Abstract
The generation of high-density lipoprotein (HDL), one of the most critical events for preventing atherosclerosis, is mediated by ATP-binding cassette protein A1 (ABCA1). ABCA1 is known to transfer cellular cholesterol and phospholipids to apolipoprotein A-I (apoA-I) for generating discoidal HDL (dHDL) particles, composed of 100–200 lipid molecules surrounded by two apoA-I molecules; however, the regulatory mechanisms are still poorly understood. Here we observed ABCA1-GFP and apoA-I at the level of single molecules on the plasma membrane via a total internal reflection fluorescence microscope. We found that about 70% of total ABCA1-GFP spots are immobilized on the plasma membrane and estimated that about 89% of immobile ABCA1 molecules are in dimers. Furthermore, an ATPase-deficient ABCA1 mutant failed to be immobilized or form a dimer. We found that the lipid acceptor apoA-I interacts with the ABCA1 dimer to generate dHDL and is followed by ABCA1 dimer–monomer interconversion. This indicates that the formation of the ABCA1 dimer is the key for apoA-I binding and nascent HDL generation. Our findings suggest the physiological significance of conversion of the ABCA1 monomer to a dimer: The dimer serves as a receptor for two apoA-I molecules for dHDL particle generation.
Keywords: membrane protein, transporter
Plasma high-density lipoprotein (HDL) is critical for preventing coronary artery disease (1). A member of the ATP-dependent transporter family of ABC proteins, ATP-binding cassette protein A1 (ABCA1) initiates the generation of discoidal HDL (dHDL), a bilayer fragment consisting of 100–200 lipids wrapped by two molecules of apolipoprotein A-I (apoA-I) (2–4), by exporting cholesterol and phospholipids to lipid-free apoA-I in serum (5). More than 70 mutations have been identified in the ABCA1 gene. Indeed, mutations in ABCA1 lead to Tangier disease, which is characterized by plasma HDL deficiency (6–10). ABCA1 has two large extracellular domains (ECDs), and the two intramolecular disulfide bonds between them are necessary for apoA-I binding and HDL formation (11–13) (Fig. 1A). Two pieces of evidence suggest that apoA-I interacts with a specific conformation of the ECDs in an ATP-dependent manner: Chemical cross-linkers can cross-link apoA-I with ABCA1, and ATPase-deficient ABCA1 mutants fail to mediate apoA-I binding and cross-linking. However, the importance of direct binding of ABCA1–apoA-I in HDL formation is still controversial and, furthermore, how a dHDL particle containing two molecules of apoA-I is formed from lipid-free apoA-I monomers and membrane lipids is unknown.
Fig. 1.
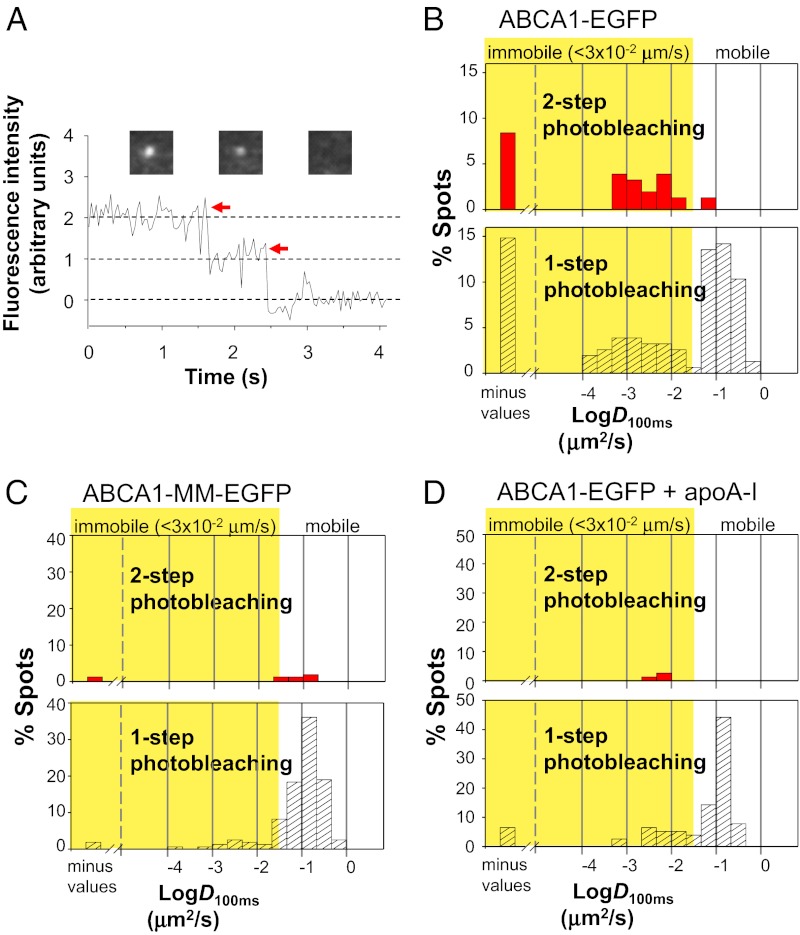
Single-molecule fluorescence tracking analysis of ABCA1. ABCA1 or ATPase-deficient ABCA1 mutant (ABCA1-MM) fused with EGFP was transiently expressed in HeLa cells, and the bottom PM was observed by TIRF microscope. (A) Putative secondary structure of human ABCA1. The two disulfide bonds formed between two ECDs are indicated (not to scale). The two lysine residues in nucleotide-binding domains (NBDs) of ABCA1-MM are replaced by methionine. (B) Representative TIRF images of single molecules of ABCA1 or ABCA1-MM fused with EGFP. (Scale bar, 5 μm.) (C) Obtained from video images (30 frames/s): typical 1-s trajectories of ABCA1 (Left), ABCA1-MM (Center), and ABCA1 at 14 min 20 s after the addition of 5 μg/mL nonlabeled apoA-I to the medium (Right) are shown. Ten trajectories that exhibited median values are shown. (D) Distributions of D100ms for ABCA1 and ABCA1-MM are shown. Percentages show the immobile fractions (yellow region of D100ms <0.032 μm2/s); for immobile versus mobile fraction classification, see Materials and Methods. Digits and arrowheads indicate the median values of D100ms and their positions, respectively.
