Abstract
Glia, including astrocytes, are increasingly at the forefront of neurodegenerative research for their role in the modulation of neuronal function and survival. Improved understanding of underlying disease mechanisms, including the role of the cellular environment in neurodegeneration, is central to therapeutic development for these currently untreatable diseases. In these endeavours, experimental models that more closely reproduce the human condition have the potential to facilitate the transition between experimental studies in model organisms and patient trials. In this review we discuss the growing role of astrocytes in neurodegenerative diseases, and how astrocytes generated from human pluripotent stem cells represent a useful tool for analyzing astrocytic signalling and influence on neuronal function.
Keywords: antioxidants, astrocytes, neuroprotection, Nrf2, oxidative stress, stem cells
Introduction
Neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease, amyotrophic lateral sclerosis (ALS) and Huntington's disease are untreatable conditions that collectively represent a major healthcare burden. Improved understanding of the biology of these diseases is required in order to develop neuroprotective and ultimately reparative treatments. While these disorders differ in their symptomatology and presentation, they share some common features: gradual clinical progression over years, implicating ongoing degenerative processes, and disturbance of the cellular environment play a key role in neuronal deterioration [1]. Moreover, these disorders share some injury mechanisms. From human pathological tissue and rodent models of neurodegeneration, it is apparent that oxidative stress, glutamate excitotoxicity and protein misfolding are involved in the progression of several disorders, both in the propagation of injury as well as potentially induction. In the context of oxidative stress, hallmarks of oxidative neuronal injury have been found in a range of disorders, including AD [2–4], ALS [5] and Parkinson's disease [6, 7]. Despite these established links between neurodegenerative disease to oxidative and nitrosative stress, trials of small molecule antioxidants or spin traps have had limited success [8]. There are many potential explanations for this, including the challenge of maintaining high concentrations of the drug in the brain in order to neutralize reactive oxygen species when they appear. Another consideration is that the antioxidant and detoxification systems of the brain are sophisticated and complex, and cannot be mimicked simply by a single small molecule. As such, researchers are turning towards the mechanisms by which the brain's intrinsic antioxidant defences are controlled, or how neurons regulate downstream effects of oxidative insults, and how these may be manipulated for therapeutic effect [9–11].
The control of a neuron's antioxidant defences and indeed multiple aspects of its function are highly dependent on its cellular microenvironment, and in particular interactions between microglia and macroglia, including astrocytes [12]. Furthermore, neuronal dysfunction in several neurodegenerative disorders may be attributable in part to damage or dysfunction in astrocytes and other glial cells [13]. Human stem cell based technology now allows direct study in vitro of glial-neuronal interaction to study mechanisms of neurotoxicity and neuroprotection [14].
The growing role of astrocytes in neurodegenerative diseases
While traditional thinking has been neuron-centric in addressing the causes of neurodegenerative diseases, there is growing evidence for the role of glia, specifically astrocytes. It is becoming increasingly evident that the astrocytic environment can be central to disease outcome and, dependent on context, can be injurious or protective [15–19].
For example, in AD, reactive astrocytes are intimately associated with amyloid beta plaques in patient pathological tissue. Experimental studies have demonstrated that astrocytes undergo chemotaxis, responding to MCP-1 found in AD lesions, and internalize amyloid beta [20–22]. Astrocyte internalization of amyloid beta is potentially an ApoE-dependent process, which further implicates astrocytes in the pathogenesis of heritable forms of AD characterized by ApoE mutations [23]. The involvement of astrocytes in disease lesions has been replicated in vivo. Monitoring the migration of transplanted e-GFP positive astrocytes in human amyloid beta-bearing transgenic mice demonstrated that transplanted astrocytes migrated to and internalized amyloid beta [24]. Whilst contributing to clearance, amyloid beta internalization appears to detrimentally alter astrocyte behaviour causing accumulation of intracellular calcium, depletion of glutathione and mitochondrial dysfunction [25, 26]. Astrocytes appear to tolerate amyloid beta-induced metabolic changes reasonably well. However, co-cultured neurons die as a result. Mechanisms implicated in neurotoxicity include NADPH oxidase and PI3Kinase dysregulation [26, 27].
Alpha synuclein accumulation within astrocytes has been observed in the brains of Parkinson's disease patients and in vitro [28], where direct transfer of alpha-synuclein from neurons to astrocytes has been observed [29]. Expression of mutant alpha-synuclein selectively in astrocytes has been shown to result in neuronal death due to astrocytic dysfunction, evidenced by reactive gliosis, disruption of the blood–brain barrier, down-regulation of astrocyte glutamate transporters [30] and production of inflammatory cytokines [29].
