In Cambodia, the same region where chloroquine resistance developed in the 1950s, a significant delay in the Plasmodium falciparum parasite clearance rates to artemisinins has been persistently observed over the past 5 y, raising the specter of artemisinin “resistance” (1). The parasites in this region have imprints of genetic selection associated with the delay in parasite clearance rates measured by serial patient blood films (2–4). However, the traditional continuous inhibition concentration 50% (IC50) drug assays measured directly with parasites from patient’s blood indicate no significant difference in IC50s (1, 5). In PNAS, Klonis et al. (6) expand upon our knowledge of intraerythrocytic action of artemisinins, in a tour de force of P. falciparum in vitro culture work, by exposing three laboratory isolates to short 1-, 2-, 4-, or 6-h pulses of artemisinins every hour throughout the 48-h life cycle to determine pulse drug IC50. An important unique finding is that an early ring stage that is less than 8-h-old is hypersensitive to artemisinins, and that intrinsic differences preexist among decades-old laboratory P. falciparum isolates not thought to be exposed to the artemisinin drugs before isolation.
In an earlier report by Klonis et al. (7), as well as a report by ter Kuile et al. (8), the timing of artemisinin action had been demonstrated to coincide with the onset of hemoglobin ingestion during the late-ring or early trophozoite stages. This experimental window of artemisinin duration of action during the 48-h P. falciparum erythrocyte life cycle is longer than quinoline drugs, such as quinine, mefloquine, or lumefantrine, which inhibit heme crystallization (8). Many malariologists believe artemisinins work after iron activation to induce carbon-centered radical damage of nearby proteins for low 1- to 10-nM inhibition of Plasmodium trophozoite stages, which catabolize hemoglobin (9). The artemisinins also inhibit Babesia (10), Toxoplasma (11), and human cancer cells (12), albeit at 10- to 100-times higher drug concentrations. Furthermore, these microbes (cells) do not catabolize hemoglobin but either have enough bioavailable iron or heme to activate the artemisinin’s endoperoxides for radical damage, or the artemisinin molecules at the higher drug concentrations interact with specific proteins or calcium channels to kill (13, 14).
Reminiscent of bacterial antibiotic resistance discovered in an ancient isolated section of Carlsbad cave with no exposure to modern human antibiotics for millions of years (15), Klonis et al. show that older laboratory isolates of malaria have almost 10-fold different response rates to drug pulses of artemisinins during the 48-h erythrocyte cycle (6). This work correlates well with the very recent 2013 observation of altered ring-stage sensitivity to 6-h pulse artemisinins in laboratory isolates, as well as isolates from Palin, Cambodia (5), where most parasites from this region have a four- to fivefold more pulse drug IC50 for ring stage compared with the trophozoite stage. A possible troubling conclusion from this work is that the delayed artemisinin clearance phenotype may be more widespread than previously thought if an isolate from Africa-3D7 or Papua New Guinea-D10 have elevated pulse drug IC50 for artemisinins, even though the pulse drug IC50 is less than the Palin, Cambodia isolates.
