Abstract
Stem cell therapy and organ regeneration are therapeutic approaches that will, we suggest, become mainstream for the treatment of human disease. Endothelial cells, which line the luminal surface of every vessel in the body, are essential components in any organ regeneration programme. There are a number of potentially therapeutic endothelial cell types, including embryonic, adult progenitor and induced pluripotent stem cell-derived endothelial cells, as well as host vascular cells. The features (benefits as well as disadvantages) of each cell type that make them potentially useful in therapy are important to consider. The field of stem cell biology is well developed in terms of protocols for generating endothelium. However, where there is a distinct and urgent unmet need for knowledge concerning how the endothelial cells from these different sources function as endothelium and how susceptible they may be to inflammation and atherosclerosis. Furthermore, where stem cells have been used in clinical trials there is little commonality in protocols for deriving the cells (and thereby the specific phenotype of cells used), administering the cells, dosing the cells and/or in assessing efficacy attributed to the cells themselves. This review discusses these and other issues relating to stem cell-derived endothelial cells in cell therapy for cardiovascular disease.
Keywords: cardiovascular disease, endothelium, stem cell therapy
Types of stem cell-derived endothelial cells
Human embryonic stem cell-derived endothelial cells
Human embryonic stem cells (hESCs) are an attractive source of therapeutic endothelial cells because they are pluripotent (i.e. can form any cell type in the body) and can be stored in large numbers for long periods of time, are expandable and have unlimited renewal capacity. Human embryonic stem cells are derived from the inner cell mass of the preimplantation blastocyst [1], and in certain culture conditions hESCs have the ability to form a range of cell lineages, including endothelial cells. Endothelial cells were first derived from hESCs in Robert Langer's laboratory in 2002 using a cell culture technique that involved spontaneous differentiation of hESCs into mixed populations, so-called embryoid bodies [2]. Embryoid bodies were then grown in the presence of endothelial cell growth factors to support a vascular endothelial cell expression pathway. Sorting of embryoid bodies by fluorescence-activated cell sorting for endothelial cell markers subsequently allowed isolation of a cell population that expressed endothelial cell markers (CD31, VE-cadherin and von Willebrand factor (vWF)) and formed tubes when expanded in vitro. It is this cell population that is defined as hESC-endothelial cells (hESC-ECs). Whilst the definition of these cells appears consistent throughout the literature, alternative and more efficient protocols for differentiation and isolation of hESC-ECs are now available [3, 4]. Research continues to improve the stability and expandability of these cells, and strategies exist for circumventing immunogenicity issues with these cells [5].
Whilst no human clinical trial data yet exist for the use of hESC-ECs, they have been shown to form vascular networks in vivo [2, 6, 7] and to improve cardiac function in animal models of ischaemic heart disease [8]. It is encouraging to note that other cell types derived from hESCs have shown some success in human clinical trials. In 2009, the first phase I clinical trial for use of hESCs by Geron was approved by the FDA (Clinical trials identifier: NCT01217008). In 2010, however, the field of embryonic stem cell therapy took a body blow because this major investor in the area announced that it was suspending its future hESC research programme (http://www.guardian.co.uk/science/2011/nov/15/geron-abandons-stem-cell-therapy). This turn of events, together with the long-standing debate surrounding ethical issues of using hESCs, adds increased momentum to the search for alternative sources of stem cell-derived endothelial cells. The important questions are, which stem cell-derived endothelial cell is most suitable, safest and most efficient for the treatment of vascular disease? The hESC-ECs are certainly a powerful cell type in terms of promoting vascular repair and show great potential in cardiovascular medicine, but for reasons of both ethics and safety (immunogenicity and tumour formation) may not prove to be the best cell choice in all situations.
Endothelial progenitor cells
In 1997, it was suggested by Asahara et al. that a population of cells exists that are derived from the bone marrow and are capable of differentiating into endothelial cells and forming new vessels at sites of ischaemia [9]. This marked a shift towards the concept of physiological postnatal neoangiogenesis, and the term endothelial progenitor cell (EPC) was introduced. A number of studies have followed that defined this novel cell population and their use as biomarkers and in cell therapy [10]. There has been controversy over their definition and their role in vascular biology. As a result, no unifying definition of these cells exists, and the term EPC in fact encompasses a number of cell types with putative roles in vascular homeostasis and disease. However, there is some agreement in the nature of ‘early’ vs. ‘late’ outgrowth endothelial cells (EPCs), which can be enriched in cultures using specific isolation protocols. The nature of these cells is described in detail elsewhere [11]. It appears, to date, that one of four EPC culture strategies is generally used to expand cells identified by expression of CD31, CD34 and Vascular endothelial growth factor receptor-2 (VEGFR2), together with vWF and/or endothelial nitric oxide synthase (eNOS). Whether variation in the isolation strategy of EPCs can result in endothelial cell populations different enough to affect clinical outcome remains unknown.