Studies of the SOD1 transgenic model of ALS have provided strong evidence that astrocytes have a role in associated neuronal injury. Expression of mutant SOD1 specifically in mouse neurons on a wild-type background was insufficient to cause motor deficits in mice [31] and reduction of expression of mutant SOD1 specifically in astrocytes on a mutant background reduced disease progression [32]. In human mutant SOD1/wild-type SOD1 chimera mice, neurons bearing the mutation underwent differential survival according to neighbouring non-neuronal cells. If neighbouring astrocytes were wild-type, the neurons survived, and if the neighbouring astrocytes bore mutant SOD1, significant neuronal loss was noted. Moreover wild-type neurons with neighbouring mutant astrocytes also bore hallmarks of degeneration [15]. Rodent in vitro disease modelling using a ‘mix and match’ co-culture of wild-type and mutant astrocytes with motor neurons has extended these insights to suggest contact and soluble mediated mechanisms of mutant astrocyte-mediated neurotoxicity [19, 33]. Similar insights were made in the human in vitro system. Human embryonic stem cell derived motor neurons were combined with rodent astrocytes [34] and human foetal astrocytes expressing SOD1 mutations [18], substantiating the ‘neurotoxic’ nature of mutant astrocytes. These studies further identified candidate molecules as mediators of injury, such as prostaglandin D2, in murine astrocytes and allowed testing of candidate therapeutic agents [34]. The recent discovery of the role of TDP43 in sporadic [35, 36] and familial ALS [37], and FUS in familial ALS [38, 39] will serve to accelerate investigation into mechanisms of ALS pathogenesis and the potential further roles of astrocytes. Both TDP43 and FUS are nucleic acid binding proteins that play a role in regulation of gene transcription and splicing, presenting the possibility of common downstream pathological mechanisms underlying these forms of ALS. The role of astrocytes in brain function and disease is dealt with more fully in a number of recent reviews [13, 40, 41]
Increasing the translatability of model studies: the issue of species specific differences
Experimental and descriptive studies in model organisms such as rodents, non-mammalian vertebrates and invertebrates have contributed greatly to our understanding of the central nervous system, both in health and disease. Conservation of gene orthologues, functional anatomy and development of the central nervous system facilitates the translation of experimental findings between model organisms and humans. As a result, insights from these studies have informed our understanding of disease mechanisms and revealed new therapeutic targets.
Nevertheless, important species specific differences have been identified at the cellular level, which have the potential to limit inferences made from rodent data to humans. Oberheim et al. reported that human astrocytes differed greatly from the rodent counterpart in vivo, with greater size, complexity of arborization, number of subtypes, GFAP expression and speed of calcium wave propagation, attesting to structural and functional differences [42]. Molecular level evidence suggests that gene regulation may differ more than expected between rodent and humans. A significant proportion of human transcription factor (TF) binding sites do not function in rodents [43]. Indeed, the conservation of TF occupancy in orthologous mouse and human gene promoters can be highly variable [44]. Of note, while interspecies differences in TF binding were large, the location of binding sites within different cellular human systems has been reported to be highly conserved [44]. Consistent with this, gene promoter sequence, as opposed to any differences in transcriptional machinery, appears to be the dominant factor directing species specific transcription [45], and strengthen the case for employing human-based systems to study transcriptional responses to enable the identification of interspecies differences and thus home in on the most human-relevant pathways. Beyond transcriptional differences, species specific differences in other aspects of molecular biology are also well-documented, including pathways relevant to neurophysiology and pathophysiology. For example, the developmental regulation of microtubule associated protein tau (MAPT, implicated in a group of disorders called tauopathies, which includes AD) isoforms differs between mice and humans, favouring four repeat tau in mouse and a combination of three and four repeat tau in humans [46], as well as differences in splicing of the Na+/Ca2+ transporter 1 (NCX1) [47], a class of pumps important for neuronal Ca2+ homeostasis under ischaemic conditions [48]. At the protein level, interaction partners, subcellular distribution and enzyme-substrate profiles can also differ. For example, the C-terminal PDZ ligand of human somatostatin receptor 3 binds the multi-PDZ-domain containing protein MUPP1 (a tight-junction protein proposed to regulate intracellular cell signalling pathways in a variety of tissues), unlike the rat receptor [49]. There exist human vs. rodent species specific differences in the way apoptotic caspases process their targets, and also in whether a protein is a substrate for caspases at all [50]. Of relevance to therapeutics is the fact that certain pharmacological compounds show species specific differences in efficacy such as those targeting the TRPV1 channel [51], the P2X2 receptor [52] and the TRPA1 receptor [53].
Several conclusions can be drawn from these studies, the most apparent being that significant differences can exist at the molecular and cellular level in humans and rodents, and that rodent-based studies may benefit from complementary approaches using human cells of the appropriate type.
Human pluripotent stem cells as an experimental model
Rodent–human disparity increases the burden of proof that a particular finding has inter-species relevance, necessitating that certain findings be replicated in human experimental models [54–56]. Human in vitro platforms offer the opportunity to investigate disease processes and potential protective mechanisms in human cells. Three sources of human neural cells are readily identifiable, embryonic stem cell-derived, foetal-derived and those derived from the adult brain. For ethical and practical reasons, including limited propagation potential, unpredictable availability and inability to direct differentiation reliably, the use of human adult brain-derived material is limited as an expandable in vitro system. Human foetal-derived neural precursor cells (NPCs) can be derived from defined areas of the central nervous system (CNS) and expanded in vitro in the presence of neuroepithelial mitogens as non-adherent neurospheres [57, 58] or as adherent cultures [59]. Despite being well characterized, human foetal-derived NPCs are not ideal for long term study on account of practical reliability of tissue procurement, potentially limited differentiation ability to specific neuronal and glial subtypes and uncontrollable variability in sample gestation age [57].
In contrast, human embryonic stem cells (HESCs) are particularly attractive for experimental study, due to their predictable responsiveness to developmental cues enabling controlled and scaleable directed differentiation to neuronal and glial cell types. Following the isolation of HESCs [60], many methodologies now exist for successful maintenance of HESCs, generation of neural precursor cells and subsequent differentiation to defined neuronal sub-types [61–64], also comprising adherent and non-adherent neurosphere based systems. Adherent systems and selective propagation appear to allow greater cell yield, culture consistency and purity [65–67]. These systems in turn permit in vitro modelling of human neuronal injury and, along with recent reports of astrocyte generation from HESCs, further allow study of glial–neuronal interaction [54, 65]. Indeed HESC-derived motor neurons and dopaminergic neurons have been used to model glial toxicity and both antioxidant and GDNF-mediated neuroprotection [14, 18, 34].
