Abstract
Aim
To determine the effect of increasing adult age on predicted metabolic drug clearance.
Method
Predicted metabolic drug clearances (CLPT) were determined using in vitro-in vivo extrapolation coupled with physiological-based pharmacokinetic modelling and simulation (IVIVE-PBPK) in Simcyp®. Simulations were conducted using CYP-selective ‘probe’ drugs with subjects in 5 year age groups (20–25 to 90–95 years). CLPT values were compared with human pharmacokinetic data stratified according to age (young = 20–40 years and elderly = 65–85 years) and gender. Age-related changes in the physiological parameters used for IVIVE of CLPT were described.
Results
Predicted metabolic drug clearances decreased with increasing adult age to approximately 65–70 years: caffeine from 1.5 to 1.0 ml min−1 kg−1 (a 33% decrease), S-warfarin from 0.100 to 0.064 ml min−1 kg−1 (36%), S-mephenytoin from 4.1 to 2.5 ml min−1 kg−1 (39%), desipramine from 10.6 to 7.3 ml min−1 kg−1 (31%) and midazolam from 5.4 to 3.9 ml min−1 kg−1 (27%). Except for S-mephenytoin, predictions were within 3.5-fold of clearances from clinical studies when stratified by age and gender. A trend towards higher CLPT was observed in females, but this was only statistically significant in larger virtual trials. Physiological parameters that determine CLPT decreased with increasing adult age: mean microsomal protein g–1 of liver, liver weight, hepatic blood flow and human serum albumin concentration.
Conclusion
Decreased metabolic clearance in the elderly was predicted by Simcyp® and was generally consistent with limited clinical data for four out of five drugs studied and the broader literature for drugs metabolized by CYP enzymes. IVIVE-PBPK may be increasingly useful in predicting metabolic drug clearance in the elderly.
Keywords: ageing, clearance, in vitro-in vivo extrapolation, modelling and simulation, physiologically-based pharmacokinetics, Simcyp®
What is Already Known about this Subject
The clearance of drugs metabolized by CYP enzymes is decreased in the elderly.
In vitro-in vivo extrapolation coupled with physiological-based pharmacokinetic (IVIVE-PBPK) modelling and simulation is used widely to predict metabolic drug clearance.
What this Study Adds
Similar to the broad body of clinical literature for drugs metabolized by CYP enzymes, IVIVE-PBPK modelling and simulation using Simcyp® predicts decreased weight-normalized metabolic clearance of 20–40% in the elderly.
This prediction is a direct consequence of age-related decreases in the physiological parameters used for IVIVE of metabolic drug clearance; mean microsomal protein g–1 of liver, liver weight, hepatic blood flow and human serum albumin concentration.
Predictions of decreased metabolic drug clearance with increasing adult age are similar for drugs with different hepatic extraction ratios and protein binding characteristics, and appear to be independent of drug-specific physiochemical parameters, the CYP enzymes responsible for metabolism and their fractional contribution to metabolic clearance.
Decreased clearance in the elderly may be similar for all drugs eliminated exclusively by CYP enzymes.
Introduction
Clearance is the most important pharmacokinetic parameter to consider in clinical practice because it determines steady-state drug concentration at a given maintenance dose. Elderly people, defined as those over 65 years of age, are the largest users of drugs and the most susceptible to adverse drug reactions (ADRs) [1]. Decreased clearance resulting in high drug concentrations is a major cause of ADRs. In particular, it is estimated that metabolic clearance by cytochrome P450 enzymes (CYP) is approximately 30–50% lower in older compared with younger people [2–5].
Studies with high hepatic extraction ratio (EH) drugs (‘flow-limited’) consistently show decreased clearance in the elderly as a result of decreased hepatic blood flow. The literature is also consistent for low EH drugs (‘capacity-limited’) with low protein binding (PB), for which decreases in clearance largely reflect reduced intrinsic clearance by the liver (CLint.liver). However, data for capacity-limited drugs with high PB are inconsistent, with studies showing decreased, unchanged, and even increased clearance in the elderly.
A possible reason for this inconsistency is variable consideration of renal function in studies that use urinary drug : metabolite ratios and urinary recovery of metabolites to measure differences in metabolic clearance i.e. these indices of enzyme activity are potentially confounded by the effect of renal function [6, 7]. Another plausible explanation is the use of total drug concentration (bound + free drug) to estimate clearance (referred to as ‘total drug clearance’) [2, 8]. If clearance is exclusively metabolic, decreases in total drug clearance from reduced CLint.liver may be masked by increases in the free fraction in blood, which may occur for some, but not all drugs, in the elderly [9, 10]. This is illustrated hypothetically in Figure 1. However, when the clearance of capacity-limited drugs with high PB is estimated from free drug concentrations, ‘free drug clearance’ is reduced in the elderly to a similar degree as total clearance (30–50%) for drugs with other EH and PB characteristics [2]. This is because the free clearance of capacity-limited drugs with high PB is exclusively dependent on CLint.liver and not confounded by changes in binding protein concentrations (note that ‘free drug clearance’, ‘whole liver intrinsic clearance’, ‘free metabolic clearance’ and ‘hepatic intrinsic clearance’ are used inter-changeably in the literature). Thus, although total drug clearance is a valid measure for examining changes in clearance for flow-limited drugs and capacity-limited drugs with low PB, it has limitations when applied to capacity-limited drugs with high PB [2].
