Abstract
Newly licensed direct acting antivirals for hepatitis C virus HCV are able to cure up to 75% of patients chronically infected with genotype-1 infection, which is the predominant HCV strain in Europe and North America. Emerging antiviral therapies promise further increases in virological response, as well as improved tolerability, reduced duration of therapy, and will potentially eliminate the need for interferon use. This review highlights the main therapeutic agents used in current standard of care, including telaprevir and boceprevir. It goes on to evaluate the mechanisms of emerging drugs, their stage of development and response rates seen in research to date. Finally, it projects into the not too distant future to consider treatment strategies involving combinations of agents and interferon-free therapies, and in which patients they might prove most successful.
Keywords: antiviral therapy, direct acting antiviral, hepatitis C virus, polymerase inhibitors, protease inhibitors
Introduction
Approximately 170 million people are infected with hepatitis C virus (HCV) and it is the leading cause of liver transplantation and second most common cause of liver cancer globally [1–3]. Despite receiving less attention than other blood borne viruses, HCV recently surpassed HIV in terms of attributable deaths in the United States [4]. The sequelae of chronic hepatitis C (CHC) infection are preventable by viral eradication. Peginterferon-alpha (PEG-IFN) and ribavirin (RBV) have been standard of care therapy for the past decade. Unfortunately, PEG-IFN and RBV will cure only at best 50% of patients infected with genotype-1 HCV, the predominant HCV strain in Europe and North America. Use is also limited by significant toxicities, including psychiatric morbidity, influenza-like symptoms and cytopenias, mandating careful patient selection and monitoring [5–8]. Newly licensed directly acting agents (DAA) for HCV, telaprevir and boceprevir, both used in combination with PEG-IFN and RBV, dramatically improve effectiveness and can cure up to 75% of patients chronically infected with genotype-1 HCV. Emerging antiviral therapies promise further improvements in virological response, improved tolerability, reduced duration of therapy, and may potentially eliminate the need for IFN use.
This review highlights the main therapeutic agents used in current standard of care and their limitations, and includes the two newly available DAAs, telaprevir and boceprevir. It evaluates the mechanisms of emerging drugs with the greatest promise, including the next wave of HCV protease inhibitors, as well as the HCV polymerase inhibitors, NS5A inhibitors and cyclophilin inhibitors, outlining their stage of development and response rates seen in clinical trials to date. Finally, it projects into the not too distant future to consider individualized treatment strategies involving combinations of agents and interferon-free therapies, and for which patients they might prove most appropriate.
The current backbone of HCV therapy: peginterferon-alpha and ribavirin
PEG-IFN and RBV were the standard of care treatment for chronic HCV infection until 2011 and remain so for non-genotype-1 HCV. In treatment naïve individuals it results in a sustained viral response (SVR) around 45% in HCV genotype-1 infection, compared with 75% in genotypes-2 and −3 infection (Table 1) [9]. With the introduction of telaprevir and boceprevir therapy, SVR rates up to 75% can also be achieved in genotype-1, but PEG-INF/RBV continue to play an important role to prevent the emergence of resistance-associated HCV variants. Treatment response rates in acute HCV infection are very high, with SVR rates 70–90% using PEG-IFN monotherapy. Unfortunately acute HCV infection is normally asymptomatic, and the diagnosis is not made until chronicity is established. In the minority of patients diagnosed acutely, IFN-based therapy is often contra-indicated by comorbidities. Treatment of acute HCV inection has recently been reviewed in detail elsewhere [10, 11].
Table 1.
Currently licensed therapies for chronic HCV
| Drug | Mechanism | Sustained virological response | Main limitations | |
|---|---|---|---|---|
| Treatment naïve | Re-treatment | |||
| Pegylated interferon alpha-2a/b | Non-specific antiviral agent | 45% genotype-1 and -4 65–80% genotype-2 and 3 (EASL 2011 [21]) | Relapsers: 20–29% genotype-1 (Bacon et al. 2011 [39], Zeuzem et al. 2011 [42]) 40% genotypes-2 and -3 Ghany et al. 2009 [84] | Injectable agent Psychiatric side effects 24–48 weeks for standard therapy |
| Ribavirin | Non-specific antiviral agent | Non-responders: 5–7% genotype-1 (Bacon et al. 2011 [39], Zeuzem et al. 2011 [42]) 15–20% genotypes-2 and -3 (Ghany et al. 2009 [84]) | Anaemia | |
| Boceprevir | NS3 protease inhibitor | 67–68% genotype-1 with PEG/RBV vs. 40% PEG/RBV alone (non-Black patients) (Poordad et al. 2011 [35]) | Null responders: 23–30% Partial responders: 40–52% Relapsers: 69–75% (Bacon et al. 2011 [39]) | Anaemia, dysgeusia Only active in HCV genotype-1 Requires PEG/RBV backbone |
| Telaprevir | NS3 protease inhibitor | 69–75% genotype-1 with PEG/RBV vs. 44% PEG/RBV alone (non-Black patients) (Jacobson et al. 2011 [43]) | Null-responders: 29–33% Partial responders: 54–59% Relapsers: 83–88% (Zeuzem et al. 2011 [42] | Rash, anaemia, gastrointestinal side effects Only active in HCV genotype-1 Requires PEG/RBV backbone |
PEG, Pegylated interferon alpha-2a/b; RBV, ribravirin.
IFN has both direct and indirect antiviral effects. Direct effects are mediated through the induction of interferon stimulated genes which code for effector proteins and cytokines that inhibit virus replication and generate an antiviral state. Indirect effects are mediated through up-regulation of major histocompatibility complex class 1 genes in antigen presenting cells, which leads to cytotoxic T-cell clearance of HCV infected cells [7]. Polyethylene glycol (PEG) polymer chains covalently attached to IFN reduce renal and hepatic clearance, allow for weekly administration and superior SVR over standard interferon [12, 13].
The mechanism of action of ribavirin in HCV treatment is not completely understood. There are multiple hypotheses. Viral kinetic studies suggest that HCV mutagenesis leading to error catastrophe and consequent lowering of HCV fitness is an important mechanism of action [8, 14]. RBV is also a guanosine analogue, and may act as a chain terminator through incorporation of ribavirin into the HCV genome during viral replication [8]. Ribavirin may also modulate host T-cell immunity as viral infection.
Side effects of PEG-IFN/RBV are common and are a major limitation of current therapy. Important PEG-IFN side effects include influenza-type symptoms and fatigue, psychiatric morbidity and bone marrow suppression [6, 7]. Psychiatric side effects, including depression or aggression, mandate careful patient selection and monitoring, and may preclude some patients from accessing current PEG-IFN based therapies. Ribavirin causes haemolysis and anaemia [8], and on-treatment cytopaenias due to both agents are more common in the setting of cirrhosis. Developing HCV treatment regimens with less toxicity is clinically important, and should expand the number of people appropriate for treatment and successfully completing therapy.
Factors predicting IFN-based treatment response
Host genetics are highly predictive of PEG-IFN/RBV treatment outcome. Genome-wide studies have confirmed an association between a polymorphism in the region of the IL-28B gene and response to HCV treatment [15, 16]. Individuals who carry a favourable IL-28B genotype have a two to three times increase in response to PEG-IFN/RBV [17, 18]. Other important predictors of PEG-IFN response include pre-treatment viral load, liver fibrosis state and insulin resistance [19].
On-treatment virological response is the most accurate predictor of SVR, and time to viral clearance has been adopted as a guide to treatment duration (response-guided therapy, RGT) or futility. A rapid virological response (RVR, undetectable HCV RNA at week 4) is 86–100% predictive of SVR [20], regardless of HCV genotype, and is achieved in approximately 10–27% of genotype-1 and 64–76% of genotype-2/-3 infections [21]. Patients with a low baseline viral load who achieve an RVR can be considered for short duration therapy. Genotype-1 patients with complete early virological response (EVR, undetectable HCV RVA at week 12) have a 68–84% rate of SVR [20]. Patients with a slow but persistent virological decline may be considered for extended therapy with PEG-IFN/RBV. Week 12 and 24 HCV RNA measurements are important for predicting treatment failure. Treatment should be abandoned if HCV RNA has declined by <2 log10 IU ml−1 at week 12, or is still detectable at week 24, given an expected SVR of 1–3% [22, 23].
