Abstract
We investigated the role of prostaglandin D2 (PGD2) signaling in acute lung injury (ALI), focusing on its producer–effector interaction in vivo. Administration of endotoxin increased edema and neutrophil infiltration in the WT mouse lung. Gene disruption of hematopoietic PGD synthase (H-PGDS) aggravated all of the symptoms. Experiments involving bone marrow transplantation between WT and H-PGDS–deficient mice showed that PGD2 derived from alveolar nonhematopoietic lineage cells (i.e., endothelial cells and epithelial cells) promotes vascular barrier function during the early phase (day 1), whereas neutrophil-derived PGD2 attenuates its own infiltration and cytokine expression during the later phase (day 3) of ALI. Treatment with either an agonist to the PGD2 receptor, DP, or a degradation product of PGD2, 15-deoxy-Δ12,14-PGJ2, exerted a therapeutic action against ALI. Data obtained from bone marrow transplantation between WT and DP-deficient mice suggest that the DP signal in alveolar endothelial cells is crucial for the anti-inflammatory reactions of PGD2. In vitro, DP agonism directly enhanced endothelial barrier formation, and 15-deoxy-Δ12,14-PGJ2 attenuated both neutrophil migration and cytokine expression. These observations indicate that the PGD2 signaling between alveolar endothelial/epithelial cells and infiltrating neutrophils provides anti-inflammatory effects in ALI, and suggest the therapeutic potential of these signaling enhancements.
Keywords: vascular permeability, pneumonia, lipid mediator, respiratory infection
Acute lung injury (ALI) and its severe manifestation, acute respiratory distress syndrome (ARDS), represent a clinical syndrome that results from multiple causes, including microbial infection and toxic inhalation. The most characteristic pathological findings of ALI/ARDS are neutrophil accumulation and alveolar edema caused by endothelial/epithelial barrier disruption (1). No specific pharmacologic therapies are currently available, and the mortality rate for ALI/ARDS remains very high (1). Thus, there is an urgent need to elucidate in detail the underlying pathogenesis of ALI/ARDS to develop new drugs against it.
Cyclooxygenase (COX), particularly COX-2 and its metabolites, the prostaglandins (PGs), play critical roles in the inflammatory response. Elevated PG levels have been reported in bronchoalveolar lavage (BAL) fluid obtained from patients with ARDS (2). In experimental models, Hinshaw et al. (3) originally found that COX inhibition prevents the development of sepsis and improves survival rates in dogs. Another group reported that COX-2 inhibition attenuated carrageenan-induced rat ALI (4). These findings suggest that COX-mediated production of PGs is crucial for the initiation and progression of lung inflammation. However, in a clinical study, treatment with the nonselective COX inhibitor ibuprofen did not reduce the incidence of ARDS in patients with sepsis (5). These observations suggest that PGs play a multifaceted role in the pathophysiology of airway inflammation, with both proinflammatory and anti-inflammatory components. The bioactions of each class of PGs are influenced by multiple causes. Types of PG-producing cells and their effector cells vary with both the causative pathogen and the stage of disease. The overall response of effector cells is determined by both receptors and cellular events. Thus, determining the precise contribution by an individual class of PGs regarding the functional partnership of its producer and effector cells at each disease stage is indispensable for future management of ALI/ARDS.
PGD2 is one of the COX metabolites reported to mediate an inflammatory response (6). The hematopoietic-type PGD2 synthase (H-PGDS) is expressed mainly in hematopoietic lineage cells, such as mast cells and Th2 cells. PGD2 displays its bioactivity through the G protein-coupled receptor DP and/or the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2). Several previous studies have demonstrated that DP signal activation inhibits the migration and/or activation of eosinophils, basophils, dendritic cells, and Th2 cells (6). On the other hand, CRTH2 signal activation results in the migration of eosinophils and Th2 cells (7, 8). The dehydration product of PGD2, 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2), also modulates inflammatory responses via peroxisome proliferator-activated receptor (PPAR)-γ–dependent or –independent signal activation (9). Like the other PGs, PGD2 presumably changes its pathophysiological contribution through these signal pathways depending on the target tissue, type of stimulus, and stage of the disease.
In a previous study, we focused on the physiological function of PGD2-DP signaling in neovascular endothelial cells of tumors, and found that stimulation of this pathway enhanced endothelial barrier function and suppressed tumor angiogenesis (10). Given that vascular endothelial cells and alveolar epithelial cells form their respective barriers in the lung, which is a crucial determinant of lung inflammation, these observations led us to hypothesize that PGD2 might play a protective role in ALI/ARDS. Consequently, in the present study we attempted to explore the pathophysiological implications of PGD2 biosynthesis in the development ALI/ARDS by focusing on the interaction between alveolar endothelial/epithelial cells and infiltrating neutrophils.
Results
Host H-PGDS Deficiency Accelerates Lung Inflammation.
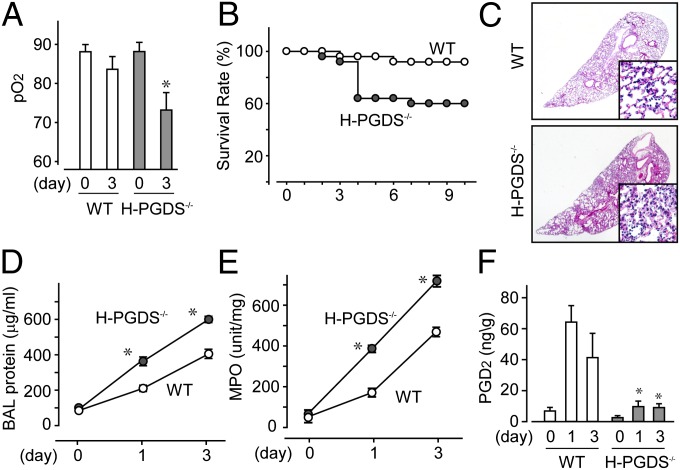
Under normal conditions, H-PGDS–deficient (H-PGDS−/−) mice did not display any functional defect in respiratory gas exchange, as quantified by arterial partial pressure of oxygen (pO2) values (Fig. 1A). Intratracheal administration of lipopolysaccharide (LPS), 3.75 mg/kg for 3 d, impaired respiratory function in both WT and H-PGDS−/− mice. Compared with the WT mice, the H-PGDS−/− mice exhibited more severe lung damage and a lower survival rate (Fig. 1B).