Furthermore, insights from HESCs based experiments also inform and benchmark human induced pluripotent stem cell based studies. The development of induced pluripotent cells (iPSCs) permits the generation of defined neural cells from readily accessible patient material such as fibroblasts for study of hereditary disorders on the pre-existing human genetic background and as a potential source of patient-specific cells for autologous cell-replacement therapies. An example of in vitro adult brain disease modelling using patient specific material is that of Parkinson's disease-causing LRRK2 mutation carrying neurons having increased vulnerability to oxidative injury as well as morphological defects [68, 69]. This and other proof of concept studies illustrate the potential of human iPSC lines to accelerate disease understanding and drug development in a variety of neurological disorders [70–77].
The utilization of human embryonic stem cell lines with a known genetic background is of tremendous benefit with experimental reproducibility. However, it insufficiently reflects the genetic diversity inherent in the living human population. Similarly, genetic homogeneity within mouse strains, while inherently useful for knock-out studies and wild-type comparisons, insufficiently reflects natural population heterogeneity and observations magnified in a homogeneous genetic background may be diminished upon translation to heterogeneous populations, an observation conceptually distinct from human-non human model disparity as discussed earlier. Using a range of human in vitro lines may begin to address human genetic heterogeneity, and generate a platform observation rather than cell line specific observation. However, the range of cell lines required to account for human population variation completely is currently unknown, and may need to be developed on a sub-population basis. Overall, human stem cell-based approaches have distinct advantages and disadvantages compared with rodent systems (Table 1) and studies involving both systems may offer the best way of limiting confounds associated with any one experimental system.
Table 1.
A summary of the strengths and weaknesses of rodent primary culture models compared to human embryonic cell line-based systems
| Cell culture based in vitro system | Strengths | Weaknesses |
|---|---|---|
| Rodent culture | Comparative ease of derivation of mature cell types | Differences in biology may limit translation for human disease modelling |
| Genetic manipulation | ||
| Animal models allow transplantation studies | ||
| Human embryonic stem cell (HESC) | Human genome adds relevance | Technically difficult |
| Expandable in culture | Currently enriched derivation of all mature neural cell types is limited | |
| Can potentially derive many/all neural cell types in a single line | Heterogeneity between different HESC cell lines | |
| Functional validation necessary |
Generating functional astrocytes from human stem cells
As outlined earlier, astrocytes perform key physiological processes vital to combating oxidative, excitotoxic and other forms of injury, and evidence suggests that dysfunction and impairment of these physiological processes can significantly contribute to disease progression and outcome. Accordingly, modulating the cellular environment and specifically astrocyte function may play a role in slowing or even reversing neurological injury. Pre-requisites for detailed studies of neuronal–glial interaction in the context of human experimental models of neurological disease are characterized populations of enriched and functional human pluripotent stem cell (HPSC) derived neurons and astrocytes. Previous studies have focussed predominantly on the differentiation of HPSCs into non-specific and regionalized neurons for isolated study of neuronal injury. A range of systems has been described, from suspension to adherent culture methods [61, 78], and recent advances have made it possible to derive enriched neuron-biased neural precursor cells from HESCs, which can be readily specified to specific neuronal sub-types [79, 80]. In contrast, derivation of enriched functional human astrocytes from HESCs has historically received lesser attention, due to comparatively poor understanding of astrocyte specification during development and poor specificity of astrocyte precursor markers [81].
Progressive developments in technologies that allow maintenance and propagation of high quality neural precursors derived from HPSCs [61, 78, 80, 82, 83] and noting the temporal regulation of gliogenesis have established an experimental platform for scaleable generation of human astrocytes. Astrocyte differentiation is determined temporally by both intrinsic temporally mediated mechanisms and exogenous factors, including BMP4 and LIF [84]. LIF and other members of the Il-6 cytokine family have been shown to drive synergistically astroglial differentiation in conjunction with BMPs, STAT3 and SMAD1 converging upon CBP/p300 respectively [85]. Notch intracellular signalling also appears to have a contributory role [86]. However, while BMP and LIF signalling can drive astrocyte differentiation in glial-competent NPCs, their limited capacity to do so in early NPCs suggests that other intrinsic factors govern glial competence. The glial competence of NPCs increases over time, with early precursors being predominantly neuronal in fate and later precursors generating glia. This gliogenic switch appears to be regulated by epigenetic changes resulting in chromatin remodelling and DNA methylation around the GFAP promoter and other astrocyte specific genes, to promote astrogliogenesis [87, 88]. The relevance of SMAD and STAT signalling to astroglial differentiation of HESC-derived neural precursor cells in vitro, however, remains unproven. The use of BMP4 in astroglial differentiation from human foetal-derived neural precursor cells provides some evidence that complementary pathways may exist [89]. Therefore, we systematically tested the ability of combinations of BMPs and LIF to drive astroglial conversion. Upon withdrawal of mitogens, drawing the neural precursor cells out of the cell cycle, application of combined Smad and Stat signalling mediators drove efficient differentiation of neural precursor cells to the astroglial lineage 65. Other groups have similarly used prolonged culture of neural precursor cells and CNTF-mediated Stat signalling for astroglial conversion from HESCs 90. Critically, glial-appropriate functionality of these in vitro human astrocytes was further demonstrated. Studies in rodent systems have demonstrated the role of astrocytes in glutamate clearance, expression of key astrocyte-specific proteins and the ability to modulate the synaptic maturation of co-cultured neurons. HESC-derived astrocytes accordingly demonstrated the acquisition of functional properties including glutamate uptake and expression of astrocyte markers GFAP, EAAT1, AQP4 and S100β 65, calcium wave propagation and induction of neuronal synapses 90. Critically, demonstration of these properties, shared with their in vivo counterparts, confirms astrocyte differentiation techniques and permits the investigation of interactions between human astrocytes and neurons. Future studies will be required to address whether HESC astrocytes also secrete the range of proteins expressed by murine astrocytes, including clusterin and thrombospondin [91–93]. Recent advances have demonstrated a panoply of astrocytic functions in rodent systems, including functional gliotransmission with consequent modulation of homosynaptic and heterosynaptic neurotransmission in hippocampal networks by astrocytes [94], and direct control of sleep pressure [95] and regulation of breathing in response to acidaemia by astrocytes [96]. It remains to be seen whether human embryonic stem cell derived astrocytes recapitulate these and other in vivo properties and are able to integrate functionally into these networks upon transplantation, a key property ahead of potential transplantation therapies.
