Abstract
Male preponderance in autistic behavioral impairment has been explained in terms of a hypothetical protective effect of female sex, yet little research has tested this hypothesis empirically. If females are protected, they should require greater etiologic load to manifest the same degree of impairment as males. The objective of this analysis was to examine whether greater familial etiologic load was associated with quantitative autistic impairments in females compared with males. Subjects included 3,842 dizygotic twin pairs from the Twins Early Development Study (TEDS) and 6,040 dizygotic twin pairs from the Child and Adolescent Twin Study of Sweden (CATSS). In both samples, we compared sibling autistic traits between female and male probands, who were identified as children scoring in the top 90th and 95th percentiles of the population autistic trait distributions. In both TEDS and CATSS, siblings of female probands above the 90th percentile had significantly more autistic impairments than the siblings of male probands above the 90th percentile. The siblings of female probands above the 90th percentile also had greater categorical recurrence risk in both TEDS and CATSS. Results were similar in probands above the 95th percentile. This finding, replicated across two nationally-representative samples, suggests that female sex protects girls from autistic impairments and that girls may require greater familial etiologic load to manifest the phenotype. It provides empirical support for the hypothesis of a female protective effect against autistic behavior and can be used to inform and interpret future gene finding efforts in autism spectrum disorders.
Keywords: epidemiology, genetics, family studies
Female risk for autism spectrum disorders (ASDs) is less than four times that of males, but the explanation for this difference is unknown (1, 2). Recent studies, however, have suggested that the rare genetic insults associated with ASDs in female cases are, on average, larger and more functionally disruptive than those in male cases (3, 4). This phenomenon would be consistent with the presence of a female protective effect (FPE)—a component of female sex that protects girls from ASDs. The possibility of such an effect has been suggested (5, 6), but the hypothesis has limited empirical support.
A female protective effect against autistic behavior is not invariably evidenced by male preponderance. The FPE model, in which girls require a greater etiologic load to manifest autistic behavioral impairment, is a different (although notably nonexclusive) concept from that driving the more extensively investigated hypothesis of male-specific ASD risk. This second possibility predominantly concentrates on factors inherent to male development, like fetal testosterone, that may influence liability toward disordered social behavior (7, 8). Both male risk and female resilience models present viable research frameworks, but the female protective effect is of particular interest given the now strong evidence of etiologic heterogeneity within ASDs (i.e., different cases of ASD are likely to reflect many different genetic and environmental causes) (9–11). Sibling studies, specifically those conducted in the general population, provide a unique opportunity to: (i) test the female protective effect hypothesis with regard to genetic and environmental risk factors that are shared within families and (ii) examine whether the FPE is adequately consistent to be detected across a representative system of familial sources of autistic behavior. This paper presents a test of these hypotheses in the general population in two nationally representative samples.
In the general population, traits typical of autism can be measured on a continuum by using behavioral scales (12, 13). Autistic trait scales capture the extent to which every individual in a population shows behaviors indicative of social impairment, communication impairment, and restricted and repetitive interests—the three behavioral domains of autism. When assessed on a continuum, autistic trait scores range from zero or very few traits to a number greater than that associated with the average diagnosis of ASD (14, 15). As with ASDs, autistic traits are moderately to highly heritable when measured continuously (16). Recently, multiple studies have evidenced a genetic relationship between the extremes of the continuum (high autistic trait scores or ASDs) and trait variation in the normal range (14, 17). In other words, evidence suggests that at least some of the genetic and environmental factors associated with ASDs are the same as those that cause individual differences in autism-like behavior below the clinical threshold.
Behavioral evidence of the link between ASDs and autistic traits has long been noted in the family members of people with concentrated autistic impairments (18, 19). In this analysis, we leverage that connection to examine the FPE in the general population. Specifically, the family members of individuals with high autistic behavior scores show a greater average number of autistic traits than the family members of individuals without high autistic behavior scores (14, 17, 20). If greater familial etiologic load is required to produce autistic impairments in girls, the family members of affected females should, on average, carry greater risk than the family members of affected males. Assuming a positive correlation between etiologic and behavioral load, the female protective effect hypothesis predicts that family members of female probands with autistic impairments should have higher autistic trait scores than the family members of male probands with the same degree of impairment.