Fig. 1.
H-PGDS deficiency worsens endotoxin-induced lung inflammation. (A and B) Arterial pO2 (A; n = 5 each) and survival rate (B; n = 30 each) were monitored. (C) Lung morphology was examined (n = 6–8). (Scale bar: 100 μm.) (D) BAL fluid was collected, and protein content was measured (n = 8–9). (E and F) MPO activity (E) and PGD2 content (F) in lung tissue homogenates were measured (E, n = 8 each; F, n = 4–5). Results are presented as the ratio of tissue dry and wet weights. *P < 0.05 compared with WT.
Morphological studies showed that the LPS challenge induced neutrophil infiltration in the lungs of WT mice by day 3 (Fig. 1C). The H-PGDS−/− mice exhibited more severe damage. The LPS challenge increased both the protein content of BAL fluid and the myeroperoxidase (MPO) activity of lung homogenate, an indicator of neutrophil infiltration (Fig. 1 D and E). These injury severity scores peaked by day 3 and normalized by day 10 (MPO score on day 10 WT, 42 ± 5 U/mg; H-PGDS−/−, 64 ± 12 U/mg; n = 4 each). Both BAL protein content and MPO activity were higher in H-PGDS−/− mice compared with WT mice throughout the test period.
LPS inhalation increased PGD2 production in the lungs of WT mice, peaking on day 1 (Fig. 1F). However, little PGD2 production was detected in the lungs of H-PGDS−/− mice, suggesting that H-PGDS is a principal source of PGD2 in this model. The foregoing observations indicate that PGD2 is an anti-inflammatory mediator in endotoxin-induced ALI.
H-PGDS Deficiency Accelerates Fluid Accumulation, Vascular Permeability, and Cytokine Expression in Inflamed Lung.
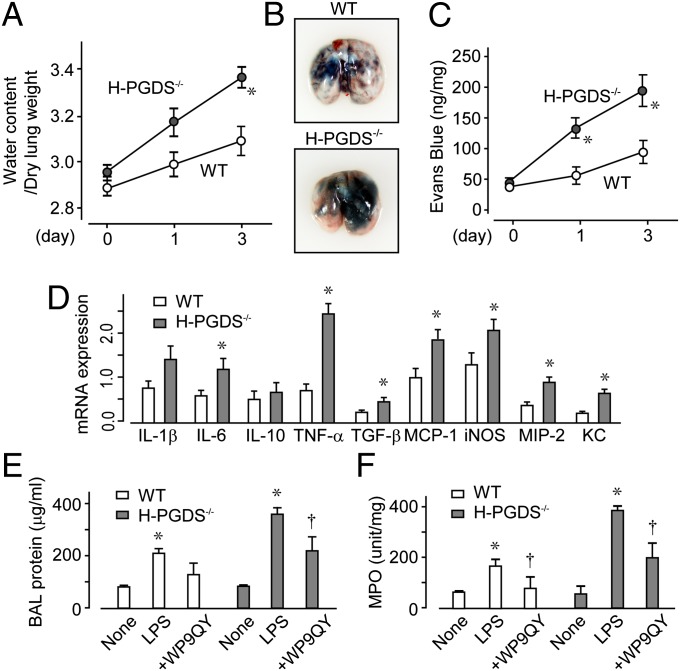
An LPS challenge induces inflammatory mediators that inhibit barrier formation of alveolar endothelial/epithelial cells. Without LPS inhalation, there was no difference between the WT and H-PGDS−/− mice (Fig. 2A). LPS inhalation slightly, but not significantly, increased the lung water content in the WT mice but caused obvious edema in the H-PGDS−/− mice. Dye extravasation was monitored to evaluate whether PGD2 production can directly and acutely influence alveolar vascular permeability. In both lines of mice, the LPS challenge increased lung vascular leakage (Fig. 2 B and C). In H-PGDS−/− mice, much more dye was extravasated, suggesting the importance of PGD2 in protecting the alveolar vascular barrier.
Fig. 2.
H-PGDS deficiency disrupts the vascular barrier and accelerates cytokine expression in an inflamed lung. (A) Lung water content was calculated (n = 10–12). (B) On day 3, Evans blue dye was injected i.v. and circulated for 30 min. (C) Dye content of the lung, shown as the ratio to tissue dry weight (n = 8–10). (D) mRNA expression of each cytokine in LPS-treated lungs on day 1, shown as the ratio of GAPDH (n = 5 each). *P < 0.05 compared with WT. (E and F) TNF-α inhibitory peptide WP9QY was administered to the LPS-challenged mice, and the protein content in BAL fluid (E) and MPO activity (F) were measured on day 1 (n = 6 each). *,†P < 0.05 compared with nontreated and LPS-treated mice.