The neuroprotective abilities of astrocytic Nrf2 in rodent and human models
Given the importance of oxidative injury in neurological diseases, understanding the mechanisms underlying neuronal oxidative cell death and endogenous antioxidant mechanisms may provide tractable therapeutic targets. The nuclear factor erythroid-2-related factor 2 (Nrf2, encoded by NFE2L2) pathway has been identified for its key role in mediating the cellular antioxidant protective response and thus as a potential candidate for targeted therapeutics in neurodegenerative disease. Nrf2 is a member of the cap'n'collar basic leucine zipper family of transcription family, and is widely regarded as the master regulator of antioxidant defences and drug metabolizing enzymes [97]. Under normal conditions Nrf2 is bound in the cytoplasm by Keap1 and targeted for degradation. However inhibition of Keap1-dependent degradation allows Nrf2 to translocate to the nucleus and activate transcription of antioxidant response element (the cis-acting promoter element, ARE) containing genes [98]. Various mechanisms appear to underlie Nrf2 activation, both direct and indirect. Nrf2 can be activated directly by oxidative stress caused by peroxide treatment and oxygen-glucose deprivation [54] and indirectly. Loss of p62 has been associated with development of AD-like pathology in rodent models; p62 knock-out mice accumulate markers of oxidative damage with age, and age-correlated accumulation of p62 promoter damage has been demonstrated in human and murine samples [99]. p62 has been shown to associate with Keap1 and cause disinhibition of Nrf2 [100]. As such heritable or acquired loss of p62 may cause accumulation of cellular oxidative injury due to dysregulation of Nrf2 activity.
Important ARE-containing genes include phase II detoxifying and antioxidant enzymes, which are largely dependent on Nrf2-mediated activation upon injury. Critical enzymes include haem oxygenase 1, sulfiredoxin, peroxiredoxins and those central to glutathione (GSH) synthesis and metabolism including GCL and GST [101, 102]. Activation of Nrf2 by genetic manipulation, hypoxia/ischemia or small chemical activators has been shown to abate neurological injury and disease progression in rodent systems [54, 103–109]. The Nrf2 pathway is particularly active in astrocytes, compared with neurons (although activation of Nrf2 in neurons is possible [110]), and activation of astrocytic Nrf2 is sufficient to confer neuroprotection on nearby neurons via a mechanism proposed to involve the release of glutathione [103, 105, 111] (Figure 1). Although driving Nrf2 expression in neurons is strongly neuroprotective [112], astrocytic expression of Nrf2 confers neuroprotection in models of Parkinson's disease, ALS and ischaemia [103, 104, 111, 113].
Figure 1.
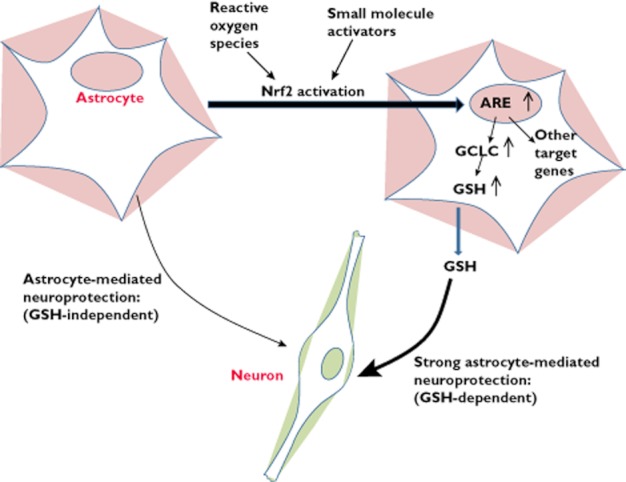
Humanembryonic stem cell-derived astrocytes mediate non-cell autonomous neuroprotection in part via activation of Nrf2-mediated gene expression. See text and reference 65 for details
The use of small molecule activators of Nrf2, which act by antagonizing Keap1-mediated Nrf2 degradation, represents a promising form of antioxidant therapy. Previous therapeutic strategies have primarily focussed on compounds that directly combat ROS, such as natural antioxidants (e.g. vitamin E) and synthetic spin traps [8]. These have enjoyed considerable efficacy in animal model studies but have been translationally disappointing, potentially due to the difficulty in getting sufficient quantities of antioxidants into the human brain to have an effect [8]. Rather than acting as free radical scavengers or spin traps themselves, these molecules act by boosting Nrf2-regulation, thus up-regulating the intrinsic antioxidant defences of cells. Among small molecule Nrf2 inducers, the series of synthetic oleanane triterpenoids, such as derivatives of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) developed by Michael Sporn (Dartmouth Medical School), have received much attention due to their potency as well as low toxicity in animal studies [114, 115]. Outside of the CNS, CDDO compounds are potently protective in a variety of disorders, including inflammatory lung disorders, cancer (prevention and treatment), renal and hepatic toxicity, and diabetes [116]. Equally impressive is their efficacy in models of a variety of neurodegenerative disorders. In mouse models of Huntington's disease chronic administration of CDDO-ethylamide and CDDO-trifluoroethylamide (CDDOTFEA) upregulated Nrf2 target genes, attenuated striatal atrophy and improved the in vivo phenotype exhibited, including motor performance by rotorod testing and survival [107]. Three month oral administration of CDDO reduced plaque and microglial burden in a mutant APP transgenic mouse model of AD, with concomitant improvement in spatial memory by Morris water maze [117]. Moreover, CDDO compounds also have demonstrable protective effects in in vivo models of Huntington's disease and Parkinson's disease [118] as well as the G93A SOD1 model of ALS [116].