In the context of tissue repair, the role of EPCs vs. flanking vascular endothelial cells has been widely discussed and debated [12, 13]. In vivo evidence for EPC-driven vascular repair came in 2003, when it was found that EPCs could populate the new endothelium in vein grafts on carotid arteries of mice [14] and that injection of these cells could reverse vascular dysfunction [15]. The mechanism by which EPCs function to repair damaged vasculature is unclear. Some groups have shown in similar in vivo models that the flanking vasculature endothelial cells rather than bone marrow-derived progenitor cells repair local damaged endothelium [16, 17]. The potential for improved vascular function by injection of healthy EPCs still stands nonetheless.
In health, EPCs are thought to be critical for vascular homeostasis [18–20]. As such, levels of EPCs are negatively correlated with cardiovascular diseases, including hypertension, pulmonary hypertension, diabetes mellitus, carotid artery disease, sepsis and heart failure [21, 22]. Taken together, these studies suggest that dysfunctional and/or low levels of circulating EPCs can contribute to disease. With this in mind, as discussed below in 'data from the clinic', there is a potential therapeutic utility in administering EPCs and/or mobilizing EPCs from the bone marrow using drugs.
Finally, EPCs are attractive in the area of organ regeneration for transplant. They offer benefits over ESC-ECs as a source of endothelium because they can be grown from the patient's own tissue, thereby avoiding issues of rejection. Furthermore, the efficacy and safety of pure autologous EPC injections have been assessed in patients with idiopathic pulmonary hypertension [26]. However, it should also be remembered that endothelial cells are not terminally differentiated and can be isolated and expanded from donor vessels. The benefit of endothelium from host EPCs vs. host vessels in the context of tissue and organ regeneration remains to be established.
Induced pluripotent stem cell-derived endothelial cells
Human induced pluripotent stem cells (iPSCs), first engineered by Yamanaka's group in 2007 [27], are adult human cells that have been reprogrammed into a pluripotent phenotype. In the original paper, Yamanaka and coworkers showed how iPSCs expressed the pluripotency-associated genes OCT3/4, REX1, NANOG and Sox-2 and could be differentiated into cardiac and neuronal cells [27]. It was later shown by the same group [28] that human iPSCs can also be differentiated into endothelial cells. Human iPSC-ECs have now been studied in preclinical models of ischaemia and found to be capable of forming vascular networks and increasing blood perfusion of the hindlimbs of SCID mice [29]. The iPSC-ECs, like EPCs, also hold the potential for autologous therapy.
Data from the clinic
Tissue-engineered vessel grafting
Vascular grafts are used to bridge blood flow between two blood vessels, either to bypass an occluded area, such as an atherosclerotic coronary artery, or to repair a congenital vascular defect. Typically, materials for vascular grafts are autologous vessels, such as saphenous vein or internal mammary artery. However, where tissue from the patient is not suitable, grafts can been ‘made’ from decellularized human (allogenic) or nonhuman (xenogenic) vessels or artificial materials. Allogenic and xenogenic conduits have been used predominantly as grafts for pulmonary arteries or to repair venous/arterial circulation defects [30, 31]. In all cases, postoperative complications are limiting and include thrombosis, restenosis, calcification and lack of durability. Given these well-documented complications, there is an impetus to find new approaches to generate suitable conduits using the best source of endothelial cells. This was reflected in 2000 when the first tissue-engineered vascular graft, consisting of a biodegradable scaffold seeded with autologous endothelial cells from the donor saphenous vein, was transplanted into a 4-year-old girl with single right ventricular and pulmonary atresia [32]. The procedure was successful, with no postoperative complications. It has been recognized that accessibility of autologous vascular endothelial cells from patients is restricted and the biopsy required to remove the vessel is distressing for the patient [31, 32]. As such, stem cell sources of endothelium represent a viable alternative.