There are several advantages to examining this predicted pattern by using quantitative traits in the general population. First, high scoring individuals are selected from a representative sample. This strategy reduces the influence of variables that predict entry into clinical cohorts (ascertainment factors). In the case of ASDs, there is evidence that sex itself is an ascertainment factor, especially among individuals with intelligence quotients (IQs) in the normal range (21, 22). Specifically, girls with high autistic trait scores are diagnosed with an ASD less frequently than boys with the same trait scores (23). Likely tied to this pattern, girls with diagnoses of ASDs often have lower average IQs and are more severely affected than boys with diagnoses of ASDs (1). This difference may be important in the context of etiologic heterogeneity. In the event that causes of ASDs may relate to sex-dependent ascertainment factors, like IQ, the female protective effect may be misestimated in clinical samples (24, 25). In other words, it is difficult to compare the family members of boys and girls with ASDs if, on average, boys and girls diagnosed with ASDs might be systematically different themselves.
To at least partially address this problem, quantitative trait scores can be used to examine whether greater etiologic load is required for females to reach equivalent thresholds of impairment. Girls are protected from autistic behaviors across their distribution (26, 27). Accordingly, the 90th percentile of the female-specific distribution, for example, will be at a less impaired position than that of the male-specific distribution. The purpose of this analysis, however, is to understand whether, on average, more familial factors are required to push female scores to the same position as males. In other words, can an increase in familial etiologic load neutralize female advantage?
Sex-equivalent cutoffs must be used to provide insight into the ASD sex ratio, as the impairment requirements for a clinical diagnosis are not sex specific (28). We do, however, include analyses that use sex-specific high scoring thresholds to ensure that our findings apply regardless of whether a non–sex-specific or sex-specific threshold is used. This study presents a test of the female protective effect when the threshold used to define impairment has been quantitatively defined, an empirically based approach to phenotypic equivalency.
In addition to providing insight into the ASD sex ratio, an understanding of familial risk in boys versus girls with autistic impairments could be informative for future genetic association efforts. Specifically, if the family members of female probands have, on average, higher autistic trait scores than the family members of male probands, one would predict that a greater inherited genetic load is associated with equivalent autistic impairments in females. This hypothesis could be used to structure the design and interpretation of genetic studies in ASD.
Methods
The Twins Early Development Study (TEDS) and the Child and Adolescent Twin Study of Sweden (CATSS), both nationally representative cohorts, are the largest twin samples in the world in which autistic behavior has been assessed. CATSS has an 80% response rate and is highly representative of the Swedish population. The 60.6% of TEDS families who returned the autistic traits survey at age 12 were highly representative of the United Kingdom (29). In each cohort, the analyses were limited to dizygotic (DZ) twins so that pairs had a consistent genetic relationship no more similar than that of regular siblings. Both same sex and opposite sex DZ twins were used for this analysis. Participants were further excluded from the analyses based on the medical exclusion criteria established within each study. In TEDS, pairs were excluded if either twin had a noted non-ASD syndromic condition (e.g., Down Syndrome, chromosomal abnormalities) or if their birth involved substantial pregnancy or perinatal complications (n = 106 pairs; 2.4%). In CATSS, pairs were excluded if either twin had a known chromosomal abnormality or brain injury (e.g., cerebral palsy; 112 pairs, 1.7%). All remaining DZ twins from TEDS (n = 3,842 dizygotic pairs) and CATSS (n = 6,040 dizygotic pairs) in which both members of the pair had measured autistic traits scores were used in this analysis. TEDS and CATSS have ethical approval. This specific set of analyses was approved by the Harvard School of Public Health Institutional Review Board.