Quantitative RT-PCR demonstrated that the LPS challenge elevated the mRNA expression of multiple proinflammatory cytokines in the lungs of WT mice on day 1 (Fig. 2D). Host H-PGDS deficiency further exacerbated these results. Of particular note, TNF-α expression was elevated almost 3.3-fold in H-PGDS−/− mice. We previously reported that H-PGDS deficiency resulted in elevated TNF-α production, which in turn further stimulated inflammation in a tumor microenvironment (11). As expected with these observations, intranasal treatment with the TNF-α inhibitory peptide WP9QY (10 mg/kg) inhibited the inflammatory response in the H-PGDS−/− mice (Fig. 2 E and F and Fig. S1A). The enhanced inflammatory responses seen in H-PGDS−/− mice can be attributed, at least in part, to the increased TNF-α production.
Contribution of Hematopoietic Cell-Derived PGD2 in Lung Inflammation.
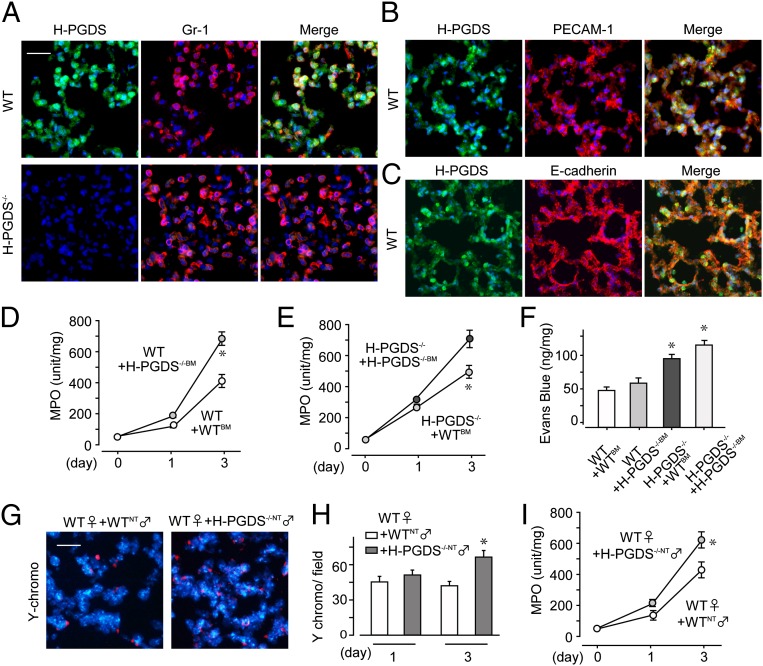
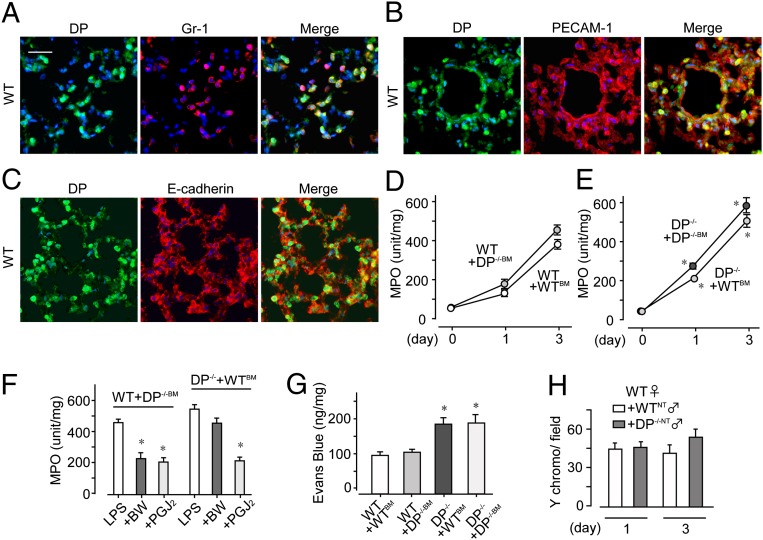
Immunostaining revealed that infiltrating Gr-1–positive neutrophils strongly expressed H-PGDS in inflamed WT mouse lung on day 3 (Fig. 3A, Upper). Platelet endothelial cell adhesion molecule (PECAM)-1–positive endothelial cells and E-cadherin–positive epithelial cells also expressed H-PGDS (Fig. 3 B and C), but at relatively low levels. No signal was detectable by anti–H-PGDS antibody in inflamed H-PGDS−/− lung (Fig. 3A, Lower).
Fig. 3.
Alveolar nonhematopoietic cell-derived PGD2 contributes to form a barrier in early-phase ALI, and leukocyte-derived PGD2 autocrinely inhibits infiltration in later-phase ALI. (A–C) H-PGDS expression was detected in neutrophils (A), endothelial cells (B), and epithelial cells (C) on day 3 (n = 5–6 each). (Scale bar: 50 μm.) (D and E) After bone marrow transplantation, MPO activity was measured in WT (D) and H-PGDS−/− (E) mice (n = 8 each). (F) Dye extravasation was monitored on day 1 (n = 8–10). (G–I) Female WT mice injected with male mouse-origin H-PGDS−/− or WT neutrophils were subjected to LPS inhalation. (G) Y chromosomes were labeled in lung sections on day 3. (Scale bar: 50 μm.) (H) Number of Y chromosome-positive neutrophils in fields (n = 8 each). (I) MPO activity (n = 8 each). *P < 0.05 compared with WT+WTBM (D and F), H-PGDS−/− +H-PGDS−/−BM (E), or WT+WTNT (H and I).
