Abstract
Most opsins selectively bind 11-cis retinal as a chromophore to form a photosensitive pigment, which underlies various physiological functions, such as vision and circadian photoentrainment. Recently, opsin 3 (Opn3), originally called encephalopsin or panopsin, and its homologs were identified in various tissues including brain, eye, and liver in both vertebrates and invertebrates, including human. Because Opn3s are mainly expressed in tissues that are not considered to contain sufficient amounts of 11-cis retinal to form pigments, the photopigment formation ability of Opn3 has been of interest. Here, we report the successful expression of Opn3 homologs, pufferfish teleost multiple tissue opsin (PufTMT) and mosquito Opn3 (MosOpn3) and show that these proteins formed functional photopigments with 11-cis and 9-cis retinals. The PufTMT- and MosOpn3-based pigments have absorption maxima in the blue-to-green region and exhibit a bistable nature. These Opn3 homolog-based pigments activate Gi-type and Go-type G proteins light dependently, indicating that they potentially serve as light-sensitive Gi/Go-coupled receptors. We also demonstrated that mammalian cultured cells transfected with the MosOpn3 or PufTMT became light sensitive without the addition of 11-cis retinal and the photosensitivity retained after the continuous light exposure, showing a reusable pigment formation with retinal endogenously contained in culture medium. Interestingly, we found that the MosOpn3 also acts as a light sensor when constituted with 13-cis retinal, a ubiquitously present retinal isomer. Our findings suggest that homologs of vertebrate Opn3 might function as photoreceptors in various tissues; furthermore, these Opn3s, particularly the mosquito homolog, could provide a promising optogenetic tool for regulating cAMP-related G protein-coupled receptor signalings.
Keywords: rhodopsin, phototransduction, nonvisual photoreception, opsin diversity
Most opsins bind 11-cis retinal as a chromophore to form a photosensitive pigment (opsin-based pigment) that serves as a light-sensitive G protein-coupled receptor (GPCR). The 11-cis to all-trans isomerization of the chromophore in an opsin-based pigment upon light absorption triggers G protein activation (1–4), and the photoreception of the opsin-based pigment initiates vision and nonvisual functions, including circadian entrainment and pupil responses. Several thousand opsins have been identified, and they are phylogenetically classified into eight groups based on the members that have existed early in animal evolution (5). The phylogenetic classification of each opsin also roughly corresponds to its molecular function. The Gt-coupled opsin group contains vertebrate visual pigments, which activate transducin (Gt)-type G proteins, and some nonvisual pigments, such as pinopsin, VA-opsin, parapinopsin, and parietopsin (1–4). The Gq-coupled opsin group is composed of the visual pigments from many invertebrates, such as insects and cephalopods, and the vertebrate opsin 4 (Opn4) homologs (melanopsins), which function as circadian photoreceptors in mammals (6). The scallop Go-coupled rhodopsin and amphioxus rhodopsin comprise the Go-coupled opsin group (7, 8), and vertebrate opsin 5 (Opn5) was revealed to activate Gi-type G proteins (9, 10). In addition to these bilaterian opsins, we discovered a Gs-coupled opsin in the visual cells of prebilaterian jellyfish (5). In contrast to these opsins, the members of the retinochrome and peropsin groups preferentially bind all-trans retinal, and light absorption causes all-trans to 11-cis isomerization (8, 11–13). Therefore, the members of these two groups are considered to be retinal photoisomerases that produce 11-cis retinal. Collectively, opsins from seven of the eight phylogenetic groups have been characterized to date.
The Opn3 group is the final distinct group, containing mammalian ospin 3 (Opn3), originally called encephalopsin or panopsin, teleost multiple tissue (TMT) opsin, insect pteropsin, and annelid c-opsin (14–17). Despite the wide distribution of Opn3 homologs from vertebrates to invertebrates, which is similar to that of Gq-coupled opsins, the molecular properties of these proteins have not yet been revealed. Therefore, the characterization of the Opn3 group is the last remaining requirement for an overall understanding of the diversity of opsin-based pigments. In addition, the molecular properties of members of the Opn3 group are important for an understanding of the evolution of the Gt-coupled opsins, including vertebrate visual pigments, because the Opn3 group forms the sister group to the Gt-coupled opsin group. More interestingly, Opn3 and its homologs are expressed in various tissues, such as the brain (human, mouse, pufferfish, zebrafish, honey bee, and annelid), liver (human and pufferfish), kidney (human and zebrafish), and heart (zebrafish), in addition to the eye (human, pufferfish, and zebrafish) (14–17). Because Opn3 homologs are expressed in tissues that are not considered photosensitive, it will be important to determine whether they form photopigments. Recently, it was reported that the introduction of the zebrafish TMT gene to cavefish fin cells, which were suggested to have originally been photosensitive for circadian photoentrainment but to have lost their photosensitivity during evolution, restored the circadian photoentrainment capacity of the cells, suggesting that Opn3 homologs can serve as light-sensor proteins (18). Therefore, the molecular properties of Opn3 homologs should be investigated to know their potential functionality for light sensors in nonphotoreceptor cells. Here, we report that Opn3 homologs of vertebrate and invertebrate form functional blue- and green-sensitive photosensitive pigments with 11-cis retinal, respectively, and activate Gi- and Go-type G proteins in a light-dependent manner. Furthermore, we found that mammalian cultured cells transfected with the Opn3 homologs became light sensitive without the addition of 11-cis retinal, indicating that the Opn3 homologs formed pigments by binding retinal endogenously present in the culture medium. In addition, the invertebrate Opn3 homolog acts as a photopigment when bound to 13-cis retinal, which is thermally equilibrated with all-trans retinal and, therefore, ubiquitously present in animals (19, 20). These results suggest that Opn3 homolog-expressing tissues may be photosensitive. Based on these unique properties, we also propose that Opn3 homologs, especially the invertebrate homolog, may have an optogenetic potential for regulating cAMP-related GPCR signaling.