In TEDS, autistic traits were measured in 12-y-old twins by using the Childhood Autism Spectrum Test (CAST) (30), a parent-rated autism-like behavior scale. The CAST is a 31-item, yes/no scale that captures the three core domains of autistic behavior (social impairments, communication impairments, and restrictive and repetitive behaviors and interests) (28). One of the items, which inquired about pretend play, was not used at age 12 because it was not age appropriate. In the TEDS sample, the CAST displays adequate overall internal consistency (Kuder–Richardson 20 = 0.74) (14) and strong within-individual correlations across a 4-y period from ages 8–12 (r = 0.64) (31). In CATSS, autistic traits were assessed in 9- and 12-y-old twins by using the autism–tics, attention deficit-hyperactivity disorder, and other comorbidities inventory (A-TAC) (32), a telephone interview with parents in which behavior scores are assigned by a trained rater. Thirteen items were used to assess behavior from each of the three ASD domains (33). Response options for each item were “yes” (“2”), “yes, to some extent” (“1”), and “no” (“0”). The 13 items have good internal consistency reliability (0.81) (33) and show good test-retest reliability (0.83–0.94) (34). There is no evidence of ceiling effects on either the CAST or A-TAC. The highest possible values for both measures are more than 4 SDs from their respective population means; their highest possible scores also exceed the average scores of individuals with clinically defined ASDs (14, 17).
Both the CAST and A-TAC assess autistic traits on a continuum, meaning scores range from zero or few autism-like behaviors to a number greater than the average associated with clinically diagnosed ASD. Twin analyses of the CAST in TEDS (17) and the A-TAC in CATSS (19) have recently suggested etiologic consistency across levels of autistic trait impairment (e.g., the top 10%, 5%, and 1%), meaning genetic and environmental influences on severe autistic behavior also influence subclinical or typical variation in those traits. Etiologic consistency across severity levels suggests that a female protective effect should operate at multiple points along the continuum as well. We accordingly examined the female protective effect at two quantitative levels of symptom severity: the 90th and 95th percentiles of the overall trait distributions. In both studies, one twin from each pair was selected at random to be the index case. The other twin in the pair was the sibling. For example, a proband in the 90th percentile analyses was an index twin scoring in the top 10% of impairment on the CAST or A-TAC. Similarly, a proband in the 95th percentile analyses was an index twin scoring the top 5% of impairment. These analyses did not have the power to analyze the top 1% extreme-scoring groups (as done in the twin analyses referenced above) after the necessary exclusion of monozygotic twins.
We first examined the female protective effect in TEDS and CATSS independently; then, we combined the samples to examine the consistency of the effect across high scoring thresholds. Specifically, the 90th percentile analyses contained an adequate number of female probands (nTEDSfemale90 = 136; nCATSSfemale90 = 230) to conduct independent tests of the female protective effect in TEDS and CATSS. The top 10% level was therefore used as the impairment threshold for independent replication. We then combined TEDS and CATSS and examined the consistency of the FPE across the top 10% and top 5% impairment levels. In other words, the number of female probands in the combined 90th percentile group was nTEDSfemale90 + nCATSSfemale90 = 366, and the number in the combined 95th percentile group was, as would be expected, approximately one-half that (nTEDSfemale95 + nCATSSfemale95 = 192).
In both the independent and combined analyses, autistic impairments in siblings of male and female probands were compared by using two statistical methods. The first comparison estimated the mean difference between the autistic trait scores of siblings of female and male probands. The outcome variable was a sex- and zygosity-specific z-score: [(sibling’s trait score) − (mean trait score for sibling’s sex and zygosity group)/(SD for sibling’s sex and zygosity group)]. This z-score allowed us to compare across male and female siblings and between the two studies. Analysis of covariance (ANCOVA) with Dunnett’s T3 post hoc test was used to examine whether mean sibling z-scores differed among (i) siblings of female probands, (ii) siblings of male probands, and (iii) siblings of nonprobands (all index individuals scoring below the cutoff), controlling for twin age. Dunnett’s T3 method was used as a constant variance could not be assumed (35). The P values shown in Fig. 1 are corrected for multiple testing within cohort (TEDS or CATSS).
