Abstract
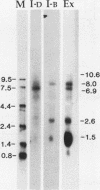
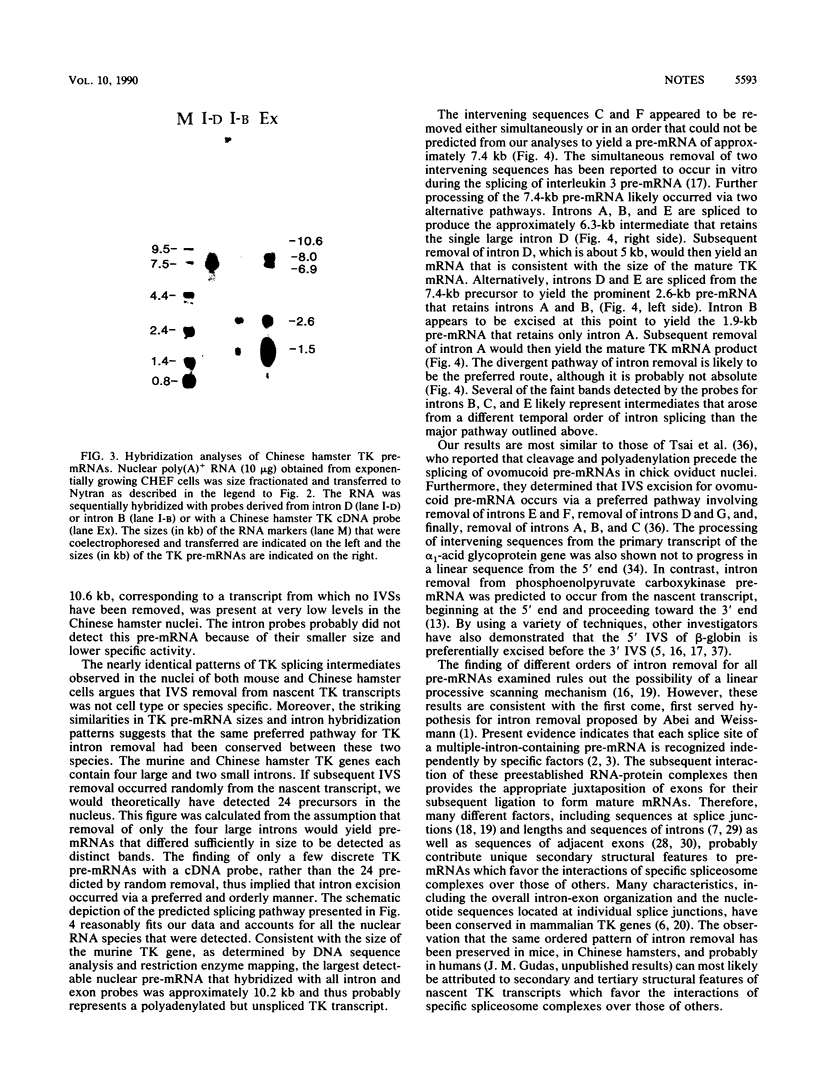
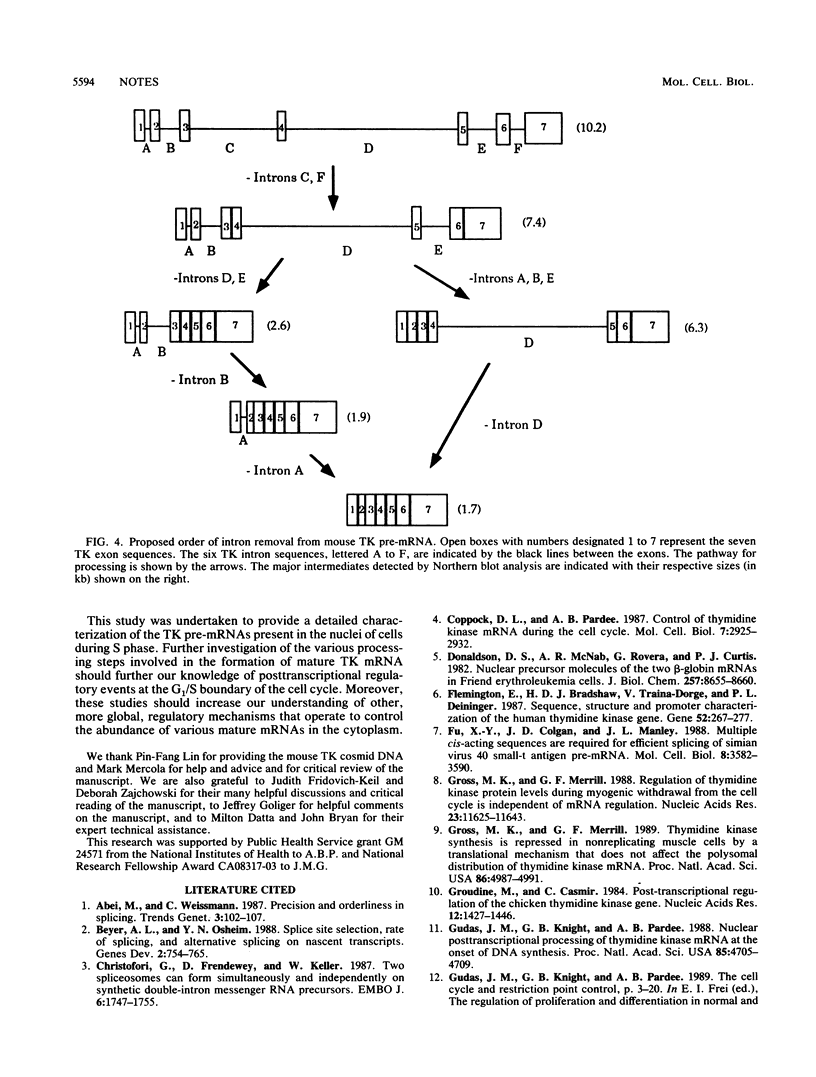
Concomitant with the onset of S phase, a series of thymidine kinase (TK) splicing intermediates as well as mature TK mRNA accumulates in the nucleus of BALB/c 3T3 cells. Most of the TK splicing intermediates are retained by oligo(dT)-cellulose chromatography, and, therefore, 3' end formation and polyadenylation probably precede the splicing of TK pre-mRNAs. We have further characterized the TK pre-mRNAs that are present in the nuclei of S-phase cells by using specific probes derived from each of the six TK intervening sequences. Based on the sizes of the pre-mRNAs and their patterns of hybridization with these intron probes, we propose a pathway for intron removal from nascent TK transcripts. Intron excision occurred by a preferred, but not necessarily obligatory, order which appears to have been conserved in mouse and Chinese hamster cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Christofori G., Frendewey D., Keller W. Two spliceosomes can form simultaneously and independently on synthetic double-intron messenger RNA precursors. EMBO J. 1987 Jun;6(6):1747–1755. doi: 10.1002/j.1460-2075.1987.tb02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppock D. L., Pardee A. B. Control of thymidine kinase mRNA during the cell cycle. Mol Cell Biol. 1987 Aug;7(8):2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson D. S., McNab A. R., Rovera G., Curtis P. J. Nuclear precursor molecules of the two beta-globin mRNAs in Friend erythroleukemia cells. J Biol Chem. 1982 Aug 10;257(15):8655–8660. [PubMed] [Google Scholar]
- Flemington E., Bradshaw H. D., Jr, Traina-Dorge V., Slagel V., Deininger P. L. Sequence, structure and promoter characterization of the human thymidine kinase gene. Gene. 1987;52(2-3):267–277. doi: 10.1016/0378-1119(87)90053-9. [DOI] [PubMed] [Google Scholar]
- Fu X. Y., Colgan J. D., Manley J. L. Multiple cis-acting sequence elements are required for efficient splicing of simian virus 40 small-t antigen pre-mRNA. Mol Cell Biol. 1988 Sep;8(9):3582–3590. doi: 10.1128/mcb.8.9.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. K., Merrill G. F. Regulation of thymidine kinase protein levels during myogenic withdrawal from the cell cycle is independent of mRNA regulation. Nucleic Acids Res. 1988 Dec 23;16(24):11625–11643. doi: 10.1093/nar/16.24.11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M. K., Merrill G. F. Thymidine kinase synthesis is repressed in nonreplicating muscle cells by a translational mechanism that does not affect the polysomal distribution of thymidine kinase mRNA. