Abstract
Cryptochrome (CRY) is the primary circadian photoreceptor in Drosophila. It resets the circadian clock by promoting light-induced degradation of the clock proteins Timeless and Period, as well as its own proteolysis. The E3 ligases that ubiquitylate Timeless and Period before degradation are known and it is known that Drosophila (d) CRY is degraded by the ubiquitin-proteasome system as well. To identify the E3 ligase for dCRY we screened candidates in S2 cells by RNAi. Knockdown of each of the 25 putative F-box proteins identified by bioinformatics did not attenuate the light-induced degradation of dCRY. However, knockdown of a WD40 protein, Bromodomain and WD repeat domain containing 3 (Brwd3) (CG31132/Ramshackle) caused strong attenuation of dCRY degradation following light exposure. We found that BRWD3 functions as a Damage-specific DNA binding protein 1 (DDB1)- and CULLIN (CUL)4-associated factor in a Cullin4-RING Finger E3 Ligase (CRL4) that mediates light-dependent binding of dCRY to CUL4-ROC1-DDB1-BRWD3, inducing ubiquitylation of dCRY and its light-induced degradation. Thus, this study identifies a light-activated E3 ligase complex essential for light-mediated CRY degradation in Drosophila cells.
Keywords: circadian rhythm, sensory flavoprotein, photocycle
Cryptochrome (CRY) is a photosensory flavoprotein (1, 2) that functions as the primary circadian photoreceptor in Drosophila (3). The Drosophila circadian clock is generated by a transcription–translation feedback loop in which the dCLOCK-dCYCLE heterodimer constitutes the positive arm (transactivator) and the Drosophila Period (dPER)–Drosophila Timeless (dTIM) complex constitutes the negative arm (repressor) of the autoregulatory circuit. The phase of the circuit can be reset by light and it has been shown that Drosophila Cryptochrome (dCRY)-mediated degradation of dTIM is a key step in light-resetting of the phase of the clock (4). In addition to dTIM degradation, light also induces dCRY itself to degrade, thus enabling newly synthesized dTIM and dPER to accumulate and reestablish the repressive phase of the circadian clock circuitry (5). The ubiquitin–proteasome system (UPS) mediates the light-dependent degradation of dTIM (6, 7) and light-independent degradation of dPER (8, 9). These degradations occur through SCF (Skp1/Cullin/F-box protein) ubiquitin E3 ligase complexes [Cullin1-RING Finger E3 Ligase (CRL1)], which are responsible for targeting substrates to the UPS (10, 11). There are at least 25 F-box proteins encoded by the Drosophila genome and two of these, SLIMB (12, 13) and JETLAG (JET) (14, 15) are involved in the degradation of circadian clock proteins, dPER and dTIM, respectively.
Initial studies with the proteasome inhibitor MG-132 indicated that dCRY as well is degraded by the UPS (7, 16). However, subsequent studies to identify the E3 ligase responsible for ubiquitylation of dCRY have yielded conflicting results. Using a genome-wide RNAi screen in Drosophila S2 cells followed by validation in mutant flies, two E3 ligases were reported to play essential roles in light-dependent dCRY degradation: BRUCE, which is a composite E2-E3 ligase, and CG17735, which is presumed to be a HECT domain-containing E3 ligase (17). However, a subsequent study concluded that in syngenic flies, BRUCE and CG17735 do not contribute to light-dependent degradation of dCRY and that the effects reported in the previous study were attributable to the eye-color differences between mutants and controls (15). Furthermore, it was demonstrated that dCRY interacts with the F-box protein JET and that this interaction was followed by proteolysis of dCRY in flies and in S2 cells (15). Therefore, it was suggested that JET functions as a substrate receptor for a CRL1 E3 ligase complex that ubiquitylates dCRY (14, 15). In support of this model, an in vitro study with purified JET and dCRY proteins revealed that blue light induces a conformational change in the C-terminal extension of dCRY (15, 18, 19), and this conformational change facilitated the binding of JET to dCRY (18). However, whereas these studies clearly showed that light-induced conformational change in dCRY enabled it to bind to both dTIM and JET, they did not prove that JET was responsible for ubiquitylation and eventual degradation of dCRY.
