Abstract
Aim
To assess reasons that prevent Alzheimer's disease (AD) patients from being included in clinical trials.
Methods
In 2009, we reviewed the Lille Memory Clinic's case database to identify patients suitable for inclusion in four AD clinical trials. An initial selection was made on the basis of four criteria: (i) a diagnosis of AD (with or without white matter lesions [WML]), (ii) age, (iii) mini mental state examination (MMSE) score and (iv) symptomatic treatment of AD (cholinesterase inhibitors/memantine). Next, data on patients fulfilling these criteria were reviewed against all the inclusion/exclusion criteria for four clinical trials performed in 2009 at the Memory Clinic. Reasons for non-inclusion were analyzed.
Results
Two hundred and five patients were selected according to the four initial criteria. Reasons for subsequently not including some of patients in clinical trials were abnormalities on MRI (56.9%, 88.9% of which were WML), unauthorized medication (37.3%), the lack of a study partner/informant (37.1%), the presence of a non-authorized disease (24.4%), contraindication to MRI (9%), a change in diagnosis over time (3.9%), visual/auditory impairments (2.9%), alcohol abuse (2%) and an insufficient educational level (1%).
Conclusion
A high proportion of AD patients presented with vascular abnormalities on MRI. This was not unexpected, since the patients were selected from the database and, as shown in epidemiologic studies, cerebrovascular diseases are frequently associated with AD. The presence of a study partner is essential for enabling a patient to participate in clinical trials because of the need to record reliably primary and secondary outcomes.
Keywords: Alzheimer's disease, clinical trial
What is Already Known about This Subject
Alzheimer's disease (AD) is the most common progressive neurodegenerative disorder and a leading cause of dementia in the elderly. There is an urgent need to develop new AD therapies, because cholinesterase inhibitors and memantine offer modest symptomatic benefit. However, AD clinical trials face a lot of challenges in the recruitment of subjects.
What this Study Adds
No study has evaluated the main medical and non-medical factors that explain the non-inclusion of AD patients in clinical trials. Many barriers to participation in AD clinical trial are not modifiable, particularly the lack of a study partner. However, when designing large studies in AD, the main factors capable of increasing the number of inclusions are: (i) the presence of white matter lesions on MRI and (ii) add-on symptomatic treatment with a cholinesterase inhibitor and/or memantine.
Introduction
Over 24 million people worldwide suffer from dementia [1]. Alzheimer's disease (AD) is the leading cause of dementia and accounts for two thirds of all cases of this condition [1]. Indeed, the prevalence of AD increases with age and reaches between 24% and 33% in populations over the age of 85 years [1]. Cholinesterase inhibitors (ChEI) and memantine offer modest symptomatic benefit but, as yet, no clear evidence of a disease-modifying effect. Hence, there is an urgent need to develop new AD therapies. Randomized, double-blind, placebo-controlled clinical trials (RCTs) are essential for establishing the efficacy of medications intended to improve cognition and function in AD patients. However, RCTs in AD are challenging because of the nature of the illness and that of the patient population. Twelve key difficulties in recruiting subjects for RCTs in AD are underlined [2]: potential risks of intervention, uncertainty of benefits of intervention, protocol restrictions (including inclusion and exclusion criteria), comorbid conditions, subjects′ background, the need for caregiver participation, transportation problems, concern over study drug adverse effects, lengthy duration of trial, cynicism among minority groups, media influence and lack of awareness of clinical trials.
‘Comorbid conditions’ is a particularly important exclusion factor because AD patients are mostly over the age of 65 years. This implies a busier medical history, more associated diseases, vascular risk factors, medication use, etc. Moreover, participation in RCTs in AD requires commitment from both the patient and a well-informed study partner who often lives with the patient. This person must accompany the patient to the different study visits and may be required to fill out questionnaires or ensure that the study drug has been taken, which can constitute a burden on the caregiver. Hence, many factors act as barriers to patient inclusion in RCTs in AD.