The success rate of re-treatment with PEG-IFN/RBV after failing initial therapy has been disappointing, estimated at a pooled SVR of 16% in a meta-analysis [24]. Prior ‘relapsers’ (individuals who achieved an undetectable HCV RNA at the end of treatment, but did not achieve an SVR) with genotype-1 infection have a 15–25% SVR rate with re-treatment. Previous ‘null-responders’ (defined as <2 log10 IU ml−1 decline in HCV RNA viral load after 12 weeks of therapy) with genotype-1 infection have a 4–14% SVR on re-treatment [25, 26].
The presence of HIV co-infection marginally reduces the effectiveness of PEG-IFN/RBV treatment: the APRICOT study reported an SVR of 29% among genotype-1 infection and 62% among genotype-2/-3 infection [27]. In contrast, active injecting drug use does not affect treatment response. In a systematic review of chronic HCV treatment with PEG-IFN/RBV, median SVR among people who inject drugs was 54% (range 18%-94%), compared with 54%–63% among non-injectors [28]. Acute HCV treatment is also effective among people who inject drugs [29].
The moderate effectiveness of PEG-IFN/RBV for chronic HCV treatment, and the poorer SVR in populations with advanced liver disease, who previously failed therapy and HIV co-infection, demonstrates the need for improvements in treatment efficacy.
New and emerging direct acting antiviral therapy
Improved understanding of HCV replication has allowed for the development of a plethora of new therapeutic agents that target enzymes directly (Figure 1). HCV is a flavivirus with an RNA genome encoding a polyprote in [30]. After HCV enters hepatocytes, translation takes place to produce the structural polyprotein which must then be cleaved into functional proteins [31]. Several non-structural proteins (NS2-NS5) mediate these intracellular functions and have proven promising therapeutic targets for DAAs [32, 33]. Host target inhibitors include the cyclophilin inihibitors, a host enzyme intimately involved in HCV replication, and the antagomir targeting miR-122. Novel immunomodulators, new interferons or ribavirin analgoues are not HCV specific but generate an anti-viral state. A recent systematic review found more than 50 molecules currently in development to treat chronic HCV, divided across various therapeutic classes [34]. In light of the rapidly evolving literature, we have selected the more promising agents from each major class as illustrations of drugs in development (Table 2). It is worth noting that most of these drugs are in the early stages of development, have been studied in small cohorts, and have been presented in conference form only. Therefore their results need to be considered with some caution.
Figure 1.
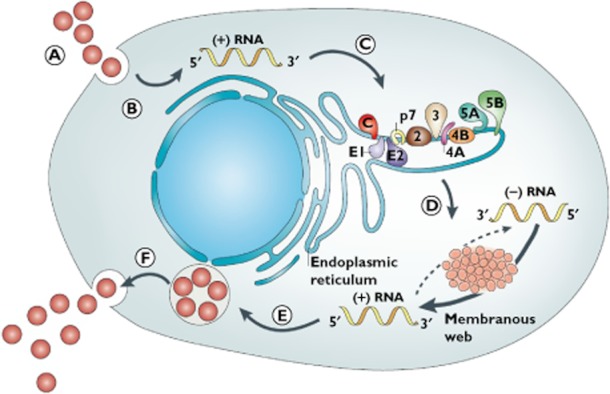
HCV viral life cycle and therapeutic targets for new drug classes (A) HCV virus binding and entry via receptor-mediated endocytosis (targeted by entry inhibitors); (B) RNA release into the cytoplasm; (C) Translation into a polypeptide on the ribosome, and processing into viral proteins that form structural components of the virus (targeted by protease inhibitors); (D) RNA replication in the endoplasmic reticulum (targeted by protease, polymerase, NS5A and cyclophilin inhibitors, and antagomirs); (E) RNA packaging and assembly (targeted by NS5A inhibitors); and (F) virion maturation and release (targeted by glycosylation inhibitors). (Figure reproduced with permission from Nature Publishing Group, Moradpour et al. 2007 [31], copyright)
Table 2.
Class leading agents in development for chronic HCV treatment
| Class | Mechanism | Drug | Trial phase | Patients | End point, virological response | Reference |
|---|---|---|---|---|---|---|
| NS5A inhibitor | NS5A inhibitor | Daclatasvir (BMS-790052) | II | Genoytpe-1, naïve | +PEG-IFN/RBV: SVR12 83–92% | Pol et al. 2011 [59] |
| II | Genoytpe-1, null responders | +protease inhibitor: SVR 36% +protease inhibitor + PEG-IFN/RBV: SVR 90% | Lok et al. 2012 [58] | |||
| Polymerase inhibitors | NS5B inhibitor | GS-7977 | II | Genotype-2 and -3, naïve | +PEG-IFN/RBV: SVR 100% +RBV: SVR 100% | Gane et al. 2011 [51] |
| II | Genotype-1, naïve | +PEG-IFN/RBV: ETR 100%, SVR12 88–91%, SVR24 93% | Lawitz et al. 2011 [53] | |||
| NS5B inhibitor | Mericitabine, (RG7128) | II | Genoytpe-1, naïve | +PEG-IFN/RBV: SVR12 76% | Pockros et al. 2011 [54] | |
| II | Genoytpe-1, naïve | +protease inhibitor: Day14 viral load decline 5.1 log10 IU ml−1 | Gane et al. 2010 [55] | |||
| II | Genoytpe-1, nullnresponders | +protease inhibitor: Day14 viral load decline 4.9 log10 IU ml−1 | Gane et al. 2010 [55] | |||
| Non-nucleoside | Tegobuvir (GS-9190) | II | Genoytpe-1, naïve | +PEG-IFN/RBV: EVR 67% +protease inhibitor + PEG-IFN/RBV: EVR 92% | Lawitz et al. 2011 [85] Foster et al. 2011 [86] | |
| NS3/4a Protease inhibitors | NS3/4A inhibitor | TMC435 | II | Genotype-1, naïve Genotype-1, experienced | +PEG-IFN/RBV: SVR 89–97% +PEG-IFN/RBV: Week 24 treatment response 84–91% | Fried 2011 [48] Zeuzem et al. 2011 [87] |
| Asunaprevir (BMS-650032) | II | Genoytpe-1, naïve | +PEG-IFN/RBV: EVR 83–100% | Bronowicki et al. 2011 [88] | ||
| II | Genoytpe-1, experienced | +polymerase inhibitor: SVR 36% +polymerase inhibitor + PEG-IFN/RBV: SVR 90% | Lok et al. 2012 [58] | |||
| Danoprevir (RG7227) | II | Genoytpe-1, naïve | +PEG-IFN/RBV: SVR 60–71% +polymerase inhibitor: Day14 viral load −5.1 log10 IU ml−1 | Larrey et al. 2011 [89] Gane et al. 2010 [55] | ||
| Genoytpe-1, experienced | +polymerase inhibitor: Day14 viral load −4.9 log10 IU ml−1 | Gane et al. 2010 [55] | ||||
| Quadruple therapy | Interferon +ribavirin +NS5A +NS3 inhibitor | PEG-IFN/RBV +daclatasvir +asunaprevir | II | Genotype-1, experienced, non-responders | SVR 90% | Lok et al. 2012 [58] |
| Host-targeted agents | Cylcophilin inhibitor | Alisporivir (Debio025) | II | Genotype-1, naïve | +PEG-IFN/RBV: SVR 76% compared to 55% standard therapy | Flisiak et al. 2011 [65] |
| Antagomirs | Micro-RNA (miR-122) antagomir | Miravirsen | II | Genotype-1, naïve | +PEG-IFN/RBV: 10 weeks HCV RNA decline 2.7 log10 IU ml−1 | Janssen et al. 2011 [68] |
| Ribavirin analogues | Ribavirin analogues | Taribavirin (Viramidine) | III | All genotypes | +PEG-IFN: SVR 38% (inferior to RBV) | Benhamou et al. 2009 [77] Marcellin et al. 2010 [78] |
| Interferons | PEG-interferon lambda | PEG-IFN-lamba | II | Genotypes-1–4 | +RBV: EVR 40–71% | Zeuzem et al. 2011 [75] |
| Immunomodulators | Nitazoxadide | Nitazoxadide | II | Genotypes-1, -2 and -4, naïve | +PEG-IFN: SVR 61% +PEG-IFN/RBV: SVR 79% | Rossignol et al. 2010 [69] |
| Toll-like Receptor-7 agonist | ANA733 | II | Genotype-1, naïve | Monotherapy: 10 days viral load decline 1.3 log10 IU ml−1 | Bergmann et al. 2011 [71] | |
| Toll-like Receptor-9 agonist | IMO-2125 | II | Genotype-1, null responder | Increased serum IFN-alpha, IP-10, cytokines | Rodriguez-Torres et al. 2010 [72] | |
| Therapeutic vaccination | GI-5005 | 2 | Genotype-1, naïve | +PEG-IFN/RBV: SVR 58% | McHutchison et al. 2010 [73] Jacobson et al. 2010 [74] |
PEG-IFN, pegylated interferon alpha-2a/b; RBV, ribavirin; Gt, HCV genotype; EVR, early virological response 12 weeks on-therapy SVR12, sustained virological response 12 weeks post-therapy; SVR, sustained virological response 24 weeks post-therapy.