Figure 1.
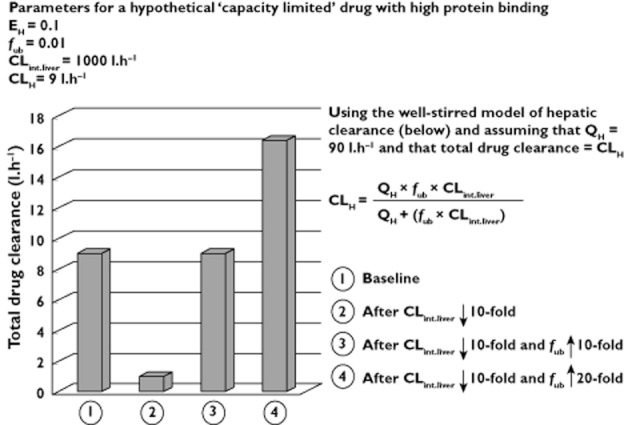
How total drug clearance can decrease, increase or remain unchanged following decreases in whole liver intrinsic clearance
In vitro-in vivo extrapolation (IVIVE) coupled with physiological-based pharmacokinetic (PBPK) modelling and simulation is used widely to predict the metabolic clearance of drugs [11]. Simcyp®, an IVIVE-PBPK programme, predicts clearance across populations and not just in individuals [12–14]. Inter-individual variability in clearance may be assessed rapidly for new drug candidates, thus aiding the appropriate selection of drugs and dosing regimens for clinical studies [15]. Given that Simcyp® is used routinely in the pharmaceutical industry, the validity of its performance in predicting clearance in different populations (e.g. poor metabolizers, paediatrics, the elderly etc.) is an important consideration when evaluating in silico data during preclinical drug development. However, an assessment of Simcyp® in predicting metabolic drug clearance with increasing adult age has not been conducted.
Thus, the primary aim of this study was to determine the effect of increasing adult age on predicted metabolic drug clearance using IVIVE-PBPK models in Simcyp®. The secondary aims were to investigate the effect of gender on predicted metabolic drug clearance, and to compare the predicted clearances with observed clearances from human pharmacokinetic studies.
Methods
Drugs used in simulations and IVIVE-PBPK of clearance
IVIVE-PBPK predicted metabolic drug clearances were determined using Simcyp® (V10). Five drugs were chosen for simulation studies based on their established use as selective in vivo ‘probes’ of the major drug metabolizing CYP enzymes [16], and because together they cover a range of EH and PB characteristics: caffeine for CYP1A2 (low EH with low PB), S-warfarin for CYP2C9 (low EH with high PB), S-mephenytoin for CYP2C19 (high EH with low PB), desipramine for CYP2D6 (intermediate EH with high PB) and midazolam for CYP3A (low EH with high PB) [17]. As the fraction excreted unchanged in urine (fe) for each of these drugs is <0.05, total drug clearance was considered to equate to metabolic clearance.
In Simcyp®, IVIVE of CLint.liver for caffeine, S-mephenytoin, S-warfarin and midazolam is based on in vitro kinetic data from recombinant CYP using the following equation:
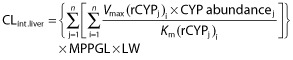 |
where Vmax (maximum reaction velocity) and Km (Michaelis-Menten constant) describe the kinetics of metabolite formation by each recombinant CYP enzyme (j) involved in a particular metabolic pathway (i), CYP abundance is the amount of active enzyme per mg of microsomal protein, MPPGL is the mean microsomal protein per g of liver tissue and LW is liver weight [18]. For desipramine, the extrapolation in Simcyp® is conducted from human liver microsomal data using the above equation but without the CYP abundance term [18]. The well-stirred model shown in Figure 1 is then used to ‘scale-up’ CLint.liver to metabolic clearance by the liver. A model that predicts human serum albumin (HSA) concentration based on age, gender, body mass index and pregnancy status is incorporated in Simcyp® to estimate the value of fub used in the well-stirred model. Since no covariates have been identified that may be modelled to determine accurately α1-acid glycoprotein concentration, variability in its concentration is randomly assigned in Simcyp® (Dr Zoe Barter, personal communication). Variability in drug clearance across populations and between population subgroups is estimated based on known variability in CYP abundance, MPPGL, LW, QH and fub [12–14].
Simulations
The ‘PK Profiles’ mode in Simcyp® was used to run virtual trials of single oral doses of each CYP-selective probe drug; 150 mg caffeine, 10 mg S-warfarin, 200 mg S-mephenytoin, 50 mg desipramine and 5 mg midazolam. North European Caucasian subjects (1:1 males to females) were used for all simulations. ‘Duration of study’ was set to record the area under the plasma concentration–time curve for each drug from the time of dose to infinity (AUC(0,∞)). To determine the effect of increasing adult age on clearance, simulations were conducted for sequential 5 year age groups (20–25, 25–30, 30–35 etc.) up to 90–95 years. Simulations were repeated for each age group using three trial sizes; 1 trial ×10 subjects (n = 10), 10 trials ×10 subjects (n = 100), and 10 trials ×100 subjects (n = 1000).