Here, we have used RNAi screening and yeast two-hybrid assays to identify the E3 ligase responsible for light-induced dCRY ubiquitylation and proteolysis. We found that the knockdown of each of 25 F-box proteins that could potentially mediate dCRY ubiquitylation by CRL1 E3 ligases did not attenuate light-induced degradation of dCRY. A yeast two-hybrid assay with mammalian cryptochrome 1 had identified a homolog of BRWD3, which we considered as a potential candidate for mediating dCRY ubiquitylation. BRWD3 has WD40 motifs, and it is known that many WD-motif bearing proteins function as substrate receptors [or Damage-specific DNA binding protein 1 (DDB1) and Cullin (CUL)4-associated factors] in CRL4 E3 ligases (20). Knockdown of BRWD3 (21) markedly attenuated the light-induced degradation of dCRY. Furthermore, in vitro experiments with purified proteins showed that dCRY binds to BRWD3 in a light-dependent manner and is ubiquitylated by the BRWD3/DDB1/CUL4/ROC1 E3 ligase. Thus, we conclude that dCRY is ubiquitylated by a CRL4 E3 ligase in which BRWD3 functions as a light-dependent dCRY receptor.
Results
Identification of the E3 Ligase for dCRY.
In view of the uncertainty regarding the identity of the E3 ligase responsible for light-dependent ubiquitylation of dCRY (15, 17), we set out to identify and screen all known F-box proteins encoded by the Drosophila melanogaster genome. A total of 25 F-box proteins were identified through literature and database searches, with 23 confirmed by S2 cell microarray data analysis based on the dataset of Cho et al. (22) (Table S1). A derivative of S2 cell line (16) constitutively expressing Luciferase (LUC)-dCRY (pAc-LUC-dCRY/S2) was treated with dsRNA for each of these genes and then subjected to a one-hour light pulse. Luciferase assays from 25 candidates (including SLIMB and JET) revealed no attenuation in fusion protein degradation caused by knockdown of any of the known F-box proteins (Fig. S1).
In our long-standing work on human CRYs, we had previously detected a hCRY1-WDR9 interaction in a yeast two-hybrid assay (23). Although this result was not further analyzed, it raised the possibility that a WDR9 homolog in Drosophila may also interact with the cognate CRY. We note that BRWD3 (24) is the Drosophila homolog of the human WDR9 gene (25) (also known as BRWD1), and the mutation of BRWD3 in Drosophila is known as ramshackle (21). Several members of this family of proteins are known to function as substrate receptors for CRL4 E3 ligases (20, 26). We first established that BRWD3 interacted with dCRY in yeast two hybrid in a light-dependent manner. Similar to the light-dependent interaction between dCRY and dTIM, dCRY, and BRWD3 formed a hybrid only when yeast were grown in light (Fig. S2). dsRNA knockdown of BRWD3 in S2 cells markedly reduced light-induced LUC-dCRY degradation in S2 cells, with 84% of original luciferase activity remaining after 10 min (compared with 52% in untreated cells) and 65% (versus 22% in untreated cells) after 60 min of constant light (Fig. 1A). This degradation inhibition matched that observed following treatment with the proteasome inhibitor MG-132, which yielded 57% luciferase remaining after 60 min (Fig. 1B). Incubation with both MG-132 and BRWD3 RNAi showed 58% of original activity after 60 min of light exposure (Fig. 1C), indicating that the effects are not additive. Thus, MG-132 and BRWD3 RNAi act through the same mechanism by inhibiting dCRY proteolysis through the UPS system.
Fig. 1.