Because PGD2-mediated signaling appeared to be activated in both endothelial/epithelial cells and neutrophils, we next investigated the contributions of these two PGD2 sources to the progression of ALI. Transplantation of H-PGDS−/− bone marrow (BM) exacerbated ALI, particularly in the later phase (day 3), in WT mice (compare WT+H-PGDS−/−BM and WT+WTBM; Fig. 3D). Conversely, transplantation of WTBM diminished later-phase ALI in H-PGDS−/− mice (compare H-PGDS−/−+WTBM and H-PGDS−/−+H-PGDS−/−BM; Fig. 3E). These findings demonstrate the importance of BM-derived hematopoietic cells, mostly neutrophils, as a functional source of PGD2 at the progression stage of ALI. At an early phase of ALI (day 1), H-PGDS deficiency in mice of nonhematopoietic lineage (H-PGDS−/−+WTBM/H-PGDS−/−+H-PGDS−/−BM; Fig. 3 D and E) was associated with increased ALI scores regardless of the genotype of hematopoietic lineage cells (compare with WT+H-PGDS−/−BM/WT+WTBM). These observations show the functional impact of PGD2 produced by nonhematopoietic alveolar cells (i.e., endothelial cells and epithelial cells) at the initiation of ALI.
We next investigated how PGD2 derived from hematopoietic cells and PGD2 derived from nonhematopoietic cells affect vascular barrier formation in the lung. Hematopoietic lineage-specific H-PGDS deficiency (WT+H-PGDS−/−BM) did not substantially affect vascular permeability on day 1 (compare with WT+WTBM; Fig. 3F), but nonhematopoietic lineage-specific H-PGDS deficiency (H-PGDS−/−+WTBM) disrupted the vascular barrier. This result suggests that PGD2 production in cells of nonhematopoietic origin is crucial for protection of the vascular barrier in the current model of ALI.
We performed a neutrophil invasion assay to evaluate the importance of neutrophils in PGD2-mediated immunosuppression. Neutrophils isolated from male H-PGDS−/− mice (H-PGDDS−/−NT) or WT mice (WTNT) were injected additively into female WT mice subjected to LPS inhalation, and infiltration of Y chromosome-positive neutrophils was assessed. During the early phases of ALI, no differences in either invasiveness (Fig. 3H) or MPO score (Fig. 3I) were detected in the two groups of mice; however, in a later phase of ALI, the H-PGDS−/−-NT mice demonstrated a greater degree of invasiveness (Fig. 3 G and H) and thus elevated MPO scores (Fig. 3I). At this later phase, neutrophil-derived PGD2 is presumed to suppress cell invasiveness following an autocrine signaling mechanism.
Roles of the PGD2-DP and PGD2-PGJ2 Axes in ALI.
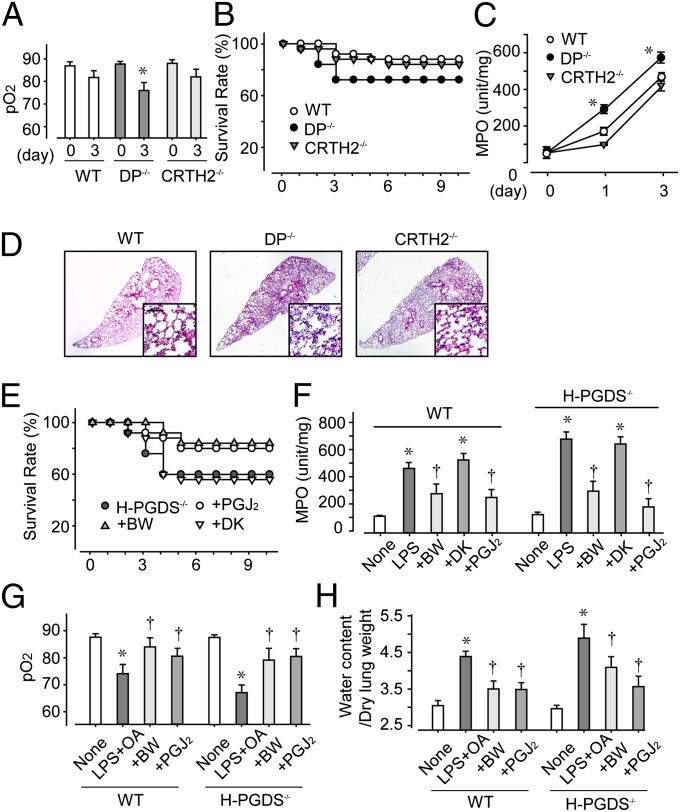
We next investigated the involvement of each PGD receptor in PGD2-mediated ALI suppression. In response to LPS inhalation, the lungs of DP-deficient (DP−/−) mice, but not those of CRTH2-deficient (CRTH2−/−) mice, demonstrated less efficient gas exchange (Fig. 4A), resulting in a lower survival rate (Fig. 4B). The lungs of DP−/− mice consistently showed both elevated MPO scores (Fig. 4C) and severe histological damage (Fig. 4D); however, these symptoms were still not as severe as those seen in the H-PGDS−/− mice (MPO score on day 3: DP−/−, 592 ± 11; H-PGDS−/−, 734 ± 23). This result implies a role for signal pathways other than the PGD2-DP receptor axis in PGD2-mediated immunosuppression.
Fig. 4.
(A–D) PGD2-DP and PGD2-PGJ2 axes contribute to ALI suppression. LPS was administered to WT, DP−/−, and CRTH2−/− mice, and pO2 (A; n = 5 each), survival rate (B; n = 30 each), and MPO activity (C; n = 8–10 each) were monitored. (D) H&E staining on day 3. (Scale bar: 100 μm.) *P < 0.05 compared with WT. (E–H) DP agonism or PGJ2 signal enhancement is beneficial against ALI/ARDS. BW245C or 15d-PGJ2 was administered to LPS-challenged mice and LPS+OA-challenged mice. Survival rate (E; n = 25–30) and MPO activity (F; n = 8–10) were monitored in LPS-challenged mice, and pO2 (G; n = 5 each) and lung tissue water (H; n = 8–10) levels were monitored in LPS+OA-challenged mice at 2 h after the challenge. *,† P < 0.05 compared with LPS/LPS+OA-treated and nontreated mice.