Drug clearances from clinical studies
The human clinical pharmacokinetic studies on caffeine, S-warfarin, desipramine and midazolam that were cited by the major review articles on decreased metabolic clearance in the elderly [2–4] were retrieved from the literature. An extensive literature search (Medline and GoogleScholar®) was also conducted to identify additional studies that could provide further clinical data for the five drugs studied. A total of seven studies were identified for which mean total plasma drug clearances (CLobs) could be estimated for each probe drug in the following groups: young female (20–40 years), elderly female (65–85 years), young male (20–40 years) and elderly male (65–85 years). The basic characteristics of these studies are shown in Table 1. For the purpose of the comparisons with simulations, preference was given to data published in studies specifically designed to compare the pharmacokinetics of drugs in the young and elderly (e.g. the single studies on this topic for caffeine [19], desipramine [20] and midazolam [21]). Although there are numerous studies with clinical data on S-warfarin clearance in different age groups, data for the comparisons with simulations were taken from a recent publication for which access to the original data was possible [22] (see discussion for further details). Clearance data for S-mephenytoin was only available for young males and females [23], and data for the clearance of caffeine in young females were retrieved from two studies [24, 25]. All the clearance data from clinical studies were then normalized for mean total body weight and the units standardized to ml min−1 kg−1.
Table 1.
Characteristics of the clinical studies used to estimate observed clearances in the young and elderly
| Drug | Subjects identified | Study location | Ethnicity of subjects | Reference |
|---|---|---|---|---|
| Caffeine | 8 young males and 8 elderly males | UK | Caucasian | [19] |
| 18 young females | USA | 17 Caucasian, 1 African American | [24] | |
| 3 young males and 6 young females | Germany | NR | [25] | |
| S-warfarin | 10 young males, 10 young females, 10 elderly males, 10 elderly females | New Zealand | ∼90% New Zealand Europeans | [22] |
| S-mephenytoin | 7 young males and 5 young females (23–49 years) | USA | NR | [23] |
| Desipramine | 12 young males, 7 young females, 7 elderly males, 9 elderly females | USA | NR | [20] |
| Midazolam | 10 young males, 10 young females, 9 elderly males, 11 elderly females | USA | NR | [21] |
NR, not reported.
Data analysis
Mean predicted total drug clearances (CLPT), ‘systemic plasma clearance’ in Simcyp®, were recorded from the ‘Clearance Trials SS’ tab on the Excel® output from Simcyp®. Mean predicted free drug clearances (CLPF) were estimated by calculating ‘time-averaged CLint.liver’ from the ‘CLint Profiles’ tab according to a previously published approach [15]. All predicted clearances were normalized for total body weight and the units standardized to ml min−1 kg−1.
To allow comparison between the predicted and observed clearances (CLPT and CLobs) based on age and gender, mean CLPT values were calculated from the results of simulation studies (n = 100) for each probe drug in the following groups: young female (20–40 years), elderly female (65–85 years), young male (20–40 years) and elderly male (65–85 years). The data were then normalized for mean total body weight and the units standardized to ml min−1 kg−1. The mean CLPT that was stratified in this way was then compared with mean CLobs using the mean fold error (MFE):
Differences in mean clearance between groups were analyzed using t-tests (R Program Suite 2.8). The significance level of all tests was P < 0.05.
The mean unbound drug fraction in blood (fub) for virtual individuals was calculated from the unbound drug fraction in plasma (fup) and the ratio of blood to plasma drug concentration (BP) according to the relationship fub = fup/BP. Mean MPPGL, LW and HSA concentration were calculated for each age group in simulations using caffeine (n = 100 trial design). Mean hepatic blood flow (QH) in each age group was estimated by multiplying mean cardiac output for females by 0.285 and mean cardiac output for males by 0.255.
Results
Simulations of CLPT with increasing adult age
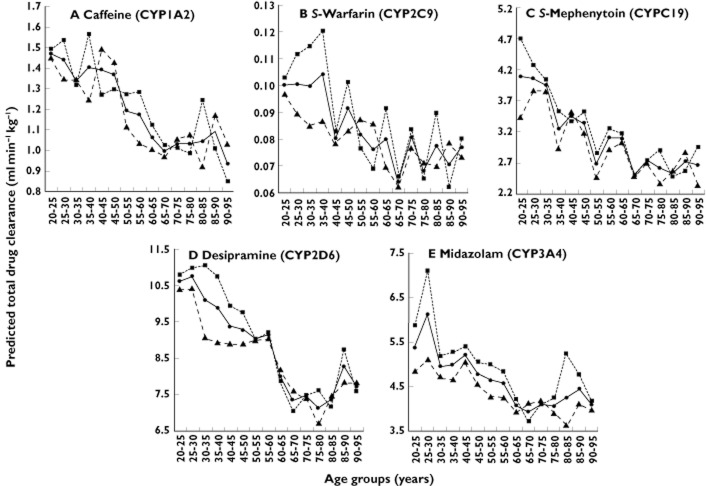
Figures 2(A) to (E) show the effect of increasing adult age on CLPT. These simulations used a trial size of n = 100. The same trends were observed using n = 10 and n = 1000 (data not shown). For all drugs, mean CLPT decreased with increasing adult age until approximately 65–70 years and was similar in magnitude for drugs with different EH and PB characteristics: caffeine from 1.5 to 1.0 ml min−1 kg−1 (a 33% decrease), S-warfarin from 0.100 to 0.064 ml min−1 kg−1 (36%), S-mephenytoin from 4.1 to 2.5 ml min−1 kg−1 (39%), desipramine from 10.6 to 7.3 ml min−1 kg−1 (31%) and midazolam from 5.4 to 3.9 ml min−1 kg−1 (27%). Women generally had slightly higher mean CLPT than men.