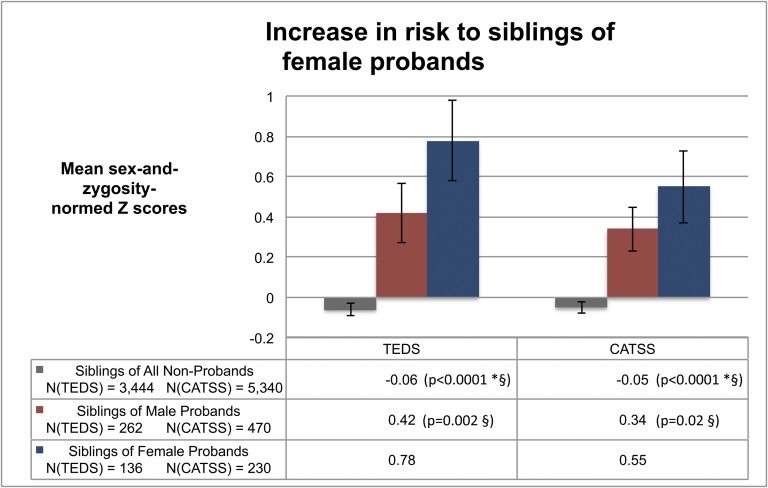
Fig. 1.
Increase in risk to siblings of female probands above the 90th percentile. Probands were 34.2% female in TEDS and 32.9% female in CATSS. Error bars indicate 95% confidence interval around group mean. P values indicate the multiple-testing–corrected strength of the difference between the group mean noted in the cell and the group mean in one or more of the cohort-specific rows below, indicated by the symbols (*, comparison with male probands; §, comparison with female probands). For example, “0.42 (p=0.002 §)” indicates that, in the TEDS cohort, the P value for the difference in means between the siblings of male (0.42) and female (0.78) probands was 0.002.
The second comparison estimated the difference in categorical recurrence risk (e.g., probability of the sibling also being above the cutoff) for the siblings of affected females versus affected males, adjusting for sibling sex, using a Mantel–Haenszel risk ratio (MH-RR). In both sets of analyses, we first examined TEDS and CATSS separately at the top 10% impairment level, then combined the cohorts for an estimate of the combined effect at both the top 10% and 5% levels.
To ensure that our results did not depend on the use of universal high scoring thresholds, we conducted additional combined-cohort analyses with sex-specific cutoffs. In other words, in the top 10% sex-specific analyses, probands were identified as male index twins scoring in the top 10% of the male-specific trait distribution and female index twins scoring in the top 10% of the female-specific trait distribution. At both the 10% and 5% sex-specific levels, we conducted the combined-cohort mean difference and categorical recurrence analyses as otherwise described above.
Results
Independent Evaluations in TEDS and CATSS.
In both population samples, there was strong evidence for greater aggregation of autistic symptomology in the siblings of female probands than in the siblings of male probands. Within each cohort, the omnibus ANCOVA (F) test was significant at the top 10% level (P < 0.0001). As shown in Fig. 1, the scores of female probands’ siblings were significantly more deviated from their sex- and zygosity-normed means compared with the scores of male probands’ siblings in both TEDS (P = 0.002) and CATSS (P = 0.02). After the main effects analysis, we included an interaction term in the models to test whether the proband sex effect differed by sex of the sibling. There was no evidence of effect modification by sibling sex in either TEDS (F = 0.05, df = 1, P = 0.82) or CATSS (F = 0.30, df = 1, P = 0.59).
Presented in Table 1, the siblings of female probands also had increased categorical recurrence risk in both TEDS and CATSS at the top 10% level. The risk ratios were 1.50 and 1.29 in TEDS and CATSS, respectively, although the risk ratio in CATSS was not statistically significant (95% CI 0.97–1.72). The Breslow–Day test for homogeneity did not suggest variation in the proband sex effect on categorical recurrence by sex of the sibling in either TEDS (χ2 = 0.22, df = 1, P = 0.64) or CATSS (χ2 = 0.12, df = 1, P = 0.73).
Table 1.
High scoring recurrence in TEDS and CATSS
| Cohort | TEDS | CATSS |
| Siblings of male probands in top 10%, % | 20.23 | 20.00 |
| Siblings of female probands in top 10%, % | 31.62 | 24.24 |
| Mantel- Haenszel recurrence risk ratio for siblings of female v. male probands (95% CI) | 1.50 (1.07–2.10) | 1.29 (0.97–1.72) |
Combined Cohort Analyses.