Down-regulation of BRWD3 by RNAi prevents light-induced degradation of dCRY in S2 cells. (A) Knockdown of BRWD3 suppressed LUC-dCRY degradation, with 84 ± 0.4% of original luciferase activity remaining after 10 min (compared with 51.6 ± 3% in untreated cells) and 64.5 ± 5% (compared with 22.4 ± 2.3% in untreated cells) after 60 min of constant light. (B) MG-132 inhibits light-induced degradation of dCRY. Incubation of cell with MG-132 during light exposure results in 57.3 ± 2% luciferase activity remaining after 60 min (compared with 22.4 ± 2.3% in control cells). (C) Effects of BRWD3 RNAi and MG-132 are not additive. Incubation with both showed 58.4 ± 4% of original luciferase activity remaining after 60 min of light. Error bars represent SEM of three independent experiments. D, dark control; L, light-exposed (16).
Comparison of the Effects of BRWD3 and JET Knockdown on Light-Induced dCRY Degradation.
JET has been proposed to be the E3 ligase responsible for ubiquitylation and eventual degradation of dTIM (14). JET binds to both dCRY and dTIM in a light-dependent manner, and light exposure of S2R+ cells expressing JET, dCRY, and dTIM leads to rapid degradation of dTIM followed by degradation of dCRY at a slower rate (14). Given our observed effect of knockdown of BRWD3 on dCRY degradation, we wished to directly compare the effects of JET and BRWD3 knockdown on light-induced degradation of dCRY. The homozygote ramshackle mutation is embryonic lethal in Drosophila (21); hence, it is not possible to study the effect of BRWD3 mutation at the organism level. Therefore, the comparative analysis of JET and BRWD3 down-regulation on dCRY degradation was carried out in S2 cells.
S2 cells were transfected with dsRNA for JET coding sequence (CDS) or BRWD3 CDS or 3′ untranslated region (UTR) and then exposed to blue light for 10–60 min and the amount of dCRY was determined by Western blotting. The results are shown in Fig. 2 A–C. As seen in Fig. 2A, JET RNAi reduces the level of Jetlag transcript to about 10% of the control without affecting the level of Brwd3 transcript. Conversely, knockdown of CDS and UTR of BRWD3 reduce the level of Brwd3 transcript to 10% and 2%, respectively, of the control without affecting the level of Jetlag transcript. When these cells were analyzed for light-dependent dCRY degradation, it was found that whereas JET down-regulation did not inhibit dCRY degradation, down-regulation of BRWD3 resulted in the inhibition of light-induced dCRY degradation at a level proportional to the degree of down-regulation; in cells with severe BRWD3 down-regulation (attributable to UTR RNAi), light-induced dCRY degradation was completely eliminated over the course of 60 min of irradiation (Fig. 2 B and C). Moreover, the degradation of dCRY was similar in JET down-regulated and mock-treated dCRY-V5H/S2 cells (Fig. S3). Because of a previous report implicating JET in dCRY degradation (15), we wished to confirm these findings by analyzing the effects of overexpressed JET on dCRY degradation. As seen in Fig. 3, S2 cells overexpressing JET do not degrade dCRY at a faster rate than the control, supporting the conclusion that (despite binding dCRY in a light-dependent manner), JET is neither necessary nor sufficient for light-dependent dCRY ubiquitylation and degradation.
Fig. 2.
Knockdown of BRWD3 but not of JET inhibits light-dependent degradation of dCRY in S2 cells. A S2 cell line expressing dCRY-V5H under the Ac5 promoter was used. The S2-dCRY-V5H cells were treated with 1 μg of dsRNAs every other day four times and exposed to light for the indicated times. Then, aliquots were analyzed for Jet and Brwd3 expression by RT-PCR and for dCRY by immunoblotting. (A) Knockdown of Jet and Brwd3. Aliquots from dsRNA-treated cells were used to analyze the knockdown of Brwd3 and Jet mRNA by quantitative real-time PCR and normalized to Gapdh expression. Error bars represent SEM of three independent experiments. (B) Light-induced proteolysis of dCRY-V5H following Jet and Brwd3 (CDS and UTR) down-regulation analyzed by immunoblotting. Actin was used as a loading control. (C) Quantitative analysis of three dCRY proteolysis experiments including the one shown in B. Error bars represent the SEM of three independent experiments.