Figure 2.
Effect of increasing adult age on the predicted total drug clearance of CYP-selective probe drugs in Simcyp®. Data points represent mean clearances for all subjects (•), for female subjects only (▪) and for male subjects only (▴) in the n = 100 simulations
Comparison of CLPT with CLobs
Mean CLPT values stratified according to age (young vs. elderly) and gender were within 3.5-fold of estimated CLobs values for caffeine, S-warfarin, desipramine and midazolam (Table 2). The mean fold errors of these predictions are shown in brackets next to the predicted clearances in Table 2. The mean CLPT of S-mephenytoin was <10% that of the CLobs in young men and women (Table 2).
Table 2.
Comparison of predicted total drug clearance (CLPT) with observed clearance from clinical studies (CLobs) when stratified according to age (young = 20–40 years and elderly = 65–85 years) and gender. The mean fold error between observed and predicted total drug clearance is given in brackets next to the predicted value
| CYP-selective probe drug | Young female (ml min−1 kg−1) | Elderly female (ml min−1 kg−1) | Estimated reduction in clearance (female) | Young male (ml min−1 kg−1) | Elderly male (ml min−1 kg−1) | Estimated reduction in clearance (male) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predicted (CLPT) | Clinical (CLobs) | Predicted (CLPT) | Clinical (CLobs) | Predicted | Clinical | Predicted (CLPT) | Clinical (CLobs) | Predicted (CLPT) | Clinical (CLobs) | Predicted | Clinical | |
| Caffeine | 1.48 (0.87) | 1.75 [24] 1.66*[25] | 1.07 (NA) | NA | 28.7% | NA | 1.35 (0.83) | 1.42 [19] 1.88* [25] | 1.01 (0.63) | 1.60 [19] | 25.2% | –12.7 to 14.9% |
| S-warfarin | 0.112 (1.47) | 0.076† [22] | 0.076 (1.90) | 0.040† [22] | 32.2% | 48.3% | 0.090 (1.88) | 0.048† [22] | 0.070 (1.15) | 0.061† [22] | 22.2% | –27.1% |
| S-mephenytoin | 4.14 (0.09) | 59.0‡ (CL/F) [23] | 2.66 (NA) | NA | 35.8% | NA | 3.53 (0.06) | 59.0‡ (CL/F) [23] | 1.54 (NA) | NA | 56.4% | NA |
| Desipramine | 10.9 (0.49) | 22.0 (CL/F) [20] | 7.32 (0.35) | 20.9 (CL/F) [20] | 33.3% | 6.0% | 9.72 (0.34) | 28.7 (CL/F) [20] | 7.27 (0.42) | 17.3 (CL/F) [20] | 25.2% | 39.7% |
| Midazolam | 5.86 (0.63) | 9.39 [21] | 4.33 (0.58) | 7.50 [21] | 26.2% | 20.2% | 4.84 (0.62) | 7.75 [21] | 3.97 (0.90) | 4.41 [21] | 18.0% | 43.1% |
Data are mean values excluding subjects 4, 9 and 12 (>40 years).
Reported CL/F taken as CL since F is assumed to be complete [36].
Data are mean values for 12 volunteers (23–49 years) after weight normalization using 70 kg. NA, data not available.
The effects of age and gender on CLPT
The differences in mean CLPT with age (young vs. elderly) were statistically significant for all drugs even in virtual trials of 10 subjects (P < 0.05). Larger virtual trials (n = 100 and 1000) were required to demonstrate statistically significant differences in CLPT between genders (data not shown).