The combined cohort analyses suggested that the FPE operates consistently at multiple impairment levels. In the combined top 10% analysis, the average score of siblings of female probands was 0.64 SDs above the mean; the average score of siblings of male probands was 0.37 SDs above the mean (mean difference 0.27 SDs, P < 0.0001). Results were similar in the combined analyses at the top 5% level. The average score of siblings of female probands (n = 192) was 0.69 SDs above the mean; the average score of siblings of male probands (n = 360) was 0.47 SDs above the mean (mean difference 0.22 SDs, P = 0.03).
The increase in categorical recurrence risk was also similar across high scoring thresholds. At the top 10% level, the probability of the sibling also scoring in the top 10% was 38% higher for siblings of female versus male probands (MH-RR 1.38, 95% confidence interval 1.11–1.72) in the combined cohort analysis. At the combined top 5% level, the probability of the sibling also scoring in the top 5% was 37% higher for female versus male probands (MH-RR 1.37, 95% confidence interval 0.90–2.09). Despite the nearly identical effect size to the combined top 10% analysis, the categorical comparison was not statistically significant at the top 5% level after the reduction in sample size with increasingly extreme group definition.
Sex-Specific Analyses.
The combined-cohort results were similar when analyzed by using sex-specific cutoffs. In the combined top 10% sex-specific analysis, the average score of siblings of female probands (n = 673) was 0.54 SDs above the mean; the average score of siblings of male probands (n = 676) was 0.36 SDs above the mean (P = 0.003). In the top 5% sex-specific analysis, the average scores of siblings of female (n = 263) and male (n = 321) probands were 0.70 and 0.36 SDs above the mean, respectively (P = 0.02).
The siblings of female sex-specific probands were also more likely to exceed sex-specific high scoring thresholds themselves. At the combined 10% level, the siblings of female probands were 35% more likely to meet high scoring criteria than the siblings of male probands (χ2 = 11.02; df = 1; P = 0.0009). Siblings of female probands were 14% more likely to meet high scoring criteria at the combined top 5% sex-specific level, although the difference was not statistically significant after the reduction in effect and sample sizes (χ2 = 0.48; df = 1; P = 0.49).
Discussion
Autistic impairments are far less frequent in females than males, but there has been little data to explain this sex bias. This study provides empirical evidence, replicated across two population samples, indicating that a comparatively greater familial etiologic load may be associated with autistic impairments in females—a pattern consistent between impairment levels and robust to sex-specific proband definition. In other words, males may display autistic impairments more frequently because on average they require fewer familial risk factors to reach an equivalent impairment threshold. This finding is consistent with previous suggestions of a female protective effect (3–6, 36).
Alternate Explanations for the Data.
There are alternate models by which the siblings of female probands would have higher autistic trait scores than the siblings of male probands. In presenting several of them below, we aim to consider other possible explanations for these data in light of ASDs’ etiologic and phenotypic heterogeneity.
The first alternate possibility is that, on average, different types of genes and environments may be influencing autism-like behavior in boys and girls. Autistic behaviors, like ASDs, are hypothesized to reflect a diverse causal system, in which affectedness can be influenced by inherited genetic effects, de novo genetic effects, environmental effects, and likely often a combination thereof (11, 16, 37). If etiologic structure varies between affected males and affected females, particularly with regard to the fraction of cases with familial causes, sibling recurrence estimates that differ by the sex of the proband become difficult to interpret. For example, the lower risk to siblings of male probands seen here could also be explained by reduced familiality of autistic behaviors in affected boys. Under this scenario, one would expect the siblings of male probands to have lower average autistic trait scores than the siblings of female probands because the male probands’ conditions are less likely to arise from genes or environments that are shared by their family members.
In the context of ASDs, de novo mutations are the most clearly identified source of nonfamilial etiology (9, 37). De novo mutations that have been associated with autism are infrequent in the general population—it is accordingly unlikely that their distribution within affected individuals is relevant when probands are defined as top 10% or 5% scorers in a community sample, as done here. This assumption cannot necessarily be made, however, for clinical ASD samples. A higher frequency of identified de novo mutations among girls with ASDs has been noted in multiple studies (4, 38). Although this difference is in and of itself difficult to interpret given that diagnosed females are often, on average, more severely affected than diagnosed males (1, 21, 22), an excess burden of de novo events among female probands could nonetheless influence sibling recurrence estimates (albeit in the opposite direction to the pattern seen here). Bias could also occur based on the differential distribution of processes associated with de novo events (e.g., paternal age) (39).
