Abstract
Objectives
For the purpose of blocking transmission of Plasmodium falciparum malaria from humans to mosquitoes, a single dose of primaquine is recommended by the WHO as an addition to artemisinin combination therapy. Primaquine clears gametocytes but causes dose-dependent haemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Evidence is needed to inform the optimal dosing of primaquine for malaria elimination programmes and for the purpose of interrupting the spread of artemisinin-resistant malaria. This study investigates the efficacy and safety of reducing doses of primaquine for clearance of gametocytes in participants with normal G6PD status.
Methods and analysis
In this prospective, four-armed randomised placebo-controlled double-blinded trial, children aged 1–10 years, weighing over 10 kg, with haemoglobin ≥8 g/dl and uncomplicated P falciparum malaria are treated with artemether lumefantrine and randomised to receive a dose of primaquine (0.1, 0.4 or 0.75 mg base/kg) or placebo on the third day of treatment. Participants are followed up for 28 days. Gametocytaemia is measured by quantitative nucleic acid sequence-based analysis on days 0, 2, 3, 7, 10 and 14 with a primary endpoint of the number of days to gametocyte clearance in each treatment arm and secondarily the area under the curve of gametocyte density over time. Analysis is for non-inferiority of efficacy compared to the reference dose, 0.75 mg base/kg. Safety is assessed by pair-wise comparisons of the arithmetic mean (±SD) change in haemoglobin concentration per treatment arm and analysed for superiority to placebo and incidence of adverse events. Ethics and dissemination Approval was obtained from the ethical committees of Makerere University School of Medicine, the Ugandan National Council of Science and Technology and the London School of Hygiene and Tropical Medicine.
Results
These will be disseminated to inform malaria elimination policy, through peer-reviewed publication and academic presentations.
Keywords: Infectious diseases, Parasitology, Public health
Article summary.
Article focus
Single-dose primaquine, administered together with artemisinin combination therapy, blocks transmission of Plasmodium falciparum malaria by clearing gametocytes.
Primaquine, an 8-aminoquinoline, causes dose-dependent haemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Evidence is lacking on the safety and efficacy of lower doses of primaquine.
This is the protocol of a dose-finding trial being conducted in eastern Uganda.
Key messages
Dose-finding is a priority for the use of primaquine in malaria elimination programmes and to block the spread of artemisinin-resistant malaria.
This trial is designed to investigate the efficacy and safety of reducing doses of primaquine for gametocytocidal action.
This paper highlights the unique trial design issues that are relevant for investigating the efficacy and safety of antimalarials targeted against the sexual stages of malaria for blocking transmission rather than clinical cure.
Strengths and limitations of this study
For ethical reasons, in this trial, dose-finding is conducted in children with normal G6PD status, but, ultimately, information is needed on the safety of lower doses in people with G6PD deficiency.
This trial measures primaquine's transmission-blocking potential by assessing gametocyte clearance. Endpoints of mosquito transmission at multiple time points could be usefully assessed but on smaller numbers of individuals.
Background
Sustained deployment of vector control measures and accessible, effective drug therapy has reduced the transmission of Plasmodium falciparum in many endemic countries. However, further scaling-up of currently available malaria control measures is unlikely to achieve malaria elimination in most settings.1 Moreover, the emergence of resistance to artemisinin in Southeast Asia,2 3 and the development of insecticide resistance and adaptive behaviour in the mosquito vector4––6 present significant threats to the current trend of declining malaria burden. Malaria elimination initiatives and artemisinin-resistance containment strategies both require additional tools that are specifically aimed at reducing the transmission of malarial parasites.7 8
Antimalarial drugs are designed primarily to target the asexual stages of the parasite that cause morbidity and mortality. The effect of antimalarial drugs on gametocytes, the transmission stages, has for decades been seen as ancillary. P falciparum gametocytes undergo complex development that is characterised by five morphologically distinct stages of maturation.9 The immature gametocyte stages (I–IV) are sequestered in the reticuloendothelial system and bone marrow.10––12 Mature stage V gametocytes typically appear approximately 12 days after the onset of patent asexual bloodstream infection, and are the only gametocyte stage that circulates in the peripheral blood and is infective to biting female Anopheles mosquitoes.13 14 The majority of antimalarial drugs, including artemisinins, lumefantrine and piperaquine, have some efficacy against immature gametocytes.15 16 These drugs have the potential to reduce transmission at a population level because asexual parasites are cleared, preventing de novo development of gametocytes, and fewer of the immature gametocytes that are present upon initiation of treatment survive to maturity. However, the vast majority of symptomatic cases have measurable and transmissible levels of mature gametocytes at presentation.17 18 These persist after treatment with all antimalarials that are currently implemented as first-line treatment, including artemisinin combination therapy (ACT). Gametocytes that persist after ACT have repeatedly been shown to be infectious to mosquitoes.17 19 20 This post-treatment gametocyte carriage frequently occurs at low densities, commonly below the microscopic threshold for detection,21 22 but is sufficiently high for efficient mosquito infection.17 23