Although the general public is increasingly aware of AD and its consequences, few patients may be qualified to participate in RCTs. Most studies of RCT participation have analyzed the socioeconomic and personal reasons why patients participate in RCTs [3, 4]. Some researchers have suggested new RCT designs and assessed the latter's efficacy in increasing participation by AD patients and their caregivers. The most attractive design (permitting participation of 60% of patients and caregivers) combined home visits, a low risk protocol and a 2:1 active: placebo randomization plan [5].
However, to the best of our knowledge, no-one has evaluated the main medical and non-medical factors that explain the non-inclusion of AD patients in RCTs.
Our university hospital's Memory Clinic has been performing RCTs in the field of AD for over 30 years, with an average of nine RCTs on-going at any one time and 19 new patients included per year. However, we had never previously assessed the trial inclusion rate for AD patients consulting at the Lille Memory Clinic.
Hence, the study's objective was to establish why a proportion of our AD patients were not participating in RCTs during 2009.
Methods
Patients
Patients were selected from the case file database at Lille University Hospital's Memory Clinic in northern France. Since 1991, all patients (regardless of their initial diagnosis) referred to either of the Clinic's two sites (Lille and Bailleul) have been collated in a computer database (4D software, 4D SAS, Clichy-la-Garenne, France). The database has been declared to the Commission Nationale de l'Informatique et des Libertés (CNIL, the French National Data Protection Commission). This standardized database contains demographic and medical data, including age, clinical diagnoses (according to the International Classification of Disease), year of disease onset, mini mental state examination (MMSE) score, changes over time, medications (antithrombotics, antihypertensive drugs, antidiabetic drugs, ChEIs, memantine, medication for mood or anxiety disorders, antipsychotics, epilepsy medicines, etc.), associated disorders (epilepsy, alcohol consumption, cardiovascular or psychiatric diseases, etc.), family history and stated willingness to participate in RCTs or not.
Clinical trials
Four on-going RCTs in AD at Lille Memory Clinic were selected for this study. All were actively recruiting during 2009 but had not necessarily started recruiting in that year. They were chosen from among the seven RCTs in AD being run in 2009. Two were not chosen because they were trial extensions and one had been terminated prematurely.
For reasons of confidentiality, the four RCTs studied here will be referred to as trials A, B, C and D. All were multicentre, double-blind, placebo-controlled, parallel-group, randomized studies evaluating the efficacy, safety and tolerability of various medications in AD. RCTs A and D were phase III studies and RCTs B and C were phase II studies. Our Memory Clinic's inclusion targets were 10 patients for RCT A (over a 24 month period), 10 for RCT B (over a 30 month period), 4 to 5 for RCT C (over a 14 month period) and 10 for RCT D (over a 12 month period). Hence, a total of 35 patients should have been included over a 30 month period.
All four trials had very similar main inclusion criteria (a diagnosis of probable AD according to the NINCDS-ADRDA criteria [6], age range, MMSE score, a modified Hachinski ischaemic scale (mHIS) score ≤ 4 [7] and the presence of study partner), as reported in Table 1. The study start-ups were supposed to be staggered but our centre was activated late in two cases. RCTs A and B did not prohibit the use of ChEIs or memantine. RCT C prohibited these medications and RCT D authorized donepezil only.
Table 1.
The main inclusion criteria for the four clinical trials
| Clinical trial A | Clinical trial B | Clinical trial C | Clinical trial D | |
|---|---|---|---|---|
| Diagnosis | Probable AD | Probable AD | Probable AD | Probable AD |
| Age range (years) | 50–80 | 50–85 | 55–85 | ≥50 |
| MMSE score | 16–26 | 16–26 | 15–26 | 12–24 |
| mHIS score | ≤4 | ≤4 | ≤4 | ≤4 |
| Study partner | Yes | Yes | Yes | Yes |
| Cholinesterase inhibitor or memantine authorized | Yes | Yes | No | Only donepezil |
MMSE, mini mental state examination; mHIS, modified Hachinski ischaemic scale.