Current HCV protease inhibitors
Two HCV protease inhibitors (PIs), boceprevir and telaprevir, are now licensed for chronic HCV treatment. The HCV non-structural (NS)-3/4A HCV protease is responsible for cleaving the HCV viral polyprotein into mature proteins. Both drugs bind reversibly to the NS3 active site, blocking polyprotein cleavage and preventing HCV replication [35]. In addition to this direct antiviral action, inhibition of NS3 protease may also act to restore the hepatocyte interferon-signalling pathways [36]. Both drugs were designed using genotype-1 HCV-specific in vitro systems, and have limited activity against other HCV genotypes. When used as monotherapy, virological resistance develops rapidly to boceprevir and telaprevir [37, 38], necessitating combination with PEG-IFN/RBV.
The pivotal trials of boceprevir or telaprevir triple therapy demonstrated significantly increased rates of SVR compared with PEG-IFN/RBV in both treatment-naïve and treatment experienced HCV genotype-1 infected patients.
Boceprevir
Boceprevir treatment consists of 800 mg (4 × 200 mg capsules) administered orally, every 8 h, and is introduced after a 4 week lead-in of standard PEG-IFN/RBV therapy in patients with genotype-1 HCV infection. Treatment naïve patients receive boceprevir for a further 24 weeks (if HCV RNA was undetectable from week 8) or 44 weeks (if HCV RNA was detectable at week 8), accompanied by PEG-IFN/RBV [35]. Treatment experienced patients receive the same dose for 44 weeks after the 4-week PEG-IFN/RBV lead-in [39]. The rationale for a lead-in period was to lower HCV RNA levels prior to boceprevir treatment, thereby reducing the risk of viral breakthrough or resistance [40]. The lead-in allows assessment of IFN responsiveness – if HCV RNA decreases <1 log10 IU ml−1 by week 4, predicted SVR with PEG-IFN/RBV alone is 5% vs. 29–39% with the addition of boceprevir.
Among treatment naïve patients, SVR improved when boceprevir was added to PEG-IFN/RBV from 40% to 67–68% in non-Black patients, and from 23% to 42–53% in Black patients (Table 1) and 44% of patients were eligibile for short duration therapy using RGT. Anaemia occurred nearly twice as often in boceprevir patients as in controls (49% vs. 29%), but there was no significant difference in the frequency of treatment discontinuation due to adverse events [35]. Erythropoietin use to treat anaemia was more frequent among patients on boceprevir (43% vs. 24%). Dysgeusia also occurred more than twice as often in boceprevir patients than controls (37% vs. 18%). Among patients who had previously failed PEG-IFN/RBV, boceprevir improved SVR from 29% to 75% among previous relapsers, and improved SVR from 7% to 52% among partial responders [39]. Prior null responders had an SVR rate of 38% with boceprevir therapy [41].
The registration studies for both boceprevir [35, 39] and telaprevir [42, 43] were well designed and powered randomized control trials. Their main limitations stem from generalizability from the trial to clinical settings. Firstly, boceprevir's 4 week lead-in period in their study design offers advantages in predicting overall outcome based on week 4 RVR. However the lead-in contributes to treatment complexity in practice. Secondly, the high treatment completion rates and corresponding high SVR rates for both PIs may also have been augmented by erythropoietin administration used to manage anaemia during the trials in >40% of participants which may not be available outside of a research setting. Finally, recent data from a French observational cohort on the use of HCV PIs in clinical practice found high rates of serious adverse events (38–49%) compared with the boceprevir and telaprevir clinical trials (9–14%) [44]. However, there are no data available yet on whether the SVR observed in trials will differ in practice.
Telaprevir
The telaprevir treatment paradigm involves 750 mg (2 × 375 mg capsules) administered orally for 12 weeks, concurrently with PEG-IFN for 24 weeks (with RVR and EVR) or 48 weeks (for patients who do not achieve EVR) in treatment naïve patients. In the registration trial, telaprevir treatment for 8 or 12 weeks in addition to PEG-IFN/RBV for 24–48 weeks improved SVR from 44% to 69–75% in previously untreated patients [43]. Rash and anaemia were higher in the groups that received telaprevir, and discontinuation of treatment was more frequent (7–11% telaprevir group vs. 3% control group). Rashes were primarily eczematous and reversed on discontinuation of telaprevir. However one rare case of Stevens-Johnson syndrome and DRESS syndrome have been reported. Erythropoietin use was not permitted. Gastrointestinal side effects (nausea, diarrhoea, anorectal pain and haemorrhoids) were also more common with telaprevir compared wiht controls (40–43% vs. 31%).
Telaprevir also improves SVR rates in HCV genotype-1 patients who have previously failed PEG-IFN/RBV. Telaprevir used for 12 weeks with 48 weeks of PEG-IFN/RBV, with or without a 4 week lead-in phase, improved SVR from 24% to 83–88% among prior relapsers, from 15% to 54–59% in partial responders, and from 5% to 29–33% among null responders [42].
Patients with cirrhosis had much improved SVR when treated with either telaprevir (62% vs 33%) or boceprevir (50% vs. 39%, non-Black cohort) combination therapy but treatment toxicities were more challenging with higher discontinuation rates (15% vs. 11% among non-cirrhotics) [45]. In a sub-analysis of the telaprevir registration study, rash, pruritus and anaemia were more frequent in patients with cirrhosis (43%, 55% and 44%, respectively) than in those who received PEG-IFN/RBV (27%, 35% and 27%, respectively).
Protease inhibitor drug interations
Drug interactions between HCV PIs and other medications introduce extra treatment complexity. HCV PIs seem to exert strong, reversible inhibition of CYP34A. However other data indicate that another non-CYP34A pathway is involved in boceprevir metabolism and excretion [46]. PI concentrations can vary with co-administration of other cytochrome P450 metabolized drugs including HIV combination antiretroviral therapy, with implications most important for HIV/HCV co-infected patients [47].
Novel HCV protease inhibitors
Beyond telaprevir and boceprevir, a number of new NS3A PIs are being developed in phase II/III trials. The next PI to market will likely be TMC-435, which is dosed once daily, offering a benefit over current generation NS3/4A PIs. A phase II trial of treatment naïve, genotype-1 individuals used TMC-435 with PEG-IFN/RBV for 24 of 48 weeks total therapy, guided by HCV RNA at weeks 4 to 20. 68–76% of patients achieved RVR, of whom 88–95% achieved SVR [48]. 79–86% of patients were eligible for short duration (24 weeks) therapy. The control group in this study also had a high SVR response, so the overall virological response in this cohort may have been over-estimated. It had a favourable side effect profile, with similar rates of rash and anaemia compared with the control group.