To address these issues, we performed single-molecule fluorescence imaging (14, 15) of ABCA1 and apoA-I on the plasma membrane (PM) via a total internal reflection fluorescence (TIRF) microscope in living cells. We examined the dynamic behaviors of ABCA1 as well as the interaction of ABCA1 with apoA-I, and found that ABCA1 forms an immobile dimer on the PM; the ABCA1 dimer then dissociates into diffusing monomers upon interaction with apoA-I, finally generating HDL. We determined that the interconversion of ABCA1 between dimers and monomers depends on its interaction with apoA-I.
Results
ABCA1 Is Immobilized on the Plasma Membrane Depending on Its Function.
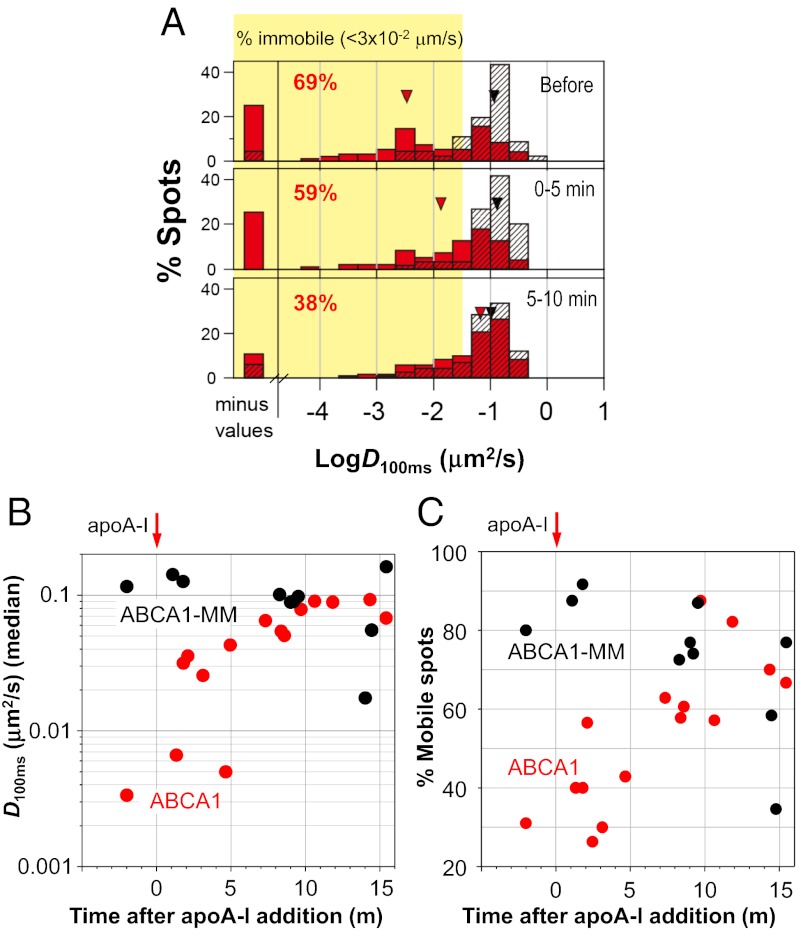
To examine ABCA1 dynamics on the PM, ABCA1, fused with enhanced green fluorescent protein (EGFP) at its carboxyl terminus, was transiently expressed in HeLa cells (Fig. 1B), which do not express endogenous ABCA1 at levels detectable by immunoblotting. As a nonfunctional control molecule, an ATPase-deficient ABCA1 mutant (ABCA1-MM), in which two lysine residues essential for ATP hydrolysis (11) were replaced by methionines, was used for comparison (Fig. 1A). Strikingly, before apoA-I application, many ABCA1-EGFP molecules expressed on the PM exhibited immobilization (Fig. 1C and Movies S1 and S2), which could be conveniently characterized by the diffusion coefficient in a time window of 100 ms, D100ms (Fig. 1D). Because the “maximal” operational D100ms (by noise) for purified EGFP attached to the cover glass was 0.032 μm2/s, we classified molecules exhibiting D100ms < 0.032 μm2/s as “immobile” (meaning that the diffusion was slow and/or undetectable compared with the noise). We found that about two-thirds (69%) of the ABCA1 spots were classified as immobile, with a median D100ms of 0.0034 (Fig. 1D and Table 1). In contrast, only 20% of ABCA1-MM spots were classified as immobile, with a median D100ms of 0.12 (Fig. 1D, Table 1, and Movies S3 and S4).