Selection of patients for clinical trials
The database was reviewed in order to select patients suitable for inclusion in RCTs in AD. In 2009, 2339 patients with a diagnosis of neurodegenerative or vascular dementia or mild cognitive impairment were in the active case file. Of these, we assessed the 1833 patients who had consulted at the Lille site (at which RCTs are performed) rather than the Bailleul site.
By applying the 4D database management software, an initial selection from among these 1833 patients was made on the basis of four criteria: (i) a diagnosis of probable AD according to the NINCDS-ADRDA criteria (with or without white matter lesions [WML]), (ii) age, (iii) MMSE score and (iv) current symptomatic treatment of AD (ChEIs and/or memantine). A diagnosis of AD and concomitant cerebrovascular disease (i.e. ‘mixed dementia’) was a non-inclusion criterion.
Patients in this initial selection could then be selected for one or more RCTs. Their data were reviewed (by ARS and LB) with respect to all the inclusion and exclusion criteria for RCTs A to D. By definition, inclusion criteria are used to define the population (in terms of age, gender, pathology, severity…) and exclusion or non-inclusion criteria are used to eliminate patients with a particular risk (including associated disorder, associated treatment …) or for regulatory reason. Both the presence of inclusion criteria and absence of exclusion criteria are necessary to include patients.
Medical data were obtained from the patients′ medical records and included history, past and current treatments, life habits, clinical diagnosis, magnetic resonance imaging (MRI) results, cerebrospinal fluid assay results, etc. In this second selection, only medical data reported before the end of 2009 were considered. The various reasons for non-inclusion (including inclusion and exclusion criteria) in a RCT were reported. These included: (1) prior or current neurological or central nervous system disorders, (2) psychiatric disorder (including a history of schizophrenia, schizoaffective disorder, bipolar disorder, major depression or any other psychiatric condition which could have significantly interfered with the subject's cooperative participation), (3) abnormalities on MRI (WML, territorial or lacunar infarcts, cerebral lesions, etc.), (4) contraindication to MRI, (5) the presence of associated disorders (cardiovascular disease, infections, immune, metabolic or endocrine disorders, a history of cancer, etc.), (6) use of prohibited medication, (7) the lack of a study partner, (8) an insufficient educational level, (9) insufficient visual or auditory acuity for performing cognitive tests, (10) residence in an institution, (11) alcohol abuse and (12) biological abnormality (for creatinine clearance, liver enzymes, folic acid or B12 concentrations, etc.). A change in diagnosis (if any) was also reported. When noted in the medical file, willingness to participate in RCTs was recorded. The number of patients finally included in each trial A to D was reported.
Statistical analysis
Qualitative data were expressed as percentages. Percentages were calculated from patients selected by the first selection. The exact 95% confidence interval was computed using the Clopper-Pearson method. All statistical analyses were performed with SAS software (version 9.2, SAS Institute Inc., Cary, NC, USA).
Results
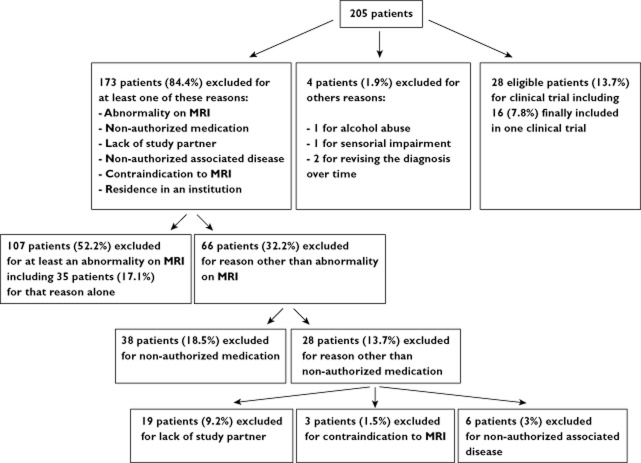
Of the 1833 patients in the database, 205 met the four main criteria for the four RCTs (Figure 1). The mean ± SD age was 76.3 ± 8.7 (range 50–91) years. The number of patients from the first selection then selected for each RCT is summarized in Figure 1. Patients were potentially eligible for several RCTs. Permission to take ChEIs or memantine in the RCT increased the number of patients fulfilling the four main criteria (Figure 1).