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4987–4991. doi: 10.1073/pnas.86.13.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Casimir C. Post-transcriptional regulation of the chicken thymidine kinase gene. Nucleic Acids Res. 1984 Feb 10;12(3):1427–1446. doi: 10.1093/nar/12.3.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas J. M., Knight G. B., Pardee A. B. Nuclear posttranscriptional processing of thymidine kinase mRNA at the onset of DNA synthesis. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4705–4709. doi: 10.1073/pnas.85.13.4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou M., Sekeris C. E., Hanson R. W. Processing of phosphoenolpyruvate carboxykinase (GTP) RNA in vivo. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4346–4350. doi: 10.1073/pnas.82.13.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer R., Müllner E., Seiser C., Wintersberger E. Cell cycle regulated synthesis of stable mouse thymidine kinase mRNA is mediated by a sequence within the cDNA. Nucleic Acids Res. 1987 Jan 26;15(2):741–752. doi: 10.1093/nar/15.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight G. B., Gudas J. M., Pardee A. B. Coordinate control of S phase onset and thymidine kinase expression. Jpn J Cancer Res. 1989 Jun;80(6):493–498. doi: 10.1111/j.1349-7006.1989.tb01664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K. M., Spritz R. A. In vitro splicing pathways of pre-mRNAs containing multiple intervening sequences? Mol Cell Biol. 1987 Oct;7(10):3428–3437. doi: 10.1128/mcb.7.10.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K. M., Spritz R. A. RNA splice site selection: evidence for a 5' leads to 3' scanning model. Science. 1983 Jun 24;220(4604):1351–1355. doi: 10.1126/science.6304877. [DOI] [PubMed] [Google Scholar]
- Lear A. L., Eperon L. P., Wheatley I. M., Eperon I. C. Hierarchy for 5' splice site preference determined in vivo. J Mol Biol. 1990 Jan 5;211(1):103–115. doi: 10.1016/0022-2836(90)90014-D. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Matkovich D. A. Genetic determinants of growth phase-dependent and adenovirus 5-responsive expression of the Chinese hamster thymidine kinase gene are contained within thymidine kinase mRNA sequences. Mol Cell Biol. 1986 Jun;6(6):2262–2266. doi: 10.1128/mcb.6.6.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A. Structure and expression of the Chinese hamster thymidine kinase gene. Mol Cell Biol. 1986 Jun;6(6):1998–2010. doi: 10.1128/mcb.6.