Table 1.
Statistics of ABCA1 and ABCA1-MM dynamics
| Molecule + addition | n (no. of cells) | Median of D100ms, μm2/s | % immobile |
| ABCA1 | 96 (5) | 0.0034* | 69 |
| +latrunculin A | 212 (2) | 0.025† | 52 |
| +apoA-I (0–5 min) | 95 (5) | 0.013‡ | 59 |
| +apoA-I (5–10 min) | 121 (3) | 0.067§ | 38 |
| ABCA1-MM | 46 (4) | 0.12¶ | 20 |
| +apoA-I (0–5 min) | 60 (1) | 0.13|| | 10 |
| +apoA-I (5–10 min) | 116 (5) | 0.10** | 23 |
P < 0.0001, between * and ¶; P = 0.0001, between * and †; P = 0.1797, between * and ‡; P < 0.0001, between * and §; P = 0.1676, between ¶ and ||; P = 0.4023, between ¶ and ** (Mann–Whitney U test). Numbers of spots examined (n), median values of D100ms for all of the examined spots, and percentages of the immobile fraction under each experimental condition are shown. Digits in parentheses represent the number of cells examined.
Immobile ABCA1 Molecules Are Dimers.
We found that many immobile ABCA1-EGFP molecules are dimers. Because immobile fluorescent spots can be easily tracked, we examined the time-dependent changes of the fluorescent signal intensity of each individual immobile spot (Fig. 2A). Among 155 spots in 11 cells examined, 38% (35 out of 91) of immobile spots exhibited two-step photobleaching (Fig. 2B and Movie S5), suggesting that the spots are dimers, whereas 97% (62 out of 64) of mobile spots were photobleached in a single step (Fig. 2B). Because the actual fluorescent fraction of ABCA1-EGFP was 62.8 ± 3.7% under our expression conditions, many dimers should be pairs of fluorescent and nonfluorescent molecules and thus would show single-step photobleaching. The true dimer fraction of immobilized ABCA1 molecules was determined by correcting with the actual fluorescent fraction of GFP as previously described by Kasai et al. (15) (SI Materials and Methods). We calculated that 88.6 ± 12.0% of immobile ABCA1 molecules are involved in dimers. In contrast, among 158 spots of ABCA1-MM–EGFP in 16 cells examined here, 94% of the spots were photobleached in a single step (Fig. 2C). In summary, ABCA1 molecules with lipid-transporting activity form dimers and are immobilized, whereas nonfunctional molecules tend to remain monomers and are mobile. Furthermore, the diffusion of ABCA1-MM was unaffected by the simultaneous expression of ABCA1 in the same cell (Fig. S1); this suggests that ABCA1 immobilization is directly linked to its lipid transport activity and not to lipid composition changes in the PM by ABCA1 activity.
Fig. 2.
Immobile ABCA1 exists as dimers. (A) Time-dependent changes of the fluorescence intensity of a representative spot of ABCA1 fused with mEGFP in the PM showing two-step photobleaching. As EGFP is reported to have a weak tendency to dimerize (33), we examined whether EGFP is involved in ABCA1 dimerization. The ratio of two-step photobleaching of immobile spots of ABCA1 fused with EGFP was not significantly different (P = 0.3613) compared with that of ABCA1 fused with mEGFP, suggesting that ABCA1 dimerization is not due to EGFP. Representative images of an individual spot before and after photobleaching are shown above the graph. Arrows indicate photobleaching dips in the fluorescence intensity. (B–D) Distributions of D100ms for ABCA1-EGFP spots (B), ABCA1-MM–EGFP spots (C), and ABCA1-EGFP spots 10–15 min after apoA-I addition (D).
Partial depolymerization of actin filaments by latrunculin A treatment decreased the immobile fraction to 52% and significantly (P = 0.0001) increased the median D100ms to 0.025 μm2/s (Table 1 and Fig. S2). These results suggest that actin cytoskeletons underneath the PM (actin-based membrane skeleton) are involved in ABCA1 dimer immobilization; however, it is likely that there are additional mechanisms for immobilizing ABCA1 dimers.
ApoA-I Increases ABCA1 Mobility and Releases ABCA1 Dimer to Monomers.
We found that the addition of a physiological concentration (5 μg/mL, 0.17 μM) of apoA-I released ABCA1 from an immobilized state (Fig. 1C and Movie S6). The immobile fraction of ABCA1 decreased from 69% to 38%, and the distribution of D100ms for ABCA1 became similar to that for ABCA1-MM in 10 min (Fig. 3A and Table 1). There was also a concomitant increase in the median D100ms (P < 0.0001) (Fig. 3B and Table 1) and mobile fraction (Fig. 3C) of ABCA1, whereas apoA-I addition did not influence the dynamics of ABCA1-MM (P = 0.40). In addition, 10–15 min after apoA-I application, 96% (74 out of 77) of ABCA1 spots exhibited single-step photobleaching (Fig. 2D). This timescale of 10 min is comparable to that of apoA-I binding to cells expressing ABCA1 (16). These results suggest that apoA-I, upon binding to ABCA1, induces dissociation of ABCA1 dimers into fast-diffusing monomers. This monomer dynamic behavior is similar to that of ATPase-deficient ABCA1-MM, which does not translocate lipids.