Fig. 3.
Overexpression of JET does not enhance the light-dependent degradation of dCRY. (A) S2-dCRY-V5H cells transfected with pAc5.1/V5His A (mock) or pAc5.1V5-JET, were exposed to blue light for the indicated times, and then were analyzed for dCRY and JET by immunoblotting. Actin was used as a loading control. (B) Quantitative analysis of two independent experiments. Error bars represent the SD of two independent experiments.
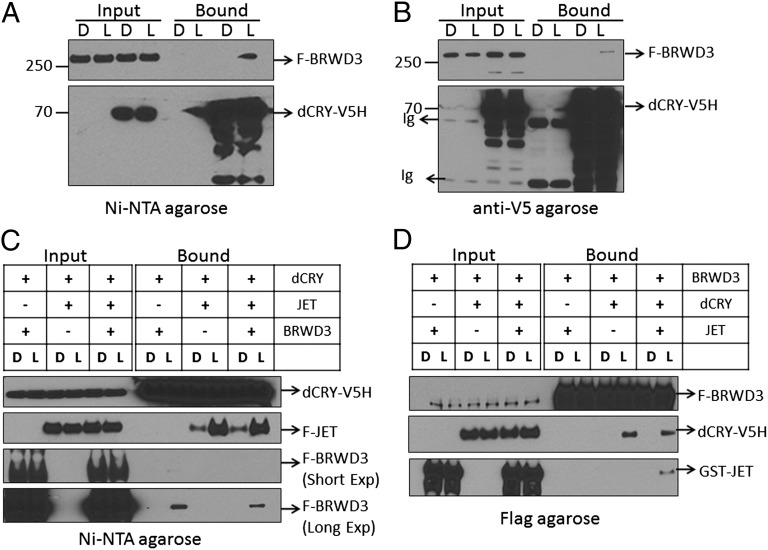
Effect of Light on dCRY-BRWD3 Interaction in Vitro.
The finding that light-induced dCRY degradation is dependent on BRWD3 suggested that light-induced conformational change in dCRY (18) may enable it to bind to BRWD3, which might function as substrate receptor of a putative E3 ligase responsible for light-dependent ubiquitylation of dCRY. Hence, we investigated the effect of light on interaction of dCRY with BRWD3 using purified proteins. We also used JET in these experiments because it has been shown that light stimulates dCRY-JET binding (15, 18). The results are shown in Fig. 4. With immobilized dCRY, which was bound either to nickel-nitrilotriacetic acid (Ni-NTA) beads (Fig. 4A) or V5-agarose beads (Fig. 4B), we found that light promotes BRWD3-dCRY interaction. When tested in parallel with JET, it appears that light-activated dCRY binds to JET more strongly than it does to BRWD3 (Fig. 4C). Next, we wished to determine whether light-activated dCRY could bind to both JET and BRWD3 simultaneously to form a ternary complex. To this end, JET and dCRY were added to immobilized BRWD3, and then the binding of JET and dCRY to BRWD3 in the absence or presence of light was assessed by immunoblotting. As apparent from Fig. 4D, whereas dCRY binds to BRWD3 upon light exposure in the absence or presence of JET, only in the presence of dCRY and light can JET bind to the BRWD3 resin. Thus, we conclude that dCRY in the active (“lit”) conformation can bind two different substrate receptors that target the cognate substrates to their respective E3 ligases.
Fig. 4.