The only class of drugs that are effective against mature P falciparum gametocytes is the 8-aminoquinolines. Primaquine is the most widely available drug in this class. The exact mechanism for this gametocytocidal activity is unknown, but it is probably dependent on oxidative damage to the intraerythrocytic parasite by primaquine metabolites.24 Primaquine as a single dose of 0.75 mg base/kg added to standard ACT has superior gametocytocidal activity to ACT alone.25––27 All doses of primaquine described hereafter refer to the dose of primaquine base per unit weight. There are indications that doses of primaquine lower than 0.75 mg/kg may be equally efficacious. A Thai study showed that both 0.5 and 0.25 mg/kg of primaquine administered with ACT to adults infected with malaria effectively and indistinguishably reduced the proportion of mosquitoes that became infected after a blood meal.28 In small numbers of adults, total doses of 30 mg and 15 mg have shown comparable efficacy to a 45 mg dose in reducing mosquito infection rates.29 30
The efficacy of primaquine when given as a single low dose is important in the light of concerns over the haematological safety of primaquine. There is conclusive evidence for primaquine-induced haemolysis in glucose-6-phosphate dehydrogenase (G6PD) deficient individuals.31 32 G6PD deficient individuals are vulnerable to oxidative stress because their erythrocytes do not have alternative pathways for G6PD-dependent nicotinamide adenine dinucleotide phosphate production, which is essential to maintain antioxidant defences. There is conflicting evidence on the risk of haemolysis after a single dose of primaquine. A single dose of 45 mg primaquine administered to a Vanuatan adult caused life-threatening haemolysis.33 In G6PD-deficient Tanzanian children, the mean fall in haemoglobin after a single dose of 0.75 mg/kg primaquine was 2.5 g/dl (95% CI 1.2 to 3.8 g/dl), though no associated severe adverse events were recorded and haemolysis was transient.34 On the other hand, primaquine was reported to be well tolerated when 0.75 mg/kg was given without prior G6PD testing in large studies in Myanmar, Sudan, Russia, Cambodia and China.27 31 35 36
Because primaquine-induced haemolysis is dose-dependent,29 and because gametocytocidal efficacy may be retained with primaquine doses lower than 0.75 mg/kg, the WHO-recommended dose in its 2010 Guidelines for the Treatment of Malaria, dose-finding studies are needed urgently. This trial tests the hypothesis that lower doses of primaquine have a substantially lower risk of, or an absence of, adverse effects but that their gametocytocidal efficacy is retained.
Methods and analysis
Study design
The study is a prospective, randomised, parallel arm, placebo-controlled, double-blinded clinical trial of reducing doses of primaquine administered with artemether lumefantrine (AL) for the treatment of uncomplicated clinical P falciparum malaria infection in children aged 1–10 years of age. The study uses a non-inferiority design to evaluate the efficacy and a superiority design to evaluate the safety of 0.1 and 0.4 mg/kg primaquine compared with 0.75 mg/kg when added to AL.
Study objectives
To evaluate the efficacy of 0.1, 0.4 and 0.75 mg/kg primaquine when administered together with the fifth dose of AL as measured by gametocyte prevalence and density.
To evaluate the safety of 0.1, 0.4 and 0.75 mg/kg primaquine when administered together with the fifth dose of AL as measured by change in mean haemoglobin, prevalence of severe anaemia (haemoglobin <5 g/dl) and evidence of black urine (haemoglobinuria).
To assess the safety of different doses of 0.1, 0.4 and 0.75 mg/kg primaquine when administered together with the fifth dose of AL as measured by prevalence/incidence of adverse events and tolerability.
Participants and enrolment
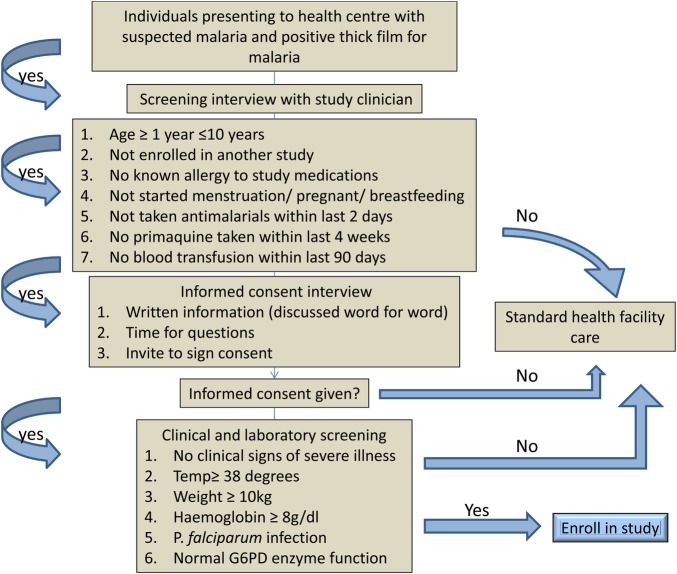
The study is conducted at Walukuba Health Centre IV in Walukuba subcounty, Jinja district, in eastern Uganda. In this area, malaria transmission is year-round with two seasonal peaks. The entomological inoculation rate (EIR) was estimated at 7 infectious bites per person per year in Walukuba.37 Study participants are recruited from children attending the Health Centre IV with suspected malaria (figure 1). Inclusion criteria are age 1–10 years, weight over 10 kg, fever (tympanic temperature >38°C) or history of fever in the last 24 h, P falciparum mono-infection with a parasite density <5 00 000/µl and normal G6PD enzyme function. Exclusion criteria are evidence of severe illness/danger signs, known allergy to study medications, haemoglobin <8 g/dl, started menstruation, pregnancy or breastfeeding, antimalarials taken within the last 2 days, primaquine taken within the last 4 weeks and blood transfusion within the last 90 days.