Fig. 3.
Time-dependent increase in ABCA1 mobility after apoA-I addition. ABCA1 or ABCA1-MM fused with EGFP was transiently expressed in HeLa cells. Nonlabeled apoA-I (5 μg/mL) was added to the medium and observed by TIRF microscope. (A) Distributions of D100ms for ABCA1 (red bars) and ABCA1-MM (black hatched bars) before and after apoA-I addition are shown. Percentages of immobile spots of ABCA1 are also shown. Red (ABCA1) and black (ABCA1-MM) arrowheads show the positions of the median value of D100ms. (B) Time-dependent changes in the D100ms median value of ABCA1 (red circles) or ABCA1-MM (black circles). (C) Time-dependent changes in the percentage of mobile spots of ABCA1 (red circles) or ABCA1-MM (black circles).
Lipid-Free ApoA-I Interacts with ABCA1.
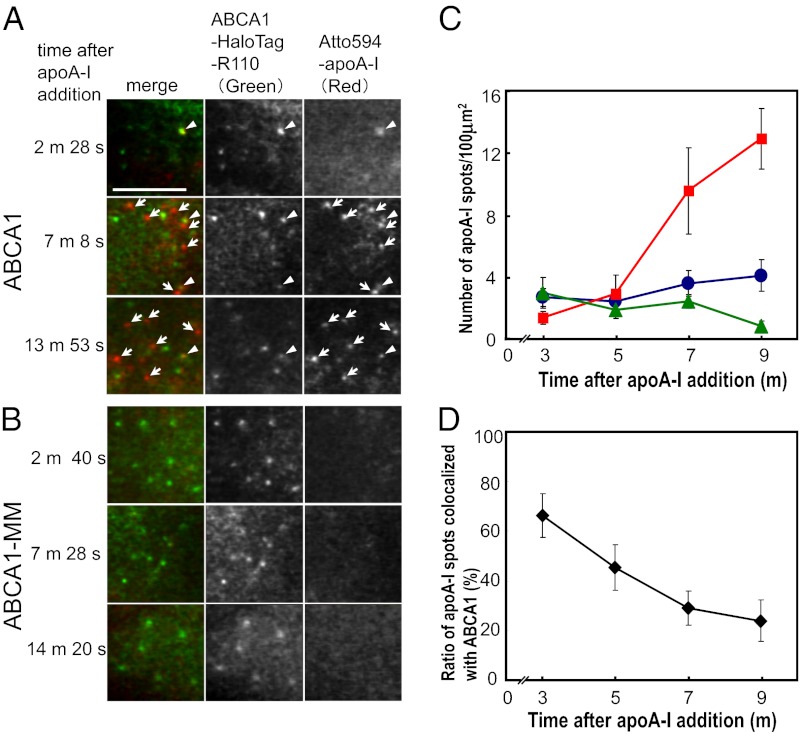
We confirmed apoA-I binding to ABCA1 at the level of single molecules. This observation was conducted in the upper PM via oblique illumination (rather than total internal reflection illumination; see Materials and Methods for details). Within 2–4 min after the addition of 3 nM Atto594–apoA-I to the medium, 66.5 ± 8.8% of apoA-I spots observed on the PM were colocalized with immobile ABCA1, which are most likely to be dimers (Fig. 4D; arrowheads in Fig. 4A). ApoA-I did not bind to cells expressing ABCA1-MM at levels similar to ABCA1 (Fig. 4B; green triangles in Fig. 4C); that is, apoA-I only binds to functional ABCA1.
Fig. 4.
Colocalization of ABCA1 and lipid-free apoA-I on the PM. (A and B) Simultaneous two-color single-molecule fluorescence imaging of ABCA1 (A) or ABCA1-MM (B) with apoA-I. ABCA1 was fused with Halo protein to its carboxyl terminus and labeled with rhodamine 110 (ABCA1-HaloTag-R110). ApoA-I was labeled with Atto594 (Atto594–apoA-I). Arrowheads indicate colocalization of ABCA1 and apoA-I. Arrows indicate apoA-I only (i.e., not colocalized with ABCA1). (Scale bar, 5 μm.) (C) Time-dependent changes in the number of apoA-I spots not colocalized with ABCA1 (red squares) and of apoA-I spots colocalized with ABCA1 (blue circles) on the PM of ABCA1-expressing cells per unit area (100 μm2). The total number of apoA-I spots on the PM of ABCA1-MM–expressing cells per unit area (100 μm2) is also shown (green triangles). ApoA-I spots (mean ± SE) at designated intervals (e.g., 2–4 min, plotted at 3 min; 4–6 min, plotted at 5 min) after apoA-I addition were observed (ABCA1, 6–8 cells; ABCA1-MM, 2–6 cells). (D) Time-dependent changes in the percentage of apoA-I spots colocalized with ABCA1 on the upper PM surface (mean ± SE) (see C for x-axis details).