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman H. B., Lin P. F., Yeh D. B., Ruddle F. H. Transcriptional and posttranscriptional mechanisms regulate murine thymidine kinase gene expression in serum-stimulated cells. Mol Cell Biol. 1988 Dec;8(12):5280–5291. doi: 10.1128/mcb.8.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Lieberman H. B., Yeh D. B., Xu T., Zhao S. Y., Ruddle F. H. Molecular cloning and structural analysis of murine thymidine kinase genomic and cDNA sequences. Mol Cell Biol. 1985 Nov;5(11):3149–3156. doi: 10.1128/mcb.5.11.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson K. E., Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9774–9777. doi: 10.1073/pnas.86.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Reed R. The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing. Nature. 1987 Feb 19;325(6106):673–678. doi: 10.1038/325673a0. [DOI] [PubMed] [Google Scholar]
- Merrill G. F., Hauschka S. D., McKnight S. L. tk Enzyme expression in differentiating muscle cells is regulated through an internal segment of the cellular tk gene. Mol Cell Biol. 1984 Sep;4(9):1777–1784. doi: 10.1128/mcb.4.9.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Reed R. The organization of 3' splice-site sequences in mammalian introns. Genes Dev. 1989 Dec;3(12B):2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiser C., Knöfler M., Rudelstorfer I., Haas R., Wintersberger E. Mouse thymidine kinase: the promoter sequence and the gene and pseudogene structures in normal cells and in thymidine kinase deficient mutants. Nucleic Acids Res. 1989 Jan 11;17(1):185–195. doi: 10.1093/nar/17.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Splicing of messenger RNA precursors. Science. 1987 Feb 13;235(4790):766–771. doi: 10.1126/science.3544217. [DOI] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Shiels B. R., Northemann W., Gehring M. R., Fey G. H. Modified nuclear processing of alpha 1-acid glycoprotein RNA during inflammation. J Biol Chem. 1987 Sep 15;262(26):12826–12831. [PubMed] [Google Scholar]
- Stewart C. J., Ito M., Conrad S. E. Evidence for transcriptional and post-transcriptional control of the cellular thymidine kinase gene. Mol Cell Biol. 1987 Mar;7(3):1156–1163. doi: 10.1128/mcb.7.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai M. J., Ting A. C., Nordstrom J. L., Zimmer W., O'Malley B. W. Processing of high molecular weight ovalbumin and ovomucoid precursor RNAs to messenger RNA. Cell. 1980 Nov;22(1 Pt 1):219–230. doi: 10.1016/0092-8674(80)90170-1. [DOI] [PubMed] [Google Scholar]
- Zeitlin S., Efstratiadis A. In vivo splicing products of the rabbit beta-globin pre-mRNA. Cell. 1984 Dec;39(3 Pt 2):589–602. doi: 10.1016/0092-8674(84)90466-5. [DOI] [PubMed] [Google Scholar]