Light promotes the interaction of dCRY with BRWD3. (A) Flag-BRWD3 (F-BRWD3) was added to the dCRY-V5H bound to Ni-NTA agarose beads. The reaction mixtures were then incubated at 24 °C either in the dark (D) or under light (L) of 1 mW⋅cm−2 fluence rate for 12.5 min. The beads were collected and washed three times in dark with 500 μL of binding buffer. Then, bound proteins were separated on 4–12% SDS/PAGE and visualized by immunoblotting using anti-FLAG antibodies for BRWD3 and anti-V5 antibodies for dCRY. (B) The experiment was performed as in A, except that recombinant dCRY-V5H was bound to V5 agarose beads instead of Ni-NTA agarose. Ig, Ig heavy and light chains. (C) Comparative affinities of JET and BRWD3 to dCRY. To dCRY-V5H on Ni-NTA agarose beads comparable amounts of Flag-JET (F-JET) and Flag-BRWD3 were added. Following incubation either in dark (D) or under light (L) for 12.5 min, the beads were extensively washed and were then probed for the indicated proteins by immunoblotting. Note that binding of both JET and BRWD3 is strongly stimulated by light and that the binding of BRWD3 to dCRY was detectable only after long exposure of the membrane to the imaging system. (D) Formation of BRWD3-dCRY-JET ternary complex. dCRY-V5H and GST-JET were added to the Flag-BRWD3 on Flag-agarose beads. The beads were kept in dark (D) or exposed to light (1 mW⋅cm−2 for 12.5 min), and then bound proteins were analyzed by immunoblotting using appropriate antibodies. Note that dCRY binds to BRWD3 only in light but in a JET-independent manner but that JET associates with BRWD3 in both light- and dCRY-dependent manner, indicating that dCRY bridges BRWD3 and JET.
BRWD3 as a Substrate (dCRY) Receptor for CRL4 E3 Ligase.
Light-dependent binding of dCRY to BRWD3, along with the inhibition of light-dependent degradation of dCRY after BRWD3 knockdown, suggested that BRWD3 might function as a substrate receptor for a CRL4 E3 ligase in a manner similar to JET functioning as a substrate receptor for CRL1 E3 ligase (14). In CRL4 E3 ligases, DDB1 functions as a linker between the Cullin 4 scaffold and a WD40–protein substrate receptor (20, 26). Indeed, when BRWD3 was expressed in human HEK293T cells and immunopurified, DDB1, CUL4A, CUL4B, and ROC1 copurified with it, indicating that BRWD3 is a substrate receptor for a CRL4 E3 ligase (Fig. 5A).
Fig. 5.
In vitro ubiquitylation of dCRY by BRWD3-DDB1-CUL4-ROC1 complex. (A) Binding of BRWD3 to the CUL4A/B-ROC1-DDB1 complex. HEK293T cells were transfected with pcDNA3 or pcDNA3.BRWD3-V5H, and the expressed protein was isolated by V5-agarose beads and probed for CRL4 member proteins by immunoblotting. The immunoprecipitate was also probed for Proliferating Cell Nuclear Antigen (PCNA) and Binding immunoglobulin Protein (BiP) as negative controls. A represents one of two independent pull-down assays. (B) Isolation of CRL4 complex. HEK293T cells were transfected with pcDNA3.Myc3-CUL4A or -CUL4B expression vectors, and the immunoaffinity purified complexes were probed for DDB1 and ROC1 by immunoblotting. (C) Ubiquitylation of dCRY by the CRL4-BRWD3 complex. All reactions contained dCRY, E1, E2, ATP, HA-ubiquitin, and CRL4 and BRWD3 where indicated. The reaction mixtures were either kept in dark (D) or exposed to light (L) for 30 min as indicated. Reaction products were separated on SDS/PAGE and analyzed by immunoblotting. The upper blot shows HA-ubiquitin (HA-Ub) immunoblot, and the lower blot shows immunoblot for BRWD3 and dCRY. C represents one of three independent in vitro ubiquitylation assays.