The number of apoA-I spots that were located on the PM but were not colocalized with ABCA1 increased over time (arrows in Fig. 4A; red squares in Fig. 4C), whereas those that were colocalized with ABCA1 spots (arrowheads in Fig. 4A; blue circles in Fig. 4C) remained constant with time. Because lipid-free apoA-I binding occurs only to functional ABCA1 and not on the general PM surface, our results suggest that apoA-I loaded with lipids by ABCA1 dissociates from ABCA1 and in turn spontaneously binds to the general PM surface (Fig. S3). This explanation is consistent with previous observations in which apoA-I dissociates from ABCA1 within 1 min after binding (16); apoA-I undergoes a conformational transition in response to lipids (17); and lipidated apoA-I can no longer interact with ABCA1 (5, 18).
Discussion
Using single-molecule fluorescence imaging methods, we succeeded in revealing dimer-to-monomer and immobile-to-mobile interconversion of ABCA1 molecules during the initial steps of HDL formation. Based on the present results, we propose the following working hypothesis for dHDL formation (Fig. 5). ABCA1 monomers that have not reserved sufficient lipids constantly translocate lipids as transporters in an ATP-dependent manner, even in the absence of apoA-I, and diffuse freely in the PM (step 1). As ABCA1 reserves cholesterol and phospholipids, perhaps within its large ECDs and/or its vicinity in the PM, the molecule undergoes conformational changes (11), consequently forming dimers. These lipidated ABCA1 dimers interact with the membrane–skeletal actin filaments and other stable structures in the PM, leading to the halt of diffusion, and become ready for apoA-I access (step 2). Lipid-free apoA-I directly binds to the ECD(s) of the ABCA1 dimers (step 3), but not that of monomers, consistent with a previous report (19), and becomes loaded with lipids reserved by ABCA1 (step 4). Because dHDL is composed of 100–200 lipid molecules surrounded by two apoA-I monomers, it is reasonable that ABCA1 would form dimers with its reserved lipids and be ready to interact with two apoA-I molecules. The conformational transition of apoA-I caused by lipid loading might facilitate its dissociation from ABCA1. By losing the reserved lipids, the ABCA1 dimer dissociates into monomers, is released from immobilization, and resumes diffusion in the PM, again to reserve lipids by constantly translocating them in an ATP-dependent manner (step 1). Meanwhile, the lipid-loaded apoA-I spontaneously interacts with the PM and is in equilibrium between the PM and the media (step 5).
Fig. 5.
Schematic drawing of HDL formation by ABCA1. ABCA1 monomer diffuses freely and translocates lipids on the PM in an ATPase-dependent manner (1). ABCA1, which reserves lipids within or nearby, is immobilized as a dimer by being tethered to actin cytoskeletons (2). Lipid-free apoA-I binds to the ABCA1 dimer (3). Lipids are loaded onto apoA-I, and the ABCA1 dimer dissociates into monomers (4). Lipidated apoA-I spontaneously binds to the PM and is in equilibrium between the PM and the media (5).
In general, receptor dimerization or oligomerization is important for many signaling pathways, often as the first step for inducing intracellular signals upon ligand binding (20, 21). Furthermore, even in the absence of extracellular stimulation, many receptors have been proposed to form dimers, including epidermal growth factor receptor (22) and a number of G protein-coupled receptors (GPCRs) (23–28), to facilitate stimulation-induced dimerization and multimerization. Therefore, dimer–monomer interconversion is important for understanding signal transduction, and the dynamic equilibrium between monomers and dimers of a GPCR has recently been fully characterized (15).
In contrast, dimer–monomer interconversion of transporters has not drawn much attention, although some transporters are known to function as oligomers. Here, by using single-molecule fluorescence imaging, we showed that a dimer–monomer interconversion cycle of ABCA1 occurs during dHDL generation and depends on ATPase activity and lipid transport. We determined that about 70% of total ABCA1-GFP spots functioning on the PM are immobilized, and estimated that about 89% of immobile ABCA1 molecules are in dimers, which dissociate into monomers after apoA-I binding. In this study, we succeeded in quantifying monomer–dimer interconversion of a transporter at the single-molecule level. The shift from immobile ABCA1 dimer to diffusing monomers after transferring lipids onto apoA-I provides insights into the dimerization mechanism of a membrane protein that depends on its lipid-interacting status. The formation of ABCA1 dimer is the key for apoA-I binding and dHDL generation. Thus, ABCA1 dimerization has physiological significance for cholesterol homeostasis in the body and, furthermore, dissecting the dimer and monomer dynamics is necessary to understand ABCA1 function. Our findings reveal a mechanism by which lipids are transported and HDL is generated by ABCA1 in the context of dimer–monomer interconversion.
