Abstract
Learning and other cognitive tasks require integrating new experiences into context. In contrast to sensory-evoked synaptic plasticity, comparatively little is known of how synaptic plasticity may be regulated by intrinsic activity in the brain, much of which can involve nonclassical modes of neuronal firing and integration. Coherent high-frequency oscillations of electrical activity in CA1 hippocampal neurons [sharp-wave ripple complexes (SPW-Rs)] functionally couple neurons into transient ensembles. These oscillations occur during slow-wave sleep or at rest. Neurons that participate in SPW-Rs are distinguished from adjacent nonparticipating neurons by firing action potentials that are initiated ectopically in the distal region of axons and propagate antidromically to the cell body. This activity is facilitated by GABAA-mediated depolarization of axons and electrotonic coupling. The possible effects of antidromic firing on synaptic strength are unknown. We find that facilitation of spontaneous SPW-Rs in hippocampal slices by increasing gap-junction coupling or by GABAA-mediated axon depolarization resulted in a reduction of synaptic strength, and electrical stimulation of axons evoked a widespread, long-lasting synaptic depression. Unlike other forms of synaptic plasticity, this synaptic depression is not dependent upon synaptic input or glutamate receptor activation, but rather requires L-type calcium channel activation and functional gap junctions. Synaptic stimulation delivered after antidromic firing, which was otherwise too weak to induce synaptic potentiation, triggered a long-lasting increase in synaptic strength. Rescaling synaptic weights in subsets of neurons firing antidromically during SPW-Rs might contribute to memory consolidation by sharpening specificity of subsequent synaptic input and promoting incorporation of novel information.
Keywords: long-term depression, long-term potentiation, network plasticity, excitability
Memory requires more than recording sensory input (1–3). New sensory experience must be incorporated into a cognitive framework, or schema, that preserves temporal sequence and associates the new information together with other relevant aspects of the experience (4). This central processing requires coupling neurons into transiently stable functional assemblies and transferring information between different brain regions (2, 5). The functional coupling of neurons within assemblies is believed to be organized by network oscillations that cover multiple frequency bands and follow distinct mechanisms (6). During slow-wave sleep (SWS) and quiet wakefulness, characterized by decreased sensory and cognitive processing, hippocampal neurons fire in brief periods of high-frequency coherent oscillations (100–300 Hz), termed sharp-wave ripple complexes (SPW-Rs). SPW-Rs coincide with periods of offline replay of neural sequences learned during encoding sensory information (7, 8). Disrupting SPW-Rs, and therefore replay, impairs memory retention (9, 10), suggesting that replay of activity sequences during resting states is critical for memory consolidation. However, little is known of how synaptic strength may be affected by SPW-Rs’ that are associated with memory consolidation in the hippocampus.
Recent studies demonstrate that a distinguishing feature of individual CA1 hippocampal neurons that participate in SPW-Rs is firing of antidromic action potentials generated spontaneously in the distal region of axons in a nonclassical mode (ectopically) (11, 12). Ectopic spike generation is facilitated by GABA-mediated axonal depolarization and electrotonic coupling through axonal gap junctions (11, 13). Since ectopic action potentials may propagate antidromically into the dendritic field of CA1 neurons (14), synaptic strength may be altered in all synaptic inputs to neurons participating in SPW-Rs.
It is thought that synaptic down-scaling during SWS is necessary to prevent saturation of synapses that become potentiated during wakefulness (15), and assimilation of new memories into a schema by an increase of the signal-to-noise ratio and refinement and sharpening of previously acquired memories (3). There are some data in support of synaptic rescaling during SWS, including decreased amplitude of evoked responses (16–18) and reduced spontaneous activity (19, 20). We sought to determine whether SPW-Rs induce downscaling of synaptic strength and if so to identify the mechanism. Specifically, the causal relation that we are investigating is between the antidromic firing and changes in synaptic strength, not strictly a possible relation between the SPW-Rs and synaptic strength as network oscillations will have multiple effects. Our findings bridge between current literature seeking to understand how SPW-Rs are involved in mediating memory consolidation by identifying the cellular mechanisms (antidromic firing) for this new form of synaptic depression. Our data confirm this hypothesis and reveal a unique form of cell-wide synaptic plasticity that is initiated by antidromic action potentials.
Results
Facilitation of Spontaneous SPW-Rs Leads to Reduction in Synaptic Strength.
Spontaneous SPW-Rs can be recorded in acute hippocampal slice preparations (21). We tested whether increasing the occurrence of SPW-Rs, and associated antidromic action potential firing (11), would result in changes in synaptic strength in the hippocampal CA1 region. It has been demonstrated that GABAA receptor activation, which depolarizes axons and hyperpolarizes somatodentritic regions, facilitates antidromic spike generation in CA1 neurons during SPW-Rs (11, 12). Our studies show that local injection of muscimol, a GABAA receptor agonist, to axons in the alveus evoked depression of synaptic responses, which lasted for 90 min after muscimol withdrawal (Fig. S1A). Although GABA-mediated depolarization of axons, associated with antidromic firing during SPW-Rs, is sufficient to induce transient depression of synaptic response in the CA1 region, activation of additional mechanisms may be required to transform this depression to a long-lasting change. Alternatively, activation of nonaxonal GABAA receptors by muscimol could result in hyperpolarizing response (22, 23), and thus limit axonal depolarization and action potential propagation.