To test whether dCRY is ubiquitylated by the putative CUL4-ROC1-DDB1-BRWD3 E3 ligase, we purified the CUL4-ROC1-DDB1 complex by immunoaffinity (Fig. 5B) and recombinant BRWD3 from insect cells by immunoaffinity and conventional chromatography (Fig. 5C). Fig. 5C shows that when CRL4 was combined with purified BRWD3, along with E1 and E2 enzymes, it catalyzed the ubiquitylation of dCRY in a light-dependent manner. As a further test for the specificity of dCRY ubiquitylation by CRL4/BRWD3, we compared the activity of CRL4/BRWD3 and CRL1/JET on dCRY. We found that CRL1/JET has negligible activity on dCRY compared with CRL4/BRWD3 (Fig. S4). In agreement with a previous report (15), we find that Timeless overexpression in S2R+ cells attenuates dCRY degradation. Overexpression or down-regulation of JET does not affect light-induced dCRY degradation (Fig. S5). Thus, we conclude that dCRY is ubiquitylated by a CRL4 E3 ligase with BRWD3 as a substrate receptor and in a light-dependent manner, before its proteolysis by the proteosome.
Discussion
Proteolytic degradation of circadian clock proteins is a key event in generation of rhythmicity at the molecular and ultimately at the behavioral level. Surprisingly, even though the Drosophila circadian clock is arguably the best understood animal circadian clock (2, 5, 27), and dCRY is a key photosensory molecule for daily resetting of the clock by promoting the proteolytic degradation of TIM and of itself by the UPS system, the mechanism of dCRY ubiquitylation remained poorly understood. Here, we have provided strong evidence that light induces ubiquitylation of dCRY by a CRL4 E3 ligase in which the WD40 protein BRWD3 functions as substrate receptor for dCRY.
In light of the findings in this study, combined with previous reports on light-independent and light-dependent core clock protein degradation by the UPS system, we present the following working model for the Drosophila circadian clock under dark:dark (DD) and light:dark (LD) regimens (Fig. 6): in the dark phase, dCRY does not interact with TIM, JET, or BRWD3. Instead, PER and TIM, following phosphorylation by the appropriate kinases, are ubiquitylated by a CRL1 E3 ligase with the F-box protein SLIMB as the substrate receptor (12, 13), resulting in PER (and TIM) degradation and circadian cycling under free-running (DD) conditions. Under LD, conditions, in the light phase dCRY binds to TIM and the JET F-box protein, enabling CRL1/JET to ubiquitylate TIM and leading to its degradation by the UPS system (14, 15). Similarly, light-induced conformational change promotes dCRY-BRWD3 binding with the consequent degradation by the cognate CRL4 ligase and subsequent degradation to reset the clock. Light-dependent CRY activation of different ligases for TIM and its own degradation may explain the different degradation kinetics noted for the two proteins after light exposure in S2 cells (5).
Fig. 6.
Working model for the roles of ubiquitin E3 ligases in the Drosophila circadian clock. In the dark, PER and, ultimately, TIM are degraded by the UPS system following ubiquitylation of PER by a CRL1 E3 ligase with an F-box protein called SLIMB. This degradation provides the clock protein oscillation necessary for circadian cycling under free-running (DD) condition. Under conditions of LD cycles, in the L phase dCRY assumes its lit (active) conformation that enables it to bind to TIM, JET, and BRWD3. This binding leads to ubiquitylation of TIM by CRL1/JET E3 ligase and of dCRY by CRL4/BRWD3 E3 ligase and subsequent degradation of the ubiquitylated proteins to reset the clock. Thus, under constant darkness (DD), rhythmic transcription of clock-controlled genes (CCG) is controlled by the F-box protein SLIMB, and under the LD regimen, the rhythmicity of CCG transcription is achieved by the F-box proteins SLIMB and JET and the WD-40 protein BRWD3. E2, E2 ligase; U, ubiquitin; (Ub)n, poly-ubiquitin.