We then investigated whether PGD2-signal enhancement can protect against lung inflammation. In LPS-treated WT and H-PGDS−/− mice, intranasal administration of a DP receptor agonist, BW245C, or a degraded product of PGD2, 15d-PGJ2 (both at 100 μg/kg), enhanced the survival rate (Fig. 4E) and lowered the MPO score (Fig. 4F). Administration of a CRTH2 agonist DK-PGD2 did not produce the same improvements, however.
Given that bacterial sepsis is one of the most common causes of ALI, numerous investigators have used the administration of endotoxin to initiate ALI. LPS-induced inflammatory changes are relatively mild and transient, however. In another standard model, oleic acid (OA) is used to induce ALI, which produces significantly more severe histopathological changes, similar to those observed in ARDS. In the present study, administration of OA (150 μL/kg) in addition to LPS (1.5 mg/kg) caused very severe alveolar damage. At 2 h after OA administration to WT mice, damage to the lung was characterized by extensive neutrophil infiltration, pulmonary hemorrhage (Fig. S1 B and C), respiratory dysfunction (Fig. 4G), and alveolar edema (Fig. 4H). Preadministration of either BW245C or 15d-PGJ2 effectively diminished the injuries seen in this severe ALI/ARDS model, suggesting these agents’ strong potential for therapeutic application.
Immunostaining of LPS-challenged WT mouse lung (on day 3) revealed DP-positive signals in both Gr-1–positive neutrophils (Fig. 5A) and PECAM-1–positive endothelial cells (Fig. 5B), but not in E-cadherin–positive epithelial cells (Fig. 5C). These findings verify the functional contribution of DP receptors in ALI. Hematopoietic lineage-specific DP deficiency did not influence neutrophil infiltration in the WT lung (Fig. 5D; compared WT+WTBM and WT+DP−/−BM). DP−/−+DP−/−BM mice had significantly higher MPO scores than WT+WTBM mice throughout the test period. Interestingly, hematopoietic reconstitution using WT mice (DP−/−+WTBM) did not change the severity of inflammation in DP−/− mice (Fig. 5E). These findings suggest the importance of the DP-mediated signals in nonhematopoietic alveolar cells, presumably endothelial cells, in anti-inflammatory effects during ALI. In line with this idea, DP agonism was not seen in mice with a nonhematopoietic lineage-specific DP deficiency (DP−/−+WTBM) on day 3 (Fig. 5F). Treatment with 15d-PGJ2 was effective regardless of the hematopoietic genotype.
Fig. 5.
DP activation in endothelial cells is beneficial against ALI. (A–C) DP protein expression was detected in neutrophils (A) and endothelial cells (B), but not in epithelial cells (C), on day 3 (n = 5 each). (Scale bar: 50 μm.) (D and E) After bone marrow transplantation, MPO activity was monitored in WT, DP−/−, and CRTH2+ mice (n = 8 each). (F and G) BW245C or 15d-PGJ2 was administered to LPS-challenged mice, and MPO activity (F; n = 6–8) and dye extravasation (G; n = 6–8) were monitored on day 3. (H) The infiltrating ability of DP−/− neutrophils isolated from male mice into inflamed WT lung was monitored on day 3 (n = 8 each). *,†P < 0.05 compared with WT+WTBM (E), LPS-treated (F), and WT+WTNT (G) mice.
We next examined the impact of DP-mediated signals on vascular barrier formation and neutrophil motility. On a dye extravasation assay, mice lacking DP from a nonhematopoietic lineage (DP−/−+WTBM and DP−/−+DP−/−BM) exhibited defective vascular barrier formation on day 3 regardless of hematopoietic genotype (Fig. 5G). As demonstrated by the neutrophil infiltration assay, DP deficiency in the neutrophils of LPS-challenged WT mice did not affect the infiltrative capability of these cells on day 3 (Fig. 5H). This finding reinforces the crucial role of the PGD2-DP signal axis in nonhematopoietic alveolar cells in protecting the vascular barrier against inflammation.
DP Agonist, But Not PGJ2, Inhibits Vascular Permeability in Vitro.
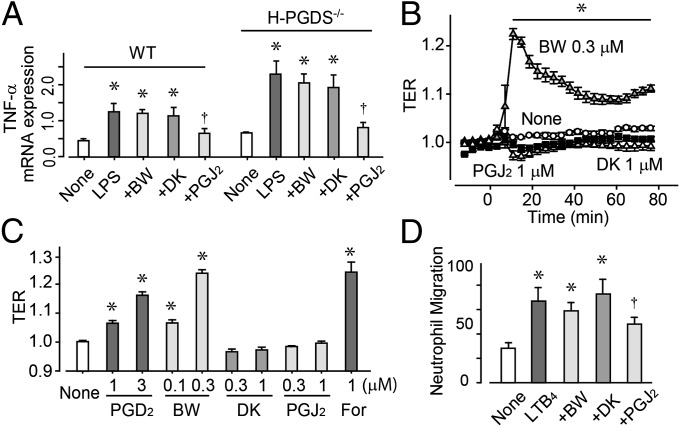
ALI progression has three main phases: cytokine expression, vascular barrier disruption, and neutrophil infiltration. In vitro LPS treatment (50 ng/mL for 6 h) of both WT and H-PGDS−/− mouse lung endothelial cells (MLECs) resulted in elevated mRNA expression of TNF-α (Fig. 6A). In line with the in vivo data, H-PGDS−/− MLECs were more responsive to LPS challenge compared with WT MLECs. Pretreatment with DP agonist BW245C (0.3 μM) or 15d-PGJ2 (0.3 μM) attenuated the elevated gene expression of TNF-α in both MLEC lines.