Figure 1.
Enrolment and selection procedures.
The fluorescent spot test38 is used for G6PD screening. This test has a cut-off of approximately 20% enzyme function, below that, there is no fluorescence. The WHO classification defines severe G6PD deficiency as 10% enzyme function.39
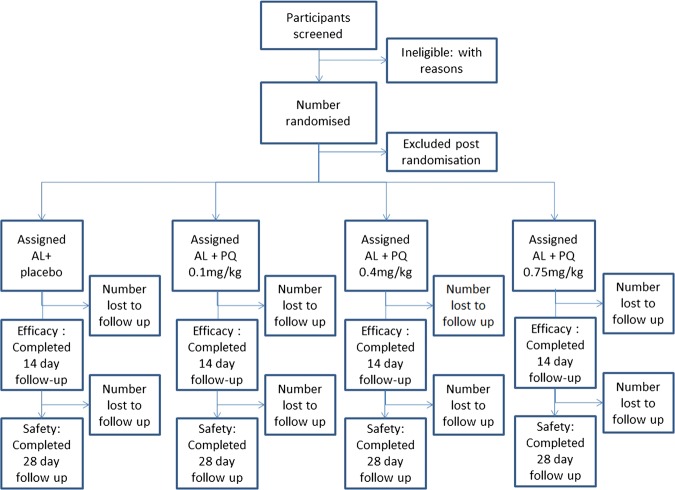
Randomisation, blinding and intervention
After enrolment (day 0), participants are randomised to a treatment arm stratified by gender (figure 2). The study pharmacist selects sequential opaque envelopes (from either the male or the female pile). Each envelope contains a predetermined treatment assignment code. The study pharmacist is the only member of the clinic team not blinded to the treatment arm and is not involved in assessing patients or assigning outcomes. All study site staff who administer drugs, assess patients and process laboratory samples do not have access to the randomisation code breaker.
Figure 2.
Participant flow diagram.
All participants receive a 3 day course of artemether lumefantrine according to Ugandan national treatment guidelines for uncomplicated malaria. Participants are randomised to receive a placebo or a dose of 0.1, 0.4 or 0.75 mg/kg primaquine in addition to the AL treatment. The dose of primaquine/placebo is given at the same time as the fifth dose of AL, in the morning of day 2. To preserve the accuracy of lower weight-based doses, all primaquine doses are administered in aqueous solution and measured using a sterile syringe. The placebo is aqueous solution alone. All doses including placebo are mixed with glucose-based syrup that masks the colour and taste of primaquine. All treatments are directly observed. A snack with approximately 5 g of fat is administered prior to both AL and primaquine administration to optimise absorption of AL and minimise gastrointestinal side effects with primaquine. Participants are observed for 30 minutes; treatment is readministered in any case of vomiting within this period. Repeated vomiting (>3 times) leads to exclusion from the study and treatment as complicated malaria according to national guidelines.
Follow-up measurements
Study participants are reviewed on days 0, 1, 2, 3, 7, 10, 14, 21 and 28 after enrolment or on any day of illness. On each of the scheduled visit days they are assessed clinically with standardised adverse event recording and blood samples are taken for microscopical detection of asexual parasites and gametocytes, molecular detection of gametocytes and haemoglobin measurements (table 1).
Table 1.
Summary of outcome measures
| Outcome measure | Description | |
|---|---|---|
| Efficacy | ||
| Primary | Mean number of days to gametocyte clearance (GCT) | Mean number of days per treatment arm for gametocytes to become undetectable using submicroscopic molecular testing methods (QT-NASBA). Reappearance of gametocytes after day 14 will be considered as re-infection and excluded |
| Secondary | Mean (±SD) area under the curve of gametocyte density per day during 14 days of follow-up | Total number of gametocytes (measured by QT-NASBA) seen over follow-up, averaged per day of follow-up (days 0–14) |
| Density of gametocytes on days 7, 10 and 14 | Mean number of gametocytes (measured by QT-NASBA) per treatment arm on days 7, 10 and 14 | |
| Proportion (%) of participants with gametocytes on each day of follow-up | For each treatment arm, percentage of participants with gametocytes (measured by QT-NASBA) on each day of follow-up from days 0–14 | |
| Safety | ||
| Primary | Mean (± SD) maximal fall (±) in Hb (haemoglobin, g/dl) from enrolment to day 28 of follow-up | Mean maximal greatest negative difference in Hb (measured by HemoCue) from enrolment value per treatment arm over 28 days follow-up |
| Secondary | Follow-up day of Hb nadir | Mean day of follow-up (day 0–28) per treatment arm of lowest Hb measurement (by HemoCue) |
| Maximal percentage fall in Hb level compared to enrolment value | Size of maximal Hb drop (by HemoCue) during follow-up (day 0–28) from enrolment value, divided by enrolment value, *100 | |
| Percentage of participants with Hb<5 g/dl during follow-up | Percentage (number) per treatment arm during days 0–28 | |
| Requirement for blood transfusion | Percentage (number) of children receiving blood transfusion per treatment arm during days 0–28 | |
| Evidence of black urine | Percentage (number) of children with documented black/dark urine with urine dipstick positive for Hb per treatment arm during days 0–28 | |
| Incidence of serious adverse events by sign, symptom, laboratory parameter and relationship to taking study drug | Percentage (number) per treatment arm during days 0–28 | |
| Incidence of gastrointestinal symptoms after taking study drug | Percentage (number) per treatment arm during days 2–7 | |
GCT, gametocyte clearance time; Hb, haemoglobin; QT-NASBA, quantitative real-time nucleic acid sequence-based analysis.
