Abstract
Objective
In a cohort of children less than 5 years old exposed to adult intrathoracic tuberculosis (TB) in 1996–1998, we found 66% increased mortality compared with community controls. In 2005, we implemented isoniazid preventive therapy (IPT) for children exposed to TB at home, and the present study evaluates the effect of this intervention on mortality.
Setting
This prospective cohort study was conducted in six suburban areas included in the demographic surveillance system of the Bandim Health Project in Bissau, the capital city of Guinea-Bissau.
Participants
All children less than 5 years of age and living in the same house as an adult with intrathoracic TB registered for treatment in the study area between 2005 and 2007 were evaluated for inclusion in the IPT programme.
Main outcome measures (end points)
The all-cause mortality rate ratio (MRR) between exposed children on IPT, exposed without IPT and unexposed community control children.
Results
A total of 1396 children were identified as living in the same houses as 416 adult TB cases; of those, 691 were enrolled in the IPT programme. Compared with community controls, the IPT children had an MRR of 0.30 (95%CI 0.1 to 1.2). The MRR comparing exposed children with and without IPT was 0.21 (0.0 to 1.1). The relative mortality in IPT children compared with community controls in 2005–2008 differed significantly from the relative mortality of exposed untreated children compared with the community controls in 1996–1998 (test of interaction, p=0.01).
Conclusions
In 2005–2008, exposed children on IPT had 70% lower mortality than the community control children, though not significantly. Relative to the community control children, the mortality among TB-exposed children on IPT in 2005–2008 was significantly lower than the mortality among TB-exposed children not on IPT in 1996–1998.
Article summary.
Article focus
Impact of isoniazid preventive therapy (IPT) on mortality among children exposed to an adult with intrathoracic tuberculosis (TB) at home: comparing (1) exposed children who received IPT to unexposed children and (2) exposed children who did not receive IPT to unexposed children.
Mortality in children exposed to TB who were enrolled on IPT compared with those exposed but not receiving IPT in a previous study in the same setting.
Key message
-
▪
Isoniazid preventive treatment programs can be implemented in low resource settings and saves lives. Children exposed to tuberculosis who were not enrolled in the preventive isoniazid treatment program had a 12-fold higher mortality.
Strengths and limitations of this study
Overall, evaluation of compliance showed that around 79% of the possible isoniazid pills were taken. Given the low mortality in the cohort, it was not possible to test to what extent adherence mattered for a beneficial effect of IPT.
Mortality in the study area declined dramatically between the two study periods, and the study therefore had much less power than originally expected. Nonetheless, the results were so marked that it was still possible to show the hypothesised inversion of the mortality rate ratios between TB-exposed children and community controls between the pre-IPT period and the IPT period.
In an intervention study in an area with a very mobile population as in Bissau, it is not possible to enrol all eligible children. There are always some children travelling or absent at inclusion visits. This obviously opens up to the possibility of selection biases as to who participated in the study. Owing to the current WHO recommendation, it was decided not to conduct a randomised study; hence, there are a number of theoretical biases. Furthermore, the previous study was conducted 10 years prior to the present study and many things have changed in the meantime. Another limitation was that the children were not HIV tested, which might bias the results if there were more HIV-infected children in the IPT group compared with the no IPT group. However, we would then expect higher mortality in the no IPT group compared with the community controls in the present IPT cohort.
Introduction
Childhood tuberculosis (TB) is to a large degree neglected in endemic areas with limited resources, mainly because children are considered to develop mild forms of disease and to contribute little to the maintenance of the TB epidemic.1 2 However, recent studies indicate that children contribute a significant proportion of the disease burden and suffer severe TB-related morbidity and mortality.2 In 2011, TB incidence among children was estimated at 490 000, equivalent to about 6% of the total number of 8.7 million incident cases,3 and the proportion of children in the high-burden countries is estimated to be higher.4 5
Information on the cause of death among children in developing countries is difficult to ascertain. Most childhood deaths occur at home6 and reliable medical information on causes of death is therefore lacking. According to verbal autopsy studies, acute respiratory infection is one of the most important causes of mortality among children in low-income countries.7 8 Necropsy studies conducted in Africa have shown that TB rivals acute bacterial and viral pneumonia as a major cause of death from respiratory disease in children from endemic areas.9
An intervention known to contribute to the reduction of morbidity and mortality due to TB is isoniazid preventive therapy (IPT). Isoniazid was recommended for TB preventive therapy during the 1960s after large, well-conducted randomised controlled trials that included a total of nearly 70 000 people of all ages.10 After all this time, isoniazid continues to be the drug of choice,11 and WHO recommends that all TB contacts under the age of 5 years should receive at least 6 months of IPT. No recent meta-analysis of IPT for TB-exposed children in general has been made; a recent Cochrane review concluded that there was not enough evidence for general recommendation of IPT for HIV-infected children.12 The use of isoniazid in low income countries is limited by difficulties of ruling out TB disease before initiation,13 liver toxicity14–16 and poor adherence.17 IPT has been shown to be effective in recent tuberculin skin test (TST) converters and recent contacts of identified cases of TB disease.17 18 In one study of IPT in HIV-infected individuals, a 20% reduction of mortality was found in those with positive TST.18 The effect of such preventive therapy in children, however, is not well established.