Previous reports have suggested that ABCA1 forms dimers that undergo transition into higher-order structures, such as tetramers, during the ATP catalytic cycle by native PAGE, chemical cross-linking, and fluorescence resonance energy transfer experiments (19, 29). However, the oligomeric structure is not fully resolved at the single-molecule level in living cells, and it is not clear how oligomerization of ABCA1 is regulated. Because the expression levels of ABCA1 were kept very low (<0.3 copies/μm2) for single-molecule imaging in the present study, we observed very limited numbers of oligomer-like bright spots before apoA-I application. The interconversions of ABCA1 between dimers and monomers observed here occurred on the PM, likely to be independent of the endocytic pathway (30, 31). The observation that an ATPase-deficient ABCA1 mutant, ABCA1-MM, which is unable to generate HDL, did not form dimers or show immobilization on the PM implies an obvious medical impact that dimerization of ABCA1 is physiologically critical. The inconsistency between our data and a previous report by Trompier et al. (29) in which ABCA1-MM forms a dimer, tetramer, and an even higher supramolecular assembly could be due to differences in methodologies. For instance, a protein overexpression system might affect the oligomeric state. Single-molecule fluorescence imaging allowed us to directly observe ABCA1 dimers and monomers at the level of single molecules on the PM, which could not have been achieved solely by biochemical analysis such as native PAGE or chemical cross-linking. The assembly state of another ABC protein, the cystic fibrosis transmembrane conductance regulator (CFTR), was also controversial as a result of indirect evidence that suggested CFTR could exist as monomers, dimers, or higher-order oligomers. However, fluorescence-intensity measurement and analysis of photobleaching dynamics of CFTR fused with GFP by Haggie and Verkman revealed that CFTR is monomeric (32), where single-molecule fluorescence imaging was beneficial for verifying the oligomeric state of a membrane protein as well as in our study.
Simultaneous observation of apoA-I and ABCA1 revealed that apoA-I binds to the dimer form of ABCA1 and dissociates from ABCA1 after being loaded with lipids. In turn, lipid-loaded apoA-I binds to the general PM surface independently of ABCA1. The equilibrium of lipid-loaded apoA-I between the membrane surface and the medium could lead to dHDL generation. We successfully visualized the initial steps of the dHDL generation process mediated by ABCA1 and apoA-I.
We found that ABCA1 is unique as it forms a dimer via lipid translocation and dissociates into monomers upon apoA-I binding during HDL generation. We clarified the relationship between the function and dynamics of a transporter, and succeeded in characterizing the mobility of dimeric and monomeric ABCA1. In addition to the photobleaching analysis, the mobility of the ABCA1 molecule will be an informative indicator and parameter for discerning dimers and monomers. The dimerization of ABCA1 is critical to dHDL generation; the ABCA1 dimer could serve as a scaffold for two apoA-I molecules to interact, come together, and receive lipids from ABCA1 for dHDL generation. It is known that HDL generation is regarded as the most important event for preventing atherosclerosis, and that reverse cholesterol transport mediated by HDL is the only way to retrieve excess cholesterol from peripheral tissues to the liver. Thus, our findings provide insight into the mechanism of HDL generation and may have therapeutic potential for atherosclerosis and other disorders.
Materials and Methods
Cell Culture, cDNA Construction, and Expression in HeLa Cells.
HeLa cells were cultured in minimum essential medium Eagle (Sigma) supplemented with 10% (vol/vol) FBS at 37 °C in 5% CO2. Human ABCA1 and ABCA1-MM (with two point mutations of K939M and K1952M) were fused with EGFP or monomeric (m)EGFP at their carboxyl termini, where an mEGFP mutant was modified from EGFP by introducing a mutation corresponding to an A206K monomeric mutant (33). For further experiments, HaloTag (Promega) with the appropriate linker (SRGSPGSIATINTTHYRASKAT) was used to label ABCA1 at its carboxyl terminus. HeLa cells were transfected with expression vectors encoding labeled ABCA1 or ABCA1-MM, using Lipofectamine and Plus Reagent or Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The cells were seeded in a glass-base dish (35-mm diameter with a window diameter of 12 mm, 0.15-mm-thick glass; Iwaki) and cultured for 2 d before observation.
Preparation of Atto594–ApoA-I.
Purified recombinant apoA-I expressed in Escherichia coli was incubated with Atto594 NHS ester (ATTO-TEC) in carbonate buffer (pH 8.3) (1 h) followed by fractionation using PD-10 columns (Amersham). The dye:protein ratio was calculated to be between 2.5 and 5.0 by measuring the absorbance at 280 and 601 nm. Alexa546–apoA-I was prepared as previously described (30).
Single-Molecule Fluorescence Tracking and Diffusion Analysis.
Single-molecule fluorescence tracking was performed as previously described (15, 34). In brief, a home-built, objective lens-type TIRF microscope based on a Nikon TE2000-PFS was used. HeLa cells expressing ABCA1-(m)EGFP were observed at 37 °C in HBSS supplemented with 2 mM Hepes (pH 7.4) and 0.02% lipid-free BSA. For most of our experiments, the bottom cell membrane was locally illuminated with an evanescent field (a Nikon 100×, 1.49 NA objective lens was mainly used, but an Olympus 100×, 1.49 NA objective lens was also used by combining an attachment for several experiments; total magnifications on the camera chip were 400× and 444×, respectively), as described (14, 35–37). To observe the apical membrane, we applied a highly inclined and laminated optical sheet illumination method (38). The fluorescence from labeled ABCA1 was detected on the appropriate channel on the simultaneous observation system. For simultaneous observation, the fluorescent images in each channel were projected onto a two-stage microchannel plate intensifier (C8600-03; Hamamatsu Photonics), and the lens coupled to an electron bombardment charge-coupled device camera was operated at video rate (C7190-23; Hamamatsu Photonics). The movies were recorded on a digital videotape (PDV-184ME; Sony).