Within community twin samples, differences in familiality are most commonly considered through the estimated role of the unique environment. Classically, twin studies decompose the variance of a trait into genetic effects, shared environmental effects, and unique environmental effects. Genetic effects are assumed to be inherited in twin studies; shared environmental effects are environmental influences common to twins in a pair that act to make them more similar. Familiality is then the sum of inherited genetic and shared environmental influences on a trait. The variance that remains is attributed to the unique environment, a construct that captures both things that make the twins different and measurement error (40). As nonfamilial environmental effects on a trait are definitionally unique, the data pattern seen here could also be alternately explained by greater average influence of the unique environment in male probands.
Current data from twin and family studies do not suggest that either ASDs or autistic behaviors are more influenced by unique environmental effects (are less familial) in boys than in girls (11, 14, 16, 17). However, this question is difficult to evaluate among extreme scorers in the general population (as well as in clinical ASD samples) because of the limited number of affected females. Because statistically significant differences are difficult to detect between small groups, male and female ASD heritability and environment estimates are often presented together more as a reflection of power to conduct the test than as actual evidence for consistent etiologic structure (11, 14).
A second set of alternate explanations center around sex differences in the behavioral phenotype associated with a given autistic traits score. These possibilities are numerous, but we will focus on one that could bias the data in the direction of the present findings. Specifically, if autistic trait measures better capture male manifestations of autism-like features, as has been hypothesized (41), girls may need to be more severely affected to meet high-scoring criteria. Under this scenario, quantitative trait scores for ASD could be associated with greater phenotypic severity, on an absolute scale, in girls. Were high scoring girls in actuality, on average, at a higher position on the severity distribution than high scoring boys, one would expect the autistic trait scores of girls’ siblings to be significantly more deviated from the mean than those of the boys’, as noted here.
Current evidence, however, does not suggest that autistic trait scores are associated with greater phenotypic severity in girls and, in fact, may suggest the opposite. A recent analysis from the Avon Longitudinal Study of Parents and Children (ALSPAC) found that, for any given quantitative trait score, girls are less likely to carry a diagnosis of ASD (23). Although, as the authors suggest, this finding could reflect gendered conceptualizations of the disorder and differential ascertainment, it does not support the possibility denoted in the paragraph above. Further, in another ALSPAC analysis, Skuse et al. (26) reported that autistic traits were more strongly associated with both peer problems and deficits in prosocial behavior in boys than in girls, also suggesting that autistic behavior counts are unlikely to be associated with greater phenotypic severity in females.
In sum, the etiologic and phenotypic heterogeneity of autistic behavior renders several possible interpretations of the data pattern found here. However, the possibility of a female protective effect— through which autistic behavioral impairment in females is accompanied by a significantly greater aggregation of familial etiologic factors—is more probable than many other explanations and consistent with the suggestions of previous research (3–5, 36).
Future Examinations of the Female Protective Effect.
Unselected, general population samples provide the best framework in which to search for potential protective effects. In the present case, sex differences in ascertainment factors for clinical ASD samples (e.g., intellectual disability) (1), coupled with nonrepresentative sampling, make the female protective effect difficult to examine or detect consistently in clinical cohorts using family trait and recurrence approaches (18, 36, 42). This study, by contrast, examined the female protective effect in two nationally representative community samples and found evidence for it in both cohorts.
Assessing behavior in large, representative samples, however, precludes direct observation for every participant. Accordingly, the primary limitation of this analysis was its dependence on parent survey report in TEDS and parent interview in CATSS. Although the measure used in CATSS, the A-TAC, is clinician structured, future tests of this hypothesis would benefit from a multirater or clinically measured approach while maintaining the representative framework. Although, in practice, such a replication may be difficult given the sample size requirements, different approaches may be taken to investigate the same hypothesis in the general population. These findings suggest that genetic risk factors for ASD should be associated with a greater average quantitative trait burden in males than in females, a test that can be now be conducted in individually genotyped general population cohorts where autistic traits have been assessed (43).