The present study examined the impact of IPT on mortality in children less than 5 years of age exposed to intrathoracic TB at home in an urban area of Bissau. In a previous study in the same community, we found that exposure to TB at home was associated with 66% excess mortality compared with community control children not exposed to TB at home.19 The aim of the present study was to compare mortality between exposed children on IPT and community control children, and to compare this relative mortality to the previously observed excess mortality.
Materials and methods
Setting
The study was conducted as a prospective cohort study from 1 September 2005 to 31 October 2007 in six areas covered by the Bandim Health Project (BHP), a demographic surveillance site, located in Bissau, the capital city of Guinea-Bissau, where the prevalence of HIV1 and HIV2 among adults was 4.6% and 4.4%, respectively.20 The population, which is currently around 102 000, is followed through regular censuses and registered with information on sex, ethnic background, date of birth, death and migration as well as additional data on socioeconomic factors. Information regarding hospitalisations and deaths is collected every 3 months for children under 3 years of age. Information about children older than 3 years of age and adults is obtained from general censuses carried out approximately every third year. All paediatric hospitalisations from the study area have been registered since 1990. The incidence of adult intrathoracic TB in the area is high, 471/100 000 person-years.21
Owing to difficulties in obtaining specific causes of deaths in our setting, all-cause mortality was used as the main outcome measurement of the effect of IPT.
The reliability of population and mortality data in the study setting is a huge strength, which makes this a unique and important study that would be difficult to duplicate in other TB endemic areas.
Houses and household contacts
Houses in the study area are one-storey, rectangular constructions, usually with 6–8 rooms, and are inhabited by 2–4 households (families), which can be extended families or not. The majority of houses do not have an internal ceiling, leaving a large gap between the internal walls and the roof. Households were defined as the extended family sharing the same space in the house, as well as eating from the same pot.
Recruitment of participants and patients
Identification of adult TB index cases
Since May 1996, a TB surveillance system, implemented in collaboration with the national TB hospital (‘Hospital Raoul Follereau’), has identified adult (≥15 years) intrathoracic TB cases using passive and active case finding.21 As previously described in more detail,21 an intrathoracic TB case was defined as an adult with symptoms of TB with sputum smear microscopy positive or negative for acid-fast bacilli, presenting abnormalities in the chest x-ray (CXR) with no improvement on treatment with broad spectrum antibiotics for 2 weeks.
Enrolment in the IPT cohort
Children less than 5 years of age and living in the same house when the adult TB case started treatment were eligible for inclusion in the IPT cohort. Exposure was set to start at the later of the following two dates: 3 months before treatment or date of registration. The children were followed until 5 years of age contributing follow-up time until the date of the last follow-up information. Children lost to follow-up were censored.
Prior to the initiation of IPT, the children were investigated for TB disease in a clinical examination for signs and symptoms using the Keith Edwards score.22 If the investigation suggested TB disease, the children were submitted to a careful and thorough assessment of all evidence from history, clinical examination and relevant investigations, for example, laboratory examination, including HIV testing and CXR. Broad spectrum antibiotics were administered for 10–15 days. Children who failed to improve clinically and radiologically after 2 weeks of broad spectrum antibiotics, and without other explanatory disease, were given a full TB treatment regimen according to the national protocol. Antiretroviral (ARV) treatment was not available at the time of the study and HIV testing was therefore not generally performed. It was only performed when the investigation suggested TB. Children who developed signs and symptoms suggestive of TB disease while on IPT were evaluated and treated in a similar way.
Children with TB disease, as well as those who did not give consent or were absent from the first, second or third visit or at enrolment consultation, were excluded from the IPT cohort.
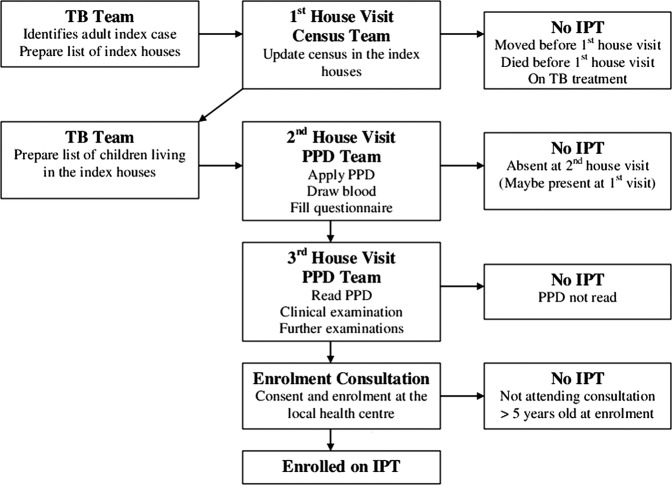
There were several steps in the enrolment procedure as depicted in figure 1. Once an adult TB case from the study area was identified, a project assistant went to the patient's house to update the census for the families living in the house, and socioeconomic and demographic information was noted on the questionnaire. Following the census update, a field assistant and a nurse visited the house to apply TSTs. After 48–72 h, the house was visited by the nurse who read the TST and referred potential TB cases for further clinical examination. Children without TB disease were eligible for enrolment in the IPT cohort regardless of the TST result and were invited to attend the enrolment visit at the local health centre. Eligible children who did not show up at inclusion were traced again. If not found, they were considered absent, but still followed up using basic census information. Owing to the limited time frame, logistic reasons and limited funding, they were not included later.