Given that the neuroprotective effects of CDDO-triterpenoids have been well established in rodent systems, we sought to use CDDO-triterpenoid mediated neuroprotection as a proof-of-concept to illustrate the utility of our HESC-derived platform to investigate astrocyte-mediated neuroprotection. We demonstrated that HESC-derived astrocytes respond physiologically to CDDOTFEA-triterpenoid treatment, upregulating glutathione-mediated antioxidant processes, and protect HESC-derived neurons from peroxide-mediated cell death by soluble factors secreted into astrocyte-conditioned medium 65. This protective effect was in addition to the basal protection mediated by conditioned medium from untreated astrocytes (Figure 1). Furthermore direct CDDOTFEA-triterpenoid treatment conferred no protective benefit to HESC-derived neurons against oxidative cell death, demonstrating the non-cell autonomous mechanisms involved in CDDOTFEA-triterpenoid mediated neuroprotection 65. These findings suggest that HESC-derived neural derivatives can be used effectively to model human neuron–astrocyte interactions, investigate neurological injury and rescue mechanisms, and have the potential to increase the translational hit of findings made in complementary non-human based systems. Moreover, similarly derived HESC-derived astrocytes also respond to mild oxidative stress by inducing Nrf2 target genes [54], raising the possibility that Nrf2 forms an endogenous adaptive protective response in the CNS as well.
Concluding remarks
Developments in HESC-based technologies permit studies of early neuronal development, modelling neural injury in vitro, and potentially cell-replacement therapies. Amongst other injury mechanisms, oxidative stress is a common theme in the aetiopathogenesis of a range of chronic neurodegenerative disorders. As a result, informed antioxidant strategies that alter redox balance effectively may contribute to novel therapies across a range of conditions, and multi-modal therapies may be of benefit. Furthermore, astrocytes have been proven to be principle sites of oxidative-stress mediated neuronal injury and neuroprotection as well as the therapeutic target for candidate drugs. As such, non-cell autonomous neuroprotection, as shown in these studies, has widespread and fundamental implications for human-based drug discovery and screening of novel neuroprotective agents. Screens focussing solely on the direct effect of compounds on isolated neurons may overlook potentially important neuroprotective processes that act via astrocytes or other non-neuronal cells. Despite these advances, inferences drawn from in vitro models to the in vivo state are limited by the cell types included in the co-culture. Pure neuron-astrocyte co-cultures lack the complexity that neighbouring oligodendrocytes and microglia would present in vivo. Furthermore, the reduced culture time and as yet unclear correlation of in vitro culture time points with in vivo development suggest that in vitro models may encounter difficulty in recapitulating aspects of long term chronic neurodegeneration. Nevertheless, human in vitro systems provide unparalleled access to human cell types and have the potential to provide previously inaccessible insights in cell–cell interaction, molecular processes and potentially tractable clinical targets. Beyond disease modelling, the generation of enriched human neurons and astrocytes offers the prospect of cell replacement therapies. Many hurdles remain, including the enriched generation of subtype-specific cells, issues surrounding tissue rejection and functional engraftment in appropriate disease models. Some experimental successes have been achieved with engraftment of HESC-derived dopaminergic neurons in rodent models of Parkinson's disease [119, 120]. Glial replacement therapies also offer some promise as neuroprotective interventions. Astrocyte replacement has already received some success in experimental rodent-based models of age-related neurodegeneration, spinal cord injury and ALS [17, 121]. The ability to alter the behaviour of these cells prior to implantation, either genetically [113, 122] or with drug treatment 65, may also enhance their neuroprotective abilities in the injured brain. Longer term strategies building on these insights that target mobilization of endogenous glial populations are an area of particularly active research.
In summary the establishment of bespoke human in vitro platforms for neurological disease modelling and drug discovery offers a major new resource to accelerate successful clinical translation of novel neuroprotective therapeutics.
Acknowledgments
KG is funded by a Wellcome Trust Clinical Training Fellowship. The work from the GH and SC laboratories described is funded by the Medical Research Council, the Biotechnology and Biological Sciences Research Council, and the Wellcome Trust. We thank Professor Michael Sporn for the gift of CDDOTFEA for use in the HESC studies.
Competing Interests
There are no competing interests to declare.