Figure 1.
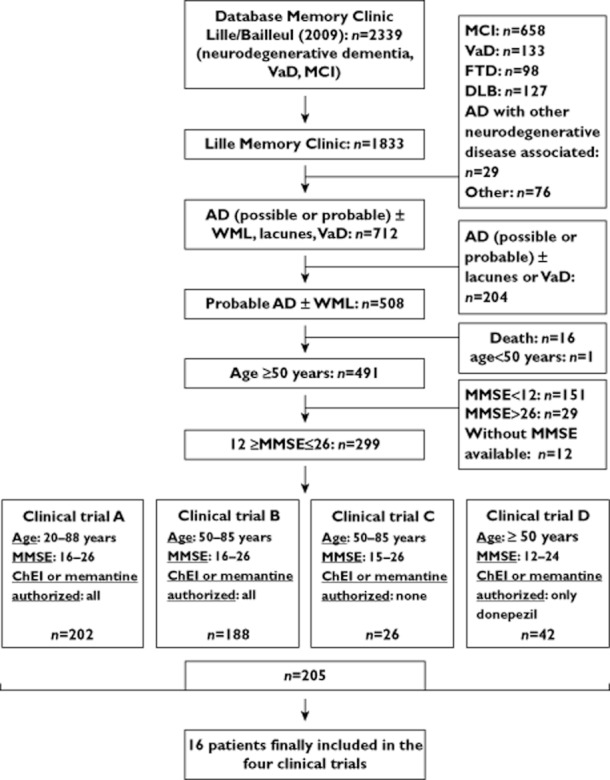
Patient selection flow chart. Dg. diagnosis; AD, Alzheimer's disease; ChEI, cholinesterase inhibitor; FTD, frontotemporal dementia; DLB, dementia with Lewy body; MCI, mild cognitive impairment; MMSE, mini mental state examination; VaD, vascular dementia; WML, white matter lesions
The reasons that prevented the inclusion of AD patients in RCTs are detailed in Table 2 and Figure 2. Frequencies were calculated relative to the number of patients in the initial selection (n = 205).
Table 2.
Reasons for non-inclusion of patients in clinical trials
| Frequency (%) | 95% CI | |
|---|---|---|
| Abnormality on MRI | 56.9 | 49.51, 64.10 |
| Non-authorized medication | 37.3 | 30.60, 44.28 |
| Lack of a study partner | 37.1 | 30.45, 4.08 |
| Non-authorized associated disease | 24.4 | 18.68, 30.86 |
| Contraindication to MRI | 9.0 | 5.42, 13.85 |
| Residence in an institution | 8.3 | 4.91, 12.95 |
| Change of diagnosis over time | 3.9 | 1.70, 7.54 |
| Visual/auditory impairments | 2.9 | 1.08, 6.26 |
| Alcohol abuse | 2.0 | 0.53, 4.92 |
| Low educational level | 1.0 | 0.012, 3.48 |
| Abnormal biochemistry results (creatinine clearance, liver enzymes, folic acid or B12 concentrations, etc.) | 0.5 | 0.001, 2.69 |
Patients may have presented more than one reason for non-inclusion. CI: confidence interval.
Figure 2.
Detail of the different proportions of each of the main factors of non-inclusion in clinical trials
The various non-authorized medications are reported in Table 3.The various medical disorders excluding patients from RCTs are reported in Table 4. We further analyzed the types of MRI abnormality reported for RCT A (Table 5).
Table 3.
Non-authorized medications
| Frequency (%) | 95% CI | |
|---|---|---|
| Ginkgo biloba or any agent intended to improve cognition* | 16.7 | 11.83, 22.50 |
| Medication for reducing cardiovascular or cerebrovascular events (e.g. warfarin) | 11.2 | 7.25, 16.36 |
| Systemic immunosuppression | 5.4 | 2.71, 9.40 |
| Anticonvulsant | 3.9 | 1.70, 7.54 |
| Medication for mood disorder | 1.5 | 0.30, 4.22 |
| Neuroleptic | 1.0 | 0.12, 3.48 |
| Medication for parkinsonian symptoms | 0.5 | 0.01, 2.69 |
Patients may have been taking several non-authorized medications. CI confidence interval.