Figure 1.
Illustration of the inclusion process.
For children enrolled in the IPT programme, isoniazid tablets were administered at 5 mg/kg/day together with pyridoxine (vitamin B6) tablets for 9 months. The vitamin B6 dosage was 25 mg for children receiving <100 mg of isoniazid and 50 mg for children receiving >100 mg of isoniazid.23 The medicine was provided at the house every 2 weeks by a field assistant. Study children were visited after 1, 4, 7 and 9 months of IPT. The follow-up visits at 1 and 7 months were performed by the research clinician at the local health centre and at 4 and 9 months by a field assistant at the child’s home. Evaluation at follow-up visits included questions about side effects and a physical assessment of signs and symptoms of hepatotoxicity, for example, jaundice and vomiting. The cohort and study routines are described in detail elsewhere.24 The initially intended IPT enrolment period was September 2005–October 2007 with 9 months of follow-up to June 2008. However, children continued to be enrolled on IPT until the end of the study period in June 2008.
Pre-IPT cohort
As previously described, children less than 5 years of age and living in the same house as an adult index TB case at the time of initiation of treatment during the period May 1996–July 1998 were retrieved from the BHP register.19 To assess the impact of TB exposure at home in the absence of IPT, their mortality was compared with the mortality of children living in the study area who had not been exposed to TB at home, during the same period.
Groups in the study
In the pre-IPT cohort, we had two groups: TB-exposed children and community control children. In the IPT cohort, we also intended to have two groups: TB-exposed children on IPT and community control children. As we failed to include all exposed children in the IPT programme, a third group arose: TB-exposed children not on IPT.
Effect-size calculation
For the pre-IPT cohort, our study protocol initially estimated 700 index cases/houses during a 4.5-year period. An average of three children <5 years of age per house and a mean follow-up time of 2.25 years (half of the 4.5-year study period) would yield 2100 children with approximately 4725 child-years of observation. We anticipated the TB surveillance system to identify an increased number of TB patients during the IPT period. An estimated 300 index cases per year during a 2.5-year period would give 750 index cases. An average of three children <5 years of age per house and a mean follow-up time of 1.25 years (half of the 2.5-year study period) would yield 2250 children with approximately 2813 child-years of observation. Assuming a 7% annual mortality for the pre-IPT cohort, we initially expected to be able to detect a 27% mortality reduction in the IPT cohort.
Several factors reduced the actual power of the study. Owing to a civil war in Guinea Bissau, we limited follow-up for the pre-IPT cohort to the period from February 1996 to June 1998 before the war. Difficulties in locating the adult TB cases became greater in the IPT cohort, lowering the number of identified children. In addition, <5-mortality dropped considerably more than we had anticipated.
Ethical approval
The legal guardians (parents) or caregivers were informed about the study in writing (Portuguese) and verbally in the common language, creole, before the child was enrolled in the IPT study. Informed consent was obtained from all the parents or caregivers before enrolment. The study protocol was approved by the Guinea-Bissau National Research Coordination and Ethics Committee.
Statistical analysis
Data regarding adult TB cases were obtained from the general TB identification system in the study area while demographic information was taken from the basic surveillance system of the BHP. Statistical analyses were conducted in STATA V.10.
Similar to the analysis of the impact of TB exposure in the absence of IPT, the average delay from onset of symptoms to initiation of TB treatment was assumed to be 3 months. Hence, children registered in the same house as an adult index TB case 0–3 months before treatment were considered exposed. The effect of exposure on mortality was evaluated by rate ratios from a Cox analysis with age as underlying time. Neonatal mortality in Guinea-Bissau is very high. To be exposed, a child had to be born and registered at the time of exposure. Consequently, only a few children were exposed before 3 months of age. Therefore, we have chosen to start the analyses at 3 months of age.
IPT treatment was initiated in September 2005. To allow exposure time before IPT, follow-up started in July 2005. IPT enrolment for the present study ended in October 2007 with treatment ending in June 2008. Thus, the study period for the present study is July 2005–June 2008. Exposed children counted as unexposed controls until the time of exposure. Exposed children never receiving IPT counted as exposed without IPT from the start of exposure to the end of follow-up. Children subsequently enrolled on IPT counted as exposed without IPT from the start of exposure until the start of IPT and then as exposed with IPT to the end of follow-up. However, enrolment into the IPT programme continued after the enrolment period of the present study. Children enrolled on IPT after October 2007 were also counted as exposed on IPT even though they did not finish the treatment within the study period. Censoring the children enrolled on IPT after October 2007 at the time of IPT had little impact on the results. Some children who were present when the TB team visited the TB case house (figure 1) were enrolled on IPT even though they were not born or registered in the house before the TB case initiated treatment. According to the epidemiological definitions, these children were not exposed and they have been counted as unexposed with IPT. A separate analysis was conducted excluding these individuals.