Because BRWD3 mutations would be expected to affect the entrainment of circadian core oscillator or its entrainment to light, it is of interest to know why BRWD3 was not identified in the numerous screens for fly clock mutations. Because BRWD3 is an essential gene required for early embryonic development, null mutations of this gene are lethal (21). Indeed, even somatic clones carrying this allele are lethal in the developing eye (21). Partial loss-of-function alleles have not been detected to date. RNAi knockdown of BRWD3 in vivo results in a pupal lethal phenotype as well (28). Our preliminary attempts at creating circadian clock cell-specific BRWD3 knockdowns have similarly yielded death of knocked-down cells. Future availability of partial loss-of-function alleles of BRWD3, or creation of CRY alleles that can no longer interact with this protein, may allow more detailed analysis and delineation of the full role of this protein in Drosophila circadian clock function in vivo.
Methods
The procedures for baculovirus preparation and protein purification were described previously (29, 30). RNAi experiments in Drosophila S2 cells were performed following established protocols (31, 32). Primers (sequences available upon request) for each of the F-box genes were designed to amplify a ∼700-bp product, adding 5′ T7 RNA polymerase binding sites to each primer (33). CRL4 complex was purified as described previously (34). A detailed protocol for the reagents, yeast two-hybrid assay, RNAi assays, protein pull-down, immunoblot, in vitro ubiquitylation, and quantitative real-time PCR are given in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Yue Xiong (The University of North Carolina at Chapel Hill) for providing the CRL4 reagents and Beezly Groh (The University of North Carolina at Chapel Hill) for advice on in vitro ubiquitylation assay. We thank Dr. Michael Young (The Rockefeller University) for providing the pIZ-Tim-YFP construct. This work was supported by National Institutes of Health Grants GM31082 (to A.S.); GM85404, P30EY1730, and an unrestricted departmental grant from Research to Prevent Blindness (to R.N.V.G.); and the Burroughs-Wellcome Clinical Scientist Award in Translational Science (to R.N.V.G and L.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303234110/-/DCSupplemental.
References
- 1.Lin C, Shalitin D. Cryptochrome structure and signal transduction. Annu Rev Plant Biol. 2003;54:469–496. doi: 10.1146/annurev.arplant.54.110901.160901. [DOI] [PubMed] [Google Scholar]
- 2.Sancar A. Regulation of the mammalian circadian clock by cryptochrome. J Biol Chem. 2004;279(33):34079–34082. doi: 10.1074/jbc.R400016200. [DOI] [PubMed] [Google Scholar]
- 3.Stanewsky R, et al. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95(5):681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosato E, et al. 2001. Light-dependent interaction between Drosophila CRY and the clock protein PER mediated by the carboxy terminus of CRY. Curr Biol 11(12):909–917.
- 5.Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304(5676):1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- 6.Ceriani MF, et al. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science. 1999;285(5427):553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- 7.Lin FJ, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol. 2001;21(21):7287–7294. doi: 10.1128/MCB.21.21.7287-7294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naidoo N, Song W, Hunter-Ensor M, Sehgal A. A role for the proteasome in the light response of the timeless clock protein. Science. 1999;285(5434):1737–1741. doi: 10.1126/science.285.5434.1737. [DOI] [PubMed] [Google Scholar]
- 9.Peschel N, Veleri S, Stanewsky R. Veela defines a molecular link between Cryptochrome and Timeless in the light-input pathway to Drosophila’s circadian clock. Proc Natl Acad Sci USA. 2006;103(46):17313–17318. doi: 10.1073/pnas.0606675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardozo T, Pagano M. The SCF ubiquitin ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5(9):739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 11.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 12.Grima B, et al. The F-box protein slimb controls the levels of clock proteins period and timeless. Nature. 2002;420(6912):178–182. doi: 10.1038/nature01122. [DOI] [PubMed] [Google Scholar]
- 13.Ko HW, Jiang J, Edery I. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature. 2002;420(6916):673–678. doi: 10.1038/nature01272. [DOI] [PubMed] [Google Scholar]
- 14.Koh K, Zheng X, Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312(5781):1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peschel N, et al. 2009. Light-dependent interactions between the Drosophila circadian clock factors cryptochrome, jetlag, and timeless. Curr Biol 19(3):241–247.