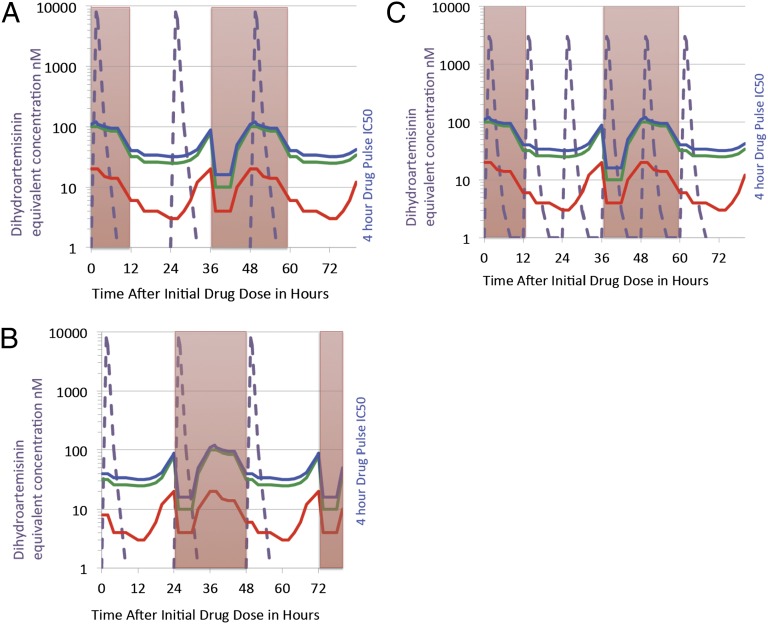
The work by Klonis et al. provides experimental evidence and a mathematical model to account for a slower clearance rate, based on the altered higher drug concentrations needed for pulse-dose short half-life artemisinins to inhibit ring stages (6). The timing of the 24-h artesunate drugs, used in many of the studies to describe delayed parasite clearance, may make a difference based on the percentage of parasites that are more susceptible to drugs. Fig. 1 depicts the model output with the high peak but short time intervals above 10-nM blood concentrations of either artesunate or artemether—dosed every 24 or 12 h—converted to the active metabolite dihydroartemisinin equivalents. As one can see from the shape of the pulse drug IC50 curves for two laboratory isolates, D10 and 7G8, as well as modeled ring-stage isolates from Palin just published as an additional work (5), the initiation of artemisinin dosing on a predominate single patient population of rings at 12 h of age (Fig. 1A) will result in two doses of the artemisinin coinciding with intrinsically higher pulse drug IC50 for ring-stage parasites. Even though only ring-stage parasites are visible on microscopy, clinicians are not able to discern if the total P. falciparum biomass at the time of microscopy is either 80% ring and 20% trophozoite stage or 20% ring and 80% trophozoites. If the same dosing regimen is initiated by chance on a predominately parasite population of 26-h trophozoites (Fig. 1B), then the dosing regimen will result in three doses coinciding with the lower-pulse drug IC50 for trophozoite stages. Another way to increase dosing on predominately trophozoite stages is to increase frequency to every 12 h, as is dosed with
Fig. 1.
Artemisinin killing varies by stage of erythrocyte life cycle. During the 48-h erythrocyte cycle, P. falciparum spends the initial 24 h as a ring stage (red shaded columns) before progressing to a heme crystal-containing trophozoite/schizont stage (white columns) for the last 24 h of the cycle. By careful short 1- to 6-h drug pulsing starting at every hour of the 48-h life cycle, Klonis et al. (6) demonstrate a unique initial hypersensitivity nadir at low nanomolar drug for rings up to 8 h, followed by a 16-h period of relative insensitivity to pulsed artemisinins with IC50 10-times as high. At the onset of the hemoglobin digestion period, the IC50 to a 4-h drug pulse decreases from the ring zenith to a range of 5–20 nM for the trophozoite stages. During the end of the 48-h cycle, when hemoglobin digestion stops, the IC50 rises at late schizont stage. Interestingly the work compared three laboratory isolates to note an intrinsic large four- to eightfold variation in the 4-h drug pulse IC50, with the isolate 7G8 (red line) being the most sensitive compared with isolates D10 or 3D7 (green line). The model described in the article takes into account the timing of short half-life artemisinins, which may result in an altered delayed parasite clearance rates in P. falciparum with artemisinin treatment, which has been described in Palin, Cambodia. The delay in clearance rate could be accounted by the intrinsic difference in pulse drug IC50 throughout the life cycle or by timing of the 24-h artesunate dosing, such that in the worst case (depicted in A) the artesunate dihydroartemisinin equivalent short-pharmacokinetic profile (dotted purple line) is initiated with parasite biomass predominately at 12-h rings, showing that most of artemisinin (two of three drug doses) drug exposure coincides with ring stages (red boxes), which require higher drug inhibitory concentrations to kill. (B) If by chance the drug dosing was to coincide with a parasite biomass predominately at early trophozoite stages, then all three 24-h doses of the drug produce high drug peaks coincident with the more susceptible trophozoite stages or hypersensitive early-ring stage. (C) An alternative way to maximize both time and peak drug concentrations above inhibition concentrations is to dose every 12 h, as is done with artemether-lumefantrine. Continuous in vitro drug inhibition assays have shown little difference in IC50 because of influence of the hypersensitive early-ring stage and trophozoite stages.