An adjusted analysis was conducted including possible confounders related to child mortality: gender, ethnicity, district, socioeconomic status, schooling of the mother, child crowding (<5 years) and crowding among older individuals (>5 years). A score for socioeconomic status was calculated adding house indicators (yes=1; no/missing=0): corrugated iron roof, electricity, television and indoor toilet. A separate ‘Missing’ category was constructed when information was missing on all four variables. Crowding was defined as the number of individuals in the house of the TB case on 1 January 2007, the mid-point of the examined period. Crowding was included in the analysis as a linear predictor.
It was further examined whether differential mortality not related to TB exposure may have existed in the exposed houses. Mortality was compared between children living in the house of the adult TB case 3 years before TB exposure began and children living in the remaining houses. As for the main study,19 the comparison was made over a 3-year period from July 2002 to June 2005. The period was chosen so as to not overlap the study period.
Results
Index TB cases and included children
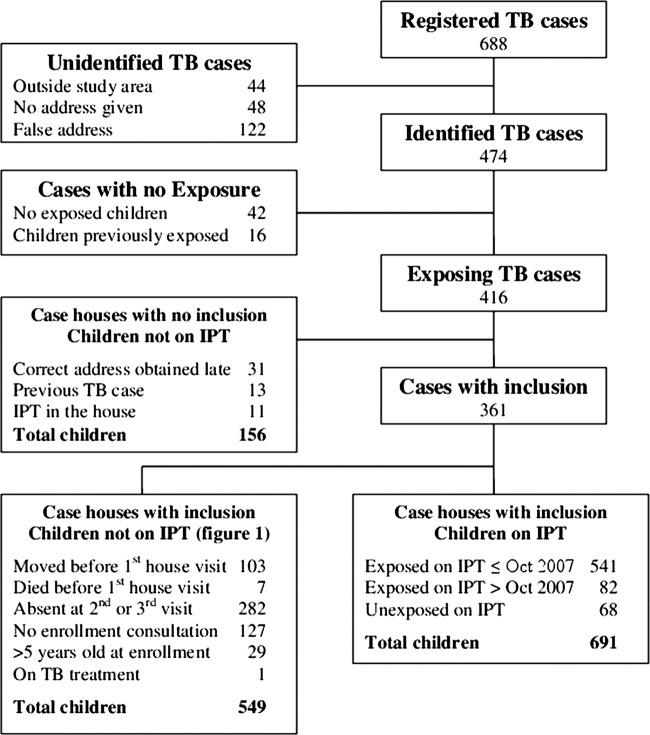
The surveillance system registered 688 adult intrathoracic TB cases from July 2005 to June 2008 (figure 2). Of these, 44 lived outside the study area, 48 gave no address and 122 gave a false address. Hence, a total of 474 TB cases were identified. For 42 identified cases, there were no eligible children less than 5 years of age and for a further 16 TB cases, the children were previously exposed before the present study period began, and were therefore not included. No inclusion was conducted for a total of 55 TB cases with 156 exposed children; for 31 cases, the correct address was only obtained long after treatment had been initiated and for 24 cases, IPT enrolment had previously been initiated in the house and a new enrolment was not initiated. Inclusion was initiated in the houses of 361 TB cases with 623 exposed and 68 unexposed children getting IPT. The latter happened when children born or registered after exposure were present at inclusion. A total of 705 exposed children never received IPT; there were 156 children from ‘case houses with no inclusion’ and 549 children from case houses with inclusion (figure 2). See baseline characteristics in online supplementary appendix table 1. Twenty-nine children were <5 years old when exposure started, but >5 years old at the time of enrolment. They did not therefore receive IPT. These children counted as exposed without IPT until the end of follow-up at 5 years of age. Follow-up ended before 3 months of age for 14 children leaving 691 to enter the analysis. A total of 21 907 children, not registered as exposed or on IPT, entered the survival analysis as controls.
Figure 2.
Flow chart of inclusion.
TB among exposed children
One child was on TB treatment at the time of inclusion. TB disease was diagnosed in two children after 3 and 5 weeks of IPT, respectively. Both diagnoses were based on clinical and CXR findings. One of these children was HIV-infected and was enrolled in an ARV programme.
TB exposure and mortality
Two children died during IPT. For a 6-month-old boy, hospital records stated the cause of death as severe malaria and anaemia. The mother of a 2-year-old girl, who died 2 months after the initiation of the IPT programme, reported that the child had had diarrhoea, cough and fever prior to death. Antibiotics and other medications had been prescribed. The research clinician had requested a CXR, but the result was never received. No further IPT children died during the follow-up period.
In all, the children on IPT (exposed and unexposed) contributed with 706 person-years of observation (PYO) in the analysis (figure 3). The children exposed but not on IPT contributed with 1023 PYO and the controls contributed with 30 713 PYO.