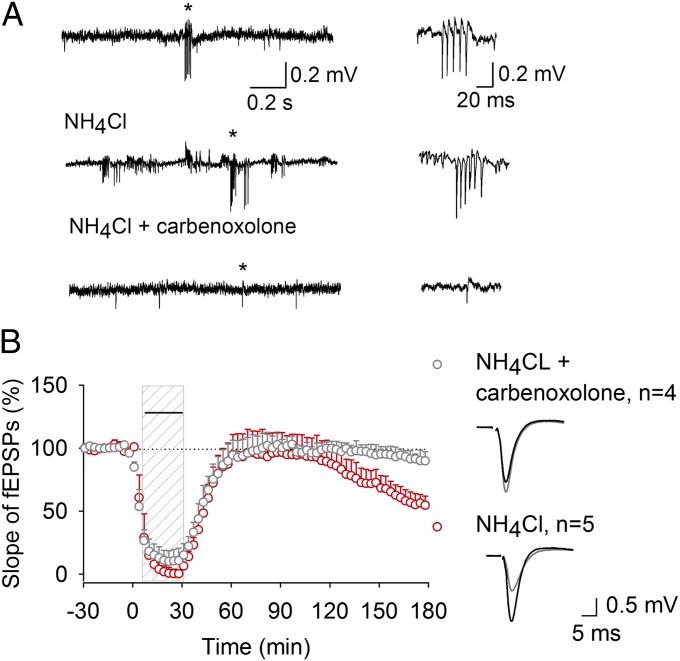
We therefore tested the effect of increasing SPW-Rs using a different method. Electrotonic coupling through gap junctions located between axons of hippocampal projection cells are crucial for very fast oscillations, including neuronal synchronization during SPW-Rs (13, 21, 24). SPW-R frequency increased 3.8 times when gap junctions were opened by intracellular alkalinization using NH4Cl (0.09 ± 0.49 before to 0.35 ± 0.10 Hz 5 min after NH4Cl application, P < 0.05, n = 6 slices), and this was accompanied by reduction of synaptic strength (Fig. S1B). Furthermore, after NH4Cl washout, long-lasting depression (LTD) of synaptic strength persisted for up to 3 h after drug application. This LTD was not due to activation of glutamate receptors during application of NH4Cl, since LTD was induced in the presence of glutamate receptor blockers. Consistent with a mechanism of ectopic spike propagation requiring opening gap junctions (13), a gap junction blocker carbenoxolone reduced SPW-R frequency 2.8 times (from 0.73 ± 0.34 before to 0.26 ± 0.10 Hz 5 min after carbenoxolone application, P < 0.05, n = 9 slices) and prevented LTD induced by NH4Cl (Fig. 1). These data indicate that facilitation of spontaneous SPW-Rs in the CA1 region of hippocampus by increasing the electrotonic coupling through gap junctions is sufficient to induce long-lasting reduction of synaptic strength.
Fig. 1.
Facilitation of spontaneous SPW-Rs leads to reduction in synaptic strength. (A) Representative field potential recording of spontaneous SPW-Rs recorded in stratum pyramidale of area CA1 (Top). Opening gap junctions with NH4Cl (10 mM) added to perfusate in the presence of glutamatergic antagonists kynurenic acid (3 mM), DL-2-Amino-5-phosphonopentanoic acid (APV) (50 μM), and (RS)-α-Methyl-4-carboxyphenylglycine (MCPG) (250 μM) increased the number and duration of spontaneous SPW-Rs in CA1 (Middle), which is prevented by gap-junction blocker carbenoxolone (100 μM) (Bottom). Corresponding expanded events are shown on the right. (B) LTD can be induced by NH4Cl in the presence of glutamatergic antagonists (dashed vertical bar) (53.8 ± 7.2%, P < 0.05) and is blocked by addition of carbenoxolone (92.5 ± 9.1%, P < 0.01). Carbenoxolone and NH4Cl application is indicated by horizontal bar. Representative synaptic responses evoked and recorded in the stratum radiatum before (black) and after (gray) treatment are shown on the right.
Antidromic Stimulation of Axons Induces LTD.
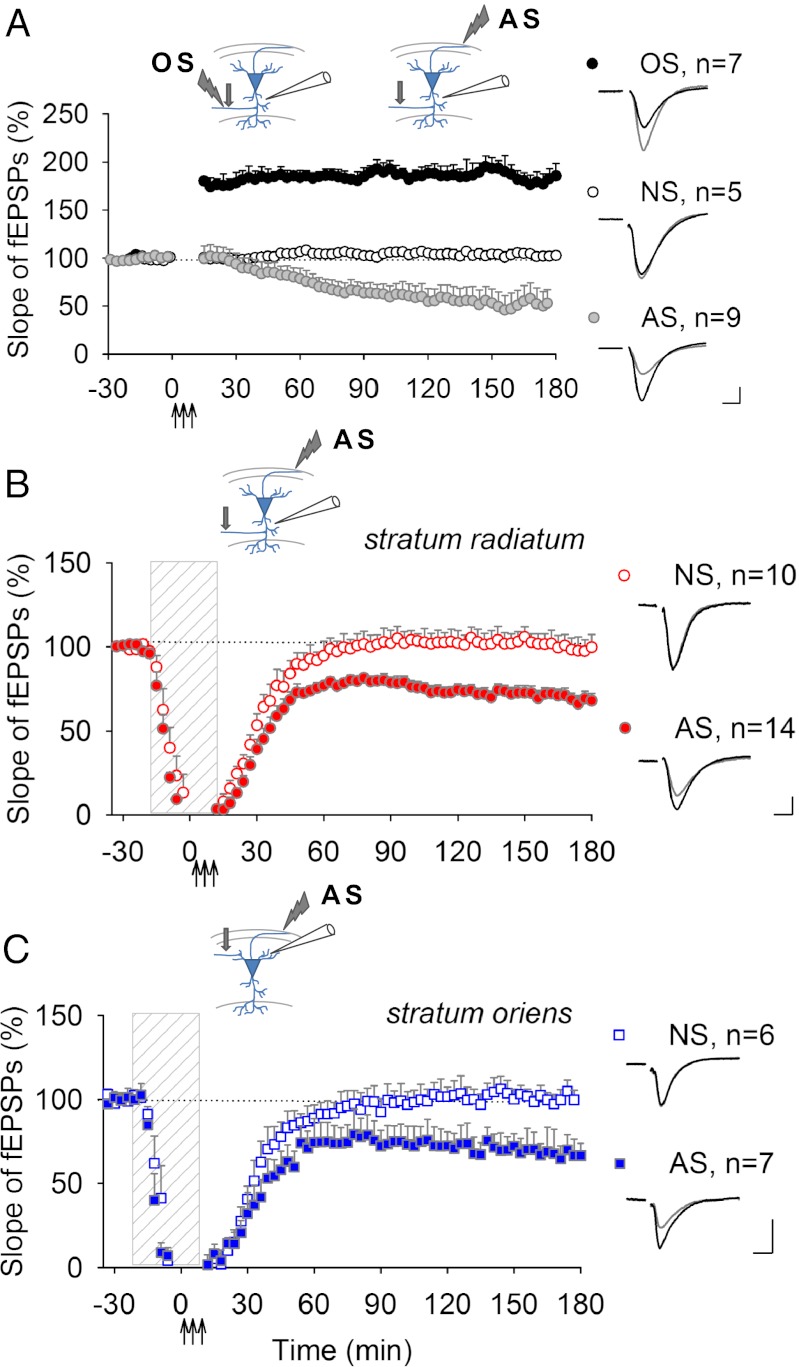