MK-5712 is a potent second generation NS3 PI in early stage development. It requires once daily dosing, and has efficacy against HCV genotypes 1–6 in vitro [49]. MK-5172 also has activity against a number of variants that are resistant to other protease inhibitors in development. There are several other PIs in development (Table 2). These new PIs will likely replace the first generation PIs due to their improved side effect profile and simplified use, regardless of any additional improvement in SVR.
HCV NS5B polymerase inhibitors
NS5B polymerase inhibitors can be classified as nucleoside inhibitors (NI) or non-nucleoside inhibitors (NNIs). NIs are potent and are active against all HCV genotypes, as the HCV catalytic site is conserved across genotypes. They have a good resistance profile and NI-resistant HCV variants have displayed very poor fitness to date [50]. The most promising NI at present is GS-7977 which has entered phase III development for genotype-1 HCV in combination with PEG-IFN/RBV [51–53]. GS-7977 has also entered phase III development as IFN-free treatment for genotype-2/-3 HCV (see below). Mericitabine is a second NI in advanced clinical development. In one study, HCV treatment naive patients infected with HCV genotypes-1/-4 received response guided mericitabine plus PEG-IFN/RBV or PEG-IFN/RBV alone for at least 24 weeks. Virological response 12 weeks post-therapy (SVR12) was 76% in the intervention group, compared with 56% in the standard therapy group [54]. Its antiviral potency (91% RVR) has been confirmed in other phase II studies [55].
NNIs bind to allosteric sites around the active site of the NS5B enzyme, induce conformational changes and down-regulate the polymerase's activity. There are multiple NNIs that have entered clinical development, including tegobuvir in phase II development, and others in earlier stages including filibuvir [56] and silibinin [57]. The class-wide limitations of NNIs to date include their relatively weak potency and rapid emergence of resistance. They may have a role in combination DAA regimens.
HCV NS5A inhibitors
NS5A replication complex inhibitors are potent, pan-genotypic antivirals. Daclatsavir is a potent NS5A inhibitor with efficacy in HCV genotype-1 treatment naïve and experienced patients [58, 59]. In a phase II study of treatment naïve patients, daclatasvir given in combination with PEG-IFN/RBV vs. standard therapy had an SVR12 of 83–92% vs. 25%, respectively [59]. Natural polymorphisms at the HCV NS5A gene conferring daclatasvir resistance have been identified from gene bank studies and have been shown to be clinically relevant in vivo [60]. Further study is underway to determine how these primary resistance mutations might affect the NS5A class.
HCV resistance associated with DAA therapy
The high replication rate and error-prone HCV polymerase give rise to naturally occurring resistance-associated variants (RAVs) [50]. In the setting of PI monotherapy, RAVs are selected within days, leading to virological breakthrough [61]. Single nucleotide substitutions have been identified that are associated with resistance to all PIs in development, and the R155/A156 substitutions are cross-resistant for all PIs. Combination with PEG-IFN/RBV can prevent mutants from emerging [62, 63] Hence current PI trials underway in phase III are using triple therapy with PEG-IFN/ RBV.
Host target inhibitors
Cyclophilin inhibitors
Cyclophilin A is required for HCV replication and this has led to the development of several cyclophilin inhibitors [54]. Alisporivir (Debio025) is a representative, non-immunosuppressive ciclosporin analogue that inhibits HCV assembly and replication by binding to the host protein, cyclophilin A [64]. In a phase II trial of HCV genotype-1 treatment naïve patients, those receiving alisporivir and PEG-IFN/RBV had superior SVR (76%) compared with PEG-IFN/RBV alone (55%) [65]. Alisporivir is now in phase III development for HCV genotype-1. Alisporivir also has activity against HCV genotypes-2/-3 and IFN-free regimens are currently being evaluated. Resistance to cyclophilin inhibitors has been described, although is thought to be rare in vivo [66].
Antagomirs
The liver-expressed microRNA-122 (miR-122) is essential for HCV accumulation in hepatocytes [67]. In animal models, an oligonucleotide complementary to miR-122 (a so called ‘antagomir’) led to prolonged suppression of HCV viraemia. In an early phase II trial, miravirsen (an miR-122 antagomir) was given in weekly subcutaneous weight-based injections to individuals with HCV genotype-1 for 1 month, followed by standard therapy [68]. A continuous and prolonged anti-viral activity was observed beyond the end of active therapy with minimal side effects.
Immunomodulators
Immunomodulatory agents improve innate immune responses indirectly promoting HCV elimination. Nitazoxanide is an anti-parasitic agent that improves SVR in combination with standard therapy, and is thought to act by improving interferon signalling [69]. In a phase II study of treatment naïve, predominantly HCV genotype-4 patients, nitazoxanide was used as a 4 week lead-in, then continued with PEG-IFN for 36 weeks, with or without ribavirin. The SVR was 79% vs. 61%, with or without ribavirin, respectively. but side effects were more frequent among those receiving nitazoxanide [69].
Anti-HCV activity can be induced by toll-like receptor (TLR)-7 and TLR-9 stimulation by mediating endogenous interferon and cytokine release [70]. A small molecule inducer of the TLR-7 pathway is in phase II development indicating anti-viral activity of −1.3 log10 IU ml−1 from baseline, compared with −0.3 log10 IU ml−1 in the placebo group [71]. A TLR-9 agonist has also completed phase-1 development [72]. Therapeutic vaccination targets are also in early development. One vaccine agent (GI-5005) expresses a protein encompassing HCV NS3 and core protein sequences, and demonstrated antiviral activity in phase II studies of genotype-1, treatment naïve patients, improving SVR from 48% to 58% when added to PEG-IFN/RBV [73, 74].
Other therapeutic approaches
There are a number of novel indirectly acting antivirals in various stages of development. Their main advantage is that by acting non-specifically, they do not engender viral resistance, but they add to treatment complexity if used additively.
Interferon analogues
Alternative interferon agents have been studied to improve tolerability and efficacy. PEG-IFN-lambda is active against all HCV genotypes and binds a more hepatocyte-specific receptor, thus reducing haematological side effects. A phase II study demonstrated fewer dose interferon reductions, less marrow toxicity and flu-like symptoms, and generally improved rapid virological response (between 40–71% dependent on HCV genotype) [75]. Albinterferon is a fortnightly preparation of PEG-IFN-alpha bound to albumin to prolong its half-life, with consequently reduce side effects. Although the SVR was 51% in non-responders with genotypes 1–3, development has been discontinued [76].
Ribavirin analogues
Ribavirin analogues have been studied in place of ribavirin primarily as a means of reducing ribavirin-associated anaemia [77]. Taribavirin is a prodrug, which preferentially targets the liver, and accumulates less in red blood cells. A phase III study of PEG-IFN with taribavirin demonstrated significantly less anaemia compared with PEG-IFN/RBV, but inferior SVR in the taribavirin group (38% vs. 52%) [78].
The promise of new combinations: with and without interferon
The challenge of new therapeutic agents and combinations is to improve virological response, shorten the length of therapy and offer treatment free of interferon. New treatments will need to be evaluated by several markers: first, in terms of their improvements in efficacy beyond the new standard of PI/PEG-IFN/RBV response; second, their reductions in side effects; third, improved treatment simplicity and pill burden; and finally cost for the individual and society. There is the real potential to have interferon free regimes available within several years with the consequent reduction in side effects and availability of treatment for IFN-intolerant individuals (Table 3). However, there may remain a selective role for IFN since quadruple therapy with two DAAs plus PEG-IFN/RBV has been shown to improve outcomes for patients with multiple poor treatment response predictors (such as previous treatment non-response, cirrhosis, genotype-1 infection and high HCV viral load). In these difficult to treat individuals, the increase in efficacy may outweigh increases in complexity and toxicity. Another area whether combination therapy is likely to improve in the next few years will be incremental changes in the current triple therapy PI/PEG-IFN/RBV as newer once daily protease inhibitors replace boceprevir and telaprevir.