Figure 3.
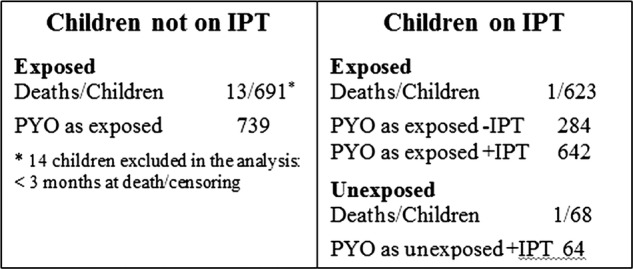
Person-years of observation (PYO).
Though not statistically significant, the exposed children receiving IPT had a lower death rate than the controls of unexposed children, the mortality rate ratio (MRR) being 0.30 (95% CI 0.1 to 1.2; table 1). This estimate changed little when controlled for background factors (tables 1 and 2). Some children had follow-up time as exposed before starting IPT and some exposed children did not receive IPT (figure 1). In this group of TB-exposed children without IPT, the MRR was 0.97 compared with the controls (95% CI 0.6 to 1.7; table 1).
Table 1.
The effect of exposure on mortality according to age
| Age (months) | On IPT* D/PYO | Exposed no IPT D/PYO | Unexposed D/PYO | MRR† IPT/unexposed | MRR‡ IPT/unexposed | MRR† No IPT/unexposed | MRR‡ No IPT/unexposed |
|---|---|---|---|---|---|---|---|
| 3–11 | 1/28 | 2/138 | 184/6204 | 1.40 (0.2 to 9.8) | 1.44 (0.2 to 11) | 0.51 (0.1 to 2.0) | 0.52 (0.1 to 2.1) |
| 12–35 | 1/306 | 10/509 | 226/14620 | 0.23 (0.0 to 1.7) | 0.23 (0.0 to 1.6) | 1.29 (0.7 to 2.4) | 1.28 (0.7 to 2.4) |
| 36–60 | 0/371 | 1/376 | 46/9888 | 0 | 0 | 0.57 (0.1 to 4.1) | 0.65 (0.1 to 4.6) |
| All | 2/706 | 13/1023 | 456/30713 | 0.30 (0.1 to 1.2) | 0.30 (0.1 to 1.2) | 0.97 (0.6 to 1.7) | 0.98 (0.6 to 1.7) |
Unexposed: community sample.
*Include unexposed children on IPT.
†MRR from a model with age as underlying time.
‡MRR from a model with age as underlying time, adjusted for gender, ethnicity, district, socioeconomic status, schooling of the mother and child crowding.
D/PYO, deaths/person-years of observation; IPT, isoniazid preventive therapy; MRR, mortality rate ratio.
Table 2.
Adjusted analysis on the overall effect of exposure on mortality from July 2005 to June 2008
| MRR | |
|---|---|
| Exposed with IPT vs controls | 0.30 (0.1 to 1.2) |
| Exposed without IPT vs controls | 0.98 (0.6 to 1.7) |
| Gender | |
| Male | 1 |
| Female | 0.93 (0.8 to 1.1) |
| Ethnicity | |
| Pepel | 1 |
| Balanta | 1.00 (0.7 to 1.4) |
| Manjaco/Mancanha | 0.84 (0.6 to 1.1) |
| Mandinga/Fula | 0.59 (0.5 to 0.8) |
| Others | 0.88 (0.7 to 1.2) |
| Missing | 2.32 (0.8 to 6.5) |
| District | |
| Bandim 1 | 1 |
| Bandim 2 | 1.01 (0.7 to 1.4) |
| Belem | 0.64 (0.4 to 1.0) |
| Mindara | 0.72 (0.4 to 1.2) |
| Cuntum 1 | 0.97 (0.8 to 1.2) |
| Cuntum 2 | 0.92 (0.6 to 1.3) |
| Socioeconomic score | |
| 1 | 1 |
| 2 | 0.89 (0.6 to 1.3) |
| 3 | 0.98 (0.6 to 1.6) |
| 4 | 0.83 (0.5 to 1.3) |
| Missing | 0.87 (0.4 to 1.9) |
| Schooling of mother (years) | |
| 0–3 | 1 |
| 4–6 | 0.93 (0.7 to 1.2) |
| 7–9 | 0.72 (0.5 to 1.0) |
| 10+ | 0.46 (0.3 to 0.7) |
| Missing | 1.00 (0.7 to 1.4) |
| Crowding <5 | 0.98 (0.9 to 1.1) |
| Crowding >5 | 1.00 (1.0 to 1.0) |
IPT, isoniazid preventive therapy; MRR, mortality rate ratio.
There were 68 children on IPT who were not formally exposed to TB because the child was born or registered after exposure occurred in the house. Excluding these from the IPT group, we observed one death from 642 PYO giving an MRR of 0.16 (0.0 to 1.1). Comparing with community controls, all exposed children, irrespective of whether they received IPT or no IPT, had an MRR of 0.71 (0.4 to 1.2).
