Summary
Encephalopathy from hypoxic-ischemic injury is a major cause of morbidity and mortality in the term infant. Magnetic Resonance Imaging (MRI) has become the gold standard test in evaluating the nature and extent of injury. While imaging this population can be challenging, clinically important information can be obtained safely. Classical patterns of injury and the likely mechanisms that cause them are reviewed. These patterns include selective neuronal necrosis, parasagittal cerebral injury, periventricular leukomalacia, and ischemic perinatal stroke. Conventional images combined with additional techniques such as diffusion-weighted imaging, diffusion-tensor, spectroscopy, and MRA/MRV provide clues to both etiology and timing as well as long term prognosis. As the possibility of altering acute neurologic damage with interventions in the acute period becomes more of a reality, MRI will have a crucial role both in delineating which infants will likely have the most to gain, and also act as a biomarker to gauge response.
Keywords: Term encephalopathy, Neuroimaging, Cerebral Injury
Introduction
Neonatal encephalopathy occurs in 3–5/1000 live births (1) and presents many challenges to the clinician. Hypoxic-ischemic encephalopathy (HIE), the most common etiology (1 per 1000 live births), continues to have significant morbidity and mortality despite advances in neonatal intensive care. As many as 10–60% infants die in the newborn period, and up to 25% of survivors go on to develop poor long-term neurodevelopmental outcome. This population accounts for 15–28% of children that develop cerebral palsy (2), often with a more severe form than those without a history of encephalopathy (3). Magnetic resonance imaging (MRI) has proven value in evaluating infants with perinatal asphyxia (4, 5). The construction of a time frame for a potential injury has critical importance both from a medical and legal perspective. While some references state that only 8–15% of term infants with encephalopathy or seizures have evidence of perinatal asphyxia (6, 7), other sources state that 90% of these infants have evidence by both MRI and histology of perinatal acquired injuries (8). Conventional MRI along with diffusion and spectroscopic MRI provides information on the nature of the injury, clues on the timing, and the later prognosis in the term encephalopathic neonate. In the following article we will address optimization of MRI in the newborn along with the common patterns of cerebral injury in the asphyxiated term newborn.
Why Undertake Neuro-imaging and which modality should I employ?
History and physical exam are not sufficient to determine the nature and extent of brain injury and have variable prognostic value. Although cranial ultrasound is frequently employed in term infants with encephalopathy, it rarely discloses the true nature of the cerebral pathology. Cranial ultrasound (CUS) provides relatively poor contrast for lesions of the brain parenchyma. Acute stroke, for example, can be difficult to detect compared with CT and MRI (9). In addition, since ultrasound images are typically obtained through the anterior fontanel, there is a limited field of view that does not “see” the cerebral convexities where cortical neuronal injury occurs. Furthermore, image detail in the posterior fossa, which is relatively far from the transducer, is often poor.
Computed tomography (CT) has been available for approximately 30 years and continues to be widely used for term encephalopathic infants due to its speed and ease of acquisition. CT provides excellent views of bone and is also very sensitive for the detection of hemorrhage, which appears bright. It allows differentiation of white and grey matter, though the contrast between these two types of tissue is relatively low in comparison with MRI. CT scans usually require that the infant be removed from the ICU, which is a disadvantage compared to CUS. On the other hand, the scan time is shorter than that of a typical MRI study. Further, the infant is more readily accessible while in the scanner, in the event of an emergency, than for an MR scan, though there is a trend in MR magnet design towards more open magnet configurations which provide better patient access.
A further issue for CT is the exposure of the infant brain to ionizing radiation. There are two main areas of concern related to this exposure – firstly the risk of future malignancy and, secondly, cognitive impairment. Recently, Hall et al. (10), suggested that even low doses of ionizing radiation, similar to those delivered by CT scans, may adversely affect brain and cognitive development. Currently, it is unclear what long-term effects, if any, low doses of cranial irradiation, such as those delivered during a cranial CT scan, may have when administered during infancy, a phase of rapid brain development. Until such data are available, it is reasonable to restrict the use of this neuro-imaging technique to selected settings in which the information obtained from the imaging study is clearly of benefit to the patient.