To determine whether LTD that develops after increasing SPW-Rs is mediated by synchronous antidromic action potential firing, we applied electrical stimulation to axons in the alveus to induce antidromic firing in CA1 neurons directly. Repeated theta-burst stimulation (TBS) delivered antidromically to axons evoked AP-LTD [LTD induced by antidromic action potentials (APs)] in CA1 stratum radiatum (Fig. 2A). When antidromic stimulation (AS) was delivered in the presence of glutamatergic antagonists, AP-LTD was not blocked (Fig. 2B), indicating that excitatory synaptic transmission is not required for AP-LTD. It has been suggested that repetitive high-frequency discharges during TBS resemble SPW-Rs (25). To test other stimulation patterns also resembling spontaneously occurring SPW-Rs (26), the same total number of pulses as used in TBS was delivered antidromically in different patterns, as described in Fig. S2. Repeated high-frequency burst stimulation (HFBS) and low-frequency stimulation (LFS) delivered to the alveus induced robust AP-LTD in stratum radiatum (Fig. S2 B and C).
Fig. 2.
Widespread depression is induced by antidromic stimulation (AS). (A) Recordings in the stratum radiatum demonstrating L-LTP in response to orthodromic stimulation (OS) (182.5 ± 11.0%, P < 0.001), but LTD was induced by AS of axons in the alveus (AS, 53.6 ± 15.3%, P < 0.05). There were no changes in nonstimulated (NS) slices (103.5 ± 5.0%, P = 0.42). (B and C) Antidromic stimulation delivered to the alveus during application of glutamatergic antagonists (dashed vertical bar) induced AP-LTD in stratum radiatum (B, 67.3 ± 3.6%, P < 0.001) and in stratum oriens (C, 64.1 ± 9.2%, P < 0.05). Transient blockade of synaptic transmission had no long-lasting effect on synaptic responses in either stratum radiatum or stratum oriens (99.7 ± 5.9%, P = 0.15 and 96.3 ± 2.3%, P = 0.77, correspondingly). The insets show electrode placement (arrow indicates position of the test stimulation electrode) and representative synaptic responses before (black) and after (gray) repetitive stimulation. (Calibration, 0.5 mV, 5 ms.)
Antidromic stimulation in the presence of carbenoxolone prevented AP-LTD induction (Fig. S3A), consistent with the known role of electrotonic coupling in promoting synchronous firing of axons (13, 27, 28). AP-LTD was facilitated by AS in the presence of muscimol and furosemide to better restrict depolarizing effects of GABA receptor activation to axons (Fig. S3B). Furosemide application did not affect synaptic responses in nonstimulated (NS) slices or the magnitude of AP-LTD (Fig. S3C). However, AP-LTD was not facilitated, but rather prevented, by AS delivered during application of muscimol in the absence of furosemide (Fig. S3B). Together the results demonstrate that increasing firing of antidromic action potentials associated with SPW-Rs evoked long-lasting depression of synaptic strength, which is independent of activation of glutamatergic receptors, requires gap junctions, and is facilitated by GABAA-mediated depolarization of axons.
LTD Induced by AS Is Widespread Within the Dendritic Tree.