Table 3.
Interferon free combination strategies in development
| Mechanism | Drugs | Trial phase | Patients | Virological response, end point | References |
|---|---|---|---|---|---|
| Nucleoside polymerase +NS3 protease inhibitor | Daclatasvir + asunaprevir | II | Genotype-1, null responders, USA | RVR 64%, SVR 36% (n = 11) | Lok et al. 2012 [58] |
| II | Genotype-1b, null reponders, Japan | SVR24 100% (n = 10) | Chayama et al. 2012 [79] | ||
| Nucleoside polymerase inhibitor + RBV | GS-7977 +RBV | II | Genotype-2 and -3, naïve | RVR 100%, SVR 100% (n = 10) | Gane et al. 2011 [51] |
| II | Genotype-1, null responders | SVR4 10% (n = 10) | Gane et al. 2012 [52] | ||
| Nucleoside polymerase +NS3 protease inhibitor +Ribavirin | BI201335 +BI207127 +RBV | II | Genotype -1, naïve | RVR 100% (n = 17) | Zeuzem et al. 2011 [90] |
| Nucleoside polymerase +NS3 protease inhibitor | Tegobuvir +GS-9256 | II | Genotype-1, naïve | EVR 80% (n = 15) +RBV: EVR 100%) (n = 13) | Foster et al. 2011 [86] |
| Nucleoside polymerase +NS3 protease inhibitor | Mericitabine +danoprevir | II | Genotype-1, experienced, non-responders | Day14 viral load −4.9 log10 IU ml−1 | Gane et al. 2010 [55] |
| II | Genotype-1, naïve | Day14 viral load −5.1 log10 IU ml−1 | Gane et al. 2010 [55] |
PEG-IFN, pegylated interferon alpha-2a/b; RBV, ribavirin; RVR, rapid virological response with HCV RNA undetectable at week 4; EVR, early virological response with HCV RNA undetectable at week 12.
Interferon-free therapy
Several studies have explored IFN-free therapy combinations in different groups (Table 3). Two agents, GS-7977 and daclatasvir, are likely to be included in the first IFN-free regimens. A study of an NS5B polymerase (GS-7977) with ribavirin among treatment naïve, genotype-2/-3 patients treated 40 participants for 12 weeks, randomly assigned PEG-IFN for different durations or not at all [51]. All participants achieved an RVR and SVR, regardless of PEG-IFN administration. Viral kinetics were no different in the IFN-free arm, and there were no cases of viral breakthrough, suggesting that the combination has a high barrier to resistance. GS-7977 monotherapy for 12 weeks among 10 genotype-2/-3 patients resulted in an end-of-treatment response for all patients. However four relapsed within 4 weeks of stopping therapy, consistent with a need for RBV in IFN-free regimens [51]. Among harder to treat, genotype-1 null responders, GS-7977 plus RBV for 12 weeks demonstrated a 100% end-of-treatment response (n = 10). However nine out of 10 relapsed within 4 weeks of stopping treatment [52]. Previous null responders may require longer DAA therapy, the addition of other DAA agents, or might require PEG-IFN.
Another phase II trial of treatment experienced, genotype-1, non-responders without cirrhosis studied daclatasvir, an NS5A polymerase inhibitor, and asunaprevir, an NS3 protease inhibitor, randomized to receive PEG-IFN/RBV vs. no PEG-IFN/RBV [58]. Amongst the IFN-free group (n = 11), 4/11 of their non-responder population achieved an SVR, 1/11 relapsed after an undetectable HCV RNA at the end of treatment, while 6/11 had viral breakthrough on therapy. When examining genotype-1 subtype, both genotype-1b infected patients achieved an SVR (n = 2), while only 2/9 genotype-1a patients achieved an SVR. Resistance mutations to both NS5A and NS3 agents occurred in patients with virological failure. It is unclear whether those mutations will affect subsequent therapy. The same IFN-free drug combination was used for 24 weeks in a small Japanese cohort (n = 10) of HCV genotype-1b, IL28B favourable, treatment experienced null responders and was demonstrated to have a 100% SVR [79]. These early studies suggest that differences in genotype-1 subtype will have implications for IFN-free treatment success.
Quadruple therapy
In a phase II study of PEG-IFN and RBV in combination with daclatasvir and asunaprevir, very high SVR rates were achieved in a null responder population [58]. Ten treatment experienced, genotype-1, non-responders without cirrhosis had undetectable HCV RNA on treatment. SVR response measured at 12, 24 and 48 weeks was 10/10, 9/10 and 9/10 respectively. These results suggest that quadruple therapy may achieve very high SVR rates in patients who respond poorly to IFN, a dramatic improvement on standard therapy which would have predicted less than 10% SVR.
Conclusions
2011 was a watershed year for HCV treatments, heralding the licensing of the first DAA for HCV, as well as proof-of-concept that HCV could be cured without IFN therapy [1]. Challenges still remain to develop effective, durable, IFN-free regimens without promoting HCV resistance, and in turn improve treatment side effects allowing those with significant psychological co-morbidities to access treatment for the first time. Few people who inject drugs, the group most at risk of HCV infection, receive HCV treatment currently [80, 81]. Improved regimens of shorter duration with fewer side effects may increase the number of patients on treatment overall, and people who inject drugs in particular. Current HCV practice is evolving rapidly and newer agents and classes of drug will add to treatment complexity in the short term. In addition to the individual benefits from curing HCV, increasing treatment uptake among people who inject drugs has the potential to reduce HCV prevalence in this high risk population [82, 83]. The rapid improvements, successes to date and the number of HCV agents in development should give hope to millions of patients living with HCV infection.
Acknowledgments
JD, AT and MH acknowledge fellowship support from the National Health and Medical Research Council. JD and MH acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Programme (Department of Health, Victoria, Australia) to the Burnet Institute.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work, JD and EA had no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, MH received research support from Roche, DL received honoraria from Abbott and Merck, and AT received research/grant support from Merck, Roche Pharmaceuticals, Gilead Sciences and speaker's fees from Merck, Roche Pharmaceuticals, Bristol-Myers Squibb, JD, EA, DL and MH had no other relationships or activities that could appear to have influenced the submitted work, and AT had a consulting/advisory capacity with Merck, Roche Pharmaceuticals, Janssen-Cilag (Johnson and Johnson) and is a co-inventor of a patent related to the IL28B-HCV discovery.