One approach to capturing endothelium from EPCs is to seed bone marrow-derived mononuclear cells (BMD-MNCs, which contain EPCs) onto biodegradable scaffolds. These types of grafts have been implanted into both animal models and into patients in human clinical trials as cavopulmonary connection graft surgeries [30, 33]. In all cases, BMD-MNCs when seeded in vitro formed endothelial cells and smooth muscle cells that expressed requisite cell markers [31, 34]. Assessment of these grafts in a canine model showed that the ‘vessels’ displayed the gold standard response of NO release in response to acetylcholine [35]. Whilst the endothelium of these vessels is clearly derived from EPCs at the time of transplant, it is not clear how long this endothelium lasts or the contribution that endothelium from flanking regions of the vessel makes post-transplant [36]. It is likely that a combination of both EPCs and recipient endothelial cells populate the graft, at least in the short term. Animal studies investigating the biology of EPC-driven endothelialization suggest that up to one-third of allogenic grafts are populated by EPCs in vivo [14]. A recent paper suggests that EPCs represent a better source of endothelium than vascular sources, because in pigs, vessels revascularized with EPCs were more patent than non-endothelialized grafts or grafts coated with mature endothelial cells [37]. In this study, EPCs remained in the vessel postimplant and had integrated with the host vasculature [37]. No human clinical trial data are available for use of tissue-engineered vessel grafts made using a clearly defined EPC population and so it is difficult at this stage to address which cell type is best in vessel engineering and, indeed, whether stem cell-derived endothelial cells are best applied in vessel engineering.
Endothelial cell therapy
Therapeutic angiogenesis is a new and exciting approach to the treatment of cardiovascular disease. It involves promotion of new vessels to treat myocardial ischaemia and/or peripheral ischaemic disease. Use of BMD-MNCs has been successful in clinical trials of patients with limb ischaemia [38]; benefits were attributable to endothelial cell progenitor cells and pro-angiogenic cytokine-secreting haematopoietic cells [9, 19, 38]. Whether the BMD-MNCs or the peripheral blood mononuclear fraction is the optimal source of cytokine-releasing cells and EPCs is unclear [38–40]. One possible reason for limitations in using peripheral blood as a source of cells is the low levels of cells in blood of patients with cardiovascular disease, as already outlined. As discussed below, this creates a need for pharmacological mobilization to ensure sufficient EPC presence in the peripheral blood. Mobilization of EPCs from the bone marrow and recruitment to sites of damage are regulated by cytokines such as vascular endothelial growth factor, CXCL12, granulocyte-colony stimulating factor (G-CSF) and s-KitL. Boyle et al. [41] in 2006 took advantage of this and used G-CSF to increase the yield of CD34+ ‘EPCs’ in blood for isolation and subsequent re-injection into five patients with chronic ischaemic heart disease, whose symptoms were improved within a 12 month follow-up period. However, this trial lacked a clear control arm. Other trials, using similar mobilization and isolation strategies, have been carried out and report improved left ventricular ejection fraction in a 6 month follow-up of patients with acute myocardial infarction [42]. It should be noted, however, that CD34+ ‘EPCs’ might represent a mixed cell population. Other potential EPC-containing cell populations, such as CD133+ bone marrow-derived cells, have been considered in clinical trials with patients with chronic ischaemic disease [43]. With multiple cell definitions in use it is difficult to pin down the relative contribution of EPCs to vessel repair and the benefits thereof. The potential for hESC-ECs and human iPCS-ECs in human cell therapy remains to be investigated, but these cell types deserve consideration, particularly for patients where EPC function is impaired [21] and autologous therapy and bone marrow or peripheral blood injections are not suitable.
Another area of clinical cell therapy is cardiomyoplasty. Cellular cardiomyoplasty refers to a technique whereby cells are injected intravenously or directly onto infarct zones to restore organ vascularization and function. This technique has proved effective in patients with transmural infarction [44], where mononuclear cells from bone marrow aspirated from the ileum of 10 patients were injected onto the infarct zone. This approach resulted in significant benefit to patients in terms of decrease in perfusion defect; however, the authors note that timing of injection is critical; early (5 days postinfarct) injections are required, and the benefit declines with time postinfarct [44].
Whilst bone marrow cells have proved useful as described above, trials to date have used non-uniform definitions of cell preparations and, in any case, the cells used have poor survival in the in vivo niche [45, 46]; thus, ex vivo modifications [47–49] and a more pharmacological approach may be required to determine parameters such as optimal dose, route of delivery, pharmacokinetics and pharmacodynamics. This kind of assessment, applied by necessity to drug therapy, appears to have been neglected in cell therapy. Other types of stem cell-derived endothelial cells may also be useful in therapeutic angiogenesis. The ESC-ECs, for example, have been shown in animal models to repair damage after myocardial infarction [50] and to repair ischaemic sites following stroke [51].