Blood smears from all visits are Giemsa-stained and 100 microscopic fields are screened for asexual parasites on days 0, 1, 2, 3, 7, 10, 14, 21 and 28. Asexual parasites are counted against 200 white blood cells (WBC) or, if fewer than 10 parasites are observed per 200 WBC, against 500 WBC. Gametocytes are recorded if observed during this screening process. On day 0, 100 microscopic fields are reread for gametocytes specifically. If gametocytes are observed, they are quantified against 500 WBC. All microscopy readings are performed by two independent microscopists, if they disagree on prevalence or if density results differ by more than 25%, a third reading is requested.
Gametocytes are quantified on days 0, 2, 3, 7, 10 and 14 using quantitative real-time nucleic acid sequence-based analysis (QT-NASBA), detecting and quantifying Pfs25 mRNA. One hundred microlitres of finger prick blood is mixed with 900 µl L6 guanidine buffer (Severn Biotech, UK) and stored at −80°C until automatic nucleic acid extraction by MagNAPure (Roche) using commercial high-yield kits. The Pfs25 QT-NASBA is specific for mature gametocytes with a sensitivity of 0.01–0.1 gametocytes/µl of blood when 50 µl blood samples are used for RNA extraction.40
Haemoglobin is measured on days 0, 1, 2, 3, 7, 10, 14, 21 and 28 using HemoCue 201+ photometers (HemoCue; Angelholm, Sweden). At each follow-up visit, study staff assess participants in an objective manner according to a clinical record form and assessment for adverse events is conducted in a prospective, systematic fashion during all visits, including the enrolment visit (eg, vomiting post-AL). All data are double-entered in real time.
Safety considerations
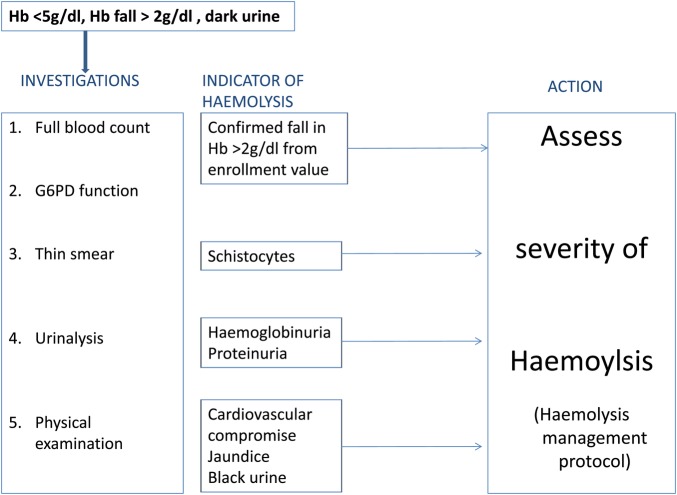
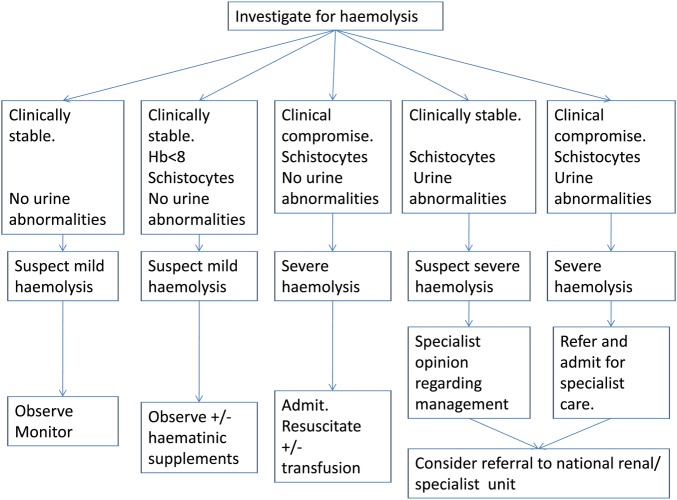
A protocol was developed in order to standardise the detection, investigation and management of severe haemolysis in this trial (figures 3 and 4). A Data Safety Monitoring Board (DSMB) has been installed; clinically relevant haemolytic events, hospital admissions, blood transfusions and deaths are reported within 72 h to this DSMB.
Figure 3.
Procedure for investigation of suspected haemolysis.
