was not markedly changed in Wwp2−/− cells in comparison with their wild-type counterparts. It is possible that in addition to a negative regulatory role in LPS-induced transcription of cytokine genes, Wwp2 is also involved in positively regulating TNFα and IL-6 protein expression and/or secretion at a posttranscriptional level following LPS stimulation in BMDMs. The combination of these functions may lead to increased mRNA levels of Tnfa and Il6 but no marked changes of secreted TNFα and IL-6 cytokines following LPS stimulation in these cells. In our experiments, we found that WWP2 did not interact with TRIF or mediate ubiquitination of TRIF following LPS stimulation in A549 epithelial cells, which seems to contradict with our observation that Wwp2 deficiency caused increased transcription of cytokine genes following LPS stimulation in BMDMs. It is possible that Wwp2 is differentially involved in LPS-induced signaling in different cell types. Alternatively, Wwp2 targets a different component rather than TRIF in LPS-induced signaling pathways. Obviously, more rigorous studies are needed to decipher the roles of Wwp2 in LPS-induced signaling in different cell types in the future.
Previously, several studies have demonstrated that WWP2 is involved in regulating transcription, embryonic stem-cell fate, cellular transport, tumorigenesis, and T-cell activation processes by targeting distinct substrates (18–20). Recently, a gene knockout study revealed a developmental role for WWP2 in chondrogenesis via mechanisms involving cartilage-specific transcription factors (24). Mice deficient in Wwp2 develop mild malformations of the craniofacial region (25). However, we have not observed such a phenotype in our Wwp2-deficient mice. It is possible that different gene knockout strategies may contribute to this minor difference. Whereas the Zou et al. (25) used a gene-trap technology to generate Wwp2-null mice by using embryonic stem cells that contained the bacterial β-galactosidase gene inserted in intron 3–4 of the Wwp2 locus, our study produced Wwp2-null mice by standard conditional knockout strategy that replaced the exon 3 with a Neomycin cassette. Because WWP2 is constitutively expressed in most tissues, it is possible that WWP2 plays divergent roles in various biological and pathological processes. Our study clearly establishes a role for WWP2 in regulation of TLR3-mediated innate immune and inflammatory responses.
Materials and Methods
Mice and Cells.
Wwp2−/− mice were generated by a conditional gene-targeting strategy. In the targeting vector, exon 3 of Wwp2 is flanked by two loxP sites and a neomycin-resistance gene cassette. An HSV-tk cassette was introduced in the targeting vector at the end of 3′ arm for negative selection of clones with random integration in the genomic DNA. Successful targeting would lead to deletion of exon 3, which corresponds to nucleotide 71–218 of the mouse Wwp2 cDNA coding sequence. Deletion of exon 3 would result in an out of frame mutation that leads to an early stop codon which gives rise to a premature mRNA encoding only 34 aa if exon 2 and 4 are spliced together. After electroporation of the Wwp2 targeting vector into embryonic stem cells (129SV/EV), G418 and gancyclovir double-resistant colonies were selected and screened by PCR. The selected Wwp2 ES clones were injected into the blastocysts of C57BL/6. Chimeric mice were mated with C57BL/6 female mice to produce heterozygotes. The heterozygotes were interbred to produce homozygous mice. The homozygous mice were then mated with mice [FVB/N-TgN(EIIa- Cre)C5379Lmgd] that expresses Cre recombinase in nearly all tissues to produce Wwp2-deficient mice. All animal experiments were performed in accordance with the Wuhan University animal care and use committee guidelines.
BMDMs were generated from mouse bone marrows as described (26). The cells (1 × 107) were cultured in RPMI medium 1640 containing 10% FBS and 10 ng/mL recombinant murine M-CSF (Peprotech) in a 100-mm dish for 5 d before experiments.
The 293 cells stably expressing TLR3 (293-TLR3) cells were generously provided by Katherine Fitzgerald (University of Massachusetts Medical School, Worcester, MA) and Tom Maniatis (Department of Molecular and Cellular Biology, Harvard University, Cambridge, MA).
Other Materials and Methods.
Other materials and methods used in this study are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Katherine Fitzgerald and Tom Maniatis for 293-TLR3 cells. This work was supported by Chinese Ministry of Science and Technology Grants 2012CB910201 and 2013CB530500; National Natural Science Foundation of China Grants 31221061, 31170792, 31270932, and 31000639; and an Academic Award for Excellent PhD Candidates funded by Ministry of Education of China.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220271110/-/DCSupplemental.
References
- 1.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008;29(2):182–191. doi: 10.1016/j.immuni.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Wang YY, Ran Y, Shu HB. Linear ubiquitination of NEMO brakes the antiviral response. Cell Host Microbe. 2012;12(2):129–131. doi: 10.1016/j.chom.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Nie Y, Wang YY. Innate immune responses to DNA viruses. Protein Cell. 2013;4(1):1–7. doi: 10.1007/s13238-012-2122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 6.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4(2):161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 8.Hoebe K, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424(6950):743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 9.Hirotani T, et al. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-beta. Biochem Biophys Res Commun. 2005;328(2):383–392. doi: 10.1016/j.bbrc.2004.12.184. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald KA, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4(5):491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 11.Sato S, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171(8):4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 12.Han KJ, et al. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-kappaB activation and apoptosis pathways. J Biol Chem. 2004;279(15):15652–15661. doi: 10.1074/jbc.M311629200. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Z, Mak TW, Sen G, Li X. Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta. Proc Natl Acad Sci USA. 2004;101(10):3533–3538. doi: 10.1073/pnas.0308496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-kappaB activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280(44):36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 15.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5(5):503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, et al. Cutting edge: A novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169(12):6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 17.Rotin D, Kumar S. Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol. 2009;10(6):398–409. doi: 10.1038/nrm2690. [DOI] [PubMed] [Google Scholar]
- 18.Chen A, et al. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol Cell Biol. 2009;29(19):5348–5356. doi: 10.1128/MCB.00407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, et al. WWP2 promotes degradation of transcription factor OCT4 in human embryonic stem cells. Cell Res. 2009;19(5):561–573. doi: 10.1038/cr.2009.31. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Serrano J, Eastman SW, Chung W, Bieniasz PD. HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J Cell Biol. 2005;168(1):89–101. doi: 10.1083/jcb.200408155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tissari J, Sirén J, Meri S, Julkunen I, Matikainen S. IFN-alpha enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J Immunol. 2005;174(7):4289–4294. doi: 10.4049/jimmunol.174.7.4289. [DOI] [PubMed] [Google Scholar]
- 22.Carty M, et al. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7(10):1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 23.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura Y, et al. Wwp2 is essential for palatogenesis mediated by the interaction between Sox9 and mediator subunit 25. Nat Commun. 2011;2:251. doi: 10.1038/ncomms1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou W, et al. The E3 ubiquitin ligase Wwp2 regulates craniofacial development through mono-ubiquitylation of Goosecoid. Nat Cell Biol. 2011;13(1):59–65. doi: 10.1038/ncb2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.