A recent Cochrane review (2012) [52] in the area of stem cell therapy for acute myocardial infarction concluded, on the basis of 33 randomized control trials, that whilst stem cell therapy appears beneficial there is significant heterogeneity in trial design. This dovetails with our suggestion to the field that a more pharmacological, interdisciplinary and unified approach for the advancement of endothelial cell stem therapy is essential. Some selected clinical trials associated with the application of cell therapy in cardiovascular disease are shown in Table 1.
Table 1.
Selected human data on endothelial progenitor cells and bone marrow-derived mononuclear cell applications as cell therapy
| Clinical trial, date and reference number | Disease | Outcome | Progenitor cell type |
|---|---|---|---|
| Wang et al. (2007) [26] | Idiopathic pulmonary hypertension | 6 min walk test ↑, mean AP ↓, PVR ↓, cardiac output ↑ | CD34+ CD133+ VEGFR2+ EPCs |
| TOPCARE-CHD (2006) [72] | Stable ischaemic heart disease. (N.B. BMD better than circulating progenitor cells) | LVEF ↑, safety | BMD-MNCs vs. VEGFR2+ CD31+ CD146+ EPCs |
| TOPCARE-AMI (2002–2011) [73][74][75] | Acute myocardial infarction | LVEF ↑, NT-proBNP ↓ | BMD-MNCs vs. VEGFR2+ CD31+ CD146+ EPCs |
| Fernández-Avilés et al. (2004) [76] | Acute myocardial infarction | LVEF, LVSV ↑, LVEDV, LVESV ↓ | BMD-MNCs |
| Kuethe et al. (2004) [77] | Acute myocardial infarction | No improvement vs. control | BMD-MNCs |
| Bartunek et al. (2005) [78] | Acute myocardial infarction | LVEF, LVESV ↑ | CD133+ BMD-MNCs |
| Boyle et al. (2006) [41] | Chronic ischaemic heart disease | Angina episodes ↓ | CD34+ PB-derived cells |
| Janssens et al. (2006) [79] | Acute myocardial infarction | LVEF (no increase vs. control), infarct size ↓ | BMD-MNCs |
| REPAIR-AMI (2006) [80] | Acute myocardial infarction | LVEF, LVEDV ↑, LVESV ↓ | BMD-MNCs |
| TCT-STAMI (2006) [81] | Acute myocardial infarction | LVEF ↑ | BMD-MNCs |
| Li et al. (2007) [42] | Acute myocardial infarction | LVEF ↑, LVESV, LVEDV ↓ | PB-MNCs (G-CSF mobilized) |
| Losordo et al. (2007) [82][83] | Coronary heart disease and angina | Angina epsiodes ↓ | CD34+ PB cells (G-CSF mobilized) |
| Stamm et al. (2007) [43][84] | Coronary ischaemic heart disease | LVEF ↑, safety | CD133+ BMD cells |
| REGENT (2008) [85] | Acute myocardial infarction | LVEF ↑, safety | CD34+ CXCR4+ BMD cells or BMD-MNCs |
| FINCELL (2008) [86] | Acute myocardial infarction | LVEF, LVEDV ↑ LVESV ↓ | BMD-MNCs |
| ASTAMI (2005, 2008) [87, 88] | Acute myocardial infarction (N.B. Safety of intracoronary cell infusion shown) | No improvement vs. control, safety | BMD-MNCs |
| BOOST (2009) [89] | Myocardial infarction | No improvement vs. control | BMD-MNCs |
| Wohrle et al. (2010) [90] | Acute myocardial infarction | LVEF ↑ | BMD-MNCs |
| STAR-heart (2010) [91] | Chronic heart failure | LVEF ↑, mortality ↓ | BMD-MNCs |
Abbreviations: BMD-MNCs, bone marrow-derived mononuclear cells; EPCs, endothelial progenitor cells; G-CSF, granulocyte-colony stimulating factor; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVSV, left ventricular stroke volume; mean AP, mean arterial pressure; NT-proBNT, N-terminal prohormone of brain natriuretic peptide; PB, peripheral blood; and PVR, pulmonary vascular resistance; VEGFR2, vascular endothelial growth factor-receptor 2. Adapted from reviews by Mingliang et al. [45] and Mund et al. [13]. Trials have been selected to illustrate that variation in outcome measure, outcome and cell type used has occurred in clinical trials.
In vitro vascularization of engineered organs
As mentioned above, stem cell-derived endothelial cells are likely to feature strongly in the field of organ regeneration where, as with vessel engineering, in vitro vascularization of engineered organs prior to transplant will be necessary to protect organs from thrombosis. A landmark study in the field of organ engineering was by Ott et al. in 2008 [53, 54], who showed how cadaveric organs when decellularized and recellularized with cardiac progenitor cells formed a three-dimensional and fully functional beating heart. However, the next step for such an approach requires techniques where vascular cells can be coupled intricately to cardiac myocytes and valve cells to form a functioning organ. This is something that is proving difficult to achieve within the field [55]. Stem cell-derived endothelial cells with their pluripotency and survival capacity could be beneficial in this area.