The generalizability of these analyses may also be limited in the event that some etiologic factors related to autistic behavior in twin individuals are unique to or disproportionally relevant in twins. Although one could construct such an argument for premature birth, which is both more common with multiples and associated with ASDs, its effect would have to disproportionately influence pairs in which one of the twins was female to bias these results. For example, the effect of premature birth on a male in the pair would have to depend on the sex of their cotwin. To our knowledge, there is no existing evidence to support such a hypothesis and there is no known reason why these results would not generalize to a nontwin population.
Conclusion
This study provides population-based evidence that familial etiologic factors relevant to autistic behavioral impairment may be more concentrated in females that manifest the phenotype. This finding suggests that there is a component of female sex that protects girls from ASDs and requires that greater familial etiologic load be present for girls to display autistic behavioral impairments. Although the hypothesis is complicated by etiologic and phenotypic heterogeneity in ASDs, these results are consistent with the expectation that a higher inherited genetic load will be associated with autistic impairments in girls. As such, they hold implications for the design and interpretation of ASD genetic association studies. An understanding of the biology underlying female advantage could greatly aid progress in understanding the phenomenology of autistic behavior and in identifying prevention factors for ASDs.
Acknowledgments
We thank the participants of the Twins Early Development Study (TEDS) and Child and Adolescent Twin Study of Sweden for making this research possible as well as Prof. Robert Plomin for use of the TEDS data. TEDS is funded by Medical Research Council Grant G0500079. CATSS is supported in part by the Swedish Council for Working Life and Social Research and the Swedish Research Council. E.B.R. was supported by the Training Program in Psychiatric Genetics and Translational Research Grant T32MH017119 at the Harvard School of Public Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4868.
References
- 1.Fombonne E. Epidemiological surveys of pervasive developmental disorders. In: Volkmar F, editor. Autism and Pervasive Developmental Disorders. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 2.Baron-Cohen S, et al. Prevalence of autism-spectrum conditions: UK school-based population study. Br J Psychiatry. 2009;194(6):500–509. doi: 10.1192/bjp.bp.108.059345. [DOI] [PubMed] [Google Scholar]
- 3.Gilman SR, et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70(5):898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy D, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70(5):886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Skuse DH, et al. Evidence from Turner’s syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387(6634):705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- 6.Skuse DH. Imprinting, the X-chromosome, and the male brain: Explaining sex differences in the liability to autism. Pediatr Res. 2000;47(1):9–16. doi: 10.1203/00006450-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Baron-Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: Implications for explaining autism. Science. 2005;310(5749):819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- 8.Auyeung B, et al. Fetal testosterone and autistic traits. Br J Psychol. 2009;100(Pt 1):1–22. doi: 10.1348/000712608X311731. [DOI] [PubMed] [Google Scholar]
- 9.Betancur C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 10.Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 11.Hallmayer J, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantino JN, Todd RD. Autistic traits in the general population: A twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 13.Skuse DH, Mandy WP, Scourfield J. Measuring autistic traits: Heritability, reliability and validity of the Social and Communication Disorders Checklist. Br J Psychiatry. 2005;187:568–572. doi: 10.1192/bjp.187.6.568. [DOI] [PubMed] [Google Scholar]
- 14.Robinson EB, et al. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%) Arch Gen Psychiatry. 2011;68(11):1113–1121. doi: 10.1001/archgenpsychiatry.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson EB, et al. Stability of autistic traits in the general population: Further evidence for a continuum of impairment. J Am Acad Child Adolesc Psychiatry. 2011;50(4):376–384. doi: 10.1016/j.jaac.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: A decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(3):255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- 17.Lundström S, et al. Autism spectrum disorders and autistic like traits: Similar etiology in the extreme end and the normal variation. Arch Gen Psychiatry. 2012;69(1):46–52. doi: 10.1001/archgenpsychiatry.2011.144. [DOI] [PubMed] [Google Scholar]