The effects of covariates on CLPT and CLPF
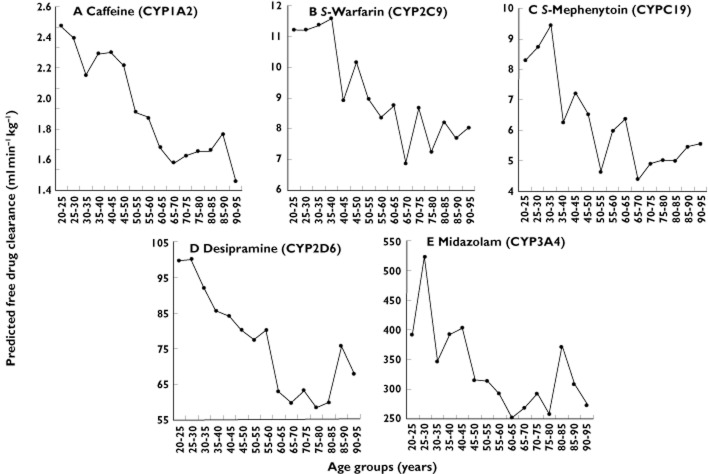
For all drugs, the decreases in mean CLPF (‘time-averaged CLint.liver’) with increasing adult age were marginally greater than the decreases in CLPT. Comparing the 20–25 and 65–70 year age groups, the decreases in CLPF were 36, 39, 47, 40 and 32% for caffeine, S-warfarin, S-mephenytoin, desipramine and midazolam, respectively (Figure 3). As stated above, corresponding decreases in CLPT were 33, 36, 39, 31 and 27% (Figure 2).
Figure 3.
Effect of increasing adult age on the predicted free drug clearance of CYP-selective probe drugs in Simcyp®. Data points represent mean clearances for all subjects in the n = 100 simulations
The physiological parameters used in Simcyp® to predict metabolic drug clearance are shown in Figure 4. Lower mean MPPGL and LW contribute to decreases in CLPF in older subjects. With respect to the determinants of CLPT, mean QH decreased from 82 to 68 l h−1 (18%). Mean HSA concentration decreased from 45.6 to 44.0 (3.5%) between 20–25 and 65–70 years (Figure 4). Consequently, the mean fub increased by 2, 7, 2 and 6% for caffeine (0.692 to 0.708), S-warfarin (0.014 to 0.015), S-mephenytoin (0.818 to 0.834) and midazolam (0.050 to 0.053), respectively. The mean fub of desipramine decreased 1% (0.218 to 0.216).
Figure 4.
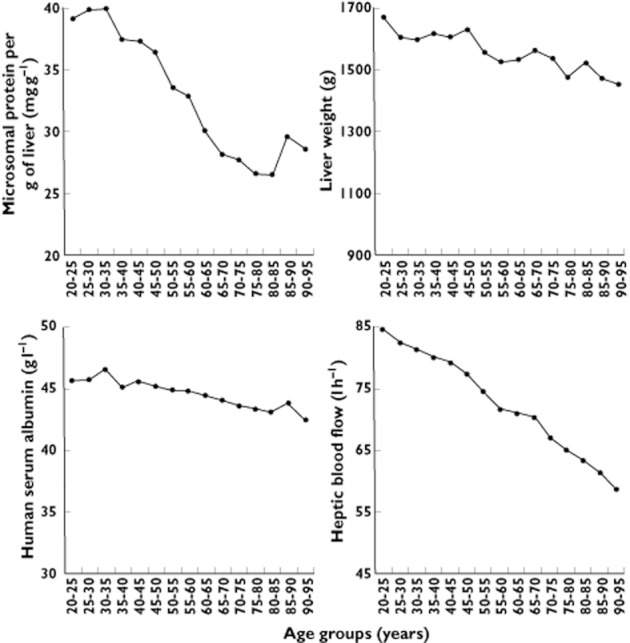
Changes with increasing adult age in the physiological parameters that determine IVIVE of metabolic drug clearance in Simcyp®. Data points represent mean values for all subjects in the n = 100 simulations with caffeine
Discussion
This is the first study to investigate systematically the effect of increasing adult age on predicted metabolic drug clearance. IVIVE-PBPK using Simcyp® predicted a weight-normalized metabolic drug clearance 20–40% lower in elderly compared with younger adults. Although simulation data should always be interpreted in the context of the limitations of IVIVE approaches, this result is generally consistent with the changes reported in clinical studies for drugs eliminated by CYP enzymes (see discussion on specific drugs below) [2–5]. Age-related changes in QH, LW and the hepatic endothelium are considered to be primarily responsible for decreased metabolic clearance with age rather than changes in CYP activity or expression [1]. This decline with age is replicated in the functions for extrapolation factors in Simcyp® – MPPGL, LW and QH. As these are physiological parameters, the clearance of any drug eliminated exclusively via CYP will be affected similarly by increasing adult age in Simcyp®. Indeed, Figures 2 and 3 show comparable percentage decreases in CLPT and CLPF for each probe drug. Clearance predictions for drugs metabolized by multiple CYP enzymes will therefore be insensitive to the fraction of drug metabolized by each enzyme (fmCYP). Thus, the current status of Simcyp® in predicting 20–40% lower metabolic clearance in elderly people is independent of drug-specific physiochemical parameters, the CYP enzymes responsible for metabolism, and their fractional contributions to clearance (e.g. specific fmCYP values).
Simulated CLPT and CLPF decreased with increasing adult age until approximately 65–70 years (Figures 2 and 3). The lack of further decreases in predicted clearances after this age appears to result from plateauing of the MPPGL decrease (Figure 4). The exact reason why MPPGL fails to decrease further with advancing age is unknown. However, this pattern may simply reflect bias in the mean MPPGL derived from liver samples in those >65 years, since they may be relatively healthy individuals who have outlived their counterparts.