Results
Bistable Photopigment Opn3.
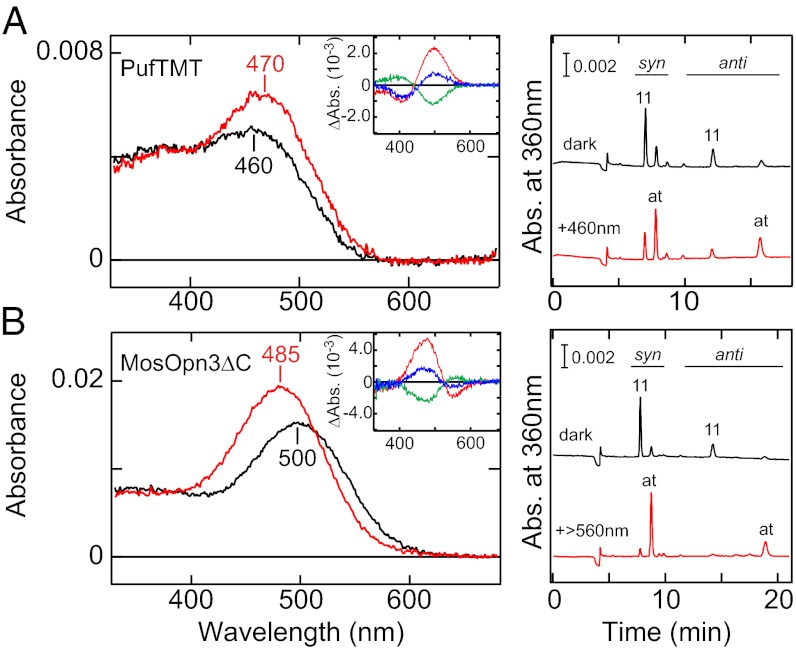
To examine whether the members of the Opn3 group form photosensitive pigments, we expressed the pufferfish (Takifugu rubripes) Opn3 homolog TMT (PufTMT) and the mosquito (Anopheles stephensi) Opn3 homolog (MosOpn3) as representatives of vertebrate and invertebrate members of the Opn3 group, respectively, in mammalian cultured cells. We succeeded in obtaining purified pigments for both Opn3 homologs after incubation with 11-cis retinal. The PufTMT-based pigment is a blue-sensitive pigment, with an absorption maximum at ∼460 nm in the dark (Fig. 1A). Irradiation with blue light resulted in a slightly red-shifted spectrum with a larger extinction coefficient and an absorption maximum at ∼470 nm, in the visible light region, which is distinct from bleaching pigments such as vertebrate visual pigments. Although the expression level of the MosOpn3-based pigment was too low to determine its absorption maximum, light irradiation caused a significant change in its absorption spectrum, indicating the successful expression of the MosOpn3-based pigment (Fig. S1). Because we had previously reported that the C-terminal truncation of long-tailed opsins increased the purification efficiency of the pigment (21), we constructed a deletion mutant of the MosOpn3 that has a shorter C terminus and expressed this mutant in HEK293 cells. The C-terminal truncation resulted in a more than 50-fold higher yield of the purified MosOpn3-based pigment, which allowed us to determine the absorption maximum of the MosOpn3-based pigment at ∼500 nm (Fig. 1B). Irradiation with green light resulted in a blue-shifted spectrum with a larger extinction coefficient and an absorption maximum at 485 nm. The difference spectrum of the dark minus irradiated MosOpn3-based pigment matched well with that of the full-length MosOpn3-based pigment (Fig. S1, Inset), indicating that the C-terminal truncation did not alter the spectroscopic features of the Opn3 homolog. Accordingly, we concluded that the MosOpn3-based pigment is a green-sensitive pigment with a peak absorbance at ∼500 nm and used the C-terminal truncated MosOpn3 for most of further studies because of its higher expression in the cultured cells.
Fig. 1.
Photochemical properties of PufTMT- and C-terminal truncated MosOpn3-based pigments. (A) PufTMT-based pigment. (B) C-terminal truncated MosOpn3 (MosOpn3ΔC)-based pigment. (Left) Absorption spectra of purified Opn3 homolog-based pigments in the dark (black curves) and after irradiation with blue light (PufTMT) or green light (MosOpn3ΔC) (red curves). (Insets) Spectral changes in lipid-containing extracts of Opn3 homolog-expressing HEK293 cells caused by the first (red curves), second (green curves), and third (blue curves) irradiations. Blue-orange-blue light and green-blue-green light were applied as the first-second-third irradiations of the PufTMT- and MosOpn3ΔC-based pigments, respectively. (Right) Chromophore configurations of purified Opn3-based pigments in the dark state (black traces) and after light irradiation (red traces). It should be noted that the peak absorbance of photoproducts of the purified Opn3 homolog-based pigments decreased within minutes, unlike the case of lipid-containing samples.