A hallmark of most forms of synaptic plasticity is that the changes in synaptic strength are specific to the activated synapse (29, 30), but since intracellular signaling would originate from the axon during antidromic action potential firing, we hypothesized that antidromic firing might induce changes in synaptic strength that are cell-wide. TBS of Schaffer collaterals, which provide synaptic input to apical dendrites of CA1 neurons, produced late form of long-term potentiation (L-LTP) in CA1 stratum radiatum as expected (Fig. 2A), which is shown to be input-specific (29). Similarly, stimulation of inputs to basal dendrites in stratum oriens reliably induces L-LTP restricted to this compartment, since no changes were observed in simultaneously recorded responses from stratum radiatum (Fig. S4A). Furthermore, when AS was delivered in the presence of glutamatergic antagonists, AP-LTD developed in two dendritic compartments of the CA1 region—stratum radiatum and stratum oriens—demonstrating widespread weakening of synapses following axonal stimulation (Fig. 2 B and C). As expected, and in contrast to AP-LTD, no changes in synaptic strength in either of these dendritic compartments could be produced by orthodromic stimulation (OS) in the presence of glutamatergic antagonists (Fig. S5). We also recorded synaptic responses in the direct entorhinal cortex–CA1 pathway in response to AS. Although AS reliably induced AP-LTD in the stratum radiatum, no significant changes in synaptic responses recorded simultaneously in the stratum lacunosum–moleculare were observed (Fig. S6). These data suggest that a set of extrahippocampal inputs to the distal compartment of CA1 dendrites respond differently to AS from intrahippocampal inputs to CA1 from CA3 (31). In summary, we demonstrate that unlike orthodromically induced input-specific LTP, which depends on glutamate receptor activation, the alterations in synaptic strength induced by AS were widespread throughout the dendrite and did not require glutamatergic transmission.
Mechanisms for AP-LTD Induction.
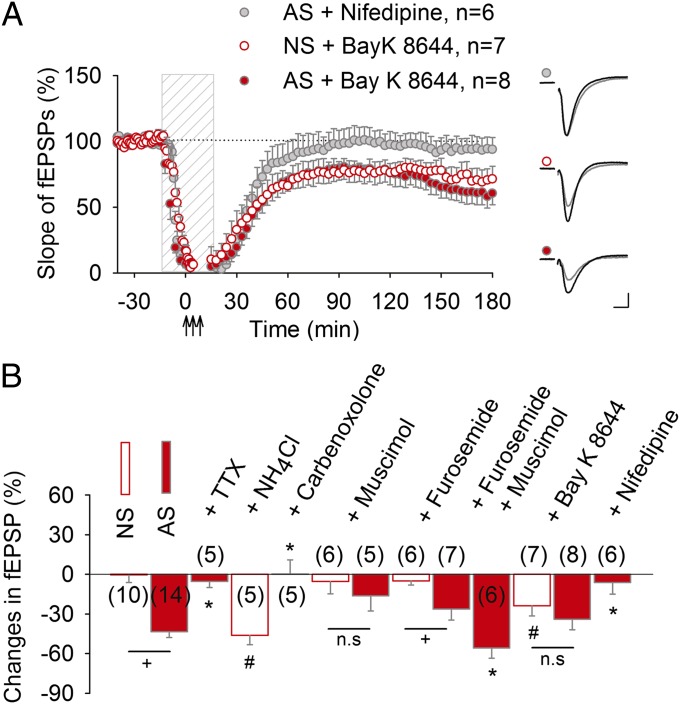
Local application of the sodium channel blocker TTX to axons during AS blocked AP-LTD in the presence of glutamatergic receptor antagonists (Fig. 3B and Fig. S3A), indicating that actively propagated action potentials are required for this form of plasticity. This is consistent with observations showing TTX-sensitive ectopic action potential firing during high-frequency oscillations in hippocampus (13, 32). We tested possible involvement of L-type voltage-dependent Ca2+ channels (L-VDCCs) in AP-LTD induction. AS in the presence of L-VDCCs antagonist nifedipine failed to evoke AP-LTD, whereas pharmacologically activating L-VDCCs with the agonist Bay K 8644 was sufficient to induce LTD (Fig. 3A). Combining AS with L-VDCC agonist treatment did not further depress synaptic strength beyond that induced by AS alone (Fig. 3A), suggesting common mechanisms acting upstream of L-VDCC activation. Together these data indicate that induction of AP-LTD is independent on glutamate receptor activation, but is facilitated by GABAA-mediated depolarization, and requires active propagation of action potentials, functional gap junctions, and L-VDCC activation. Thus, AP-LTD was induced by mechanisms implicated in SPW-R propagation and antidromic firing during SPW-Rs.
Fig. 3.
Mechanism of AP-LTD induction. (A) Either 10 μM nifedipine or 10 μM Bay K 8644 were added to block or activate L-VDCCs. Application of nifedipine abolished AP-LTD (92.2 ± 19.7%, P < 0.01). In contrast, Bay K 8644 did not affect the magnitude of AP-LTD (65.9 ± 8.0%, P = 0.89) but was sufficient to induce long-lasting fEPSPs depression (76.1 ± 7.7%, P < 0.05, comparing to NS slices). Indicated drugs were applied to NS or antidromically stimulated slices (AS) during perfusion with glutamatergic antagonists (dashed vertical bar). Representative synaptic responses evoked and recorded in the stratum radiatum before (black) and after (gray) repetitive stimulation are shown on the right. (Calibration, 0.5 mV, 5 ms.) (B) Summary data of changes in synaptic strength 160–180 min after LTD induction in the presence of indicated drugs (*P < 0.05, comparing to AS slices; # P < 0.05, comparing to NS slices; + P < 0.05 and n.s. P > 0.05, comparing NS and AS slices treated similarly; Student t test).
Interactions Between AP-LTD and L-LTP.