No patient was excluded due to only Ginkgo biloba (or any agent intended to improve cognition).
Table 4.
Diseases excluding trial participation
| Frequency % | 95% CI | |
|---|---|---|
| Cardiovascular disorder | 6.8 | 3.78, 11.19 |
| History of cancer | 5.9 | 3.06, 10.00 |
| Psychiatric disorder | 5.4 | 2.71, 9.40 |
| Neurological disorder | 4.4 | 2.03, 8.17 |
| Chronic inflammatory diseases | 3.4 | 1.38, 6.91 |
| Severe lung disorder | 2.4 | 0.80, 5.60 |
Patients could present with several disorders. CI confidence interval.
Table 5.
Type of abnormality on MRI
| Frequency % | |
|---|---|
| White matter lesion | 88.9 |
| Lacunar infarct | 11.1 |
| Territorial infarct | 6.5 |
| Meningioma | 4.6 |
| Other (microbleeds, cyst, cavernoma, etc.) | 7.4 |
Patients could present with several abnormalities on MRI.
In terms of their willingness to participate in RCTs, 58 of the 205 patients (28.3%) wished to participate and 10 (4.9%) refused. Hence, of the patients who expressed an opinion, 85.3% were willing to participate and 14.7% declined. Data were missing for the other 137 patients.
In terms of the patients who actually participated in RCTs A to D in 2009, seven (3.5%) of the 202 patients fulfilling the four main criteria for trial A were finally included. The equivalent values were four (2%) of the 188 patients fulfilling the four main criteria for trial B, four (15.4%) of the 26 patients fulfilling the four main criteria for the RCT C and one (2.4%) of the 42 patients fulfilling the four main criteria for the RCT D. The mean age of patients included in RCTs was 65.5 ± 7.6 (range 56–78) years. Two patients had already been included in other RCTs.
Discussion
To the best of our knowledge, this is the first study to have reviewed systematically the reasons for RCT non-inclusion of AD patients attending a research active university hospital memory clinic.
The main reasons for not including patients in RCTs (other than refusal which was not frequent) were: (i) abnormalities on MRI (56.9%), most of which were vascular abnormalities and WMLs, (ii) non-authorized medications (37.3%) and (iii) the lack of a reliable study partner (37.1%). Furthermore, the prohibition of add-on therapy with symptomatic drugs (i.e. ChEIs and memantine) reduced the number of patients eligible for RCTs.
A high proportion of AD patients presented with vascular abnormalities on MRI; the most common abnormalities were WMLs, followed by lacunar infarcts and territorial infarcts. This result was not unexpected, given our selection of patients from the database. We chose to select patients with a diagnosis of AD, regardless of the presence or absence of WMLs. Firstly, this choice is justified by the fact that the WMLs seen on MRI were sometimes very mild (and thus would not constitute a non-inclusion criterion for all RCTs) but were nevertheless noted in the database. However, since a diagnosis of mixed dementia was prohibited, many patients (n = 204) were excluded for this reason. Secondly, many observational epidemiological studies have highlighted the frequent association between cerebrovascular pathology and AD. Vascular risk factors are also reportedly risk factors for AD [8–10]. Most RCTs in AD reduce the likelihood of inclusion of patients with significant cerebrovascular disease by using the mHIS score [10]. They sometimes use the Fazekas or Wahlund scales to score WMLs, although this was not the case for the trials in our study [11, 12]. These precautions are supposed to limit the risk of observing a drug's confounding effect on vascular lesions rather than on AD itself, and vascular lesions were associated with adverse effects in some AD immunotherapy trials [13]. Disqualifying many potential subjects from participation in RCTs could threaten the validity and extrapolation of trial results, due to the frequent association between AD and vascular lesions. Nevertheless, the guidance from EMA recommends starting development in ‘pure’ disease forms (e.g. AD without vascular changes) and thereafter extending the scope of development to the ‘mixed’ forms. However, it recognizes that the combination between neurodegenerative disease and vascular changes is very frequent [14]. The guidance from Food and Drug Administration (FDA) for phase III studies does not mention cerebrovascular lesions. It recommends that ‘the spectrum of other conditions processes or disease (e.g. inflammation, neoplasm, infection, trauma) that may confound interpretation of the results for the disease or condition of interest also should be appropriately represented, [15]. It should be also noted that our study methodology may have overestimated the prevalence of patients with significant WMLs since we adopted the interpretation available in the medical records rather than re-analyzing the MRI data. Standardized interpretation using the Fazekas or Wahlund scales would be more appropriate for detecting patients with significant WMLs.