Figure 4.
Procedure for management of haemolysis.
Ethical considerations
The study protocol and informed consent forms were approved by the Makerere University School of Medicine Research Ethics Committee (protocol 2011–210), the Uganda National Council of Science and Technology (protocol HS1056) and the London School of Hygiene and Tropical Medicine research ethics committee (protocol 5987). The Ugandan National Drug Authority approved the protocol and importation of primaquine for the purposes of the study. The DSMB and Trial Advisory Committee for the study agreed to meet at predetermined stages of the study. Before the study began, local community stakeholders (including village health team and local council members) in Walukuba were consulted and a community advisory board meeting was held.
Sample size
For efficacy, the sample size calculation is based on non-inferiority of each of the two test dose arms to the comparator arm, the WHO-recommended dose of primaquine, 0.75 mg/kg. The primary outcome measure is number of days to gametocyte clearance. The addition of primaquine (0.75 mg/kg) to ACT in Tanzania reduced the time to gametocyte clearance from 28.6 to 6.3 days (SD 6 days).41 Allowing for a 10% loss to follow-up, a sample size of 120/arm will provide over 80% power at the 0.05 significance level to detect non-inferiority to the standard arm with a non-inferiority margin of 2.5 days, which was considered to be a clinically relevant reduction in gametocyte clearance time. This sample size also allows for an analysis of superiority of the efficacy of the two test dose arms to placebo.
For safety, the sample size calculation is based on superiority of each of the two test dose arms to the comparator arm (0.75 mg/kg). For this comparator arm, Shekalaghe et al34 found an overall mean absolute drop in haemoglobin by day 7 after treatment of 0.6 g/dl (SD 1.5). Therefore, with 80% power and at the 0.05 significance level, a sample size of 99 would be required to detect a difference in mean maximal drop in haemoglobin between treatment groups of 0.6 g/dl.
Data analysis
Data will be double entered in Microsoft Access and imported into Stata V.12.0 (Statacorp Ltd, Texas, USA). All efficacy analyses will be based on gametocyte detection by Pfs25 QT-NASBA. Gametocyte density on days 7, 10 and 14 will be compared with the comparator arm (0.75 mg primaquine/kg) by χ2 test. The mean duration of gametocyte carriage and 95% CI will be estimated in each treatment arm and compared with the comparator arm using a previously validated mathematical model.42 The area under the curve of gametocyte density over time will be calculated using the method described by Mendez et al43 For individuals who are gametocyte positive at enrolment, Kaplan-Meier survival analysis will be used to compare the decline in gametocyte prevalence.
The primary safety outcome, mean maximal fall in haemoglobin concentration during 28 days of follow-up will be assessed for each treatment arm. Pair-wise comparisons will be made between each of the treatment arms and compared with the comparator arm using unpaired t tests.
Discussion
In the 2010 edition of the Guidelines for the Treatment of Malaria, the WHO recommends that a single dose of 0.75 mg/kg primaquine is added to ACT in malaria elimination programmes and for epidemic control, provided the risks of haemolysis in G6PD-deficient patients are considered. This guidance was recently updated to recommend a lower dose of 0.25 mg/kg primaquine without G6PD testing for new malaria elimination programmes and to prevent the spread of artemisinin resistance.31 The revision was based largely on grey literature and historical data rather than on recent clinical trials and few of the data are in the public domain.44 There have been no formal dose-finding studies using contemporary tools and standards for the measurement of drug efficacy and safety for the combination of ACTs and primaquine. In the current study, we aim to provide these urgently needed efficacy data and provide safety data for individuals with normal G6PD enzyme function.
Relatively few trials have been designed specifically to test gametocytocidal drugs in vivo. Standardised protocols and trial designs for assessing the efficacy of drugs targeted against asexual parasites45 46 are not suitable to assess gametocytocidal drugs, where the main outcome is transmission-blocking activity rather than clinical or parasitological cure. There is no agreement on the best tools to quantify gametocyte carriage. Many trials have used microscopy to measure gametocytes26––28 47 48 while it has been known for decades that microscopy is notoriously insensitive for detecting gametocytes.49 Gametocytes typically circulate at densities that are ≤1% of asexual parasite densities.16 50 Nevertheless, gametocytes are often simply recorded while screening for asexual parasites. If slides are specifically read for gametocytes, the number of microscopic fields that is screened is mostly the same as that for asexual parasites.51 As a consequence, gametocytes measured microscopically by routine underestimate the total gametocyte prevalence by up to 10-fold.16 17 21 22 In the current study, gametocytes are quantified with the most widely used quantitative molecular gametocyte detection method, QT-NASBA that has an estimated sensitivity of 0.01–0.1 gametocytes/µl blood in the blood sample taken.40 The use of this sensitive molecular method will increase the power of our efficacy estimates since up to 90% of symptomatic malaria patients may harbour (submicroscopic) gametocyte densities prior to the initiation of treatment.16
Gametocyte density is associated with the likelihood of mosquito infection and some of the lowest gametocyte densities may therefore be unlikely to result in mosquito infections. In general, there are limitations to which gametocyte prevalence or density can be used to predict mosquito infection rates. The fitness or infectivity of gametocytes is variable, especially after treatment.19 52 53 Very early studies demonstrated that primaquine may render gametocytes non-infectious several days before they are cleared from the circulation.30 54 55 The only approach to directly measure transmission-blocking potential involves assessing the infectiousness of the participant's blood to mosquitoes using the membrane feeding assay or direct skin feeding assays,56 the latter being described by early malariologists.57 58 However, the capacity for mosquito feeding assays is not widely available and repeated assessments of infectiousness on the same patients have never been performed as part of clinical trials. This is partly because of ethical concerns related to repeated venous bleeding in young children, and partly because of the complexity of mosquito husbandry when large numbers of mosquitoes are required for robust transmission estimates.59 In the absence of biomarkers, using the prevalence and density of gametocytes after treatment is the most pragmatic approach to assess the transmission-blocking efficacy of drugs across a variety of malaria endemic settings.