Comparison of mortality among TB-exposed children in the absence and presence of IPT
We previously found that the excess mortality after exposure to TB only started 6 months after exposure.19 We made a similar analysis for the period 2005–2008; table 3 shows the comparison of exposed children without IPT and unexposed community control children, stratified by time since exposure and age at exposure.
Table 3.
Illustrating mortality rate ratios comparing exposed children without isoniazid preventive therapy and unexposed children
| Age at exposure (months) | ||||
| Months since exposure | 0–11 | 12–35 | 36–59 | Total |
| 0–5 months | 0 | 1.36 (0.5 to 3.7) | 0 | 0.54 (0.2 to 1.5) |
| 6–11 months | 2.06 (0.7 to 6.5) | 2.18 (0.5 to 8.8) | 0 | 1.65 (0.7 to 4.0) |
| 12+ months | 1.93 (0.6 to 6.0) | 0 | 13.1 (1.7 to 100) | 1.32 (0.5 to 3.5) |
| Total | 0.93 (0.4 to 2.1) | 1.26 (0.6 to 2.8) | 1.06 (0.1 to 7.6) | 0.97 (0.6 to 1.7) |
Exposure is stratified by time since exposure and age at exposure.
Restricted to the period after 6 months, the death rate relative to community controls in 2005–2008 was 0.32 (0.1 to 1.3) for children with IPT and 1.49 (0.8 to 2.9) for children without IPT. The MRR comparing exposed children with and without IPT was 0.21 (0.0 to 1.1).
It was furthermore examined whether the effect in the exposed house was caused by lower mortality unrelated to TB exposure and IPT. The analysis comparing mortality in the exposed houses 3 years earlier (2002–2005) showed that the overall mortality among children in the houses which later had TB cases was the same as the mortality in the control houses, the MRR being 1.04 (0.7 to 1.5; table 4).
Table 4.
Comparing children living in the house of a tuberculosis case 3 years before exposure starts
| Age months | Exposed deaths/PYO | Unexposed deaths/PYO | MRR |
|---|---|---|---|
| 3–11 | 6/151 | 286/5849 | 0.82 (0.4 to 1.8) |
| 12–35 | 20/679 | 357/14020 | 1.20 (0.8 to 1.9) |
| 36–60 | 7/710 | 127/11547 | 0.90 (0.4 to 1.9) |
| All | 33/1540 | 770/31418 | 1.04 (0.7 to 1.5) |
The period is from July 2002 to June 2005.
MRR from a model with age as underlying time.
MRR, mortality rate ratio; PYO, person-years of observation.
In a previous study in the same community, exposure to TB at home was associated with an MRR of 1.66 (1.2 to 2.3) compared with community control children.19 The present study assessed whether the excess mortality could be reduced by implementing an IPT programme. Both the MRR of 0.30 among children who received IPT and the overall MRR of 0.71 for all exposed children in the 2005–2008 period were significantly lower than the previously observed MRR of 1.66 (respective tests of interaction, p=0.01 and 0.004).
It should be noted that the general child mortality declined markedly between the two periods studied (table 5); among community controls, mortality declined by more than 50%. Given that excess mortality associated with TB exposure was 66% in the period from 1996 to 1998, the death rate directly due to TB exposure was 19/1000 of the total 48/1000. If the impact of TB exposure had been similar during the period from 2005 to 2008, the TB-exposed children without IPT should have had a mortality of 15/1000, as the unexposed children in the community, with an additional 19/1000 due to TB exposure, that is, a total of 34/1000. However, their rate was only 13/1000 (table 5).
Table 5.
Mortality rates (MR) in the two periods with studies of the impact of TB exposure at home
| MR/1000 PYO (deaths/PYO) |
||
|---|---|---|
| 1996–1998 | 2005–2008 | |
| Children without known TB exposure | 35 (526/15100) | 15 (456/30713) |
| Exposed children without IPT | 48 (41/851) | 13 (13/1023) |
| Exposed children with IPT | 3 (2/706) | |
IPT, isoniazid preventive therapy; PYO, person-years of observation; TB, tuberculosis.
Discussion
In the present study, we have shown the impact of the IPT programme on mortality among children less than 5 years of age exposed to adult TB. Children exposed to TB in 1996–1998, when IPT was not available in the area, suffered a 66% excess mortality compared with unexposed community control children. The MRR was inverted in 2005–2008 with markedly (though not significantly) lower mortality among exposed children on IPT compared with the community control children. It should be emphasised that comparing data from different time periods is not straightforward, as the conditions may have changed in many ways that cannot be completely deduced, and the results can be biased. In our situation, the child mortality has dropped markedly between these two time-periods. However, the excess mortality from 1996 to 1998 has changed to a marked trend of lower mortality in 2005–2008, which cannot solely be attributed to the drop in child mortality, and our data suggest that this is partly due to the introduction of IPT.
Unexpected observations
Mortality in the study area declined dramatically between the two-study periods and mortality declined more than expected among both exposed children receiving IPT and exposed children not receiving IPT. Based on the experience from 1996 to 1998 period, TB exposure at home in the absence of IPT should have been associated with a 19/1000 person-years excess mortality.