We also analyzed the chromophore configurations of the dark and irradiated PufTMT- and MosOpn3-based pigments. HPLC analyses showed that both the PufTMT- and MosOpn3-based pigments bound to 11-cis retinal in the dark and that light irradiation caused 11-cis to all-trans isomerization (Fig. 1). This photoisomerization profile is similar to that of the chromophores of the opsin-based pigments that activate G proteins, including visual pigments. When constituted with 9-cis retinal, the PufTMT- and MosOpn3-based pigments exhibited absorption maxima at ∼450 nm and 490 nm, respectively (Fig. S2), which are ∼10 nm blue shifted, as demonstrated for the vertebrate visual pigment rhodopsin (22).
The photoproduct properties of Opn3 homolog-based pigments are of interest because the vertebrate visual pigments in the Gt-coupled opsin group, the sister group of the Opn3 group, bleach upon light absorption; in contrast, most opsin-based pigments have bistable nature and do not bleach. We then analyzed the nature of the Opn3 homolog-based pigment photoproducts more carefully in lipid-containing extracts from Opn3 homolog-expressing HEK293 cells. Blue light irradiation of the PufTMT-based pigment caused an increase in absorbance at ∼500 nm and a decrease at ∼400 nm (Fig. 1A, Inset). Subsequent orange light irradiation caused the opposite spectral change, an increase in absorbance at ∼400 nm and a decrease at ∼500 nm. A second blue light irradiation resulted in a spectral change that was the mirror image of that caused by the orange light irradiation, clearly demonstrating the bistable nature of the pigment. The MosOpn3-based pigment also exhibited a bistable nature: green light irradiation caused an increase in absorption at ∼480 nm and a decrease at ∼530 nm, and the subsequent blue and the second green irradiation resulted in photoreactions that mirrored one another (Fig. 1B, Inset). These spectral changes demonstrated that PufTMT- and MosOpn3-based pigments are bistable photosensitive pigments.
Gi/Go Activation of Opn3 Homolog-Based Pigments.
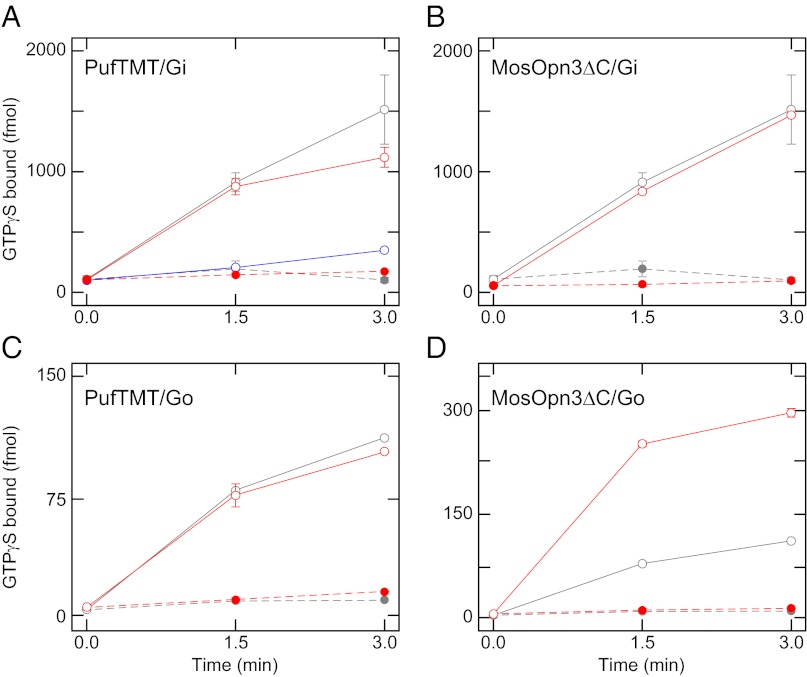
We examined the G protein activation ability of the PufTMT- and MosOpn3-based pigments because opsin-based pigments require to activate G proteins in a light-dependent manner for acting as light-sensor molecules. Because previous studies revealed that opsin-based pigments activate Gi (including Gi, Gt, and Go)-, Gq-, or Gs-type G protein, major G protein types, we investigated whether the Opn3 homolog-based pigments could activate each type of G protein. Neither Opn3 homolog-based pigment showed Gq or Gs activation ability in the experimental conditions (Fig. S3). Both Opn3 homolog-based pigments activated Gi and Go efficiently, as did bovine rhodopsin (Fig. 2, Fig. S4, and ref. 23), although the Opn3 homologs did not activate Gt efficiently in the conditions (Figs. S3 and S4).
Fig. 2.
Gi and Go activation by PufTMT- and C-terminal trunctaed MosOpn3-based pigments. The open and filled circles indicate G protein activation in the presence of the irradiated and nonirradiated pigments, respectively. (A and B) The Gi-activation efficiencies of the PufTMT- (A, blue circles) and C-terminal truncated MosOpn3 (MosOpn3ΔC)- (B, red circles) based pigment were compared with that of bovine rhodopsin (gray circles) at the same pigment concentration. The Gi activation efficiency of ∼10-fold concentration of PufTMT-based pigment is also shown (A, red circles). Note that the activity of nonirradiated PufTMT-based pigment (×1) is overlapped by that of ∼10 fold concentration of the nonirradiated pigment (red filled circles). (C and D) The Go-activation efficiencies of the PufTMT- (C, red circles) and MosOpn3ΔC- (D, red circles) based pigments were compared with that of bovine rhodopsin (gray circles) at the same pigment concentration. It should be noted that the full-length MosOpn3-based pigment exhibited virtually the same profile of G protein activation (Fig. S4), indicating that C terminus of the MosOpn3 did not markedly affect G protein selectivity.