References
- 1.Chung RT. A watershed moment in the treatment of hepatitis C. N Engl J Med. 2012;366:273–275. doi: 10.1056/NEJMe1113272. [DOI] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–538. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29:74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 4.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 6.Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M, Shiffman ML, Yurdaydin C, Dalgard O. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011;8:212–223. doi: 10.1038/nrgastro.2011.21. [DOI] [PubMed] [Google Scholar]
- 7.Noureddin M, Ghany MG. Pharmacokinetics and pharmacodynamics of peginterferon and ribavirin: implications for clinical efficacy in the treatment of chronic hepatitis C. Gastroenterol Clin North Am. 2010;39:649–658. doi: 10.1016/j.gtc.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy KR, Nelson DR, Zeuzem S. Ribavirin: current role in the optimal clinical management of chronic hepatitis C. J Hepatol. 2009;50:402–411. doi: 10.1016/j.jhep.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 10.Grebely J, Matthews GV, Dore GJ. Treatment of acute HCV infection. Nat Rev. Gastroenterol Hepatol. 2011;8:265–274. doi: 10.1038/nrgastro.2011.32. [DOI] [PubMed] [Google Scholar]
- 11.Doyle JS, Sacks-Davis R, Hellard ME. Acute hepatitis C infection: new approaches to surveillance, treatment and prevention. Curr Hepatitis Rep. 2012 doi: 10.1007/s11901-012-0143-5. in press. [Google Scholar]
- 12.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 13.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 14.Lau JY, Tam RC, Liang TJ, Hong Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. Hepatology. 2002;35:1002–1009. doi: 10.1053/jhep.2002.32672. [DOI] [PubMed] [Google Scholar]
- 15.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 16.Holmes JA, Sievert W, Thompson AJ. IL28B polymorphism and genetic biomarkers of viral clearance in hepatitis C virus infection. Biomark Med. 2011;5:461–478. doi: 10.2217/bmm.11.47. [DOI] [PubMed] [Google Scholar]
- 17.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R, Poordad F, Lawitz EJ, McCone J, Shiffman ML, Galler GW, Lee WM, Reindollar R, King JW, Kwo PY, Ghalib RH, Freilich B, Nyberg LM, Zeuzem S, Poynard T, Vock DM, Pieper KS, Patel K, Tillmann HL, Noviello S, Koury K, Pedicone LD, Brass CA, Albrecht JK, Goldstein DB, McHutchison JG. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–129. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Sarrazin C, Susser S, Doehring A, Lange CM, Muller T, Schlecker C, Herrmann E, Lotsch J, Berg T. Importance of IL28B gene polymorphisms in hepatitis C virus genotype 2 and 3 infected patients. J Hepatol. 2011;54:415–421. doi: 10.1016/j.jhep.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Vezali E, Aghemo A, Colombo M. A review of the treatment of chronic hepatitis C virus infection in cirrhosis. Clin Ther. 2010;32:2117–2138. doi: 10.1016/S0149-2918(11)00022-1. [DOI] [PubMed] [Google Scholar]
- 20.Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008;49:634–651. doi: 10.1016/j.jhep.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Berg T, Sarrazin C, Herrmann E, Hinrichsen H, Gerlach T, Zachoval R, Wiedenmann B, Hopf U, Zeuzem S. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology. 2003;37:600–609. doi: 10.1053/jhep.2003.50106. [DOI] [PubMed] [Google Scholar]
- 23.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [DOI] [PubMed] [Google Scholar]
- 24.Camma C, Cabibbo G, Bronte F, Enea M, Licata A, Attanasio M, Andriulli A, Craxi A. Retreatment with pegylated interferon plus ribavirin of chronic hepatitis C non-responders to interferon plus ribavirin: a meta-analysis. J Hepatol. 2009;51:675–681. doi: 10.1016/j.jhep.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Jensen DM, Marcellin P, Freilich B, Andreone P, Di Bisceglie A, Brandao-Mello CE, Reddy KR, Craxi A, Martin AO, Teuber G, Messinger D, Thommes JA, Tietz A. Re-treatment of patients with chronic hepatitis C who do not respond to peginterferon-alpha2b: a randomized trial. Ann Intern Med. 2009;150:528–540. doi: 10.7326/0003-4819-150-8-200904210-00007. [DOI] [PubMed] [Google Scholar]
- 26.Poynard T, Colombo M, Bruix J, Schiff E, Terg R, Flamm S, Moreno-Otero R, Carrilho F, Schmidt W, Berg T, McGarrity T, Heathcote EJ, Goncales F, Diago M, Craxi A, Silva M, Bedossa P, Mukhopadhyay P, Griffel L, Burroughs M, Brass C, Albrecht J. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology. 2009;136:1618–1628. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 27.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-García J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J, Sette H, Passe S, De Pamphilis J, Duff F, Schrenk UM, Dieterich DT. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 28.Hellard M, Sacks-Davis R, Gold J. Hepatitis C treatment for injection drug users: a review of the available evidence. Clin Infect Dis. 2009;49:561–573. doi: 10.1086/600304. [DOI] [PubMed] [Google Scholar]
- 29.Dore GJ, Hellard M, Matthews GV, Grebely J, Haber PS, Petoumenos K, Yeung B, Marks P, Van Beek I, McCaughan G, White P, French R, Rawlinson W, Lloyd AR, Kaldor JM. Effective treatment of injecting drug users with recently acquired hepatitis C virus Infection. Gastroenterology. 2010;138:123–135. doi: 10.1053/j.gastro.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979–1998. doi: 10.1053/j.gastro.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 31.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nature reviews. Microbiology. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 32.Jazwinski AB, Muir AJ. Emerging therapies in hepatitis C: dawn of the era of the direct-acting antivirals. Gastroenterol Clin North Am. 2011;40:481–494. doi: 10.1016/j.gtc.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pockros PJ. New direct-acting antivirals in the development for hepatitis C virus infection. Therap Adv Gastroenterol. 2010;3:191–202. doi: 10.1177/1756283X10363055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee LY, Tong CY, Wong T, Wilkinson M. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract. 2012;66:342–355. doi: 10.1111/j.1742-1241.2012.02895.x. [DOI] [PubMed] [Google Scholar]
- 35.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foote BS, Spooner LM, Belliveau PP. Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C. Ann Pharmacother. 2011;45:1085–1093. doi: 10.1345/aph.1P744. [DOI] [PubMed] [Google Scholar]
- 37.Susser S, Welsch C, Wang Y, Zettler M, Domingues FS, Karey U, Hughes E, Ralston R, Tong X, Herrmann E, Zeuzem S, Sarrazin C. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology. 2009;50:1709–1718. doi: 10.1002/hep.23192. [DOI] [PubMed] [Google Scholar]
- 38.Sarrazin C, Kieffer TL, Bartels D, Hanzelka B, Muh U, Welker M, Wincheringer D, Zhou Y, Chu HM, Lin C, Weegink C, Reesink H, Zeuzem S, Kwong AD. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology. 2007;132:1767–1777. doi: 10.1053/j.gastro.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 39.Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N, Rossaro L, Anderson FH, Jacobson IM, Rubin R, Koury K, Pedicone LD, Brass CA, Chaudhri E, Albrecht JK. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 41.Vierling JM. Efficacy of boceprevir in prior null responders to peginterferon/ribavirin: the PROVIDE Study. Hepatology. 2011;54(Suppl. 1):A931. [Google Scholar]
- 42.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, Ferenci P, Nevens F, Mullhaupt B, Pockros P, Terg R, Shouval D, van Hoek B, Weiland O, Van Heeswijk R, De Meyer S, Luo D, Boogaerts G, Polo R, Picchio G, Beumont M. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 44.Hezode C, Dorival C, Zoulim F, Poynard T, Mathurin P, Pol S, Larrey D, Cacoub P, Ledinghen VD, Bourlière M, Bernard PH, Riachi G, Alric L, Samuel D, Barthe Y, Fontaine H, Carrat F, Bronowicki J-P ANRS C020 CUPIC Study Group. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic non responders. First results of the French early access program (ANRS CO20-CUPIC) J Hepatol. 2012;56(Suppl. 2):S4. [Google Scholar]
- 45.Bourlière M, Khaloun A, Wartelle-Bladou C, Oules V, Portal I, Benali S, Adhoute X, Castellani P. Future treatment of patients with HCV cirrhosis. Liver Int. 2012;32:113–119. doi: 10.1111/j.1478-3231.2011.02702.x. [DOI] [PubMed] [Google Scholar]
- 46.Kasserra C, Hughes E, Treitel M, Gupta S, O'Mar E. 2011. Clinical pharmacology of boceprevir: metabolism, excretion and drug-drug interactions. Conference on Retroviruses and Opporunistic Infections, Boston.
- 47.Van Heeswijk R, Vandevoorde A, Boogaerts G, Vangeneugden T, de Paepe E, Polo R, Van Solingen-Ristea R, de Backer K, Garg V, Beumon M. 2011. Interactions with telaprevir. Conference on Retroviruses and Opporunistic Infections, Boston.