Guinea-Bissau has had one of the highest levels of child mortality in sub-Saharan Africa. However, despite the recent HIV epidemic, death rates have decreased drastically over the last decade following the same pattern observed in the other sub-Saharan African countries.25–27 The reasons for the mortality decline are not fully understood, but systematic annual vitamin A campaigns and the marked decrease in malaria incidence are likely to have contributed.
The strong trend towards less mortality among IPT-treated children could possibly be linked to increased attention to these children including easier access to other forms of treatment and more attention from the parents. However, this would be unlikely to explain why mortality also declined more than expected among the children who did not receive IPT. This may suggest that all TB-associated mortality is not directly due to clinical TB disease, but may be due to interactions with other infections. If the incidence or severity of these other infections goes down, as has happened in Bissau, the mortality associated with TB exposure would also decline.
There may be a slight trend towards better outcome in the contacts receiving IPT, although not significant. Travelling or absence was the main reason why exposed children were not enrolled in the IPT programme. These children may therefore have had little contact with the TB case. Another possible reason may be selection bias so that the group of children going through 9 months of treatment had mothers who were better able to take care of them (although no differences in education level or socioeconomic index was seen), or simply that the children at home at the time of inclusion were not exposed to the possible dangers of travelling.
Table 3 showed no difference in overall mortality between unexposed controls and exposed who did not get IPT, but did indeed show an effect among the oldest children with +12 months since exposure. With the limited number of events available, we were not able to show an overall difference in mortality which would be expected. Yet, despite the limited number of events, it was possible to show a significant effect of exposure in a subgroup of the untreated children, namely among those most likely affected, that is, the mobile children +3 years of age with the longest observation time since exposure.
Interpretation and consistency with previous studies
Children exposed to TB at home had excess mortality from around 6 months after exposure in the present study as well as in the previous study from 1996 to 1998. In both studies, we showed that the TB houses 3 years earlier had exactly the same mortality as community controls. It therefore seems unlikely that general social conditions in houses with TB cases explain the higher mortality of TB-exposed children. Furthermore, children enrolled on IPT in the IPT cohort (compared with community controls) had significantly lower mortality than the TB exposed children not receiving IPT in the pre-IPT cohort (compared with community controls). Hence, our results suggest that the use of isoniazid plays an important role in decreasing mortality in children exposed to TB.
Studies conducted in South Africa and Zambia have reported isoniazid to be highly effective in reducing the mortality and incidence of TB in HIV-infected children and adults living in an area with a high prevalence of TB.14 28 29 Our findings support those studies, as well as observations made by Dr Lincoln30 in the early 1950s showing that isoniazid chemotherapy reduced the case death from primary TB among children.
Implications and conclusions
In the period 1996–1998, an excess mortality of 66% was found in children in TB households compared with controls. This excess mortality was reduced in the cohort of children receiving IPT from 2005 to 2008, and the all-cause mortality in children from TB households was lower than the controls, though not reaching statistical significance. When comparing the mortality between the exposed children to the controls across the two different time periods, a significant difference in mortality was found. Furthermore, in 2005–2008, there was a trend that exposed that children receiving IPT had lower all-cause mortality than exposed children not receiving IPT. All our data indicate that IPT should be part of the standard TB programme and would have a large impact on child mortality in low-income countries.
Supplementary Material
Acknowledgments
We thank the staff of the Bandim Health Project in Bissau and Satens Serum Institute in Denmark, and the DANIDA's ENRECA Project, for their support, and all mothers of the children involved in this study for their collaboration.
Footnotes
Contributors: PG, PA and VFG designed the study. VFG supervised and ran the data collection; AA ran statistical analysis; CdSV, FJV and LJC ran inclusion and follow–up; IO supported data handling; CW and PG carried out adult TB study, and GL contributed to the writing of the article. VFG drafted the article and all authors contributed to the final version.
Funding: This study was supported by Sida/SAREC (Swedish International Development Cooperation Agency/Department for Research Cooperation), grant number SWE-2005–111 and Danish Agency For International Development (DANIDA), grant number 502-SSI.
Competing interests: None.
Ethics approval: Comité Nacional de Etica em Saude, Ministry of Health, Guinea-Bissau.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Marais BJ, Obihara CC, Warren RM, et al. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis 2005;9:1305–13 [PubMed] [Google Scholar]
- 2.Marais BJ, Gie RP, Schaaf HS, et al. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med 2006;173:1078–90 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Global Tuberculosis Report. WHO/HTM/TB/2012.6.