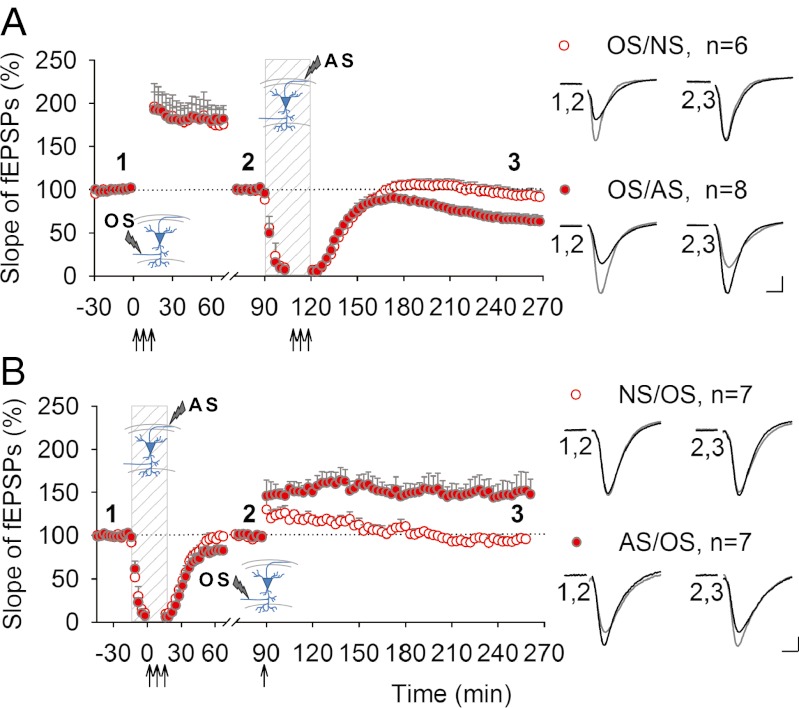
The cell-wide scope of AP-LTD would have a significant influence on information processing in the subset of neurons experiencing antidromic firing, by down-scaling all synaptic weights in these neurons. We tested whether this would include depression of previously potentiated synapses and observed that AS depotentiated previously established L-LTP (Fig. 4A). As a result of decreasing strength of all synaptic inputs to the neuron after antidromic firing, weaker synapses would become subthreshold, and neurons providing those inputs would become functionally removed from the circuit. Such tuning of receptive fields and responses to sensory experience are a common feature of learning (33–35).
Fig. 4.
AP-LTD interaction with synaptically induced L-LTP. (A) L-LTP expression was not affected by transient blockade of glutamatergic transmission (OS/NS, 92.2 ± 6.5%), but was depotentiated by AS (OS/AS, 62.8 ± 7.4%; P < 0.05). (B) Weak TBS delivered to NS slices after washout of glutamatergic antagonists induced an early form of LTP, which decayed within 3 h to prestimulated level (NS/OS, 95.1 ± 3.8%). In contrast, if the same stimulation was delivered to antidromically stimulated slices, robust LTP developed in the stratum radiatum (AS/OS, 148.7 ± 19.0%; P < 0.05). OS, orthodromic stimulation of Schaffer collaterals; AS, antidromic stimulation of axons in the alveus; NS, no stimulation was delivered. The mean slopes of fEPSPs were normalized to pre-TBS level and renormalized to 100% before the second stimulus. The insets show stimulation electrode placement. Representative synaptic responses evoked and recorded in the stratum radiatum before (black) and after (gray) stimulation are shown at the indicated time points. (Calibration, 0.5 mV, 5 ms.) Perfusion with glutamatergic antagonists marked with dashed vertical bar.
Another feature of learning is an increased sensitivity to novel stimuli and more facile incorporation of novel experiences into memory (36). Consistent with this idea, AP-LTD lowered the threshold for subsequent synaptic potentiation. Weak TBS of Schaffer collaterals in the stratum radiatum produces only a transient potentiation, but the same stimulus delivered after AS induced stable long-lasting LTP (Fig. 4B). As a consequence, synaptic strength is potentiated by subsequent synaptic input preferentially in ensembles of neurons that had fired antidromically.
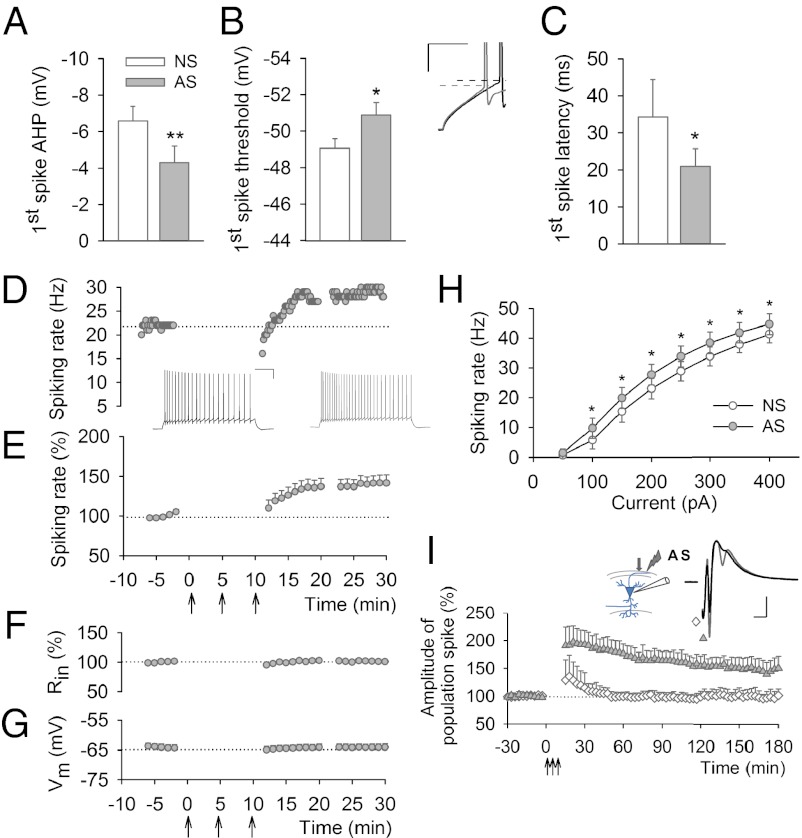
The lower threshold for LTP induction after AP-LTD could be due to the increased probability of action potential firing induced by AS. To test this hypothesis, we performed whole-cell recordings, which demonstrated an increase in neuron firing rate, decrease in spike threshold, and leftward shift in the input-output function curve (Fig. 5 A–H). This was further confirmed by extracellular recordings of the population spike of antidromic origin simultaneously with changes in synaptic responses. AP-LTD was accompanied by a long-lasting increase in the population spike amplitude of antidromic origin (Fig. 5I and Fig. S7 A and D). There was a clear leftward shift of the EPSP–spike (E–S) curve for both orthodromic and antidromic spikes following AS, indicative of E–S potentiation (Fig. S7 B and C). Since the axonal (first) component of antidromic action potential (Fig. 5I and Fig. S7 A and D) remained unchanged after AS, we would exclude that this potentiation is due to increased axonal excitability. Our extracellular and intracellular recordings rather support the view in which AS results in an increase of somatic excitability, thereby lowering the threshold for subsequent LTP induction (Fig. 4B). Thus, despite the decreased synaptic efficacy, the pyramidal cells firing antidromic action potentials have a higher probability of synchronous discharge, thus facilitating induction of stable long-lasting LTP.
Fig. 5.