Quantitative analysis of ABCA1 movement was performed based on mean square displacement (MSD) methods described previously (39–43). For each trajectory of a particle, MSD, <(Δr(Δt))2>, every time interval was calculated according to the equation (41, 44)
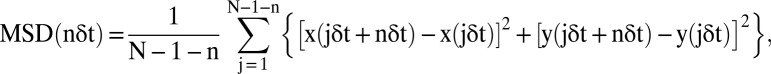 |
where δt is the time resolution, (x(jδt + nδt), y(jδt + nδt)) describes the particle’s position after a time interval Δtn = nδt after starting at position (x(jδt), y(jδt)), N is the total number of frames in the sequence, n and j are positive integers, and n determines the time increment.
Determination of the Number of ABCA1 Molecules in a Single Spot by Photobleaching Step Analysis.
Each individual fluorescent spot was identified via a homemade computer program as described previously (15, 40). Until the fluorescent spot was photobleached, the fluorescence signal intensities of distinguishable fluorescent spots were tracked frame by frame in a 740 × 740-nm2 area containing the single spot, and then normalized by subtracting the background intensity of an adjacent 740 × 740-nm2 area (14, 35, 36). The number of photobleaching steps was counted from obtained fluorescence signal intensity traces (45).
Simultaneous Observation of ApoA-I and ABCA1 at the Single-Molecule Level.
For labeling HaloTag with an organic dye, HeLa cells transiently expressing Halo-tagged ABCA1 or ABCA1-MM were incubated with a fluorescent Halo ligand (HaloTag R110 Direct; Promega) for 15 min. After washing with culture medium and incubation for 30 min, cells were again washed with HBSS. Atto594-conjugated apoA-I (obtained dye:protein ratio 2.5–5.0) was added to the observation medium at a concentration of 3 nM, and the cells were observed. To prevent nonspecific binding of apoA-I to glass and cell surfaces, HBSS was supplemented with 0.05% PEG 20K as a blocking reagent. However, because single-molecule fluorescence observation is still difficult for the basal membrane as a result of nonspecifically bound fluorescent apoA-I on the glass surface, we chose to use the apical cell membrane for this observation only and used highly inclined and laminated optical sheet illumination (38). After Atto594–apoA-I and ABCA1-HaloTag-R110 (rhodamine 110) were simultaneously observed by two individual cameras, which were separately equipped on fluorescence detection arms, the two video sequences were superimposed frame by frame after a correction factor was applied (14). The number of colocalizations of apoA-I with ABCA1 was evaluated via methodology developed by Koyama-Honda et al. (14), in which colocalization was defined by overlapping centers (within 150 nm) of the two molecules.
Statistical Analysis.
Values are presented as means ± SD. A Mann–Whitney U test was used to assess the difference between the median values of D100ms. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This research was supported in part by a Japan Society for the Promotion of Science grant (20228001); the Naito Foundation; the Program for Promotion of Basic and Applied Researches for Innovations in Bio-Oriented Industry of Japan; and the World Premier International Research Center Initiative, Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220703110/-/DCSupplemental.
References
- 1.Khera AV, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Kijac AZ, Sligar SG, Rienstra CM. Structural analysis of nanoscale self-assembled discoidal lipid bilayers by solid-state NMR spectroscopy. Biophys J. 2006;91(10):3819–3828. doi: 10.1529/biophysj.106.087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segrest JP, et al. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J Biol Chem. 1999;274(45):31755–31758. doi: 10.1074/jbc.274.45.31755. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14(9):861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 5.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275(42):33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 6.Bodzioch M, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22(4):347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 7.Rust S, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22(4):352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 8.Singaraja RR, Brunham LR, Visscher H, Kastelein JJ, Hayden MR. Efflux and atherosclerosis: The clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler Thromb Vasc Biol. 2003;23(8):1322–1332. doi: 10.1161/01.ATV.0000078520.89539.77. [DOI] [PubMed] [Google Scholar]
- 9.Oram JF, Vaughan AM. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99(10):1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 10.Brunham LR, Singaraja RR, Hayden MR. Variations on a gene: Rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu Rev Nutr. 2006;26:105–129. doi: 10.1146/annurev.nutr.26.061505.111214. [DOI] [PubMed] [Google Scholar]
- 11.Nagao K, et al. ATP hydrolysis-dependent conformational changes in the extracellular domain of ABCA1 are associated with apoA-I binding. J Lipid Res. 2012;53(1):126–136. doi: 10.1194/jlr.M019976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka AR, et al. Human ABCA1 contains a large amino-terminal extracellular domain homologous to an epitope of Sjögren’s syndrome. Biochem Biophys Res Commun. 2001;283(5):1019–1025. doi: 10.1006/bbrc.2001.4891. [DOI] [PubMed] [Google Scholar]