- 4.Donald PR. Childhood tuberculosis: out of control? Curr Opin Pulm Med 2002;8:178–82 [DOI] [PubMed] [Google Scholar]
- 5.Murray CJ, Styblo K, Rouillon A. Tuberculosis in developing countries: burden, intervention and cost. Bull Int Union Tuberc Lung Dis 1990;65:6–24 [PubMed] [Google Scholar]
- 6.Mobley CC, Boerma JT, Titus S, et al. Validation study of a verbal autopsy method for causes of childhood mortality in Namibia. J Trop Pediatr 1996;42:365–9 [DOI] [PubMed] [Google Scholar]
- 7.Etard JF, Le Hesran JY, Diallo A, et al. Childhood mortality and probable causes of death using verbal autopsy in Niakhar, Senegal, 1989–2000. Int J Epidemiol 2004;33:1286–92 [DOI] [PubMed] [Google Scholar]
- 8.Sacarlal J, Nhacolo AQ, Sigauque B, et al. A 10 year study of the cause of death in children under 15 years in Manhica, Mozambique. BMC Public Health 2009;9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 2002;60:985–90 [DOI] [PubMed] [Google Scholar]
- 10.Ferebee SH, Mount FW, Comstock G. The use of chemotherapy as a prophylactic measure in tuberculosis. Ann NY Acad Sci 1963;106:151–6 [DOI] [PubMed] [Google Scholar]
- 11.Martinez SA, Calpe Calpe JL, Llavador Ros G, et al. Primary prevention and treatment of latent tuberculosis infection with isoniazid: efficacy of a control program, 1997–2002. Arch Bronconeumol 2005;41:27–33 [DOI] [PubMed] [Google Scholar]
- 12.Gray DM, Young T, Cotton M, et al. Impact of tuberculosis preventive therapy on tuberculosis and mortality in HIV-infected children. Cochrane Database Syst Rev 2009, (1): CD006418. [DOI] [PubMed]
- 13.Lugada ES, Watera C, Nakiyingi J, et al. Operational assessment of isoniazid prophylaxis in a community AIDS service organisation in Uganda. Int J Tuberc Lung Dis 2002;6:326–31 [PubMed] [Google Scholar]
- 14.Grant AD, Charalambous S, Fielding KL, et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA 2005;293:2719–25 [DOI] [PubMed] [Google Scholar]
- 15.Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA 1999;281:1014–18 [DOI] [PubMed] [Google Scholar]
- 16.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med 2003;168:443–7 [DOI] [PubMed] [Google Scholar]
- 17.Heal G, Elwood RK, FitzGerald JM. Acceptance and safety of directly observed versus self-administered isoniazid preventive therapy in aboriginal peoples in British Columbia. Int J Tuberc Lung Dis 1998;2:979–83 [PubMed] [Google Scholar]
- 18.Churchyard GJ, Scano F, Grant AD, et al. Tuberculosis preventive therapy in the era of HIV infection: overview and research priorities. J Infect Dis 2007;196(Suppl 1):S52–62 [DOI] [PubMed] [Google Scholar]
- 19.Gomes VF, Andersen A, Wejse C, et al. Impact of tuberculosis exposure at home on mortality among children less than 5years old in Guinea-Bissau. Thorax 2011;66:163–7 [DOI] [PubMed] [Google Scholar]
- 20.da Silva ZJ, Oliveira I, Andersen A, et al. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS 2008;22:1195–202 [DOI] [PubMed] [Google Scholar]
- 21.Gustafson P, Gomes VF, Vieira CS, et al. Tuberculosis in Bissau: incidence and risk factors in an urban community in sub-Saharan Africa. Int J Epidemiol 2004;33:163–72 [DOI] [PubMed] [Google Scholar]
- 22.Narayan S, Mahadevan S, Serane VT. Keith Edwards score for diagnosis of tuberculosis. Indian J Pediatr 2003;70:467–9 [DOI] [PubMed] [Google Scholar]
- 23.Medecins Sans Frontieres Medicaments oraux. 2nd edn Medicaments Essentiels: Guide Pratique d´utilisation, 1993:78 [Google Scholar]
- 24.Gomes VF, Wejse C, Oliveira I, et al. Adherence to isoniazid preventive therapy in children exposed to tuberculosis: a prospective community-based study from Guinea-Bissau. Int J Tuberc Lung Dis 2011;15:1637–43 [DOI] [PubMed] [Google Scholar]
- 25.Delaunay V, Etard JF, Preziosi MP, et al. Decline of infant and child mortality rates in rural Senegal over a 37-year period (1963–1999). Int J Epidemiol 2001;30:1286–93 [DOI] [PubMed] [Google Scholar]
- 26.Greenwood BM, Bradley AK, Byass P, et al. Evaluation of a primary health care programme in the Gambia. II. Its impact on mortality and morbidity in young children. J Trop Med Hyg 1990;93:87–97 [PubMed] [Google Scholar]
- 27.Hill AG, MacLeod WB, Sonko ST, et al. Improvements in childhood mortality in the Gambia. Lancet 1999;354:75. [DOI] [PubMed] [Google Scholar]
- 28.Mwinga A, Hosp M, Godfrey-Faussett P, et al. Twice weekly tuberculosis preventive therapy in HIV infection in Zambia. AIDS 1998;12:2447–57 [DOI] [PubMed] [Google Scholar]
- 29.Zar HJ, Cotton MF, Strauss S, et al. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: randomised controlled trial. BMJ 2007;334:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lincoln EM. The effect of antimicrobial therapy on the prognosis of primary tuberculosis in children. Am Rev Tuberc 1954;69:682–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.