To assess the safety of the 8-aminoquinoline drugs, there must be a clear definition of the risk of haemolysis and how it should be measured.31 60 The safety profile may best be defined by the incidence of endpoints that could compromise health, such as signs of severe haemolysis, and the need for interventions such as haematinic drug administration, hospitalisation or blood transfusion. These events, however, are rare and changes in haemoglobin concentration may be a more sensitive primary safety outcome for standard clinical trials. In a recent Cochrane review of randomised controlled trials of primaquine's efficacy, only one trial25 was found to have measured the haemoglobin concentration to assess safety.61 In this current study, clinically relevant safety endpoints have been selected and a standardised procedure is in place for the investigation and management of severe haemolysis. A shortcoming of the current study is that safety data are most urgently needed in the most vulnerable group, G6PD-deficient individuals. For ethical reasons this group was excluded. The authors consider that the priority is first to determine the minimal effective dose in a G6PD normal population before G6PD-deficient individuals are exposed to this low dose of primaquine to assess safety.
The ultimate evidence for a beneficial role of primaquine in reducing malaria transmission would come from trials assessing the effect of the drug on measures of community-level transmission. Once a safe and efficacious dose of primaquine in combination with ACTs is established, the next step involves designing these community trials. Treatment of symptomatic cases could play an important role in reducing the spread of (resistant) malaria strains from symptomatic patients.62 However, because of the large pool of asymptomatic parasite carriers in all endemic settings63 and their importance in defining transmission potential, any effect of primaquine on community-wide transmission will be limited if administration is restricted to symptomatic cases. Other strategies such as pro-active screening and treatment and (focal) mass drug administration may have a larger impact in some settings.64 This trial forms the starting point for defining the optimal dose of primaquine for use in transmission-blocking interventions.
Supplementary Material
Acknowledgments
The authors would like to thank staff at the Infectious Diseases Research Collaboration (IDRC) in Uganda, notably, Mr Moses Kiggundu, Dr Catherine Maiteki-Sebuguzi, the members of the Programme for Resistance, Immunology, Surveillance and Modelling of Malaria in Uganda (PRISM) and the ACT PRIME and PROCESS research teams and also Ms Carolynne Stanley for logistical support at the London School of Hygiene and Tropical Medicine.
Footnotes
Contributors: ACE, SGS, SY, NJW, TB and CD have conceived and designed the study and participated in logistical planning together with EW and MK. EW provided the statistical support for the sample size estimates and the design of the statistical analysis. TB provided the expertise for submicroscopic gametocyte measurement. ACE organised the ethical applications, community sensitisation and study implementation and wrote the manuscript together with TB and CD. All authors have read and approved the final manuscript.
Funding: This study is funded by ACE's Clinical Fellowship from the Wellcome Trust of Great Britain (grant #090558/Z/09/Z).
Competing interests: None.
Ethics approval: Makerere University School of Medicine Research Ethics Committee (protocol 2011-210), the Uganda National Council of Science and Technology (protocol HS1056) and the London School of Hygiene and Tropical Medicine research ethics committee (protocol 5987).
Provenance and peer review: Not commissioned; externally peer reviewed.
Trial status: Recruitment began on 6 December 2011. The trial is going on .
References
- 1.Griffin JT, Hollingsworth TD, Okell LC, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med 2010;7 doi:10.1371/journal.pmed.1000324 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009;361:455–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaratunga C, Sreng S, Suon S, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis 2012;12:851–8 doi: S1473-3099(12)70181-0 [pii] 10.1016/S1473-3099(12)70181-0 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson J, St Laurent B, Lobo NF, et al. Novel vectors of malaria parasites in the Western highlands of Kenya. Emerg Infect Dis 2012;18:1547–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riehle MM, Guelbeogo WM, Gneme A, et al. A cryptic subgroup of Anopheles gambiae is highly susceptible to human malaria parasites. Science 2011;331:596–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trung HD, Bortel WV, Sochantha T, et al. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health 2005;10:251–62 [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM. Editorial commentary: single-dose primaquine as gametocytocidal treatment in patients with uncomplicated falciparum malaria. Clin Infect Dis 2013;56:694–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso PL, Brown G, Arevalo-Herrera M, et al. A research agenda to underpin malaria eradication. PLoS Med 2011;8:e1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Field JW, Shute P. The microscopic diagnosis of human malaria. II. A morphological study of the erythrocytic parasites. Studies from the Institute for Medical Research, Federated Malay States 1956.