Fig. 6.
PGJ2 signal inhibits cytokine expression in endothelial cells and neutrophil migration, and DP agonism enhances endothelial barrier formation. (A) mRNA expression of TNF-α in mouse lung endothelial cells, shown as the ratio of GAPDH (n = 5 each). (B and C) TER; typical responses are shown in B, and data are summarized in C (n = 4–6). (D) Transmembrane migration assay performed using isolated neutrophils. LTB4 was added to the lower chamber and test agents were added to both the lower and upper chambers. Data represent the number of migrating cells to lower chambers in one field (20×; n = 5 each). *,†P < 0.05 compared with nontreated or LPS-treated cells.
Treatment with PGD2 (1–3 μM) or the DP agonist BW245C (0.1–0.3 μM) increased transendothelial electrical resistance (TER), indicating decreased permeability in human pulmonary arterial endothelial cells (Fig. 6 B and C). Consistent with our previous study showing that DP agonism tightens the endothelial barrier through cAMP–protein kinase A activation in bovine aortic endothelial cells (10), treatment with the cAMP booster forskolin (1 μM) enhanced endothelial barrier formation. In contrast, treatment with the CRTH2 agonist DK-PGD2 (0.3–1 μM) or 15d-PGJ2 (0.3–1 μM) did not affect endothelial barrier formation in vitro.
In the transmembrane migration assay, isolated neutrophils migrated toward a solution of 10 nM leukotriene B4 (LTB4) added to the lower chamber (Fig. 6D). The addition of 15d-PGJ2 (1 μM) significantly inhibited LTB4-induced neutrophil migration, with no agonism resulting from the addition of BW245C (0.3 μM) or DK-PGD2 (1 μM).
The foregoing in vitro experiments suggest that PGD2 plays an anti-inflammatory role in ALI through either of two different signaling pathways: DP-dependent or PGJ2-dependent. The DP-mediated signaling is found mainly in endothelial cells, whereas PGJ2-mediated signaling is seen in various other cell types.
Discussion
The main purpose of the present study was to define the roles of PGD2-mediated signals in the pathogenesis of ALI, focusing on its producer–effector interaction in vivo. We have demonstrated that the functional source of PGD2 shifts from alveolar endothelial/epithelial cells to infiltrating neutrophils along with ALI progression, and that the PGD2 thus produced represents an anti-inflammatory response through DP-mediated signaling arising mainly in endothelial cells and PGJ2 signaling in various other types of cells (Fig. S2).
PGs play paradoxical roles, both proinflammatory and anti-inflammatory, in injured lungs (12). Major PGs, including thromboxane A2 (TXA2) and PGE2, are classified mostly as proinflammatory PGs in ALI. Treatment with TXA2 synthase inhibitor or gene deficiency of PGE receptor EP3 has been found to attenuate lung edema in mice (13, 14). In contrast, anti-inflammatory roles for lipoxin A4 and 15d-PGJ2 have been reported in mouse ALI models (15, 16). Apparently, ALI induction is intricately modulated by a variety of PGs. Given that the pathophysiological action of each PG varies with its engaged receptor subtypes, target cells, and context of activation, a detailed evaluation of the role of each PG with respect to its source and effector is indispensable to unraveling this complexity and ultimately overcoming ALI.
Our observations consistently demonstrate the anti-inflammatory effects of PGD2, as well as the related therapeutic potential of DP agonism and PGJ2 treatment in ALI/ARDS models. Some previous studies have suggested a proinflammatory role for PGD2 in certain respiratory disorders, with the mechanisms mediated through signaling pathways other than those that we have studied here. Monneret et al. (17) reported that PGD2 inhalation caused bronchoconstriction by cross-reacting with the TXA2 receptor TP. Another group reported that PGD2 causes eosinophil and basophil chemotaxis by interacting with a CRTH2 receptor (7). Like the other PGs, PGD2 appears to have a dual role, tissue-protective or tissue-invasive in the context of airway inflammation. This action may depend on the specific receptor involved, the specific type of pathogenesis occurring, and the severity and stage of the disease.
Clearly identifying the types of cells playing the greatest roles in the responses to these related respiratory pathologies is difficult, particularly in vivo. We found that hematopoietic replacement allowed us to determine the active histocellular site of PGD2 signaling in the ALI model. Using this procedure, we determined that the PGD2-DP signaling arising in lung cells of nonhematopoietic origin (i.e., endothelial/epithelial cells) is responsible for the anti-inflammatory response in early stages of ALI (Figs. 3 D–F and 5 D–G). In contrast, PGD2 produced by hematopoietic origin, most likely neutrophils, play a central role in the immunosuppressive response in the later stages of ALI. As an effector signal, the DP-mediated anti-inflammatory response of native resident lung cells is involved in all phases of ALI. Given the strong links among vascular permeability, immune cell infiltration, and cytokine induction, determining the specific contributions of DP-mediated anti-inflammatory reactions in vivo is difficult. However, our data derived from both dye extravasation experiments (Fig. 5G) and in vitro experiments clearly indicate that PGD2-DP signaling acutely and directly inhibited alveolar vascular permeability (Fig. 6 B and C). Furthermore, we found that DP-stimulation targeting mainly to endothelial cells strongly inhibited the symptoms of ALI (Fig. 5F). Teijaro et al. (18) recently reported that a proinflammatory cytokine burst in vascular endothelial cells is the major factor promoting infection-induced ALI. Although detailed investigations are needed to clarify the contributions of PGD2 signaling to epithelial immune responses, the application of endothelial suppressants might be a rational strategy against ALI/ARDS.