IVIVE-PBPK modelling of CLPT in elderly vs. young males and females was compared with clinical data that describe the clearance of caffeine, S-warfarin, desipramine and midazolam, but such a comparison was not possible for S-mephenytoin (Table 2). In each of these cases, comparisons were limited by the small sample sizes in the clinical studies. For example, the only study to investigate the effects of age on caffeine clearance showed slightly higher total clearance in elderly men, although this difference was not statistically significant [19]. In contrast, when clearance data from separate studies in young and elderly men are compared, a modest decrease in total caffeine clearance with ageing is found (14.9%) [19, 25]. However, this approach introduces additional sources of uncertainty and provides unconvincing evidence for a negative correlation between increasing adult age and caffeine clearance. Interestingly, the effects of adult age on caffeine CLPT resemble clinical data with theophylline, another capacity-limited CYP1A2-selective probe with low PB. Clinical data show that the total clearance of theophylline is significantly decreased in the elderly (22–35%) [3] despite increases in the fraction unbound (20–25%) [9]. When theophylline is studied in Simcyp®, CLPT decreases to a similar degree as shown in clinical studies (21%, simulations not shown). Thus, the effects of increasing adult age on the clearance of drugs predominantly metabolized by CYP1A2 may require consideration on a case-by-case basis.
Clinical data on S-mephenytoin clearance are scarce. The decrease in CLPT shown here (39%) is consistent with lower recovery of 4-hydroxymephenytoin (35%) and a decreased S : R enantiomeric ratio (25%) in the urine of patients ≥50 years [26, 27], although the effect of changes in renal function may contribute to this result. In the eight studies that report changes in total S-warfarin clearance with ageing, most found a significant decrease with age, although in two of the studies this was only a trend [2, 22]. The majority of these studies were not designed to investigate specifically changes in clearance with age and data are not available to allow for a direct comparison with the Simcyp® data generated here. However, for the studies in which multiple regression analysis or population pharmacokinetic modelling was used on populations of 39–306 patients, the decline in S-warfarin clearance with age was reported to be 0.3-1% per year [22], which roughly translates to a 14–45% decline between the young and elderly age groups used in the Simcyp® simulations. For one recent study that specifically investigated the influence of adult age on warfarin clearances [22], it was possible to stratify the results as shown in Table 2. This analysis showed a significant decrease (48%) in total S-warfarin clearance in women but an increase (27%), although not statistically significant, in men. When females and males were combined, a modest decrease in total S-warfarin clearance was found between the young and the elderly (<15%, data not shown). Since the CLPT of S-warfarin was decreased by 36%, this falls within the range of reported studies, although it may slightly over-predict the effects of adult age on decreased total clearance of CYP2C9 substrates (note that sub-analysis of CYP2C9 genotype was not conducted). There are two small clinical studies showing decreased total desipramine and midazolam clearance with age [20, 21], and Simcyp® was moderately successful in reproducing the observed % decreases (Table 2).
For low EH drugs with high PB, total drug clearance does not reliably assess changes in clearance and free drug clearance is preferred [2]. As shown in Figure 1, this is because decreased CLint.liver may be masked by increased fub (from decreased binding proteins) when total drug concentration is used to estimate drug clearance. In this study, the percentage decreases in CLPT were similar (27 to 39%) for all probes which have different EH and PB characteristics. Therefore, minor increases in the predicted fub of caffeine, S-warfarin, S-mephenytoin and midazolam did not significantly affect the relationship between decreased CLPT and increasing adult age (note that the non-significant increases in predicted fub, along with the small decrease for desipramine, are consistent with clinical data [19–22]). Clinical comparisons of free drug clearance between young and elderly people are limited. Of the five drugs studied here, sufficient data are available only for caffeine and S-warfarin. The free clearance of S-warfarin decreases by 0.4% per year in the absence of statistically significant decreases in PB [22], and the simulations of CLPF are consistent with this trend (Figure 3). Here, the free clearance of caffeine was accurately predicted in the young (CLPF = 2.3 vs. clinical = 2.14 ml min−1 kg−1 [19]), but the decrease in simulated caffeine free clearance with age did not correspond with the increase observed in elderly non-smoking men (CLPF = 1.6 vs. clinical = 2.55 ml min−1 kg−1 [19]).