Pharmacological mobilization
In the foregoing sections we have discussed applications of endothelial cells from stem cells that require isolation and expansion. However, stem cells can also be mobilized from the bone marrow without the need for ex vivo and in vitro manipulation. In vivo mobilization of endothelial cell stem cells to the site of injury using pharmacological agents is now an active area of research. In fact, outside the field of endothelial cell biology, mobilization of stem cells from the bone marrow of cancer patients using drugs such as G-CSF is part of standard chemotherapy [56]. Stem cell trafficking and mobilization are regulated by a cytokine axis involving CXCL12 and CXCR4 [57, 58]. Interference of this with the CXCR4 antagonist AMD 3100 (Plexifor), which is approved for use in patients with multiple myeloma, results in increased circulating levels of haematopoietic stem cells [56, 59]. Its application as a stimulant of stem cells for donation is being investigated.
Early evidence suggests that some common drugs used to treat cardiovascular disease may act in part by cardiovascular stem cell mobilization. Vasa et al. in 2001 found that patients with coronary artery disease, without myocardial infarction, when given atorvastatin had increases in circulating CD34+ VEGFR2+ EPCs [60]. Likewise, in a later study, Fadini et al. in 2010 [61] found that the dipeptidyl peptidase-4 inhibitor sitagliptin increased circulating levels of EPCs in line with levels of CXCL12, normally degraded by dipeptidyl peptidase-4. The potential of stem cell mobilization was further demonstrated in mice by Smart et al. [62], who showed that treatment of mouse epicardial explants with thymosin-β4 resulted in activation of adult epicardial stem cells and differentiation into vascular cells [62] that could restore cardiac function in a mouse model of cardiac injury [63]. Clearly, there is a real opportunity for pharmacological innovation in the field of stem cell mobilization to repair the vasculature and in organ regeneration programmes.
Enhanced endothelial cell phenotypes and ‘supercell’ engineering
Endothelial cells provide a protective lining to vessels and, by the release of vasoactive hormones, limit thrombosis, atherosclerosis and vasospasm. Ensuring that endothelium from stem cells retains a cytoprotected state and resists inflammation in sites of disease is likely to pose a challenge, not least because these cells may be prone to activation. One approach would be to engineer cells to overexpress protective genes or pathways [48]. This approach is being taken in one of the first clinical studies using EPCs from patients with pulmonary hypertension, where EPCs transfected with eNOS are being injected back into patients (clinical trial identifier, NCT00469027) [48] and into animal models of disease [48]. Another example of how endothelial cells from stem cells may express improved phenotypes comes from our group. We have shown that hESC-ECs lack function of the receptor Toll-like receptor (TLR)-4 and TLR2 [64]. The TLRs belong to the family of pattern recognition receptors and are important in innate immune responses during infection. However, TLRs also sense host molecules, including those associated with vascular inflammation and atherosclerosis [65]. Thus, TLR4 and TLR2 are thought to be critical receptors in the initiation and propagation of atherosclerosis. Engineered endothelial cells lacking TLR function may well provide a new endothelium that resists inflammatory and atherosclerotic stimuli and, in this way, be improved in comparison to endothelial cells from the host. We have also shown in pilot studies that hESC-ECs release relatively low levels of endothelin-1 66, a vascular hormone associated with hypertension and cardiovascular disease [67]. Thus, the lack of TLR function and low endothelin-1 levels present in hESC-ECs may provide this cell type with an inherent advantage over endothelial cells from other sources in the treatment of cardiovascular disease or in tissue and organ engineering.
Figure 1 summarizes the potential applications and routes of delivery of stem cell-derived endothelial cell therapy.
Figure 1.