References
- 1.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreira PI, Nunomura A, Nakamura M, Takeda A, Shenk JC, Aliev G, Smith MA, Perry G. Nucleic acid oxidation in Alzheimer disease. Free Radic Biol Med. 2008;44:1493–1505. doi: 10.1016/j.freeradbiomed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 2009;118:151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 4.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 5.Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr, Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 6.Alam ZI, Daniel SE, Lees AJ, Marsden DC, Jenner P, Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 7.Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- 8.Kamat CD, Gadal S, Mhatre M, Williamson KS, Pye QN, Hensley K. Antioxidants in central nervous system diseases: preclinical promise and translational challenges. J Alzheimers Dis. 2008;15:473–493. doi: 10.3233/jad-2008-15314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leveille F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, Tolkovsky AM, Hardingham GE. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci. 2010;30:2623–2635. doi: 10.1523/JNEUROSCI.5115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Mubarak B, Soriano FX, Hardingham GE. Synaptic NMDAR activity suppresses FOXO1 expression via a cis-acting FOXO binding site: FOXO1 is a FOXO target gene. Channels (Austin) 2009;3:233–238. doi: 10.4161/chan.3.4.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardingham GE, Lipton SA. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid Redox Signal. 2011;14:1421–1424. doi: 10.1089/ars.2010.3573. [DOI] [PubMed] [Google Scholar]
- 12.Belanger M, Magistretti PJ. The role of astroglia in neuroprotection. Dialogues Clin Neurosci. 2009;11:281–295. doi: 10.31887/DCNS.2009.11.3/mbelanger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng X, Chen J, Deng X, Liu Y, Rao MS, Cadet JL, Freed WJ. An in vitro model of human dopaminergic neurons derived from embryonic stem cells: MPP+ toxicity and GDNF neuroprotection. Neuropsychopharmacology. 2006;31:2708–2715. doi: 10.1038/sj.npp.1301125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 16.Diana V, Ottolina A, Botti F, Fumagalli E, Calcagno E, De Paola M, Cagnotto A, Invernici G, Parati E, Curti D, Mennini T. Neural precursor-derived astrocytes of wobbler mice induce apoptotic death of motor neurons through reduced glutamate uptake. Exp Neurol. 2010;225:163–172. doi: 10.1016/j.expneurol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Hampton DW, Webber DJ, Bilican B, Goedert M, Spillantini MG, Chandran S. Cell-mediated neuroprotection in a mouse model of human tauopathy. J Neurosci. 2010;30:9973–9983. doi: 10.1523/JNEUROSCI.0834-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchetto MC, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagele RG, D'Andrea MR, Lee H, Venkataraman V, Wang HY. Astrocytes accumulate A beta 42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003;971:197–209. doi: 10.1016/s0006-8993(03)02361-8. [DOI] [PubMed] [Google Scholar]
- 21.Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp Neurol. 1997;144:266–272. doi: 10.1006/exnr.1996.6373. [DOI] [PubMed] [Google Scholar]
- 22.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 23.Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 24.Pihlaja R, Koistinaho J, Malm T, Sikkila H, Vainio S, Koistinaho M. Transplanted astrocytes internalize deposited beta-amyloid peptides in a transgenic mouse model of Alzheimer's disease. Glia. 2008;56:154–163. doi: 10.1002/glia.20599. [DOI] [PubMed] [Google Scholar]
- 25.Abramov AY, Canevari L, Duchen MR. Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci. 2003;23:5088–5095. doi: 10.1523/JNEUROSCI.23-12-05088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song YJ, Halliday GM, Holton JL, Lashley T, O'Sullivan SS, McCann H, Lees AJ, Ozawa T, Williams DR, Lockhart PJ, Revesz TR. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol. 2009;68:1073–1083. doi: 10.1097/NEN.0b013e3181b66f1b. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu XL, Long CX, Sun L, Xie C, Lin X, Cai H. Astrocytic expression of Parkinson's disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain. 2010;3:1–16. doi: 10.1186/1756-6606-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pramatarova A, Laganiere J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21:3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3:637–648. doi: 10.1016/j.stem.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 36.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 37.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH., Jr Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 39.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, Swarnkar S, Goswami P, Nath C. Astrocytes and microglia: responses to neuropathological conditions. Int J Neurosci. 2011;121:589–597. doi: 10.3109/00207454.2011.598981. [DOI] [PubMed] [Google Scholar]
- 42.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dermitzakis ET, Clark AG. Evolution of transcription factor binding sites in mammalian gene regulatory regions: conservation and turnover. Mol Biol Evol. 2002;19:1114–1121. doi: 10.1093/oxfordjournals.molbev.a004169. [DOI] [PubMed] [Google Scholar]
- 44.Odom DT, Dowell RD, Jacobsen ES, Gordon W, Danford TW, MacIsaac KD, Rolfe PA, Conboy CM, Gifford DK, Fraenkel E. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet. 2007;39:730–732. doi: 10.1038/ng2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson MD, Barbosa-Morais NL, Schmidt D, Conboy CM, Vanes L, Tybulewicz VL, Fisher EM, Tavare S, Odom DT. Species-specific transcription in mice carrying human chromosome 21. Science. 2008;322:434–438. doi: 10.1126/science.1160930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D'Souza I. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol. 2008;511:788–803. doi: 10.1002/cne.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Eylen F, Bollen A, Herchuelz A. NCX1 Na/Ca exchanger splice variants in pancreatic islet cells. J Endocrinol. 2001;168:517–526. doi: 10.1677/joe.0.1680517. [DOI] [PubMed] [Google Scholar]