The second most frequent reason for not including AD patients in RCTs was the use of non-authorized medication. The main prohibited drugs were Ginkgo biloba or any other agent or supplement intended to improve cognition or reduce cognitive decline (more than 16% of the patients used these treatments). In fact, these medications are not a true contraindication to RCT participation because they can be withdrawn before patients start the trial. However, our study methodology (reporting all medications not authorized by the RCT) highlighted these medications and the surprisingly high proportion of patients who are taking them. Treatments for cardiovascular or cerebrovascular events excluded 11.2% of our patients. This result is consistent with (i) the exclusion of patients with vascular abnormalities on MRI and (ii) the presence of associated disorders as a non-inclusion criterion in 24.4% of cases (among which cardiovascular disorders were the most frequent (6.8%)). Comorbid medical conditions are inevitable in study participants. This highlights the difficulty in extrapolating RCTs results to a larger patient population.
Another important point relates to the prohibition of symptomatic treatments for AD, a criterion which reduces the number of eligible patients for RCTs. For example, RCTs B and C were very similar in terms of the four main criteria used for the initial selection. Hence, participation was possible for only 26 AD patients (i.e. those not taking any symptomatic AD treatments) in trial C vs. 188 for RCT B (in which ChEIs or memantine were authorized), i.e. 86.7% fewer patients. This restriction decreases participation when the RCT authorized donepezil alone: 202 and 188 patients were eligible for trials A and B, respectively (which each authorized any ChEI or memantine), whereas only 42 were eligible for trial D (in which only donepezil was authorized). Furthermore, restriction to a single ChEI is not really justified because the various compounds′ mechanism of action and degree of efficacy are very similar. Moreover, some studies have shown that clinical investigators may be reluctant to suggest a trial with a ‘no treatment’ arm to their patients [16].
The presence of a study partner is an essential factor in patient participation in trials because of the need to record primary and secondary outcomes. In fact, the FDA guidelines recommend an overall assessment by a clinician as one of the primary outcome measures [17]. The type of overall assessment is not specified but will require two sources: the patient and the study partner (informant). For example, the Clinical Dementia Rating (CDR) scale and the Alzheimer's Disease Cooperative Study Clinical Global Impression of Change (CGIC) both require relevant information from both informants and patients [18, 19]. Many scales assessing activities of daily living (such as the Instrumental Activities of Daily Living Scale, the Dependence Scale and the Neuropsychiatric Inventory) are scored through a caregiver in frequent contact with the patient [4, 10, 20]. Obtaining comprehensive information about the patient's level of functioning requires a high degree of cooperation from the study partner. Furthermore, the study partner is often responsible for treatment compliance and safety monitoring. Many RCTs exclude patients living in nursing homes (which was the case for 8.3% of the patients in our study), some trials exclude patients who live alone and others require the study partner to see the patients several times a week. Many factors can limit the presence of study partner in this aged population: death or illness of the spouse, family members living far away or working and the spouse's willingness to participate in a RCT [5]. In view of the FDA guidelines and the clinical consequences of AD on daily life, a study partner is an essential stakeholder in RCTs. RCTs in prodromal AD and those testing disease modifying therapies with supportive biomarker data also need study partners and would thus exclude a proportion of patients [10].