Introduction of Photosensitivity to Cultured Cells by Opn3 Homolog Expression.
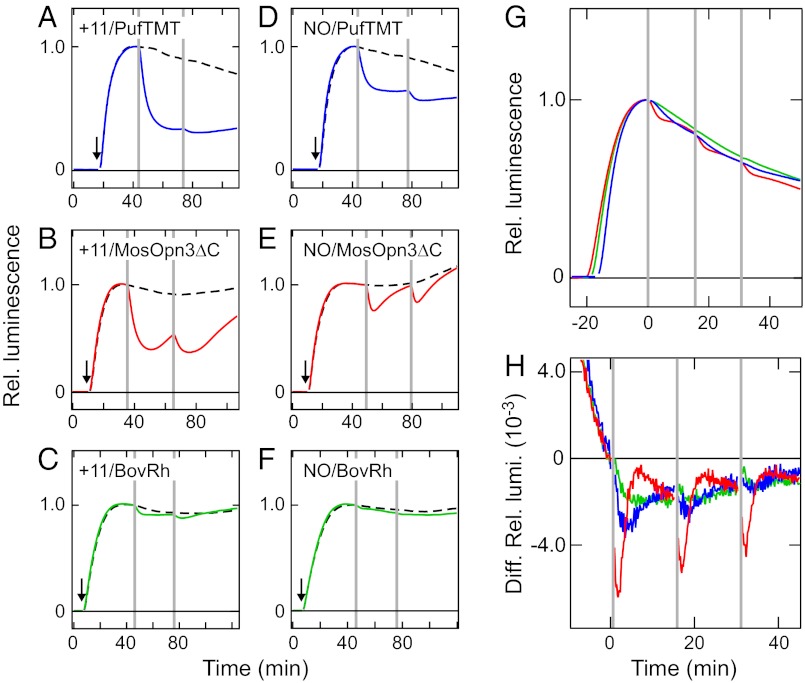
We next examined whether the expression of PufTMT and MosOpn3 can introduce photosensitivity into HEK293 cells without adding 11-cis retinal because mRNAs of Opn3 homologs are present in various extraocular “nonphotoreceptive” tissues (14–17) and their translated protein could be functional by binding to retinoids in the serum (20). As shown in Fig. 2, our in vitro experiments indicated that the Opn3 homolog-based pigments, like bovine rhodopsin, activate Gi, which generally leads to a decrease in intracellular cAMP concentration through the inhibition of adenylyl cyclase. Thus, we analyzed the changes in cAMP level in the cultured cells expressing the PufTMT or MosOpn3 upon light irradiation by using the GloSensor cAMP assay, which is based on a cAMP-dependent luciferase. When the PufTMT-expressing cells and MosOpn3-expressing cells were incubated in the serum-containing culture medium with or without 11-cis retinal in the dark, the luminescent intensity increased by forskolin treatment decreased markedly by light irradiation (Fig. 3 A, B, D, and E). However, small and faint decreases were detected in the bovine rhodopsin-expressing cells incubated with and without 11-cis retinal, respectively (Fig. 3 C and F). The significantly larger decrease in cAMP might be due to bistable nature, a sustained G protein activation, of the Opn3 homologs. Interestingly, the PufTMT- and MosOpn3-expressing cells that were maintained without the addition of 11-cis retinal in the room light also exhibited light-induced cAMP decreases (Fig. 3 G and H), showing a reusable pigment formation with retinal endogenously contained in culture medium. The efficiency of the MosOpn3 in decreasing cAMP was greater than that of PufTMT. However, the bovine rhodopsin-expressing cells showed no cAMP decrease in this condition (Fig. 3 G and H). These results suggest that PufTMT and MosOpn3 bound to the retinal that was present in the serum-containing culture medium and retained their functionality even after the continuous light exposure, possibly because of their bistable nature.
Fig. 3.
Light-induced cAMP decrease in HEK293 cells expressing PufTMT or C-terminal truncated MosOpn3. Light-induced decreases in luminescence signals, which represent the cAMP level, were observed in PufTMT-expressing (A and D, blue traces), C-terminal truncated MosOpn3 (MosOpn3ΔC)-expressing (B and E, red traces) and bovine rhodopsin (BovRh)-expressing (C and F, green traces) HEK293 cells incubated in the culture medium with (A–C) or without (D–F) 11-cis retinal in the dark. The luminescence signals of nonirradiated cells are also shown as a control (broken traces). The arrows and vertical lines indicate forskolin treatments and green light irradiations, respectively. It should be noted that the culture medium contains a small amount of “endogenous” retinal that is originally present in the serum. (G) Changes of luminescence signals upon green light irradiation of HEK293 cells expressing the PufTMT (blue trace), MosOpn3ΔC (red trace), and BovRh (green trace) maintained in the room light, without the addition of 11-cis retinal to the culture medium. (H) Differential values indicating the rate of the luminescence changes shown in G. The luminescence values were normalized to those just before the irradiations.
13-cis Retinal Binding Ability of MosOpn3.