In the present study, H-PGDS deficiency enhanced the inflammatory response to a much greater degree than DP deficiency, suggesting the existence of an alternative signaling pathway in addition to the DP-mediated pathway. Lipoxins are anti-inflammatory lipid mediators that potently inhibit neutrophil infiltration (19). In preliminary experiments, we investigated the possibility that PGD2 produces an anti-inflammatory reaction by stimulating lipoxin production, but detected no definite correlation between these two mediators (Fig. S3 A–D). It is also well known that a degraded product of PGD2, 15d-PGJ2, exerts anti-inflammatory effects through either a PPAR-γ–dependent pathway or a PPAR-γ–independent pathway. We have shown here that administration of 15d-PGJ2 produces a therapeutic action against ALI/ARDS in vivo (Fig. 4 E–H). Our in vitro experiments suggest that 15d-PGJ2–mediated suppression of both cytokine expression and neutrophil migration contribute to this action (Fig. 6 A and D). Genovese et al. (20) reported that administration of the PPAR-γ agonist rosiglitazone or 15d-PGJ2 moderates bleomycin-induced mouse lung injury. Another group demonstrated that 15d-PGJ2 is protective against carrageenan-induced ALI through a nuclear factor erythroid 2-related factor-2 (Nrf-2)-mediated transcriptional pathway (16). Rajakariar et al. (21) reported that 15d-PGJ2 is produced at sufficient levels in vivo to drive the resolution of zymosan-induced mouse peritonitis. Thus, PGD2 may produce an anti-inflammatory effect in ALI, in part through PGJ2-dependent signaling pathways.
In summary, PGD2 is an anti-inflammatory mediator in endotoxin-induced ALI, and enhancing its signal can be beneficial in the treatment of ALI/ARDS. The site of PGD2 activity shifts from native lung resident cells to infiltrating immune cells during the pathological progression of ALI.
Materials and Methods
Experimental Animals and ALI Induction.
All experiments were approved by the University of Tokyo’s Institutional Animal Care and Use Committee. H-PGDS−/−, DP−/−, and CRTH2−/− mice (C57BL/6) were generated and bred as described previously (22–24). For BM transplantation, 5-wk-old female mice received 9 Gy irradiation for BM ablation. BM cells (2 × 106) freshly isolated from donor mice were injected into the tail vein of the recipient. The mice were used for the experiments at 6 wk after transplantation.
To induce ALI, female mice (25 g) were anesthetized with 1.5% isoflurane, and Escherichia coli endotoxin LPS (O55:B5; 3.75 mg/kg) was instilled intratracheally. Intranasal administration of WP9QY (10 mg/kg), BW245C (100 μg/kg), DK-PGD2 (100 μg/kg), or 15d-PGJ2 (100 μg/kg) was started 10 min before the LPS challenge and then repeated every 3 h for WP9QY or every 12 h for the other agents. OA (0.15 mL/kg) was administered i.v. at 30 min after the instillation of LPS (1.5 mg/kg) to provoke severe inflammation.
Analysis of BAL Fluid, Blood Gases, and Lung Edema.
BAL was collected by flushing the lung with 1 mL of saline solution although a tracheal annula. Protein concentrations in BAL were measured. For blood gas measurements, blood drawn from the abdominal aorta was analyzed with an i-STAT blood analyzer (FUSO Pharmaceutical Industries) following the manufacturer’s instructions.
To measure lung water content, the excised lungs were weighed, then dried and reweighed. Water content was calculated by subtracting the dry weight from the wet weight. For permeability assessment, Evans blue dye (30 mg/kg) was injected i.v. and circulated for 3 h. Mice were killed and perfused with saline solution. Extravasated dye into lung tissue was extracted in formamide, and the contents were quantified spectrophotometrically.
PGD2 Measurement and MPO Assay.
Dissected lungs were homogenized in ethanol containing 0.02% HCl, and the samples were separated by HPLC. MS was performed using an API 3200 triple-quadruple tandem mass spectrometer (AB SCIEX). For MPO assays, dissected lungs were homogenized in potassium phosphate buffer containing 0.3% hexadecyltrimenthyl ammonium bromide. After centrifugation, supernatant was collected. Then 0.5 mM o-dianisidine dihydrochloride (MP Biochemicals) and 0.05% hydrogen peroxide were added to the supernatant, and optical density was measured at 460 nm.
Morphological Studies.
Paraffin-embedded sections (4 μm) were used for H&E staining. Cryosections (5 μm) were used for all other stainings. Primary antibodies included anti–H-PGDS, DP (Cayman Chemicals), anti–Gr-1, anti-CD31, and anti–E-cadherin (BD Biosciences) antibodies. In some experiments, cryosections were labeled with biotin-labeled DNA probes against the Y chromosome (Chromosome Science), following the manufacturer’s protocol.
Isolation of MLECs and Quantitative RT-PCR.
Lung tissue was dissected, minced in 0.1% collagenase, and homogenized. Magnetic beads (Dynal) coated with anti–ICAM-2 antibodies (BD Pharmingen) were added to the cells. After a 1-h incubation, endothelial cells were collected using a magnetic holder, washed, and plated and passaged for use. After 48 h, serum-starved endothelial cells were used for experiments.
Total RNA was isolated and reverse-transcribed into cDNA. Subsequent quantitative PCR using platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) and specific primers was performed with an ABI Prism 7000 (Applied Biosystems).
TER Measurement.