Antidromically induced increase in excitability. (A–C) Whole-cell recording revealed an increase in excitability associated with decreased first spike after hyperpolarization (AHP) [A; 6.6±0.8 mV before (NS) and 4.3±0.9 mV after (AS) stimulation], first spike threshold (B; NS, –49.1 ± 0.5 mV; AS, –50.9 ± 0.7 mV) and first spike latency (C; NS, 34.2 ± 10.2 ms; AS, 21.0 ± 4.7 mV), after AS (*P < 0.05, **P < 0.01, paired t test). (D and E) Antidromically induced increase in the spiking rate of pyramidal CA1 neurons (141.1 ± 9.5%; P < 0.01, n = 7): time course of spiking rate in a representative example (D) and pooled data (E). Note that normalized input resistance (F), and resting membrane potential (G), remained stable, indicating that the passive membrane properties did not change after AS. (H) A leftward shift in the average input/output curve in response to AS (*P < 0.05, paired t test). (I) Extracellular recordings of population spikes of antidromic origin demonstrated that AS results in a long-lasting increase in amplitude of the somato-dendritic component (filled triangle) of the population spikes (164.9 ± 16.5%, P < 0.01, n = 6), while the axonal component (diamond) remained unaltered throughout the recording duration (97.2 ± 11.3%, P = 0.77, n = 5). The insets show electrode placement (arrow indicates position of the test stimulation electrode) (I) and representative traces illustrating the decrease in the first spike threshold (dashed line), first spike AHP and first spike latency (B), increase in the spiking rate (D), and antidromic population spike (I) before (black) and after (gray) AS. [Calibrations, 20 ms, 10 mV (B); 200 ms, 20 mV (D); 0.5 mV, 5 ms (I).]
Discussion
The results show that antidromic action potential firing that occurs during intrinsic high-frequency network oscillations (11, 12) induces a form of synaptic plasticity that reduces the strength of synapses in a cell-wide manner. From a neuronal information-processing perspective, action potential firing is a most reasonable reference for regulating synaptic strength in a neuron globally since the action potential threshold is the ultimate binary output of the neuron that results from all synaptic integration that is provided by thousands of afferent inputs to it (37). In these respects, a cellular process of regulating synaptic strength globally that is based not on glutamate receptor activation but instead on voltage-dependent sodium and calcium channels activated by axonal firing seems reasonable.
Despite decades of research, it is still unclear how postsynaptic action potential firing regulates strengthening or weakening of synapses (38). Our results show that whether the action potential is generated antidromically or orthodromically is a critical factor in neuronal plasticity that has not been considered previously. These findings are not in conflict with synapse-specific mechanisms of synaptic plasticity. Previous studies of spike-timing–dependent plasticity (STDP) have in general not explored the consequences of ectopic action potentials, and the studies typically examine synaptic plasticity on the temporal domain of tens of milliseconds from action potential firing (39). The comparatively slow time course of AP-LTD, developing tens of minutes after action potential firing, is typically outside the scope of STDP studies.
Axonal properties and ectopic action potential generation can be modified concomitantly with, or in response to, neuronal plasticity. The generation of SPW-Rs in hippocampus is facilitated by LTP (26) and after learning (40). Putative ectopic spikes occur in CA1 in vivo during exploration (41) and in the distal axon of interneurons in response to natural firing patterns in vitro (27). Similarly, as we observed for AP-LTD, previous reports show antidromic spiking persisted in the presence of glutamate receptor blockers (13, 27), is facilitated by GABAA-mediated depolarization of axons (11), and relies on electrotonic coupling through gap junctions (13, 27). Interestingly, activity-dependent down-regulation of the K+- Cl− cotransporter (KCC2) is a common mechanism for the reduction of GABAergic inhibition in certain physiological states (42), and following LTP induction (43), suggesting that GABAA-mediated facilitation of ectopic action potential firing may be enhanced by a positive shift in ECl.