Gender difference in drug metabolism is a controversial topic that has been extensively reviewed in the literature [28–32]. In the simulations, women generally had higher CLPT than men (Figure 2). This results from differences between women and men in the physiological parameters that determine IVIVE of metabolic clearance, although analysis of these differences was not undertaken here (see Chetty et al. for more on this topic [33]). Most investigators concur that a small but statistically significant increase in the size adjusted clearance of CYP3A substrates, including midazolam, occurs in women [34]. Similarly for S-mephenytoin, women have significantly lower S : R enantiomeric ratios in urine (35%) as a result of greater CYP2C19 activity [27]. However, for the other CYP-selective probes studied here, increased CLPT in women is not supported by available clinical data. For example, a recent population pharmacokinetic analysis of S-warfarin in predominantly older patients showed men to have 12% higher clearance than women [35]. Our sub-analysis of the elderly in Jensen et al. [22] showed a trend of an even greater difference, although the opposite gender difference for S-warfarin is seen in the young (Table 2). Lower CYP1A2 and CYP2D6 activities in women have also been suggested [20, 25]. It must be emphasized, however, that many studies on gender difference in clearance are problematic, as many are underpowered to consider large inter-individual variability in clearance, they employ different probes with varying degrees of selectivity, they use total drug concentration to estimate clearance in the absence of PB information, and urinary drug : metabolite ratios are measured to assess differences in metabolic clearance without considering the impact of renal function [6, 7]. Therefore, there are no simple answers on whether particular CYPs differ significantly between men and women. We noted that large numbers of virtual subjects (n = 100 and 1000) were required to show statistically significant gender differences in predicted metabolic drug clearance (data not shown). Thus, in the absence of superior clinical studies, it is difficult to assess accurately the current performance of Simcyp® in predicting gender differences in the clearances of caffeine, S-mephenytoin and desipramine.
In conclusion, the primary aim of this study was to investigate the effect of increasing adult age on predicted metabolic drug clearance. IVIVE-PBPK using Simcyp® predicted 20–40% lower weight-normalized metabolic drug clearance in the elderly. This is generally consistent with limited clinical data for four out of five drugs studied here and the broader literature on drugs metabolized by CYP enzymes [2–5]. Decreased metabolic clearance with increasing adult age was similar for probe drugs of major CYP enzymes with different EH and PB characteristics. Observed decreases in predicted drug clearance with age were attributable to the physiological changes of ageing. IVIVE-PBPK modelling and simulation may be used with increasing confidence to predict metabolic drug clearance in the elderly and to inform clinical study design.
Acknowledgments
We thank Dr Zoe Barter and Professor Amin Rostami-Hodjegan for thoughtful review and comments on the manuscript.
Funding
No funding was received to support this study.
Competing Interests
Part of this work was published as a poster at the Annual Scientific Meeting of the Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT) in Perth, Australia, 4–7 December 2011.
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Hilmer SN, McLachlan AJ, Le Couteur DG. Clinical pharmacology in the geriatric patient. Fundam Clin Pharmacol. 2007;21:217–230. doi: 10.1111/j.1472-8206.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 2.Butler JA, Begg EJ. Free drug metabolic clearance in elderly people. Clin Pharmacokinet. 2008;47:297–321. doi: 10.2165/00003088-200847050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Durnas C, Loi CM, Cusack BJ. Hepatic drug metabolism and aging. Clin Pharmacokinet. 1990;19:359–389. doi: 10.2165/00003088-199019050-00002. [DOI] [PubMed] [Google Scholar]
- 4.Le Couteur DG, McLean AJ. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet. 1998;34:359–373. doi: 10.2165/00003088-199834050-00003. [DOI] [PubMed] [Google Scholar]
- 5.Schmucker DL. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging. 2001;18:837–851. doi: 10.2165/00002512-200118110-00005. [DOI] [PubMed] [Google Scholar]
- 6.Rostami-Hodjegan A, Kroemer HK, Tucker GT. In vivo indices of enzyme activity: the effect of renal impairment on the assessment of CYP2D6 activity. Pharmacogenetics. 1999;9:277–286. doi: 10.1097/00008571-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TN, Tucker GT, Rostami-Hodjegan A. Development of CYP2D6 and CYP3A4 in the first year of life. Clin Pharmacol Ther. 2008;83:670–671. doi: 10.1038/sj.clpt.6100327. [DOI] [PubMed] [Google Scholar]
- 8.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163–184. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 9.Grandison MK, Boudinot FD. Age-related changes in protein binding of drugs. Clin Pharmacokinet. 2000;38:271–290. doi: 10.2165/00003088-200038030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chin PKL, Jensen BP, Larsen HS, Begg EJ. Adult age and ex vivo protein binding of lorazepam, oxazepam and temazepam in healthy subjects. Br J Clin Pharmacol. 2011;72:985–989. doi: 10.1111/j.1365-2125.2011.04036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang SM, Lesko LJ. Authors' response. J Clin Pharmacol. 2009;49:370. [Google Scholar]