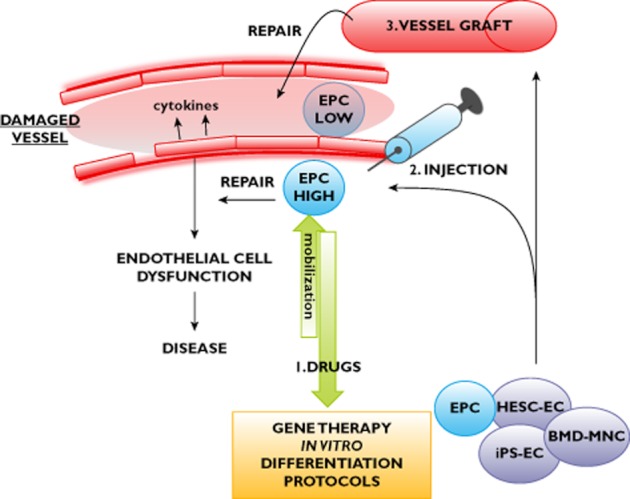
Summary of potential applications of bone marrow-derived mononuclear cells (BMD-MNCs), endothelial progenitor cells (EPCs), human embryonic stem cell-derived endothelial cells (HESC-ECs) and induced pluripotent stem cell-derived endothelial cells (iPS-ECs). In the damaged vessel, or in cardiovascular disease, there is endothelial cell dysfunction, EPC numbers are decreased, and function is impaired. Possible therapeutic uses are as follows: (1) bone marrow-derived progenitors, including EPCs, could be mobilized with drugs to stimulate endogenous repair; (2) mobilized cells or HESC-ECs or human iPS-ECs could be expanded in vitro, combined with gene therapy approaches to enhance cell function, and injected at sites of vascular injury; and (3) EPCs, HESC-ECs, human iPS-ECs or BMD-MNCs could be used to engineer vessels for grafting at sites of damage
Potential for stem cell therapy to cause tumours
Along with efficacy, the safety aspect of stem cell therapy is currently an active area of research. Owing to the pluripotent nature of stem cells the potential for them to cause cancer has been of concern, and it is clear that undifferentiated embryonic stem cells [68] or iPSCs [71].
Summary
Endothelial cells are more than simple lining cells, and cardiovascular health relies on a fully functional endothelium. Endothelial cells must be able to react rapidly to injury to release protective mediators, such as NO, and resolve infection through innate immune pathways. These and other functions of the endothelium must be considered and preserved in any cell therapy programme. Endothelial cells can be expanded from host blood vessels, differentiated embryonic stem cells (hESC-ECs), EPCs and iPSCs. Each of these sources represents viable options for cell therapy and in organ regeneration programmes. However, there are many important questions that need to be addressed using a multidisciplinary approach, with input from cell biology, pharmacology, bioengineering and clinical trials, before the full and optimal utility of stem cell endothelial cells can be realized. The ultimate goal would be to have a source of endothelial cells that would effectively repair or regenerate the vasculature and function as healthy endothelium, and that could be stored centrally and made available for use in clinical trials and therapy, just as with drugs. The role of the pharmacologist is essential in achieving this.
Competing Interests
There are no competing interests to declare.
References
- 1.Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones J. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Levenberg S, Golub J, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levenberg S, Ferreira LS, Chen-Konak L, Kraehenbuehl TP, Langer R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat Protoc. 2010;5:1115–1126. doi: 10.1038/nprot.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James D, Nam H, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, Zaninovic N, Rosenwaks Z, Rabbany S, Rafii S. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinnemo KH, Sylvén C, Hovatta O, Dellgren G, Corbascio M. Immunogenicity of human embryonic stem cells. Cell Tissue Res. 2008;331:67–78. doi: 10.1007/s00441-007-0486-3. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira L, Gerecht S, Shieh H, Watson N, Rupnick M, Dallabrida S, Vunjak-Novakovic G, Langer R. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 7.Cho S, Moon S, Lee S, Kang S, Kim J, Lim J, Kim H, Kim B, Chung H. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Wilson K, Smith B, Kraft D, Jia F, Huang M, Xie X, Robbins R, Gambhir S, Weissman I, Wu J. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS ONE. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13:87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mund JA, Ingram DA, Yoder MC, Case J. Endothelial progenitor cells and cardiovascular cell-based therapies. Cytotherapy. 2009;11:103–113. doi: 10.1080/14653240802714827. [DOI] [PubMed] [Google Scholar]
- 14.Xu Q, Zhang Z, Davison F, Hu Y. Circulating progenitor cells regenerate endothelium of vein graft atherosclerosis, which is diminished in ApoE-deficient mice. Circ Res. 2003;93:e76–86. doi: 10.1161/01.RES.0000097864.24725.60. [DOI] [PubMed] [Google Scholar]
- 15.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 16.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 17.Hibino N, Villalona G, Pietris N, Duncan DR, Schoffner A, Roh JD, Yi T, Dobrucki LW, Mejias D, Sawh-Martinez R, Harrington JK, Sinusas A, Krause DS, Kyriakides T, Saltzman WM, Pober JS, Shin'oka T, Breuer CK. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25:2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 19.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 21.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 22.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 23.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 24.Rafat N, Hanusch C, Brinkkoetter PT, Schulte J, Brade J, Zijlstra JG, van der Woude FJ, van Ackern K, Yard BA, Beck GCh. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med. 2007;35:1677–1684. doi: 10.1097/01.CCM.0000269034.86817.59. [DOI] [PubMed] [Google Scholar]