We also found that AD patients who were included in RCTs are younger than average (with a mean age of 65.5 years, compared with around 80 years for those in the active case file). This is probably because they have fewer comorbid conditions and fewer associated treatments and are more likely to have a study partner, when compared with older AD patients.
In contrast, individual refusals to participate in a RCT do not appear to be a real barrier for overall trial accrual. We found that 58 (28.3%) of the patients with data on this matter agreed to participate and 10 (4.9%) declined. This result must be considered with caution because of the high proportion of missing data. Of the 58 patients who agreed to participate, 16 were included in one of trials A to D and two were included in another RCT. These data are probably biased towards high participation because the Lille Memory Clinic is a tertiary centre. Many of the patients referred here are aware of its involvement in clinical research.
Finally, many barriers to participation in RCT in AD are not modifiable and include the absence of a study partner, contraindication to MRI, visual or auditory impairments, use of some types of medication, low education level and comorbid conditions. However, when designing studies some factors capable of increasing the number of those included could be taken into account depending of the stage of development. Indeed, at the early stage of development of a new drug, patient selection must be restricted (‘pure disease’, no concomitant associated disorder or treatment which could interfere with the new drug) as it is recommended by the EMA guidance [14]. The permission to take symptomatic treatment of AD is questionable [21]. Excluding them could be acceptable in short studies. Furthermore, promoters should provide a cerebrovascular lesions scale like Fazekas or Wahlund scales (which is already the case in some RCTs) to prevent inappropriate screening. Then, prohibition of medications or non-authorized associated disorders must be well justified (potential interaction with tested drug for example) and it should not be done by excess. Furthermore, patients under 55 years should not to be excluded from RCTs. These propositions are summarized in Table 6.
Table 6.
Factors that may improve patients’ selection in clinical trials
| Early development of a new drug (proof of concept), phase I, phase IIa clinical trial | No age limitation but use of biomarkers to enrich the population (Probable AD dementia with evidence of the AD pathophysiological process [22]) |
| Pure disease | |
| Short duration | |
| Large clinical trial: phase IIb or phase III clinical trial | No age limitation |
| Symptomatic treatment of AD (all ChEI or memantine) authorized with stable dose | |
| Cerebrovascular lesions scale for MRI interpretation | |
| Restricted prohibited medication | |
| Restricted prohibited associated disorder |
However, besides improving the accessibility of patients to RCTs, e.g. by simplifying the selection criteria, many questions arise concerning the limitations of the RCTs results when mapped on a standard population (see Table 7 for some examples).
Table 7.
Examples of the limitations of trials results when mapped to a standard clinical population
| Clinical trial population | ‘Standard’ clinical population |
|---|---|
| Mandatory MRI | - Exclusion of all patients with contraindication to perform MRI, e.g. claustrophobia |
| - Availability for the patients? (based on location of living – country, region, institution-) | |
| - Frequency of MRI? | |
| - Cost? | |
| - Long scanning times | |
| Exclusion of vascular lesion on imaging (microbleeds, white matter lesion, lacunar infarct ….) | - Need of MRI (with all limits previously named) |
| - Standardization of the results interpretation (using vascular scale, training rater…) | |
| - Exclusion of numerous patients (particularly, old patients, patients with amyloïd angiopathy…) | |
| Need of genetic status (APOE) | - Acceptability for patients? |
| Exclusion of anticoagulant or antithrombotic therapy (like clopidrogrel or higher dose of aspirin) | - Exclusion of patients with cardiovascular pathology (infarct, atrial fibrillation…) for example |
| Mandatory inclusion of biomarker like CSF biomarkers or amyloïd PET imaging | - Availability? (differences based on location of living – country, region, institution-) |
| - Costs? | |
| - Contraindication for lumbar puncture? | |
| - Acceptance (for lumbar puncture)? | |
| - Standardization of the results interpretation (use of and experience with different image analysis) | |
| Monitoring of the patient | - Frequency? |
| - Acceptance? | |
| - Cost? |
In conclusion, when designing large studies, the main factors capable of increasing the number of inclusions are: (i) the presence of WMLs and (ii) add-on symptomatic treatment with a ChEI and/or memantine.