- 10.Smalley ME, Abdalla S, Brown J. The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Trans R Soc Trop Med Hyg 1981;75:103–5 [DOI] [PubMed] [Google Scholar]
- 11.Rogers NJ, Hall BS, Obiero J, et al. A model for sequestration of the transmission stages of Plasmodium falciparum: adhesion of gametocyte-infected erythrocytes to human bone marrow cells. Infect Immun 2000;68:3455–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson JGRA. The structure and development of Plasmodium falciparum gametocytes in the internal organs and peripheral circulation. Trans R Soc Trop Med Hyg 1935;29:31–4 [Google Scholar]
- 13.Smalley ME, Sinden RE. Plasmodium falciparum gametocytes: their longevity and infectivity. Parasitology 1977;74:1–8 [DOI] [PubMed] [Google Scholar]
- 14.Lensen A, Bril A, van de Vegte M, et al. Plasmodium falciparum: infectivity of cultured, synchronized gametocytes to mosquitoes. Exp Parasitol 1999;91:101–3 [DOI] [PubMed] [Google Scholar]
- 15.Adjalley SH, Johnston GL, Li T, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci USA 2011;108:E1214–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 2011;24:377–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bousema JT, Schneider P, Gouagna LC, et al. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis 2006;193:1151–9 [DOI] [PubMed] [Google Scholar]
- 18.Ali E, Mackinnon MJ, Abdel-Muhsin AM, et al. Increased density but not prevalence of gametocytes following drug treatment of Plasmodium falciparum. Trans R Soc Trop Med Hyg 2006;100:176–83 [DOI] [PubMed] [Google Scholar]
- 19.Targett G, Drakeley C, Jawara M, et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J Infect Dis 2001;183:1254–9 [DOI] [PubMed] [Google Scholar]
- 20.Drakeley CJ, Jawara M, Targett GA, et al. Addition of artesunate to chloroquine for treatment of Plasmodium falciparum malaria in Gambian children causes a significant but short-lived reduction in infectiousness for mosquitoes. Trop Med Int Health 2004;9:53–61 [DOI] [PubMed] [Google Scholar]
- 21.Schneider P, Bousema T, Omar S, et al. (Sub)microscopic Plasmodium falciparum gametocytaemia in Kenyan children after treatment with sulphadoxine-pyrimethamine monotherapy or in combination with artesunate. Int J Parasitol 2006;36:403–8 [DOI] [PubMed] [Google Scholar]
- 22.Mens PF, Sawa P, van Amsterdam SM, et al. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar J 2008;7:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouedraogo AL, Bousema T, Schneider P, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS ONE 2009;4:e8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tekwani BL, Walker LA. 8-Aminoquinolines: future role as antiprotozoal drugs. Curr Opin Infect Dis 2006;19:623–31 [DOI] [PubMed] [Google Scholar]
- 25.Shekalaghe S, Drakeley C, Gosling R, et al. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS ONE 2007;2:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutanto I, Suprijanto S, Kosasih A, et al. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin-piperaquine in South Sumatra, Western Indonesia: an open-label, randomized, controlled trial. Clin Infect Dis 2012;56:685–93 doi:10.1093/cid/cis959 [published Online First: Epub Date] [DOI] [PubMed] [Google Scholar]
- 27.Smithuis F, Kyaw MK, Phe O, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 2010;10:673–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pukrittayakamee S, Chotivanich K, Chantra A, et al. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob Agents Chemother 2004;48:1329–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alving AS, Johnson CF, Tarlov AR, et al. Mitigation of the haemolytic effect of primaquine and enhancement of its action against exoerythrocytic forms of the Chesson strain of Piasmodium vivax by intermittent regimens of drug administration: a preliminary report. Bull World Health Organ 1960;22:621–31 [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess RW, Bray RS. The effect of a single dose of primaquine on the gametocytes, gametogony and sporogony of Laverania falciparum. Bull World Health Organ 1961;24:451–6 [PMC free article] [PubMed] [Google Scholar]
- 31.White NJ, Qiao LG, Qi G, et al. Rationale for recommending a lower dose of primaquine as a Plasmodium falciparum gametocytocide in populations where G6PD deficiency is common. Malar J 2012;11:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charles LJ. Observations on the haemolytic effect of primaquine in 100 Ghanaian children. Ann Trop Med Parasitol 1960;54:460–70 [DOI] [PubMed] [Google Scholar]
- 33.Reeve PA, Toaliu H, Kaneko A, et al. Acute intravascular haemolysis in Vanuatu following a single dose of primaquine in individuals with glucose-6-phosphate dehydrogenase deficiency. J Trop Med Hyg 1992;95:349–51 [PubMed] [Google Scholar]
- 34.Shekalaghe SA, ter Braak R, Daou M, et al. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A-) individuals. Antimicrob Agents Chemother 2010;54:1762–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song J, Socheat D, Tan B, et al. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J 2010;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Sayed B, El-Zaki SE, Babiker H, et al. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS One 2007;2:e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okello PE, Van Bortel W, Byaruhanga AM, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg 2006;75:219–25 [PubMed] [Google Scholar]