- 25.Cribbs SK, Martin GS, Rojas M. Monitoring of endothelial dysfunction in critically ill patients: the role of endothelial progenitor cells. Curr Opin Crit Care. 2008;14:354–360. doi: 10.1097/MCC.0b013e3282fc216d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49:1566–1571. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Taura D, Sone M, Homma K, Oyamada N, Takahashi K, Tamura N, Yamanaka S, Nakao K. Induction and isolation of vascular cells from human induced pluripotent stem cells – brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- 29.Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, Gambhir SS, Cooke JP. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–436. doi: 10.1016/j.jtcvs.2009.09.057. 36.e1–2. [DOI] [PubMed] [Google Scholar]
- 31.Hibino N, Shin'oka T, Matsumura G, Ikada Y, Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg. 2005;129:1064–1070. doi: 10.1016/j.jtcvs.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 33.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura G, Miyagawa-Tomita S, Shin'oka T, Ikada Y, Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 35.Matsumura G, Ishihara Y, Miyagawa-Tomita S, Ikada Y, Matsuda S, Kurosawa H, Shin'oka T. Evaluation of tissue-engineered vascular autografts. Tissue Eng. 2006;12:3075–3083. doi: 10.1089/ten.2006.12.3075. [DOI] [PubMed] [Google Scholar]
- 36.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci U S A. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T Investigators TAuCTTS. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 39.Minamino T, Toko H, Tateno K, Nagai T, Komuro I. Peripheral-blood or bone-marrow mononuclear cells for therapeutic angiogenesis? Lancet. 2002;360:2083–2084. doi: 10.1016/s0140-6736(02)11977-5. author reply 84. [DOI] [PubMed] [Google Scholar]
- 40.Moriya J, Minamino T, Tateno K, Shimizu N, Kuwabara Y, Sato Y, Saito Y, Komuro I. Long-term outcome of therapeutic neovascularization using peripheral blood mononuclear cells for limb ischemia. Circ Cardiovasc Interv. 2009;2:245–254. doi: 10.1161/CIRCINTERVENTIONS.108.799361. [DOI] [PubMed] [Google Scholar]
- 41.Boyle AJ, Whitbourn R, Schlicht S, Krum H, Kocher A, Nandurkar H, Bergmann S, Daniell M, O'Day J, Skerrett D, Haylock D, Gilbert RE, Itescu S. Intra-coronary high-dose CD34+ stem cells in patients with chronic ischemic heart disease: a 12-month follow-up. Int J Cardiol. 2006;109:21–27. doi: 10.1016/j.ijcard.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 42.Li ZQ, Zhang M, Jing YZ, Zhang WW, Liu Y, Cui LJ, Yuan L, Liu XZ, Yu X, Hu TS. The clinical study of autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute myocardial infarction (AMI) Int J Cardiol. 2007;115:52–56. doi: 10.1016/j.ijcard.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, David A, Liebold A, Nienaber C, Zurakowski D, Freund M, Steinhoff G. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 44.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 45.Mingliang R, Bo Z, Zhengguo W. Stem cells for cardiac repair: status, mechanisms, and new strategies. Stem Cells Int. 2011;2011:310928. doi: 10.4061/2011/310928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 47.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 48.Ward MR, Thompson KA, Isaac K, Vecchiarelli J, Zhang Q, Stewart DJ, Kutryk MJ. Nitric oxide synthase gene transfer restores activity of circulating angiogenic cells from patients with coronary artery disease. Mol Ther. 2011;19:1323–1330. doi: 10.1038/mt.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart DJ, Mei SH. Cell-based therapies for lung vascular diseases: lessons for the future. Proc Am Thorac Soc. 2011;8:535–540. doi: 10.1513/pats.201105-035MW. [DOI] [PubMed] [Google Scholar]
- 50.Kraehenbuehl TP, Ferreira LS, Hayward AM, Nahrendorf M, van der Vlies AJ, Vasile E, Weissleder R, Langer R, Hubbell JA. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oyamada N, Itoh H, Sone M, Yamahara K, Miyashita K, Park K, Taura D, Inuzuka M, Sonoyama T, Tsujimoto H, Fukunaga Y, Tamura N, Nakao K. Transplantation of vascular cells derived from human embryonic stem cells contributes to vascular regeneration after stroke in mice. J Transl Med. 2008;6:54. doi: 10.1186/1479-5876-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD006536.pub3. CD006536. [DOI] [PubMed] [Google Scholar]
- 53.Taylor DA. From stem cells and cadaveric matrix to engineered organs. Curr Opin Biotechnol. 2009;20:598–605. doi: 10.1016/j.copbio.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 54.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 55.Phelps EA, García AJ. Engineering more than a cell: vascularization strategies in tissue engineering. Curr Opin Biotechnol. 2010;21:704–709. doi: 10.1016/j.copbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keating GM. Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma. Drugs. 2011;71:1623–1647. doi: 10.2165/11206040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 57.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 59.Dugan MJ, Maziarz RT, Bensinger WI, Nademanee A, Liesveld J, Badel K, Dehner C, Gibney C, Bridger G, Calandra G. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010;45:39–47. doi: 10.1038/bmt.2009.119. [DOI] [PubMed] [Google Scholar]