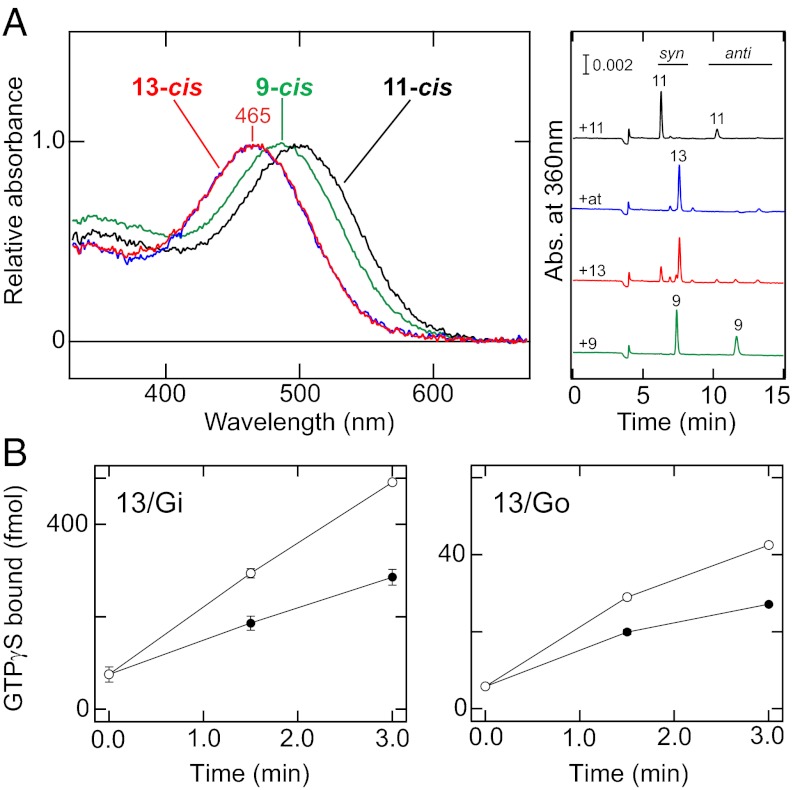
It is widely accepted that tissues other than photoreceptor organs, eyes, and pineal organs contain less 11-cis retinal that binds to opsin to form a photosensitive G protein-coupled pigment because 11-cis retinoids are generated by enzymes specifically present in the photoreceptor organs, i.e., photoisomerase retinochrome/retinal G protein-coupled receptor (RGR), and/or retinal pigment epithelium-specific protein 65 kDa (RPE65) (11, 12, 24, 25). Accordingly, one of the most important requirements for functioning of an opsin in extraocular tissues is that it forms photosensitive pigments with the other retinal isomers. Because all-trans retinoids are ubiquitously present in animals (20), we first analyzed whether the PufTMT and MosOpn3 can bind to all-trans retinal and form pigments. After incubation with all-trans retinal, the MosOpn3 formed a pigment with an absorption maximum at 465 nm (Fig. 4A), whereas the PufTMT did not exhibit obvious pigment formation. Surprisingly, however, HPLC analyses of the chromophore of the MosOpn3-based pigment revealed that the pigment bound predominantly to 13-cis retinal as the chromophore and contained only a small amount of all-trans retinal, although 11-cis retinal-bearing and 9-cis retinal-bearing pigments are generated when incubated 11-cis and 9-cis forms, respectively (Fig. 4A). Because it is well known that all-trans retinal is in thermal equilibrium with the 13-cis form (19), this result suggests that MosOpn3 binds efficiently to 13-cis retinal rather than all-trans retinal. In fact, the MosOpn3 formed a photopigment with 13-cis retinal, and its absorption spectrum was similar to that of the pigment produced by incubation with all-trans retinal (Fig. 4A and Fig. S5). These results indicate that the MosOpn3 selectively binds to 13-cis retinal rather than all-trans retinal. We also investigated the G protein activation ability of the 13-cis retinal-bearing MosOpn3. The 13-cis retinal-bearing MosOpn3 activated Gi and Go in a light-dependent manner, although the Gi/Go activation rate in the dark was higher than that of the 11-cis retinal-binding form, as shown in Fig. 2 (Fig. 4B). The light-dependent activation ability suggests that the MosOpn3 might serve as a light-sensor protein in various kinds of tissues that do not contain 11-cis retinal.
Fig. 4.
The characteristics of C-terminal truncated MosOpn3-based pigment bearing 13-cis retinal. (A) Absorption spectra and chromophore configurations of the MosOpn3ΔC-based pigments constituted by incubation with 11-cis (black curve and trace), all-trans (blue curve and trace), 9-cis (green curve and trace), and 13-cis (red curve and trace) retinals, respectively. The absorption spectrum of the MosOpn3ΔC-based pigment constituted by 13-cis retinal (red curve) was calculated to exclude the contribution of the contaminated 11-cis retinal-bearing MosOpn3ΔC-based pigment based on the chromophore configuration (red trace) (Fig. S5). Note that the MosOpn3ΔC-based pigment constituted by incubation with all-trans retinal selectively binds 13-cis retinal, which was thermally generated from the all-trans form. (B) The Gi and Go activation abilities of the 13-cis retinal-bearing MosOpn3ΔC-based pigment. The open and filled circles indicate G protein activation in the presence of the irradiated and nonirradiated pigments, respectively.
Discussion
Several thousand opsins have been identified, and they are divided into eight groups (1–4). Because the whole genome sequences of many animals representing most phyla, including humans, have been determined, the present dataset provides an overview of the diversity of opsins in the animal kingdom. The basic molecular properties of seven of the eight groups have been described to date; the Opn3 group, composed of Opn3, TMT-opsin, and c-opsin, remains the last group to be investigated. In this report, we clearly showed that the Opn3 homologs of vertebrate and invertebrate act as photosensitive pigments and activate Gi- and Go-type G proteins in a light-dependent manner (Figs. 1–3). Accordingly, we have designated the Opn3 group as Gi/Go-coupled opsins. Our findings emphasize the overall diversity of opsin-based pigments as light-sensing GPCRs: the Gt-coupled, Gq-coupled, Go-coupled, Gs-coupled, Gi-coupled, and Gi/Go-coupled opsins might have evolved from an ancestral opsin early in animal evolution.