Klonis et al. show that older laboratory isolates of malaria have almost 10-fold different response rates to drug pulses of artemisinins during the 48-h erythrocyte cycle.
artemether-lumefantrine (Fig. 1C) (15). A recent clinical study in Palin, which compared every 12- vs. 24-h artesunate dosing, showed a small but not statistically different trend to faster clearance with every 12-h dosing of artesunate (16, 17). Unfortunately, changing the dosing does not fully reverse the delayed clearance rate seen in Cambodia. This work could also explain in the published genetic studies why single “twin” P. falciparum isolates have a variance in parasite clearance rate (3) from different human patients, which also could either be from timing of artemisinin dosing with predominate age in hours of the parasite biomass or from host factors, such as hemoglobin E (18).
Pulse-dose drug-inhibition studies at synchronized ring stages, as shown by Klonis et al. (6), are now the best way at present to segregate the phenotype of delayed parasite clearance rate, as continuous 48-h drug inhibition studies do not segregate parasites. A practical implication is that artemisinins dosed more frequently or with a longer half-life will improve time above inhibition concentration (16) as long as the toxic effects of bone marrow suppression do not appear (19). Another implication is that the mechanism of action or activation of drug may be different at different times in the erythrocyte life cycle, which would explain the time-dependent pulse drug IC50. Pharmacodynamics is still everything.
Footnotes
The author declares no conflict of interest.
See companion article on page 5157.
References
- 1.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361(5):455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheeseman IH, et al. A major genome region underlying artemisinin resistance in malaria. Science. 2012;336(6077):79–82. doi: 10.1126/science.1215966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson TJ, et al. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J Infect Dis. 2010;201(9):1326–1330. doi: 10.1086/651562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takala-Harrison S, et al. Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci USA. 2013;110(1):240–245. doi: 10.1073/pnas.1211205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkowski B, et al. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother. 2013;57(2):914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klonis N, et al. Altered temporal response of malaria parasites determines differential sensitivity to artemisinin. Proc Natl Acad Sci USA. 2013;110:5157–5162. doi: 10.1073/pnas.1217452110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klonis N, et al. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci USA. 2011;108(28):11405–11410. doi: 10.1073/pnas.1104063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: In vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76(1):85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem. 2004;47(12):2945–2964. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 10.Goo YK, et al. Artesunate, a potential drug for treatment of Babesia infection. Parasitol Int. 2010;59(3):481–486. doi: 10.1016/j.parint.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Jones-Brando L, D’Angelo J, Posner GH, Yolken R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother. 2006;50(12):4206–4208. doi: 10.1128/AAC.00793-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal AS, et al. Malaria-infected mice are cured by a single oral dose of new dimeric trioxane sulfones which are also selectively and powerfully cytotoxic to cancer cells. J Med Chem. 2009;52(4):1198–1203. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagamune K, Moreno SN, Sibley LD. Artemisinin-resistant mutants of Toxoplasma gondii have altered calcium homeostasis. Antimicrob Agents Chemother. 2007;51(11):3816–3823. doi: 10.1128/AAC.00582-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckstein-Ludwig U, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424(6951):957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 15.Bhullar K, et al. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS ONE. 2012;7(4):e34953. doi: 10.1371/journal.pone.0034953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saralamba S, et al. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 2011;108(1):397–402. doi: 10.1073/pnas.1006113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das D, et al. Effect of high-dose or split-dose artesunate on parasite clearance in artemisinin-resistant falciparum malaria. Clin Infect Dis. 2013;56(5):e48–e58. doi: 10.1093/cid/cis958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amaratunga C, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: A parasite clearance rate study. Lancet Infect Dis. 2012;12(11):851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepniewska K, et al. Population pharmacokinetics of artesunate and amodiaquine in African children. Malar J. 2009;8:200. doi: 10.1186/1475-2875-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]