Membrane depolarization and L-VDCC activation can occur subsequently to GABAA receptor activation (44), and involvement of L-VDCCs in different types of LTD is well documented in the literature (45–48). It has been suggested that a transient influx of Ca2+ into the postsynaptic cell is a sufficient trigger for inducing LTD (48). Prolonged/sustained mild depolarization provided by LTD-inducing stimulation could be optimal for relatively slow activation kinetics of L-VDCC (49). Our data are well in line, demonstrating dependence of AP-LTD induction on L-VDCC. We suggest that firing of action potentials of axonal origin during SPW-Rs may provide the optimal depolarization necessary for LTD induction.
Our data suggest that the widespread rescaling of synaptic weight in a neuron by SPW-R–associated antidromic firing would have multiple consequences on neuronal circuits. First, it would prevent saturation of synapses strengthened according to synapse-specific rules of learning that are based on coincident activity of the pre- and postsynaptic membrane (50). In agreement with that, we found that antidromic firing is capable of downscaling synaptic weights in previously potentiated synapses. Another important consequence of reducing synaptic weights globally within a neuron is that weak synaptic inputs to the neuron would become subthreshold, thus removing these inputs functionally from the neuronal circuit. AP-LTD induced by antidromic action potentials associated with SPW-Rs may reflect a process of active uncoupling of neuronal ensembles to allow the emergence of new ensembles, which may be necessary to proceed from one cognitive state to another (51).
In support of this we observed that weak synaptic input to the neuron that would otherwise fail to induce long-lasting potentiation results in L-LTP after antidromic firing. These results showed that subsequent synaptic potentiation could be facilitated in neurons that had experienced antidromic firing. On a neuronal circuit level, these results suggest that neurons in functional assemblies that have participated in coordinate antidromic firing would become sensitized to subsequent weak synaptic input. As a consequence, new functional input would more easily strengthen synapses and become preferentially incorporated into existing circuits or schema (3, 52). Remarkably, the cellular mechanism for the increased sensitivity of neurons to subsequent weak synaptic input is also global, rather than a synapse-specific process—namely, increased excitability of the neuron after firing antidromic action potentials in coherent oscillations with other neurons. Altogether, our data suggest that active and dynamic circuitry modifications, induced by antidromic firing, may ensure the plastic state of synapses, which may underlie cognitive flexibility.
We suggest that firing of antidromic action potentials in subsets of neurons that participate in coherent activity during SPW-Rs at sleep and periods of quite restfulness would provide a cellular basis for SPW-R–associated memory replay and reactivation (3, 53). The reduced sensory input during such periods, when the brain is not processing external input, would be favorable for replay of neuronal assemblies induced intrinsically by antidromic firing and thus contribute to memory consolidation. Whereas, activation of sensory-evoked input to the CNS transmitted through orthodromic firing during exploratory activity is necessary to engage specific ensembles of CA1 neurons in a relatively labile form (54). The importance of two behavior stages in memory consolidation as well as their physiological sequences has been emphasized before (1). Our results expand this view and exploit concepts of frequency-dependent multiplexing to separate forms of plasticity induced by different modes of firing.
The synaptic plasticity induced by antidromic action potentials described here may have practical significance for human therapy. For example, synaptic depression induced by antidromic firing may provide insight on the neurological mechanisms by which deep brain stimulation, used as a treatment for Parkinson disease, obsessive–compulsive disorder, depression, and addiction (55), exerts effects on brain tissue.
Materials and Methods
For full details, see SI Materials and Methods.
All experiments were conducted in accordance with animal study protocols approved by the National Institutes of Child Health and Human Development Animal Care and Use Committee. Intracellular recording of intrinsic excitability and extracellular recordings of spontaneous SPW-Rs, field excitatory postsynaptic potentials (fEPSPs), and population spike were done in CA1 region of hippocampal slices prepared from adult (7–10-wk-old) male Sprague–Dawley rats.
Supplementary Material
Acknowledgments
We thank K. A. Pelkey, A. Dityatev, H. Wake, A. Morozov, and T. Coate for critically reading the manuscript and National Institute of Child Health and Human Development intramural funding for support.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210735110/-/DCSupplemental.