- 48.Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38(2 Suppl):674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- 49.Liew CW, Vockel M, Glassmeier G, Brandner JM, Fernandez-Ballester GJ, Schwarz JR, Schulz S, Buck F, Serrano L, Richter D, Kreienkamp HJ. Interaction of the human somatostatin receptor 3 with the multiple PDZ domain protein MUPP1 enables somatostatin to control permeability of epithelial tight junctions. FEBS Lett. 2009;583:49–54. doi: 10.1016/j.febslet.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 50.Ussat S, Werner U, Adam-Klages S. Species-specific differences in the usage of several caspase substrates. Biochem Biophys Res Commun. 2002;297:1186–1190. doi: 10.1016/s0006-291x(02)02358-6. [DOI] [PubMed] [Google Scholar]
- 51.Phillips E, Reeve A, Bevan S, McIntyre P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J Biol Chem. 2004;279:17165–17172. doi: 10.1074/jbc.M313328200. [DOI] [PubMed] [Google Scholar]
- 52.Tittle RK, Hume RI. Opposite effects of zinc on human and rat P2X2 receptors. J Neurosci. 2008;28:11131–11140. doi: 10.1523/JNEUROSCI.2763-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR. Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain. 2007;3:39. doi: 10.1186/1744-8069-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, Tsujita T, Chowdhry S, Patani R, Chandran S, Horsburgh K, Hayes JD, Hardingham GE. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A. 2011;108:E1–2. doi: 10.1073/pnas.1015229108. author reply E3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hardingham GE, Patani R, Baxter P, Wyllie DJ, Chandran S. Human embryonic stem cell-derived neurons as a tool for studying neuroprotection and neurodegeneration. Mol Neurobiol. 2010;42:97–102. doi: 10.1007/s12035-010-8136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Chandran S, Compston A, Jauniaux E, Gilson J, Blakemore W, Svendsen C. Differential generation of oligodendrocytes from human and rodent embryonic spinal cord neural precursors. Glia. 2004;47:314–324. doi: 10.1002/glia.20011. [DOI] [PubMed] [Google Scholar]
- 58.Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Pollard S, Conti L, Toselli M, Biella G, Parkin G, Willatt L, Falk A, Cattaneo E, Smith A. Long-term tripotent differentiation capacity of human neural stem (NS) cells in adherent culture. Mol Cell Neurosci. 2008;38:245–258. doi: 10.1016/j.mcn.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 61.Joannides AJ, Fiore-Heriche C, Battersby AA, Athauda-Arachchi P, Bouhon IA, Williams L, Westmore K, Kemp PJ, Compston A, Allen ND, Chandran S. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells. 2007;25:731–737. doi: 10.1634/stemcells.2006-0562. [DOI] [PubMed] [Google Scholar]
- 62.Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 64.Zhang XQ, Zhang SC. Differentiation of neural precursors and dopaminergic neurons from human embryonic stem cells. Methods Mol Biol. 2010;584:355–366. doi: 10.1007/978-1-60761-369-5_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta K, Patani R, Baxter P, Serio A, Story D, Tsujita T, Hayes JD, Pedersen RA, Hardingham GE, Chandran S. Human embryonic stem cell derived astrocytes mediate non-cell-autonomous neuroprotection through endogenous and drug-induced mechanisms. Cell Death Differ. 2012;19:779–787. doi: 10.1038/cdd.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharif A, Prevot V. Isolation and culture of human astrocytes. Methods Mol Biol. 2012;814:137–151. doi: 10.1007/978-1-61779-452-0_11. [DOI] [PubMed] [Google Scholar]
- 67.Wanner IB. An in vitro trauma model to study rodent and human astrocyte reactivity. Methods Mol Biol. 2012;814:189–219. doi: 10.1007/978-1-61779-452-0_14. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, Kee K, Schule B, Dolmetsch RE, Langston W, Palmer TD, Pera RR. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di, Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals JM, Memo M, Alberch J, Lopez-Barneo J, Vila M, Cuervo AM, Tolosa E, Consiglio A, Raya A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, Rodolfa CT, Dimos JT, Mikkilineni S, MacDermott AB, Woolf CJ, Henderson CE, Wichterle H, Eggan K. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145:831–834. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 74.Song B, Sun G, Herszfeld D, Sylvain A, Campanale NV, Hirst CE, Caine S, Parkington HC, Tonta MA, Coleman HA, Short M, Ricardo SD, Reubinoff B, Bernard CC. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. 2012;8:259–273. doi: 10.1016/j.scr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 75.Han SS, Williams LA, Eggan KC. Constructing and deconstructing stem cell models of neurological disease. Neuron. 2011;70:626–644. doi: 10.1016/j.neuron.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Marchetto MC, Gage FH. Modeling brain disease in a dish: really? Cell Stem Cell. 2012;10:642–645. doi: 10.1016/j.stem.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, Monni E, Tornero D, Ahlenius H, Ladewig J, Brustle O, Lindvall O, Kokaia Z. Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells. 2012;30:1120–1133. doi: 10.1002/stem.1104. [DOI] [PubMed] [Google Scholar]