- 38.Beutler E, Mitchell M. Special modifications of the fluorescent screening method for glucose-6-phosphate dehydrogenase deficiency. Blood 1968;32:816–18 [PubMed] [Google Scholar]
- 39.Beutler E, Duparc S, Group GPDW. Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg 2007;77:779–89 [PubMed] [Google Scholar]
- 40.Schneider P, Schoone G, Schallig H, et al. Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification. Mol Biochem Parasitol 2004;137:35–41 [DOI] [PubMed] [Google Scholar]
- 41.Bousema T, Okell L, Shekalaghe S, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J 2010;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okell LC, Drakeley CJ, Bousema T, et al. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med 2008;5:e226; discussion e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez F, Munoz A, Plowe CV. Use of area under the curve to characterize transmission potential after antimalarial treatment. Am J Trop Med Hyg 2006;75:640–4 [PubMed] [Google Scholar]
- 44.von Seidlein L. Mini primaquine? Controversy and uncertainty surround WHO guidelines for the antimalarial primaquine. PLoS Blogs 2012 [Google Scholar]
- 45.Laufer MK. Monitoring antimalarial drug efficacy: current challenges. Curr Infect Dis Rep 2009;11:59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Organization WH Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: World Health Organization, 2007 [Google Scholar]
- 47.Chomcharn Y, Surathin K, Bunnag D, et al. Effect of a single dose of primaquine on a Thai strain of Plasmodium falciparum. Southeast Asian J Trop Med Public Health 1980;11:408–12 [PubMed] [Google Scholar]
- 48.Kaneko A, Kamei K, Suzuki T, et al. Gametocytocidal effect of primaquine in a chemotherapeutic malaria control trial in North Sumatra, Indonesia. Southeast Asian J Trop Med Public Health 1989;20:351–9 [PubMed] [Google Scholar]
- 49.Boudin C, Olivier M, Molez JF, et al. High human malarial infectivity to laboratory-bred Anopheles gambiae in a village in Burkina Faso. Am J Trop Med Hyg 1993;48:700–6 [DOI] [PubMed] [Google Scholar]
- 50.Talman AM, Domarle O, McKenzie FE, et al. Gametocytogenesis: the puberty of Plasmodium falciparum. Malar J 2004;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilian AH, Metzger WG, Mutschelknauss EJ, et al. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Health 2000;5:3–8 [DOI] [PubMed] [Google Scholar]
- 52.Hallett RL, Dunyo S, Ord R, et al. Chloroquine/sulphadoxine-pyrimethamine for Gambian children with malaria: transmission to mosquitoes of multidrug-resistant Plasmodium falciparum. PLoS Clin Trials 2006;1:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fofana BDA, Sagara I, Dao A, et al. Impact of artemisinin-based combination therapy on malaria transmission in Mali. 5th MIM Pan-African Malaria Conference; Nairobi, Kenya, 2009 [Google Scholar]
- 54.Gunders AE. The effect of a single dose of pyrimethamine and primaquine in combination upon gametocytes and sporogony of Laverania falcipara (Plasmodium falciparum) in Liberia. Bull World Health Organ 1961;24:650–3 [PMC free article] [PubMed] [Google Scholar]
- 55.Jeffery GM, Young MD, Eyles DE. The treatment of Plasmodium falciparum infection with chloroquine, with a note on infectivity to mosquitoes of primaquine- and pyrimethamine-treated cases. Am J Hyg 1956;64:1–11 [DOI] [PubMed] [Google Scholar]
- 56.Bousema T, Churcher TS, Morlais I, et al. Can field-based mosquito feeding assays be used for evaluating transmission-blocking interventions? Trends Parasitol 2013;29:53–9 [DOI] [PubMed] [Google Scholar]
- 57.Mackerras MJ, Ercole QN. Observations on the action of quinine, atebrin and plasmoquine on the gametocytes of Plasmodium falciparum. Trans R Soc Trop Med Hyg 1949;42:455–63 [DOI] [PubMed] [Google Scholar]
- 58.Rieckmann KH, McNamara JV, Frischer H, et al. Gametocytocidal and sporontocidal effects of primaquine and of sulfadiazine with pyrimethamine in a chloroquine-resistant strain of Plasmodium falciparum. Bull World Health Organ 1968;38:625–32 [PMC free article] [PubMed] [Google Scholar]
- 59.Bousema T, Dinglasan RR, Morlais I, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE 2012;7:e42821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eziefula AC, Gosling R, Hwang J, et al. Rationale for short course primaquine in Africa to interrupt malaria transmission. Malar J 2012;11:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graves PM, Gelband H, Garner P. Primaquine for reducing Plasmodium falciparum transmission. Cochrane Database Syst Rev 2012;9:CD008152. [DOI] [PubMed] [Google Scholar]
- 62.Dondorp AM, Fairhurst RM, Slutsker L, et al. The threat of artemisinin-resistant malaria. New Eng J Med 2011;365:1073–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okell LC, Bousema T, Griffin JT, et al. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun 2012;3:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okell LC, Griffin JT, Kleinschmidt I, et al. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS ONE 2011;6:e20179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.