- 13.Hozoji M, Kimura Y, Kioka N, Ueda K. Formation of two intramolecular disulfide bonds is necessary for ApoA-I-dependent cholesterol efflux mediated by ABCA1. J Biol Chem. 2009;284(17):11293–11300. doi: 10.1074/jbc.M900580200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama-Honda I, et al. Fluorescence imaging for monitoring the colocalization of two single molecules in living cells. Biophys J. 2005;88(3):2126–2136. doi: 10.1529/biophysj.104.048967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasai RS, et al. Full characterization of GPCR monomer-dimer dynamic equilibrium by single molecule imaging. J Cell Biol. 2011;192(3):463–480. doi: 10.1083/jcb.201009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagao K, Zhao Y, Takahashi K, Kimura Y, Ueda K. Sodium taurocholate-dependent lipid efflux by ABCA1: Effects of W590S mutation on lipid translocation and apolipoprotein A-I dissociation. J Lipid Res. 2009;50(6):1165–1172. doi: 10.1194/jlr.M800597-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson WS, Thompson TB. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem. 2007;282(31):22249–22253. doi: 10.1074/jbc.R700014200. [DOI] [PubMed] [Google Scholar]
- 18.Mulya A, et al. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler Thromb Vasc Biol. 2007;27(8):1828–1836. doi: 10.1161/ATVBAHA.107.142455. [DOI] [PubMed] [Google Scholar]
- 19.Denis M, et al. Characterization of oligomeric human ATP binding cassette transporter A1. Potential implications for determining the structure of nascent high density lipoprotein particles. J Biol Chem. 2004;279(40):41529–41536. doi: 10.1074/jbc.M406881200. [DOI] [PubMed] [Google Scholar]
- 20.Weiss A, Schlessinger J. Switching signals on or off by receptor dimerization. Cell. 1998;94(3):277–280. doi: 10.1016/s0092-8674(00)81469-5. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Holowka D, Craighead HG, Baird B. Visualization of plasma membrane compartmentalization with patterned lipid bilayers. Proc Natl Acad Sci USA. 2004;101(38):13798–13803. doi: 10.1073/pnas.0403835101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung I, et al. Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature. 2010;464(7289):783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- 23.Angers S, et al. Detection of beta 2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET) Proc Natl Acad Sci USA. 2000;97(7):3684–3689. doi: 10.1073/pnas.060590697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harding PJ, et al. Constitutive dimerization of the G-protein coupled receptor, neurotensin receptor 1, reconstituted into phospholipid bilayers. Biophys J. 2009;96(3):964–973. doi: 10.1016/j.bpj.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones KA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396(6712):674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 26.Kaupmann K, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396(6712):683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 27.Meyer BH, et al. FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc Natl Acad Sci USA. 2006;103(7):2138–2143. doi: 10.1073/pnas.0507686103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White JH, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396(6712):679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 29.Trompier D, et al. Transition from dimers to higher oligomeric forms occurs during the ATPase cycle of the ABCA1 transporter. J Biol Chem. 2006;281(29):20283–20290. doi: 10.1074/jbc.M601072200. [DOI] [PubMed] [Google Scholar]
- 30.Azuma Y, et al. Retroendocytosis pathway of ABCA1/apoA-I contributes to HDL formation. Genes Cells. 2009;14(2):191–204. doi: 10.1111/j.1365-2443.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi Y, Smith JD. Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc Natl Acad Sci USA. 1999;96(20):11358–11363. doi: 10.1073/pnas.96.20.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haggie PM, Verkman AS. Monomeric CFTR in plasma membranes in live cells revealed by single molecule fluorescence imaging. J Biol Chem. 2008;283(35):23510–23513. doi: 10.1074/jbc.C800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296(5569):913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 34.Tokunaga M, Kitamura K, Saito K, Iwane AH, Yanagida T. Single molecule imaging of fluorophores and enzymatic reactions achieved by objective-type total internal reflection fluorescence microscopy. Biochem Biophys Res Commun. 1997;235(1):47–53. doi: 10.1006/bbrc.1997.6732. [DOI] [PubMed] [Google Scholar]
- 35.Iino R, Koyama I, Kusumi A. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys J. 2001;80(6):2667–2677. doi: 10.1016/S0006-3495(01)76236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakoshi H, et al. Single-molecule imaging analysis of Ras activation in living cells. Proc Natl Acad Sci USA. 2004;101(19):7317–7322. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakada C, et al. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat Cell Biol. 2003;5(7):626–632. doi: 10.1038/ncb1009. [DOI] [PubMed] [Google Scholar]
- 38.Tokunaga M, Imamoto N, Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat Methods. 2008;5(2):159–161. doi: 10.1038/nmeth1171. [DOI] [PubMed] [Google Scholar]
- 39.Powles JG, Mallett MJD, Rickayzen G, Evans WAB. Exact analytic solutions for diffusion impeded by an infinite array of partially permeable barriers. Proc R Soc Lond A. 1992;436(1897):391–403. [Google Scholar]
- 40.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157(6):1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kusumi A, Sako Y, Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys J. 1993;65(5):2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sako Y, Kusumi A. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J Cell Biol. 1994;125(6):1251–1264. doi: 10.1083/jcb.125.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomishige M, Sako Y, Kusumi A. Regulation mechanism of the lateral diffusion of band 3 in erythrocyte membranes by the membrane skeleton. J Cell Biol. 1998;142(4):989–1000. doi: 10.1083/jcb.142.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian H, Sheetz MP, Elson EL. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 1991;60(4):910–921. doi: 10.1016/S0006-3495(91)82125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulbrich MH, Isacoff EY. Subunit counting in membrane-bound proteins. Nat Methods. 2007;4(4):319–321. doi: 10.1038/NMETH1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.