- 18.Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constantino JN, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163(2):294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 20.Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57(6):655–660. doi: 10.1016/j.biopsych.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Skuse DH. Is autism really a coherent syndrome in boys, or girls? Br J Psychol. 2009;100(Pt 1):33–37. doi: 10.1348/000712608X369459. [DOI] [PubMed] [Google Scholar]
- 22.Dworzynski K, Ronald A, Bolton P, Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. 2012;51(8):788–797. doi: 10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Russell G, Steer C, Golding J. Social and demographic factors that influence the diagnosis of autistic spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 2011;46(12):1283–1293. doi: 10.1007/s00127-010-0294-z. [DOI] [PubMed] [Google Scholar]
- 24.Smoller JW, Lunetta KL, Robins J. Implications of comorbidity and ascertainment bias for identifying disease genes. Am J Med Genet. 2000;96(6):817–822. doi: 10.1002/1096-8628(20001204)96:6<817::aid-ajmg25>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984;41(12):1178–1182. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- 26.Skuse DH, et al. Social communication competence and functional adaptation in a general population of children: Preliminary evidence for sex-by-verbal IQ differential risk. J Am Acad Child Adolesc Psychiatry. 2009;48(2):128–137. doi: 10.1097/CHI.0b013e31819176b8. [DOI] [PubMed] [Google Scholar]
- 27.Ronald A, et al. Genetic heterogeneity between the three components of the autism spectrum: A twin study. J Am Acad Child Adolesc Psychiatry. 2006;45(6):691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association 2000. Diagnostic Criteria from DSM-IV-TR (Am Psych Assoc, Washington, DC)
- 29.Robinson EB, et al. A multivariate twin study of autistic traits in 12-year-olds: Testing the fractionable autism triad hypothesis. Behav Genet. 2012;42(2):245–255. doi: 10.1007/s10519-011-9500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams J, et al. The CAST (Childhood Asperger Syndrome Test): Test accuracy. Autism. 2005;9(1):45–68. doi: 10.1177/1362361305049029. [DOI] [PubMed] [Google Scholar]
- 31.Hoekstra RA, Happé F, Baron-Cohen S, Ronald A. Limited genetic covariance between autistic traits and intelligence: Findings from a longitudinal twin study. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(5):994–1007. doi: 10.1002/ajmg.b.31066. [DOI] [PubMed] [Google Scholar]
- 32.Larson T, et al. The autism—tics, AD/HD and other comorbidities inventory (A-TAC): Further validation of a telephone interview for epidemiological research. BMC Psychiatry. 2010;10:1. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronald A, Larsson H, Anckarsater H, Lichtenstein P. A twin study of autism symptoms in Sweden. Mol Psychiatry. 2011;16(10):1039–1047. doi: 10.1038/mp.2010.82. [DOI] [PubMed] [Google Scholar]
- 34.Hansson SL, et al. Psychiatric telephone interview with parents for screening of childhood autism - tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): Preliminary reliability and validity. Br J Psychiatry. 2005;187:262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- 35.Field A. Discovering Statistics Using SPSS for Windows. London: Sage; 2002. [Google Scholar]
- 36.Szatmari P, et al. Sex differences in repetitive stereotyped behaviors in autism: Implications for genetic liability. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(1):5–12. doi: 10.1002/ajmg.b.31238. [DOI] [PubMed] [Google Scholar]
- 37.Malhotra D, Sebat J. CNVs: Harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sebat J, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong A, et al. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488(7412):471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purcell S. 2008. Statistical methods in behavioral genetics. Behavioral Genetics, eds Plomin R, DeFries J, McClearn G, McGuffin P (Worth, New York), 5th Ed.
- 41.Kopp S, Gillberg C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): An instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res Dev Disabil. 2011;32(6):2875–2888. doi: 10.1016/j.ridd.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 42.Ozonoff S, et al. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St Pourcain B, et al. Association between a high-risk autism locus on 5p14 and social communication spectrum phenotypes in the general population. Am J Psychiatry. 2010;167(11):1364–1372. doi: 10.1176/appi.ajp.2010.09121789. [DOI] [PMC free article] [PubMed] [Google Scholar]