References
- 1.Buzsáki G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 3.Lewis PA, Durrant SJ. Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn Sci. 2011;15(8):343–351. doi: 10.1016/j.tics.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Nader K, Schafe GE, LeDoux JE. The labile nature of consolidation theory. Nat Rev Neurosci. 2000;1(3):216–219. doi: 10.1038/35044580. [DOI] [PubMed] [Google Scholar]
- 5.Chrobak JJ, Buzsáki G. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J Neurosci. 1996;16(9):3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 7.Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19(21):9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29(1):145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 9.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12(10):1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 10.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20(1):1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bähner F, et al. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci USA. 2011;108(35):E607–E616. doi: 10.1073/pnas.1103546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papatheodoropoulos C. A possible role of ectopic action potentials in the in vitro hippocampal sharp wave-ripple complexes. Neuroscience. 2008;157(3):495–501. doi: 10.1016/j.neuroscience.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz D, et al. Axo-axonal coupling. A novel mechanism for ultrafast neuronal communication. Neuron. 2001;31(5):831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 14.Leung LS, Peloquin P. GABA(B) receptors inhibit backpropagating dendritic spikes in hippocampal CA1 pyramidal cells in vivo. Hippocampus. 2006;16(4):388–407. doi: 10.1002/hipo.20168. [DOI] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 17.Huber R, et al. TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep. PLoS ONE. 2007;2(3):e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hulse BK, et al. A postsleep decline in auditory evoked potential amplitude reflects sleep homeostasis. Clin Neurophysiol. 2011;122(8):1549–1555. doi: 10.1016/j.clinph.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vyazovskiy VV, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct evidence for wake-related increases and sleep-related decreases in synaptic strength in rodent cortex. J Neurosci. 2010;30(25):8671–8675. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draguhn A, Traub RD, Schmitz D, Jefferys JG. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature. 1998;394(6689):189–192. doi: 10.1038/28184. [DOI] [PubMed] [Google Scholar]
- 22.Romo-Parra H, Treviño M, Heinemann U, Gutiérrez R. GABA actions in hippocampal area CA3 during postnatal development: Differential shift from depolarizing to hyperpolarizing in somatic and dendritic compartments. J Neurophysiol. 2008;99(3):1523–1534. doi: 10.1152/jn.01074.2007. [DOI] [PubMed] [Google Scholar]
- 23.Traub RD, et al. GABA-enhanced collective behavior in neuronal axons underlies persistent gamma-frequency oscillations. Proc Natl Acad Sci USA. 2003;100(19):11047–11052. doi: 10.1073/pnas.1934854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traub RD, Schmitz D, Jefferys JG, Draguhn A. High-frequency population oscillations are predicted to occur in hippocampal pyramidal neuronal networks interconnected by axoaxonal gap junctions. Neuroscience. 1999;92(2):407–426. doi: 10.1016/s0306-4522(98)00755-6. [DOI] [PubMed] [Google Scholar]
- 25.Buzsáki G, Haas HL, Anderson EG. Long-term potentiation induced by physiologically relevant stimulus patterns. Brain Res. 1987;435(1-2):331–333. doi: 10.1016/0006-8993(87)91618-0. [DOI] [PubMed] [Google Scholar]
- 26.Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8(11):1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- 27.Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci. 2011;14(2):200–207. doi: 10.1038/nn.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munro E, Kopell N. Subthreshold somatic voltage in neocortical pyramidal cells can control whether spikes propagate from the axonal plexus to axon terminals: A model study. J Neurophysiol. 2012;107(10):2833–2852. doi: 10.1152/jn.00709.2011. [DOI] [PubMed] [Google Scholar]
- 29.Andersen P, Sundberg SH, Sveen O, Swann JW, Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89(10):4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leão RN, et al. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15(11):1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stasheff SF, Wilson WA. Increased ectopic action potential generation accompanies epileptogenesis in vitro. Neurosci Lett. 1990;111(1-2):144–150. doi: 10.1016/0304-3940(90)90359-h. [DOI] [PubMed] [Google Scholar]
- 33.Ekerot CF, Jörntell H. Parallel fiber receptive fields: A key to understanding cerebellar operation and learning. Cerebellum. 2003;2(2):101–109. doi: 10.1080/14734220309411. [DOI] [PubMed] [Google Scholar]
- 34.Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learn Mem. 2007;14(1-2):1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiele A. Perceptual learning: Is V1 up to the task? Curr Biol. 2004;14(16):R671–R673. doi: 10.1016/j.cub.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Tse D, et al. Schemas and memory consolidation. Science. 2007;316(5821):76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- 37.Paulsen O, Sejnowski TJ. Natural patterns of activity and long-term synaptic plasticity. Curr Opin Neurobiol. 2000;10(2):172–179. doi: 10.1016/s0959-4388(00)00076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisman J, Spruston N. Postsynaptic depolarization requirements for LTP and LTD: A critique of spike timing-dependent plasticity. Nat Neurosci. 2005;8(7):839–841. doi: 10.1038/nn0705-839. [DOI] [PubMed] [Google Scholar]
- 39.Dan Y, Poo MM. Spike timing-dependent plasticity: From synapse to perception. Physiol Rev. 2006;86(3):1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 40.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19(10):4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Epsztein J, Lee AK, Chorev E, Brecht M. Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science. 2010;327(5964):474–477. doi: 10.1126/science.1182773. [DOI] [PubMed] [Google Scholar]
- 42.Rivera C, et al. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24(19):4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W, Gong N, Xu TL. Downregulation of KCC2 following LTP contributes to EPSP-spike potentiation in rat hippocampus. Biochem Biophys Res Commun. 2006;343(4):1209–1215. doi: 10.1016/j.bbrc.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Ari Y. Excitatory actions of gaba during development: The nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 45.Christie BR, Schexnayder LK, Johnston D. Contribution of voltage-gated Ca2+ channels to homosynaptic long-term depression in the CA1 region in vitro. J Neurophysiol. 1997;77(3):1651–1655. doi: 10.1152/jn.1997.77.3.1651. [DOI] [PubMed] [Google Scholar]
- 46.Christie BR, Abraham WC. L-type voltage-sensitive calcium channel antagonists block heterosynaptic long-term depression in the dentate gyrus of anaesthetized rats. Neurosci Lett. 1994;167(1-2):41–45. doi: 10.1016/0304-3940(94)91023-5. [DOI] [PubMed] [Google Scholar]
- 47.Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264(5162):1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 48.Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16(4):825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 49.Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20(1):266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hebb DO. The organization of behavior. 1949. (New York: Wiley)
- 51.Rodriguez E, et al. Perception’s shadow: Long-distance synchronization of human brain activity. Nature. 1999;397(6718):430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 52.Morris RG. Elements of a neurobiological theory of hippocampal function: The role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23(11):2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10(2):180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 54.Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15(7):827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 55.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: From neurology to psychiatry? Trends Neurosci. 2010;33(10):474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.