- 16.VanVickle-Chavez SJ, Van Gelder RN. Action spectrum of Drosophila cryptochrome. J Biol Chem. 2007;282(14):10561–10566. doi: 10.1074/jbc.M609314200. [DOI] [PubMed] [Google Scholar]
- 17.Sathyanarayanan S, et al. Identification of novel genes involved in light-dependent CRY degradation through a genome-wide RNAi screen. Genes Dev. 2008;22(11):1522–1533. doi: 10.1101/gad.1652308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozturk N, Selby CP, Annayev Y, Zhong D, Sancar A. Reaction mechanism of Drosophila cryptochrome. Proc Natl Acad Sci USA. 2011;108(2):516–521. doi: 10.1073/pnas.1017093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zoltowski BD, et al. Structure of full-length Drosophila cryptochrome. Nature. 2011;480(7377):396–399. doi: 10.1038/nature10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson S, Xiong Y. CRL4s: The CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci. 2009;34(11):562–570. doi: 10.1016/j.tibs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Costa A, Reifegerste R, Sierra S, Moses K. The Drosophila ramshackle gene encodes a chromatin-associated protein required for cell morphology in the developing eye. Mech Dev. 2006;123(8):591–604. doi: 10.1016/j.mod.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Cho Y, Griswold A, Campbell C, Min KT. Individual histone deacetylases in Drosophila modulate transcription of distinct genes. Genomics. 2005;86(5):606–617. doi: 10.1016/j.ygeno.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Van Gelder RN, Sancar A. 2003. Cryptochromes and inner retinal non-visual irradiance detection. Novartis Found Symp 253:31–42.
- 24.Müller P, Kuttenkeuler D, Gesellchen V, Zeidler MP, Boutros M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005;436(7052):871–875. doi: 10.1038/nature03869. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, et al. 2003. Expression of the Wdr9 gene and protein products during mouse development. Dev Dyn 227(4):608–614.
- 26.Sarikas A, Hartmann T, Pan ZQ. The cullin protein family. Genome Biol. 2011;12(4):220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev. 2003;103(6):2203–2237. doi: 10.1021/cr0204348. [DOI] [PubMed] [Google Scholar]
- 28.Mummery-Widmer JL, et al. Genome-wide analysis of Notch signalling in Drosophila by transgenic RNAi. Nature. 2009;458(7241):987–992. doi: 10.1038/nature07936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oztürk N, Song SH, Selby CP, Sancar A. Animal type 1 cryptochromes. Analysis of the redox state of the flavin cofactor by site-directed mutagenesis. J Biol Chem. 2008;283(6):3256–3263. doi: 10.1074/jbc.M708612200. [DOI] [PubMed] [Google Scholar]
- 30.Ozturk N, et al. Comparative photochemistry of animal type 1 and type 4 cryptochromes. Biochemistry. 2009;48(36):8585–8593. doi: 10.1021/bi901043s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clemens JC, et al. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci USA. 2000;97(12):6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers SL, Rogers GC. Culture of Drosophila S2 cells and their use for RNAi-mediated loss-of-function studies and immunofluorescence microscopy. Nat Protoc. 2008;3(4):606–611. doi: 10.1038/nprot.2008.18. [DOI] [PubMed] [Google Scholar]
- 33.Van Gelder RN, et al. 1995. Extent and character of circadian gene expression in Drosophila melanogaster: Identification of twenty oscillating mRNAs in the fly head. Curr Biol 5(12):1424–1436.
- 34.Nakagawa T, Xiong Y. X-linked mental retardation gene CUL4B targets ubiquitylation of H3K4 methyltransferase component WDR5 and regulates neuronal gene expression. Mol Cell. 2011;43(3):381–391. doi: 10.1016/j.molcel.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.