- 78.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 79.Koch P, Opitz T, Steinbeck J, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A. 2009;106:3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu H, Zhang SC. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci. 2011;68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patani R, Compston A, Puddifoot CA, Wyllie DJ, Hardingham GE, Allen ND, Chandran S. Activin/Nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PLoS ONE. 2009;4:e7327. doi: 10.1371/journal.pone.0007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patani R, Hollins AJ, Wishart TM, Puddifoot CA, Alvarez S, de Lera AR, Wyllie DJ, Compston DA, Pedersen RA, Gillingwater TH, Hardingham GE, Allen ND, Chandran S. Retinoid-independent motor neurogenesis from human embryonic stem cells reveals a medial columnar ground state. Nat Commun. 2011;2:214. doi: 10.1038/ncomms1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mabie P, Mehler M, Marmur R, Papavasiliou A, Song Q, Kessler J. Bone morphogenetic proteins induce astroglial differentiation of oligodendroglial-astroglial progenitor cells. J Neurosci. 1997;17:4112–4120. doi: 10.1523/JNEUROSCI.17-11-04112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 86.Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G, Weinmaster G, Sun YE. Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res. 2002;69:848–860. doi: 10.1002/jnr.10364. [DOI] [PubMed] [Google Scholar]
- 87.Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, ten, Hoeve J, Shuai K, Sun YE. DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development. 2005;132:3345–3356. doi: 10.1242/dev.01912. [DOI] [PubMed] [Google Scholar]
- 88.Song MR, Ghosh A. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci. 2004;7:229–235. doi: 10.1038/nn1192. [DOI] [PubMed] [Google Scholar]
- 89.Obayashi S, Tabunoki H, Kim SU, Satoh J. Gene expression profiling of human neural progenitor cells following the serum-induced astrocyte differentiation. Cell Mol Neurobiol. 2009;29:423–438. doi: 10.1007/s10571-008-9338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–534. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 92.Cordero-Llana O, Scott SA, Maslen SL, Anderson JM, Boyle J, Chowhdury RR, Tyers P, Barker RA, Kelly CM, Rosser AE, Stephens E, Chandran S, Caldwell MA. Clusterin secreted by astrocytes enhances neuronal differentiation from human neural precursor cells. Cell Death Differ. 2011;18:907–913. doi: 10.1038/cdd.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tao J, Wu H, Lin Q, Wei W, Lu XH, Cantle JP, Ao Y, Olsen RW, Yang XW, Mody I, Sofroniew MV, Sun YE. Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration. J Neurosci. 2011;31:8306–8319. doi: 10.1523/JNEUROSCI.0567-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 95.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andrews NC, Kotkow KJ, Ney PA, Erdjument-Bromage H, Tempst P, Orkin SH. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci U S A. 1993;90:11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 99.Du Y, Wooten MC, Gearing M, Wooten MW. Age-associated oxidative damage to the p62 promoter: implications for Alzheimer disease. Free Radic Biol Med. 2009;46:492–501. doi: 10.1016/j.freeradbiomed.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lau A, Wang XJ, Zhao F, Villeneuve NF, Wu T, Jiang T, Sun Z, White E, Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kataoka K, Handa H, Nishizawa M. Induction of cellular antioxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by antirheumatic gold(I) compounds. J Biol Chem. 2001;276:34074–34081. doi: 10.1074/jbc.M105383200. [DOI] [PubMed] [Google Scholar]
- 102.Soriano FX, Baxter P, Murray LM, Sporn MB, Gillingwater TH, Hardingham GE. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol Cells. 2009;27:279–282. doi: 10.1007/s10059-009-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shih A, Johnson D, Wong G, Kraft A, Jiang L, Erb H, Johnson J, Murphy T. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vargas M, Johnson D, Sirkis D, Messing A, Johnson J. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. doi: 10.1017/S1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bell KF, Fowler JH, Al-Mubarak B, Horsburgh K, Hardingham GE. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:689524. doi: 10.1155/2011/689524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stack C, Ho D, Wille E, Calingasan N, Williams C, Liby K, Sporn M, Dumont M, Beal M. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic Biol Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bell KF, Hardingham GE. CNS peroxiredoxins and their regulation in health and disease. Antioxid Redox Signal. 2011;14:1467–1477. doi: 10.1089/ars.2010.3567. [DOI] [PubMed] [Google Scholar]
- 109.Yang L, Calingasan N, Thomas B, Chaturvedi R, Kiaei M, Wille E, Liby K, Williams C, Royce D, Risingsong R, Musiek E, Morrow J, Sporn M, Beal M. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soriano FX, Leveille F, Papadia S, Higgins LG, Varley J, Baxter P, Hayes JD, Hardingham GE. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. J Neurochem. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson's disease: critical role for the astrocyte. Proc Natl Acad Sci U S A. 2009;106:2933–2938. doi: 10.1073/pnas.0813361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005;65:4789–4798. doi: 10.1158/0008-5472.CAN-04-4539. [DOI] [PubMed] [Google Scholar]
- 115.Sporn MB, Liby KT, Yore MM, Fu L, Lopchuk JM, Gribble GW. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74:537–545. doi: 10.1021/np100826q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neymotin A, Calingasan NY, Wille E, Naseri N, Petri S, Damiano M, Liby KT, Risingsong R, Sporn M, Beal MF, Kiaei M. Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radic Biol Med. 2011;51:88–96. doi: 10.1016/j.freeradbiomed.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dumont M, Wille E, Calingasan N, Tampellini D, Williams C, Gouras G, Liby K, Sporn M, Nathan C, Flint, Beal M, Lin M. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L, Calingasan NY, Thomas B, Chaturvedi RK, Kiaei M, Wille EJ, Liby KT, Williams C, Royce D, Risingsong R, Musiek ES, Morrow JD, Sporn M, Beal MF. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS ONE. 2009;4:e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 121.Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