The characteristics of the Opn3 group are also important to an understanding of the evolution of Gt-coupled vertebrate visual pigments because the Opn3 group exhibits a closer relationship with the Gt-coupled opsin group than any other group (1). The photoproduct properties of vertebrate visual pigments in the Gt-coupled opsin group have been investigated exhaustively. Upon light absorption, the vertebrate visual pigment is converted to a photoproduct with an absorption maximum at ∼380 nm, in the UV region; the photoproduct eventually releases the chromophore retinal and decays. However, we showed that both vertebrate and invertebrate Opn3 homologs are bistable pigments that generate a stable photoproduct and revert to their original state upon subsequent light absorption (Fig. 1). Together with our previous finding that the nonvisual pigment parapinopsin, which is also a member of the Gt-coupled opsin group and exhibits a relatively early branching in the group, is a bistable pigment (26), the present results support the hypothesis that animal opsins were originally bistable pigments and that bleaching pigments evolved relatively later in the course of visual pigment evolution (27). In addition, the Opn3 homologs activate Gi- and Go-type G proteins but not Gt-type G protein, unlike bovine rhodopsin (Fig. 2 and Figs. S3 and S4). Therefore, the Gt activation ability appears to have been acquired by the Gt-coupled opsin group. Comparative analyses of members of these two groups may provide an opportunity to investigate how the visual system-specific opsin–G protein interaction (e.g., ref. 23) has evolved.
With respect to the molecular properties of the Opn3 homolog, the most remarkable finding is that the MosOpn3 forms a photopigment by binding to 13-cis retinal as well as 11-cis retinal and acts as a Gi/Go-coupled opsin (Fig. 4). The 11-cis retinal chromophore in dark state of an opsin-based pigment functions as an inverse agonist or antagonist of a GPCR ligand, and an all-trans retinal chromophore in a G protein-activating photoproduct functions as an agonist. Our results showed that the G protein activation efficiency by the 13-cis retinal-bearing pigment in the dark (Fig. 4B) was higher than that of the 11-cis retinal-bearing pigment and much lower than that of the all-trans retinal-bearing pigment (photoproduct) (Fig. 2), suggesting that 13-cis retinal might not suppress the opsin (apoprotein) activity of G protein activation as efficiently as 11-cis retinal does (28–30). Alternatively, 13-cis retinal might serve as a partial agonist in this case. 13-cis retinal can be easily formed by the thermal isomerization of all-trans retinal, and all-trans and 13-cis retinals are in thermal equilibrium (19). Thus, the MosOpn3 might serve as a light sensor in both the eyes and the rest of the body where abundant and a small amounts of Opn3 mRNA were detected, respectively (Fig. S6). We were not able to clearly detect the binding of 13-cis retinal to the PufTMT, but we detected an obvious light-induced cAMP decrease in the PufTMT-expressing cells without the addition of 11-cis retinal to the culture medium (Fig. 3). This result suggests that the teleost Opn3 homolog TMT may have some ability to bind to 13-cis retinal and/or all-trans retinal, which is consistent with a previous report that the introduction of zebrafish TMT provided photosensitivity to the fin cells of a cavefish, a species that had lost its photosensitivity during evolution (18).
We report here the molecular properties of the insect and teleost Opn3 homologs, which are consistent with their functionality in various tissues. The vertebrate members of Opn3 are largely divided into Opn3 and TMT in the phylogenetic tree (Fig. S7). Although the vertebrate Opn3 itself has not been successfully expressed in cultured cells and, therefore, not analyzed, the molecular properties of invertebrate Opn3 homolog and the vertebrate Opn3 homolog TMT suggest that the vertebrate Opn3 might also form a functional pigment having similar molecular properties of these homologs.
We demonstrated that the introduction of the Opn3 homologs rendered mammalian cultured cells derived from kidney photosensitive without the addition of 11-cis retinal to the culture medium, even after the continuous light exposure, whereas the introduction of bovine rhodopsin did not (Fig. 3). The photoresponsiveness of the Opn3 homolog-expressing cells incubated in the culture medium with 11-cis retinal was also higher than that of cells expressing the bleaching pigment bovine rhodopsin or another Gi-coupled bistable pigment, Opn5 (10), suggesting that the Opn3 homologs might possess unknown molecular properties that provide an efficient decrease in cAMP in cultured cells, in addition to the bistable nature. Recently, neural activities have been successfully regulated by light through the introduction of light-sensing channels, namely the channelrhodopsins and their derivatives, establishing the field of optogenetics (31, 32). However, the use of optogenetics involving a light-sensing GPCR remains limited (33), mostly due to the dependence of conventional opsins on 11-cis retinal for the formation of a G protein-coupled photopigment, although this technique has the potential to regulate a considerable number of GPCR-based physiologies by light. Therefore, the Opn3 homologs, particularly the MosOpn3, would be highly useful optogenetic tools because of their abilities to form bistable pigments, which are bleach resistant and reusable, with the ubiquitously present retinal and to decrease cAMP efficiently. We have recently reported the jellyfish Gs-coupled opsin, which can be used for up-regulating cAMP level in cultured cells (5, 34). Together with the jellyfish opsin, the MosOpn3 and its derivatives could be used for regulating not only neural responses, but also nonneuronal responses that are mediated by cAMP-related GPCR signaling in any tissues by light.