- 60.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 61.Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 63.Smart N, Bollini S, Dubé KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Földes G, Liu A, Badiger R, Paul-Clark M, Moreno L, Lendvai Z, Wright J, Ali N, Harding S, Mitchell J. Innate immunity in human embryonic stem cells: comparison with adult human endothelial cells. PLoS ONE. 2010;5:e10501. doi: 10.1371/journal.pone.0010501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edfeldt K, Swedenborg J, Hansson G, Yan Z. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 66.Reed DM, Foldes G, Ali NN, Randi AM, Starke RD, Paschalaki KE, Harding SE, Mitchell JA. Endothelin-1 release from endothelial cells from blood vessels compared with those derived from stem cell sources. The Twelfth International Conference on Endothelin, Cambridge, UK: pA2 online, 2011.
- 67.Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol. 1999;31:61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- 68.Forsberg M, Hovatta O. Challenges for the Therapeutic use of Pluripotent Stem Derived Cells. Front Physiol. 2012;3:19. doi: 10.3389/fphys.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 70.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 71.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Assmus B, Honold J, Schächinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 73.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 74.Leistner DM, Fischer-Rasokat U, Honold J, Seeger FH, Schächinger V, Lehmann R, Martin H, Burck I, Urbich C, Dimmeler S, Zeiher AM, Assmus B. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100:925–934. doi: 10.1007/s00392-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 75.Schächinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, de la Fuente L, Gómez-Bueno M, Cantalapiedra A, Fernández J, Gutierrez O, Sánchez PL, Hernández C, Sanz R, García-Sancho J, Sánchez A. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 77.Kuethe F, Richartz BM, Sayer HG, Kasper C, Werner GS, Höffken K, Figulla HR. Lack of regeneration of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans with large anterior myocardial infarctions. Int J Cardiol. 2004;97:123–127. doi: 10.1016/j.ijcard.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 79.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 80.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM, Investigators R-A. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 81.Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, Fan B, Liu X, Zhang S, Sun A, Zou Y. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT-STAMI) Heart. 2006;92:1764–1767. doi: 10.1136/hrt.2005.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 83.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA Investigators A-C. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schümichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 85.Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, Musiałek P, Piwowarska W, Nessler J, Buszman P, Grajek S, Breborowicz P, Majka M, Ratajczak MZ, Investigators R. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313–1321. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 86.Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, Savolainen ER, Ukkonen H, Pietilä M, Airaksinen JK, Knuuti J, Mäkikallio TH, Investigators F. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart J. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- 87.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K Investigators A. Autologous stem cell transplantation in acute myocardial infarction: the ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J. 2005;39:150–158. doi: 10.1080/14017430510009131. [DOI] [PubMed] [Google Scholar]
- 88.Lunde K, Solheim S, Forfang K, Arnesen H, Brinch L, Bjørnerheim R, Ragnarsson A, Egeland T, Endresen K, Ilebekk A, Mangschau A, Aakhus S. Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells: safety, clinical outcome, and serial changes in left ventricular function during 12-months’ follow-up. J Am Coll Cardiol. 2008;51:674–676. doi: 10.1016/j.jacc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 89.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978–2984. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 90.Wöhrle J, Merkle N, Mailänder V, Nusser T, Schauwecker P, von Scheidt F, Schwarz K, Bommer M, Wiesneth M, Schrezenmeier H, Hombach V. Results of intracoronary stem cell therapy after acute myocardial infarction. Am J Cardiol. 2010;105:804–812. doi: 10.1016/j.amjcard.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 91.Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010;12:721–729. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]