Acknowledgments
Adeline Rollin-Sillaire is funded by the French National Alzheimer Plan. We thank Hervé Maisonneuve and Joël Menard for their help in revising the manuscript and Nathalie Jourdan for managing the database.
Competing Interests
ARS, LB, SB, PC, VD, MAM and FP have participated in many pharmaceutical trials and FP was occasionally a member of the scientific advisory board for Eisai, Bayer, Janssen, Lilly, Sanofi and Servier.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knebl JA, Patki D. Recruitment of subjects into clinical trials for Alzheimer disease. J Am Osteopath Assoc. 2010;110(Suppl. 8):S43–49. [PubMed] [Google Scholar]
- 3.Connell CM, Scott Roberts J, McLaughlin SJ. Public opinion about Alzheimer disease among blacks, hispanic and whites: results from a national Survey. Alzheimer Dis Assoc Disord. 2007;21:232–240. doi: 10.1097/WAD.0b013e3181461740. [DOI] [PubMed] [Google Scholar]
- 4.Schneider LS. Drug developpment, clinical trials, cultural heterogeneity in Alzheimer disease: the need for pro-active recruitment. Alzheimer Dis Assoc Disord. 2005;19:279–283. doi: 10.1097/01.wad.0000190808.97878.b8. [DOI] [PubMed] [Google Scholar]
- 5.Karlawish J, Cary MS, Rubright J, Tenhave T. How redesigning AD clinical trials might increase study partners′ willingness to participate. Neurology. 2008;71:1883–1888. doi: 10.1212/01.wnl.0000336652.05779.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 7.HaChEInski VC, Lassen NA, Marshall J. Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet. 1974;27:207–210. doi: 10.1016/s0140-6736(74)91496-2. [DOI] [PubMed] [Google Scholar]
- 8.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology. 2009;73:674–680. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
- 9.Bruandet A, Richard F, Bombois S, Maurage CA, Deramecourt V, Lebert F, Amouyel P, Pasquier F. Alzheimer disease with cerebrovascular disease and vascular dementia: clinical features and course compared with Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:133–139. doi: 10.1136/jnnp.2007.137851. [DOI] [PubMed] [Google Scholar]
- 10.Knopman DS. Clinical trial design issues in mild to moderate Alzheimer disease. Cogn Behav Neurol. 2008;21:197–201. doi: 10.1097/WNN.0b013e318190cf75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 12.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P. European Task Force on Age-Related White Matter Changes. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 13.Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131(Pt 12):3299–3310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 14.Guideline on medicinal products for the treatment of Alzheimer's disease and other dementias. 2008. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003562.pdf (lase accessed 5 September 2012)
- 15.Guidance for Industry. Developing Medical Imaging Drugs and Biologics. 1998. Available at http://www.fda.gov/ohrms/dockets/98fr/980785gd.pdf (lase accessed 5 September 2012) [PubMed]
- 16.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52:1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 17.Leber P. Guidelines for the Clinical Evaluation of Antidementia Drugs, First Draft. Rockville, MD: US Food and Drug Administration; 1990. [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(Suppl. 2):S22–32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 20.Mohs RC, Kawas C, Carrillo MC. Optimal design of clinical trials for drugs designed to slow the course of Alzheimer's disease. Alzheimers Dement. 2006;2:131–139. doi: 10.1016/j.jalz.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Vellas B, Hampel H, Rougé-Bugat ME, Grundman M, Andrieu S, Abu-Shakra S, Bateman R, Berman R, Black R, Carrillo M, Donohue M, Mintun M, Morris J, Petersen R, Thomas RG, Suhy J, Schneider L, Seely L, Tariot P, Touchon J, Weiner M, Sampaio C, Aisen P Task Force Participants. Alzheimer's disease therapeutic trials: EU/US Task Force report on recruitment, retention, and methodology. J Nutr Health Aging. 2012;16:339–345. doi: 10.1007/s12603-012-0044-x. [DOI] [PubMed] [Google Scholar]
- 22.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]