Materials and Methods
Animals.
The pufferfish (T. rubripes) were obtained commercially. The mosquitoes (A. stephensi) were reared from eggs kindly provided by Hirotaka Kanuka (Jikei University School of Medicine, Tokyo) and maintained according to the protocol (35). All experiments using these animals were approved by the Osaka City University animal experiment committee.
cDNA Cloning.
Partial cDNAs of the PufTMT and MosOpn3 were obtained from RNA isolated from the brain and head, respectively, by RT-PCR. The primers used for PCR amplification were designated based on the gene sequences found in the T. rubripes and A. gambiae genome databases. The full-length cDNAs of the Opn3 homologs were obtained by using the 3′ RACE and 5′ RACE systems (Invitrogen).
Expression of the Opsin-Based Pigments and Spectroscopy.
The cDNAs of the full-length PufTMT, the full-length MosOpn3 and the MosOpn3 deletion mutant of which C-terminal 99 amino acids were deleted, were tagged with the monoclonal antibody rho 1D4 epitope sequence (ETSQVAPA). The tagged cDNA was inserted into the pcDNA3.1 vector (Invitrogen), and pigment expression in HEK293S cells and pigment purification were performed as described (8). Briefly, to constitute the pigment, the expressed proteins were incubated with 11-cis, 9-cis, 13-cis, or all-trans retinal overnight. The pigments were then extracted with 1% (weight/vol) dodecyl β-d-maltoside in 50 mM Hepes buffer (pH 6.5) containing 140 mM NaCl (buffer A). For purification, the pigments in the crude extract were bound to 1D4-agarose, washed with 0.02% (weight/vol) dodecyl β-d-maltoside in buffer A (buffer B), and eluted with buffer B that contained the 1D4 peptide. The absorption spectra of the pigments were recorded at 4 °C by using a Shimadzu UV2450 spectrophotometer. Blue, green, and orange lights were supplied by a 1-kW halogen lamp (Philips) with a 460-nm interference filter, a 500-nm interference filter, and an O56 glass cutoff filter (Toshiba), respectively.
HPLC Analysis.
The chromophore configurations of the irradiated and nonirradiated purified Opn3 homolog-based pigments were analyzed by HPLC, as described (13, 36).
Guanosine 5′-O-(3-Thiotriphosphate) Binding Assay.
G protein activation by the Opn3 homolog-based pigments was evaluated by measuring the amount of guanosine 5′-O-(3-thiotriphosphate) (GTPγS) bound to Gi, Go, Gt, Gq, or Gs, as described (21, 23). Gi, Go, and Gt were prepared as described (23). Gq was a generous gift from Tomoko Doi (37). Gs was expressed in Escherichia coli (38) and purified by using the QIAexpress expression system (Qiagen).
GloSensor Assay.
The changes in the intracellular cAMP concentration of the pigment-expressing HEK293S cells were measured by using the GloSensor cAMP assay (Promega). The expression constructs for the Opn3 homologs or bovine rhodopsin were cotransfected with the pGloSensor-22F cAMP plasmid (Promega) by using the FuGENE HD transfection reagent (Promega). The transfected cells were incubated in the culture medium containing 10% (vol/vol) FBS with or without 11-cis retinal overnight. Before the measurements, the culture medium was replaced with a CO2-independent medium containing 10% (vol/vol) FBS and 2% (vol/vol) GloSensor cAMP Reagent stock solution (Promega). After equilibration with the medium and a steady basal signal was obtained, the cells were treated with 3.5 μM forskolin, a direct activator of adenylyl cyclase, to increase the intracellular cAMP level. Luminescence, representing the amount of cAMP, was measured at 25 °C by using a GloMax 20/20n Luminometer (Promega). To measure the light-induced change in the cAMP level in the transfected cells, irradiation with a green light-emitting diode light was applied for 10 s at 30-min (for dark-incubated sample) or 15-min (for light-exposed sample) intervals. For the measurement of dark-incubated samples, the cells were kept in the dark before the measurements.
Supplementary Material
Acknowledgments
We thank Robert S. Molday (University of British Columbia) for kindly supplying the rho 1D4-producing hybridoma; Tomoko Doi (Kyoto University) for the generous gift of Gq; and Robert J. Lucas (Manchester University), Daisuke Kojima, and Yoshitaka Fukada (University of Tokyo) for technical advice on Glosensor assay. This work was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (to A.T. and M.K.) and the Naito Foundation (to A.T. and M.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Bank of Japan (DDBJ) (accession nos. AB753162 and AB753163).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219416110/-/DCSupplemental.