Endothelial barrier integrity was evaluated by measuring TER using an Xcelligence real-time cell analyzer DP system (Roche). In brief, human pulmonary arterial endothelial cells were seeded on gold electrodes and incubated overnight, after which electrical resistance across the cell layer was determined. The TER value thus derived was divided by the initial value for normalization.
Isolation of Neutrophil and Chemotaxis Assay.
Marrow cavities of the tibias and femurs of 8-wk-old mice were flushed with DMEM with 10% FCS. Neutrophils were isolated by centrifugation over discontinuous Percoll gradients. For neutrophil invasion assays, 5 × 106 neutrophils were injected into tail veins of recipient mice each day.
A modified Boyden chamber with 8-μm pores (BD Biosciences) was used for chemotaxis assays. Stimulants were added to the bottom chamber, and inhibitors were added to both the upper and bottom chambers. Isolated neutrophils (2 × 105 cells) were applied to the upper chambers. After 1 h, cells on the membrane were fixed and stained with Giemsa solution. The number of cells from five randomly chosen fields (200×) on the lower side of the membrane was counted.
Statistical Analyses.
All data are presented as mean ± SEM. Statistical differences were determined using the Student t test for two-group comparisons and one-way ANOVA with Dunnett’s test for multiple-group comparisons.
Supplementary Material
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (A) and a Grant-in-Aid for Challenging Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology, and by the Japan Society for the Promotion of Science, Takeda Science Foundation, and Pharmacological Research Foundation, Tokyo.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.N.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218091110/-/DCSupplemental.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Frank JA, Matthay MA. Leukotrienes in acute lung injury: A potential therapeutic target? Am J Respir Crit Care Med. 2005;172(3):261–262. doi: 10.1164/rccm.2505008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinshaw LB, Solomon LA, Erdös EG, Reins DA, Gunter BJ. Effects of acetylsalicylic acid on the canine response to endotoxin. J Pharmacol Exp Ther. 1967;157(3):665–671. [PubMed] [Google Scholar]
- 4.Cuzzocrea S, et al. Protective effects of Celecoxib on lung injury and red blood cells modification induced by carrageenan in the rat. Biochem Pharmacol. 2002;63(4):785–795. doi: 10.1016/s0006-2952(01)00908-x. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, et al. The Ibuprofen in Sepsis Study Group The effects of ibuprofen on the physiology and survival of patients with sepsis. N Engl J Med. 1997;336(13):912–918. doi: 10.1056/NEJM199703273361303. [DOI] [PubMed] [Google Scholar]
- 6.Kostenis E, Ulven T. Emerging roles of DP and CRTH2 in allergic inflammation. Trends Mol Med. 2006;12(4):148–158. doi: 10.1016/j.molmed.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Hirai H, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193(2):255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda K, et al. Prostaglandin D2 reinforces Th2 type inflammatory responses of airways to low-dose antigen through bronchial expression of macrophage-derived chemokine. J Exp Med. 2003;198(4):533–543. doi: 10.1084/jem.20022218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher JU, Pillinger MH. 15d-PGJ2: The anti-inflammatory prostaglandin? Clin Immunol. 2005;114(2):100–109. doi: 10.1016/j.clim.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Murata T, et al. Role of prostaglandin D2 receptor DP as a suppressor of tumor hyperpermeability and angiogenesis in vivo. Proc Natl Acad Sci USA. 2008;105(50):20009–20014. doi: 10.1073/pnas.0805171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murata T, et al. Prostagladin D2 is a mast cell-derived antiangiogenic factor in lung carcinoma. Proc Natl Acad Sci USA. 2011;108(49):19802–19807. doi: 10.1073/pnas.1110011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilroy DW, et al. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 13.Ishitsuka Y, et al. Involvement of thromboxane A2 (TXA2) in the early stages of oleic acid-induced lung injury and the preventive effect of ozagrel, a TXA2 synthase inhibitor, in guinea-pigs. J Pharm Pharmacol. 2004;56(4):513–520. doi: 10.1211/0022357023150. [DOI] [PubMed] [Google Scholar]
- 14.Göggel R, Hoffman S, Nüsing R, Narumiya S, Uhlig S. Platelet-activating factor-induced pulmonary edema is partly mediated by prostaglandin E(2), E-prostanoid 3-receptors, and potassium channels. Am J Respir Crit Care Med. 2002;166(5):657–662. doi: 10.1164/rccm.200111-071OC. [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki M, et al. Role of 15-deoxy delta(12,14) prostaglandin J2 and Nrf2 pathways in protection against acute lung injury. Am J Respir Crit Care Med. 2005;171(11):1260–1266. doi: 10.1164/rccm.200406-755OC. [DOI] [PubMed] [Google Scholar]
- 17.Spik I, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174(6):3703–3708. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 18.Teijaro JR, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 20.Genovese T, et al. Effect of rosiglitazone and 15-deoxy-Delta12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur Respir J. 2005;25(2):225–234. doi: 10.1183/09031936.05.00049704. [DOI] [PubMed] [Google Scholar]
- 21.Rajakariar R, et al. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc Natl Acad Sci USA. 2007;104(52):20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuoka T, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287(5460):2013–2017. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 23.Mohri I, et al. Prostaglandin D2-mediated microglia/astrocyte interaction enhances astrogliosis and demyelination in twitcher. J Neurosci. 2006;26(16):4383–4393. doi: 10.1523/JNEUROSCI.4531-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoh T, et al. Prostaglandin D2 plays an essential role in chronic allergic inflammation of the skin via CRTH2 receptor. J Immunol. 2006;177(4):2621–2629. doi: 10.4049/jimmunol.177.4.2621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.