- 12.Jamei M, Dickinson GL, Rostami-Hodjegan A. A framework for assessing inter-individual variability in pharmacokinetics using virtual human populations and integrated general knowledge of physical chemistry, biology, anatomy, physiology and genetics: a tale of ‘bottom-up’ vs ‘top-down’ recognition of covariates. Drug Metab Pharmacokinet. 2009;24:53–75. doi: 10.2133/dmpk.24.53. [DOI] [PubMed] [Google Scholar]
- 13.Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp® population-based ADME simulator. Expert Opin Drug Metab Toxicol. 2009;5:1–13. doi: 10.1517/17425250802691074. [DOI] [PubMed] [Google Scholar]
- 14.Rostami-Hodjegan A, Tucker GT. Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat Rev Drug Discov. 2007;6:140–148. doi: 10.1038/nrd2173. [DOI] [PubMed] [Google Scholar]
- 15.Polasek TM, Polak S, Doogue MP, Rostami-Hodjegan A, Miners JO. Assessment of inter-individual variability in predicted phenytoin clearance. Eur J Clin Pharmacol. 2009;65:1203–1210. doi: 10.1007/s00228-009-0703-y. [DOI] [PubMed] [Google Scholar]
- 16.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan LS, Grimm SW, Kao J, King SP, Miwa G, Ni L, Kumar GN, McLeod J, Obach RS, Roberts S, Roe A, Shah A, Snikeris F, Sullivan JT, Tweedie D, Vega JM, Walsh J, Wrighton SA. The conduct of in vitro and in vivo drug-drug interaction studies: a pharmaceutical research and manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–832. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 17.Obach RS, Lombardo F, Waters NJ. Trend analysis of a database of intravenous pharmacokinetic parameters in humans for 670 drug compounds. Drug Metab Dispos. 2008;36:1385–1405. doi: 10.1124/dmd.108.020479. [DOI] [PubMed] [Google Scholar]
- 18.Howgate EM, Rowland-Yeo K, Proctor NJ, Tucker GT, Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: impact of inter-individual variability. Xenobiotica. 2006;36:473–497. doi: 10.1080/00498250600683197. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard J, Sawers SJ. Comparative pharmacokinetics of caffeine in young and elderly men. J Pharmacokinet Biopharm. 1983;11:109–126. doi: 10.1007/BF01061844. [DOI] [PubMed] [Google Scholar]
- 20.Abernethy DR, Greenblatt DJ, Shader RI. Imipramine and desipramine disposition in the elderly. J Pharmacol Exp Ther. 1985;232:183–188. [PubMed] [Google Scholar]
- 21.Greenblatt DJ, Abernethy DR, Locniskar A, Harmatz JS, Limjuco RA, Shader RI. Effect of age, gender and obesity on midazolam kinetics. Anesthesiology. 1984;61:27–35. [PubMed] [Google Scholar]
- 22.Jensen BP, Chin PKL, Roberts RL, Begg EJ. Influence of adult age on the total and free clearance and protein binding of (R)- and (S)-warfarin. Br J Clin Pharmacol. 2012 doi: 10.1111/j.1365-2125.2012.04259.x. doi: 10.1111/j.1365-2125.2012.04259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao C, Kunze KL, Trager WF, Kharasch ED, Levy RH. Comparison of in vitro and in vivo inhibition potencies of fluvoxamine toward CYP2C19. Drug Metab Dispos. 2003;31:565–571. doi: 10.1124/dmd.31.5.565. [DOI] [PubMed] [Google Scholar]
- 24.Abernethy DR, Todd EL. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing contraceptives. Eur J Clin Pharmacol. 1985;28:425–428. doi: 10.1007/BF00544361. [DOI] [PubMed] [Google Scholar]
- 25.Fuhr U, Klittich K, Horst Staib A. Inhibitory effect of grapefruit juice and its bitter principal, naringenin, on CYP1A2 dependent metabolism of caffeine in man. Br J Clin Pharmacol. 1993;35:431–436. doi: 10.1111/j.1365-2125.1993.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bebia Z, Buch SC, Wilson JW, Frye RF, Romkes M, Cecchetti A, Chaves-Gnecco D, Branch RA. Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther. 2004;76:618–627. doi: 10.1016/j.clpt.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 27.May DG, Porter J, Wilkinson GR, Branch RA. Frequency distribution N-hydroxylase, a putative probe for CYP4503A4 activity, in a white population. Clin Pharmacol Ther. 1994;55:492–500. doi: 10.1038/clpt.1994.62. [DOI] [PubMed] [Google Scholar]
- 28.Cotreau MM, von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas J, Espie P, Molimard M. Gender and interindividual variability in pharmacokinetics. Drug Metab Rev. 2009;41:408–421. doi: 10.1080/10837450902891485. [DOI] [PubMed] [Google Scholar]
- 31.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24:339–346. doi: 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 33.Chetty M, Mattison D, Rostami-Hodjegan A. Sex differences in the clearance of CYP3A4 substrates: exploring possible reasons for the substrate dependency and lack of consensus. Curr Drug Metab. 2012;13:778–786. doi: 10.2174/138920012800840464. [DOI] [PubMed] [Google Scholar]
- 34.Polasek TM, Miners JO. Response to ‘Zolpidem pharmacokinetics and pharmacodynamics in metabolic interactions involving CYP3A: sex as differentiating factor. Eur J Clin Pharmacol. 2010;66:957–958. doi: 10.1007/s00228-010-0854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane S, Al-Zubiedi S, Hatch E, Matthews I, Jorgensen AL, Deloukas P, Daly AK, Park BK, Aarons L, Ogungbenro K, Kamali F, Hughes D, Pirmohamed M. The population pharmacokinetics of R- and S-warfarin: effect of genetic and clinical factors. Br J Clin Pharmacol. 2012;73:66–76. doi: 10.1111/j.1365-2125.2011.04051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breckenridge A, Orme M. Kinetics of warfarin absorption in man. Clin Pharmacol Ther. 1973;14:955–961. doi: 10.1002/cpt1973146955. [DOI] [PubMed] [Google Scholar]