References
- 1.Terakita A. The opsins. Genome Biol. 2005;6(3):213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139(2):246–264. doi: 10.1016/j.cell.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peirson SN, Halford S, Foster RG. The evolution of irradiance detection: Melanopsin and the non-visual opsins. Philos Trans R Soc Lond B Biol Sci. 2009;364(1531):2849–2865. doi: 10.1098/rstb.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terakita A, Kawano-Yamashita E, Koyanagi M. Evolution and diversity of opsins. WIREs Membr Transp Signal. 2012;1(1):104–111. [Google Scholar]
- 5.Koyanagi M, et al. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc Natl Acad Sci USA. 2008;105(40):15576–15580. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koyanagi M, Terakita A. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol. 2008;84(4):1024–1030. doi: 10.1111/j.1751-1097.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 7.Kojima D, et al. A novel Go-mediated phototransduction cascade in scallop visual cells. J Biol Chem. 1997;272(37):22979–22982. doi: 10.1074/jbc.272.37.22979. [DOI] [PubMed] [Google Scholar]
- 8.Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett. 2002;531(3):525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita T, et al. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci USA. 2010;107(51):22084–22089. doi: 10.1073/pnas.1012498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima D, et al. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS ONE. 2011;6(10):e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara T, Hara R. Regeneration of squid retinochrome. Nature. 1968;219(5153):450–454. doi: 10.1038/219450a0. [DOI] [PubMed] [Google Scholar]
- 12.Hao W, Fong HK. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem. 1999;274(10):6085–6090. doi: 10.1074/jbc.274.10.6085. [DOI] [PubMed] [Google Scholar]
- 13.Nagata T, Koyanagi M, Tsukamoto H, Terakita A. Identification and characterization of a protostome homologue of peropsin from a jumping spider. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196(1):51–59. doi: 10.1007/s00359-009-0493-9. [DOI] [PubMed] [Google Scholar]
- 14.Blackshaw S, Snyder SH. Encephalopsin: A novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19(10):3681–3690. doi: 10.1523/JNEUROSCI.19-10-03681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moutsaki P, et al. Teleost multiple tissue (tmt) opsin: A candidate photopigment regulating the peripheral clocks of zebrafish? Brain Res Mol Brain Res. 2003;112(1-2):135–145. doi: 10.1016/s0169-328x(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 16.Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306(5697):869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- 17.Velarde RA, Sauer CD, Walden KK, Fahrbach SE, Robertson HM. Pteropsin: A vertebrate-like non-visual opsin expressed in the honey bee brain. Insect Biochem Mol Biol. 2005;35(12):1367–1377. doi: 10.1016/j.ibmb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Cavallari N, et al. A blind circadian clock in cavefish reveals that opsins mediate peripheral clock photoreception. PLoS Biol. 2011;9(9):e1001142. doi: 10.1371/journal.pbio.1001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groenendijk GW, Jacobs CW, Bonting SL, Daemen FJ. Dark isomerization of retinals in the presence of phosphatidylethanolamine. Eur J Biochem. 1980;106(1):119–128. doi: 10.1111/j.1432-1033.1980.tb06002.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyagi M, Yokoyama H, Shiraishi H, Matsumoto M, Ishii H. Simultaneous quantification of retinol, retinal, and retinoic acid isomers by high-performance liquid chromatography with a simple gradiation. J Chromatogr B Biomed Sci Appl. 2001;757(2):365–368. doi: 10.1016/s0378-4347(01)00158-x. [DOI] [PubMed] [Google Scholar]
- 21.Terakita A, et al. Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J Neurochem. 2008;105(3):883–890. doi: 10.1111/j.1471-4159.2007.05184.x. [DOI] [PubMed] [Google Scholar]
- 22.Hubbard R, Wald G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952;36(2):269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terakita A, Yamashita T, Nimbari N, Kojima D, Shichida Y. Functional interaction between bovine rhodopsin and G protein transducin. J Biol Chem. 2002;277(1):40–46. doi: 10.1074/jbc.M104960200. [DOI] [PubMed] [Google Scholar]
- 24.von Lintig J, Kiser PD, Golczak M, Palczewski K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem Sci. 2010;35(7):400–410. doi: 10.1016/j.tibs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montell C. Drosophila visual transduction. Trends Neurosci. 2012;35(6):356–363. doi: 10.1016/j.tins.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyanagi M, et al. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci USA. 2004;101(17):6687–6691. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terakita A, et al. Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol. 2004;11(3):284–289. doi: 10.1038/nsmb731. [DOI] [PubMed] [Google Scholar]
- 28.Cohen GB, Yang T, Robinson PR, Oprian DD. Constitutive activation of opsin: Influence of charge at position 134 and size at position 296. Biochemistry. 1993;32(23):6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- 29.Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J. 1997;73(6):3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kono M, Crouch RK. Probing human red cone opsin activity with retinal analogues. J Nat Prod. 2011;74(3):391–394. doi: 10.1021/np100749j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, et al. The microbial opsin family of optogenetic tools. Cell. 2011;147(7):1446–1457. doi: 10.1016/j.cell.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattis J, et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9(2):159–172. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailes HJ, Zhuang LY, Lucas RJ. Reproducible and sustained regulation of Gαs signalling using a metazoan opsin as an optogenetic tool. PLoS ONE. 2012;7(1):e30774. doi: 10.1371/journal.pone.0030774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maekawa E, et al. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasit Vectors. 2011;4:10. doi: 10.1186/1756-3305-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terakita A, Hara R, Hara T. Retinal-binding protein as a shuttle for retinal in the rhodopsin-retinochrome system of the squid visual cells. Vision Res. 1989;29(6):639–652. doi: 10.1016/0042-6989(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Doi T, Sugimoto H, Arimoto I, Hiroaki Y, Fujiyoshi Y. Interactions of endothelin receptor subtypes A and B with Gi, Go, and Gq in reconstituted phospholipid vesicles. Biochemistry. 1999;38(10):3090–3099. doi: 10.1021/bi981919m. [DOI] [PubMed] [Google Scholar]
- 38.Yan SZ, Tang WJ. Expression of alpha subunit of Gs in Escherichia coli. Methods Enzymol. 2002;344:171–175. doi: 10.1016/s0076-6879(02)44713-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.