- 48.Fried MW. TMC435 in combination with peginterferon and ribavirin in treatment-naïve HCV genotype 1 patients: final analysis of the PILLAR phase IIb study. Hepatology. 2011;54(Suppl. 1):LB5. [Google Scholar]
- 49.Petry AS, Fraser IP, Van Dyck K, Nachbar RB, De Lepeleire I, Robberechts M, Han L, Palcza J, Moiseez V, Kobalava ZD, Uhle M, Wagner F, O'Mara E, Wagner JA. Safety and antiviral activity of MK-5172, a next generation HCV NS3/4A protease inhibitor with a broad HCV genotypic activity spectrum and potent activity against known resistance mutants, in genotype-1 and -3 HCV-infected patients. Hepatology. 2011;54(Suppl. 1):1364A. [Google Scholar]
- 50.Halfon P, Locarnini S. Hepatitis C virus resistance to protease inhibitors. J Hepatol. 2011;55:192–206. doi: 10.1016/j.jhep.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Gane EJ, Stedman CA, Hyland RH, Sorensen RD, Symonds WT, Hindes R, Berrey M. Once daily PSI-7977 plus RBV: pegylated interferon-alfa not required for complete rapid viral response in treatment-naive patients with HCV GT2 or GT3. Hepatology. 2011;54(Suppl. 1):377A. [Google Scholar]
- 52.Gane E, Stedman C, Anderson J, Hyland R, Hindes R, Symonds W, Berrey M. 2012. 100% Rapid Virologic Response for PSI-7977 + Ribavirin in Genotype 1 Null Responders (ELECTRON): early Viral Decline Similar to that Observed in Genotype 1 and Genotype 2/3 Treatment-naïve Patients Conference on Retroviruses and Opporunistic Infections, Seattle. 54LB.
- 53.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, Afdhal NH, Bernstein DE, DeJesus E, Freilich B, Nelson DR, Dieterich DT, Jacobson IM, Jensen DM, Abrams GA, Darling JM, Rodriguez-Torres M, Reddy KR, Sulkowski MS, Bzowej NH, DeMicco MP, Strohecker JS, Hyland RH, Mader M, Albanis E, Symonds WT, Berrey MM. Once-daily PSI-7977 plus PEG/RBV in treatment-naïve patients with HCV Gt1: robust end of treatment response rates are sustained post-treatment. Hepatology. 2011;54(Suppl. 1):472A. [Google Scholar]
- 54.Pockros P, Jensen D, Tsai N. First SVR data with the nucleoside analogue polymerase inhibitor mericitabine (RG7128) combined with peginterferon/ribavirin in treatment naïve HCV G 1 and 4 patients: interim analysis from the JUMP-C trial. J Hepatol. 2011;54(Suppl. 1):S538. [Google Scholar]
- 55.Gane EJ, Roberts SK, Stedman CA, Angus PW, Ritchie B, Elston R, Ipe D, Morcos PN, Baher L, Najera I, Chu T, Lopatin U, Berrey MM, Bradford W, Laughlin M, Shulman NS, Smith PF. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2010;376:1467–1475. doi: 10.1016/S0140-6736(10)61384-0. [DOI] [PubMed] [Google Scholar]
- 56.Larrey D, Benhamou Y, Lohse AW, Trepo C, Moelleken C, Bronowicki J-P. Safety, pharmacokinetics and antiviral effect of BI 207127, a novel HCV RNA polymerase inhibitor, after 5 days oral treatment in patients with chronic hepatitis C. J Hepatol. 2009;50:S383. [Google Scholar]
- 57.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schoniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 58.Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M, Persson A, Zhu K, Dimitrova DI, Eley T, Guo T, Grasela DM, Pasquinelli C. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 59.Pol S, Ghalib RH, Rustgi VK, Martorell C, Everson GT, Tatum HA, Hezode C, Lim JK, Bronowicki J, Abrams GA, Brau N, Morris DW, Thuluvath P, Reindollar R, Yin PD, Diva U, Hindes R, McPhee F, Gao M, Thiry A, Schnittman S, Hughes EA. First report of SVR12 for a NS5a replication complex inhibitor BMS-790052 in combination with PEG-IFNa-2a and RBV: phase 2a trial in treatment-naive HCV-genotype-1 subjects. J Hepatol. 2011;54(Suppl. 1):S544–545. [Google Scholar]
- 60.Plaza Z, Soriano V, Vispo E, Del Mar Gonzalez M, Barreiro P, Seclen E, Poveda E. Prevalence of natural polymorphisms at the HCV NS5A gene associated with resistance to daclatasvir, an NS5A inhibitor. Antivir Ther. 2012;17:921–926. doi: 10.3851/IMP2091. [DOI] [PubMed] [Google Scholar]
- 61.McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhalm NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J, Bsharat M, George S, Kauffman RS, Adda N, Di Bisceglie AM PROVE3 Study Team. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 62.Bartels DJ, Zhou Y, Zhang EZ, Marcial M, Byrn RA, Pfeiffer T, Tigges AM, Adiwijaya BS, Lin C, Kwong AD, Kieffer TL. Natural prevalence of hepatitis C virus variants with decreased sensitivity to NS3.4A protease inhibitors in treatment-naive subjects. J Infect Dis. 2008;198:800–807. doi: 10.1086/591141. [DOI] [PubMed] [Google Scholar]
- 63.Kieffer TL, Sarrazin C, Miller JS, Welker MW, Forestier N, Reesink HW, Kwong AD, Zeuzem S. Telaprevir and pegylated interferon-alpha-2a inhibit wild-type and resistant genotype 1 hepatitis C virus replication in patients. Hepatology. 2007;46:631–639. doi: 10.1002/hep.21781. [DOI] [PubMed] [Google Scholar]
- 64.Crabbe R, Vuagniaux G, Dumont JM, Nicolas-Metral V, Marfurt J, Novaroli L. An evaluation of the cyclophilin inhibitor Debio 025 and its potential as a treatment for chronic hepatitis C. Expert Opin Investig Drugs. 2009;18:211–220. doi: 10.1517/13543780802651583. [DOI] [PubMed] [Google Scholar]
- 65.Flisiak R, Pawlotsky J, Crabbé R, Calistru PI, Kryczka W, Haussinger D, Mazella G, Romero M, Purcea D, Vuagniaux G, Bao W, Avila C, Zeuzem S. Once daily alisporivir (Deb025) plus PEGIFNalfa2a/ribavirin results in superior sustained virologic response (SVR24) in chronic hepatitis C genotype 1 treatment naïve patients. J Hepatol. 2011;54(Suppl. 1):S2. [Google Scholar]
- 66.Puyang X, Poulin DL, Mathy JE, Anderson LJ, Ma S, Fang Z, Zhu S, Lin K, Fujimoto R, Compton T, Wiedmann B. Mechanism of resistance of hepatitis C virus replicons to structurally distinct cyclophilin inhibitors. Antimicrobial Agents Chemother (Bethesda) 2010;54:1981–1987. doi: 10.1128/AAC.01236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janssen HLA. 2011. A randomized, double-blind, placebo (PLB) controlled safety and antiviral proof of concept study of miravirsen (MIR), an oligonucleotide targeting MIR-122, in treatment naïve patients with genotype 1 (GT1) chronic HCV infection. 62th American Association for the Study of Liver Diseases, San Francisco.
- 69.Rossignol JF, Elfert A, Keeffe EB. Treatment of chronic hepatitis C using a 4-week lead-in with nitazoxanide before peginterferon plus nitazoxanide. J Clin Gastroenterol. 2010;44:504–509. doi: 10.1097/MCG.0b013e3181bf9b15. [DOI] [PubMed] [Google Scholar]
- 70.Lee J, Wu CCN, Lee KJ, Chuang T-H, Katakura K, Liu Y-T, Chan M, Tawatao R, Chung M, Shen C, Cottam HB, Lai MMC, Raz E, Carson DA. Activation of anti-hepatitis C virus responses via Toll-like receptor 7. Proc Natl Acad Sci U S A. 2006;103:1828–1833. doi: 10.1073/pnas.0510801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergmann JF, de Bruijne J, Hotho DM, de Knegt RJ, Boonstra A, Weegink CJ, van Vliet AA, van de Wetering J, Fletcher SP, Bauman LA, Rahimy M, Appleman JR, Freddo JL, Janssen HL, Reesink HW. Randomised clinical trial: anti-viral activity of ANA773, an oral inducer of endogenous interferons acting via TLR7, in chronic HCV. Aliment Pharmacol Ther. 2011;34:443–453. doi: 10.1111/j.1365-2036.2011.04745.x. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez-Torres M, Ghalib RH, Gordon SC, Lawitz E, Patel K, Pruitt R. IMO-2125, a TLR9 agonist, induces immune responses which correlate with reductions in viral load in null responder HCV patients. Hepatology. 2010;52(4 Suppl):336A. [Google Scholar]
- 73.McHutchison JG, Thompson AJ, Jacobson IM, Boyer TD, Schiff ER, Everson GT, Vierling JM, Shiffman ML, Brown RS, Di Bisceglie AM, Gordon SC, Lee WM, Guo Z, King TH, Armstrong B, Rodell TC, Apelian D. Pharmacogenomic analysis reveals improved virologic response in all IL-28B genotypes in naive genotype 1 chronic HCV patients treated with GI-5005 therapeutic vaccine plus PEG-IFN/ribavirin. J Hepatol. 2010;52(Suppl. 1):S457. [Google Scholar]
- 74.Jacobson IM, McHutchison JG, Boyer TD, Schiff ER, Everson GT, Pockros PJ, Chasen RM, Vierling JM, Lawitz EJ, Kugelmas M, Tsai NC, Shiffman ML, Buynak RJ, Sheikh AM, Armstrong B, Rodell TC, Apelian D. GI-5005 therapeutic vaccine plus PEG-IFN/ribavirin significantly improves virologic response and ALT normalization at end-of-treatment and improves SVR24 compared to PEG-IFN /ribavirin in genotype-1 chronic HCV patients. J Hepatol. 2010;52(Suppl. 1):S465–466. [Google Scholar]
- 75.Zeuzem S, Arora S, Bacon B, Box T, Charlton M, Diago M, Dieterich D, Mur RE, Everson G, Fallón M, Ferenci P, Flisiak R, George J, Gitlin N, Gladysz A, Gordon S, Greenbloom S, Hassanein T, Jacobson I, Jeffers L, Kowdley K, Lawitz E, Lee S, Leggett B, Lueth S, Nelson D, Pockros P, Rodriguez-Torres M, Rustgi V, Serfaty L, Sherman M, Shiffman M, Sola R, Sulkowski M, Vargas H, Vierling J, Yoffe B, Ishak L, Fontana D, Xu D, Lester J, Gray T, Horga A, Hillson J, Romas E, Lopez-Talavera JC, Muir A, EMERGE Study Group Pegylated interferon-lambda (PegIFN-lambda) shows superior viral response with improved safety and tolerability versus PegIFNα-2a In HCV patients (Gl/2/3/4): emerge Phase IIb Through Week 12. J Hepatol. 2011;54(Suppl. 1):S538–539. [Google Scholar]
- 76.Nelson DR, Rustgi V, Balan V, Sulkowski MS, Davis GL, Muir AJ, Lambiase LR, Dickson RC, Weisner RH, Fiscella M, Cronin PW, Pulkstenis E, McHutchison JG, Subramanian GM. Safety and antiviral activity of albinterferon alfa-2b in prior interferon nonresponders with chronic hepatitis C. Clin Gastroenterol Hepatol. 2009;7:212–218. doi: 10.1016/j.cgh.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 77.Benhamou Y, Afdhal NH, Nelson DR, Shiffman ML, Halliman DG, Heise J, Chun E, Pockros PJ. A phase III study of the safety and efficacy of viramidine versus ribavirin in treatment-naive patients with chronic hepatitis C: ViSER1 results. Hepatology. 2009;50:717–726. doi: 10.1002/hep.23073. [DOI] [PubMed] [Google Scholar]
- 78.Marcellin P, Gish RG, Gitlin N, Heise J, Halliman DG, Chun E, Rodriguez-Torres M. Safety and efficacy of viramidine versus ribavirin in ViSER2: randomized, double-blind study in therapy-naive hepatitis C patients. J Hepatol. 2010;52:32–38. doi: 10.1016/j.jhep.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b–infected null responders. Hepatology. 2012;55:742–748. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 80.Robotin MC, Copland J, Tallis G, Coleman D, Giele C, Carter L, Spencer J, Kaldor JM, Dore GJ. Surveillance for newly acquired hepatitis C in Australia. J Gastroenterol Hepatol. 2004;19:283–288. doi: 10.1111/j.1440-1746.2003.03270.x. [DOI] [PubMed] [Google Scholar]
- 81.Grebely J, deVlaming S, Duncan F, Viljoen M, Conway B. Current approaches to HCV infection in current and former injection drug users. J Addict Dis. 2008;27:25–35. doi: 10.1300/J069v27n02_04. [DOI] [PubMed] [Google Scholar]
- 82.Hellard M, Jenkinson R, Higgs P, Stoove M, Sacks-Davis R, Gold J, Hickman M, Vickerman P, Martin NK. Modelling antiviral treatment to prevent hepatitis C infection among people who inject drugs in Victoria, Australia. Med J Aust. 2012;196:638–641. doi: 10.5694/mja11.10981. [DOI] [PubMed] [Google Scholar]
- 83.Martin NK, Vickerman P, Foster GR, Hutchinson S, Goldberg D, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modelling analysis of its prevention utility. J Hepatol. 2011;54:1137–1144. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 84.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lawitz E, Jacobson IM, Godofsky E, Foster GR, Flisiak R, Bennett M, Ryan M, Hinkle J, Simpson J, McHutchison J, Oldach D. A phase 2b trial comparing 24 to 48 weeks treatment with tegobuvir (GS-9190)/PEG/RBV to 48 weeks treatment with PEG/RBV for chronic genotype 1 HCV infection. J Hepatol. 2011;54(Suppl. 1):S181. [Google Scholar]
- 86.Foster GR, Buggisch P, Marcellin P, Zeuzem S, Agarwal K, Manns M, Sereni D, Klinker H, Moreno C, Zarski JP, Horsmans Y, Shelton M, Arterburn S, Lee W, McHutchison J, Delaney W, Oldach D. Four-week treatment with GS-9256 and tegobuvir (GS-9190), ± RBV ± PEG, results in enhanced viral suppression on follow-up PEG/RBV therapy, in genotype 1a/1b HCV patients. J Hepatol. 2011;54(Suppl. 1):S172. [Google Scholar]
- 87.Zeuzem S, Foster GR, Fried MW, Hezode C, Hirschfleld GM, Nikitin I, Poordad F, Lenz O, Peeters M, Sekar V, Smedt GD. 1376 The Aspire Trial: TMC435 in treatment-experienced patients with genotype-1 HCV infection who have failed previous PEGIFN/RBV treatment. J Hepatol. 2011;54(Suppl. 1):S546. [Google Scholar]
- 88.Bronowicki J-P, Pol S, Thuluvath PJ, Larrey D, Martorell CT, Rustgi VK, Morris DW, Younes Z, Fried MW, Bourliere M, Hezode C, Massoud O, Abrams GA, Ratziu V, Thiry A, Llamoso C, Hughes EA, Hindes RG. BMS-650032, an NS3 inhibitor, in combination with peginterferon alpha-2a and ribavirin in treatment-naive subjects with genotype 1 chronic hepatitis C I nfection. J Hepatol. 2011;54(Suppl. 1):S472. [Google Scholar]
- 89.Larrey P, Carenco C, Guyader D, Boyer N, Benhamou Y, Daniélou H, Pageaux G-P, Rouzier R, Marcellin P. High sustained virological response (SVR) rate after danoprevir for only 14 days associated with PEG-interferon alfa-2a and ribavirin in treatment-naive chronic HCV genotype 1 patients. J Hepatol. 2011;54(Suppl. 1):S481. [Google Scholar]
- 90.Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Mullhaupt B, Gane E, Schuchmann M, Lohse A, Pol S, Bronowicki JP, Roberts S, Arasteh K, Zoulim F, Heim M, Stern JO, Kukolj G, Nehmiz G, Haefner C, Boecher WO. Efficacy of the protease inhibitor BI 201335, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology. 2011;141:2047–2055. doi: 10.1053/j.gastro.2011.08.051. [DOI] [PubMed] [Google Scholar]