In contrast, there have been no concerns over safety with magnetic resonance imaging (MRI). It is clear that MRI provides the best delineation of the pattern of injury and is the strongest predictor of neurodevelopmental outcome in the term encephalopathic newborn. Although the American Academy of Neurology recommended the utilization of MRI as the neuroimaging method of choice for term encephalopathy nearly a decade ago, it has not been universally adopted due to limited availability and access to MRI for sick infants.
How to Undertake MR Imaging in the Newborn
MRI in the encephalopathic newborn is challenging due to both the severity of illness and the limited experience by the MR technologist and neuroradiologist. All infants require a thorough search for any metallic objects that would interfere with the magnet. All MR compatible monitoring devices are placed prior to wrapping the infant. These include some form of cardio respiratory monitoring along with pulse oximetry. MRI compatible IV pumps, ventilators, and incubators are commercially available and may be necessary for critically ill infants.
To maximize signal to noise ratio, a dedicated neonatal head coil is ideal, but if not available, an adult knee coil can be used as a substitute. Specialized sequences are essential because of the high water content of the neonatal brain. The majority of centers use a 1T or 1.5T magnet, but there is an increasing use of 3T magnets in clinical research. Used correctly, it can acquire images with shorter acquisition time and greater anatomical resolution (11, 12).
To maximize the study and avoid motion artifact, infants must remain still. An organized approach can avoid wasting time on inadequate images. Feeding and bundling the infant about half an hour before the study often results in deep sleep with little movement, avoiding the need for sedation. Equipment such as vacuum papooses can be extremely helpful. Due to the noise from certain MRI sequences, ear protection is also recommended. We use neonatal earmuffs (Natus mini-muffs, Natus Medical Inc., San Carlos, CA), but other options such as moldable ear putty, or shielding with gauze are acceptable. If these techniques fail to keep the infant still, sedation can be used. Common agents used are midazolam hydrochloride (0.1 mg/kg), lorazepam (0.1mg/kg), or oral chloral hydrate (30–75 mg/kg)(5, 13–15). These medications should always be administered under the watchful eye of a physician experienced in sedating newborns.
The major patterns of brain injury in the encephalopathic term infant
The patterns of injury in the term newborn have been delineated on both MRI and neuropathology. For MRI, the common classification schemes separate lesions into either focal, multi-focal, or diffuse injury(16). In this article we review a neuropathological classification that was proposed by Volpe(1) and well visualized on MR imaging. The injury types discussed include selective neuronal necrosis, parasagittal cerebral injury, periventricular leukomalacia, and lastly, focal and multi-focal ischemic brain necrosis. While these lesions are discussed as separate discrete entities, overlap is common.
Selective Neuronal Necrosis
After exposure to ischemic injury, there is often necrosis of the neurons in a characteristic, often widespread pattern. This is the most common form of injury in the term hypoxic-ischemic infant with common overlap with other patterns of injury. Unlike parasagittal cerebral injury (see below), these lesions are not solely from hypotension. The high energy demand within neurons may account for their selective vulnerability (17). Four different patterns of neuronal necrosis are commonly seen. These patterns vary depending on the severity and timing of the initial insult.
The first form of selective neuronal necrosis that is seen is that of diffuse neuronal injury affecting the cerebral cortex, basal ganglia, hippocampus, brainstem, and cerebellum (Figure 1). Injury of this magnitude results form a severe, prolonged perinatal insult, such as total placental abruption, cord prolapse, or some form of vascular interruption. In term infants, the involvement of the cerebrum includes the peri-rolandic cortex, border zones in the cerebral cortex, the depths of the sulci, the hippocampus, and all regions of deep nuclear gray matter and thalamus. Other areas of involvement include brainstem, cerebellum (most commonly Purkinje cells), and less commonly, the anterior horn cells of the spinal cord. Not surprisingly, these diffuse lesions carry a poor prognosis.
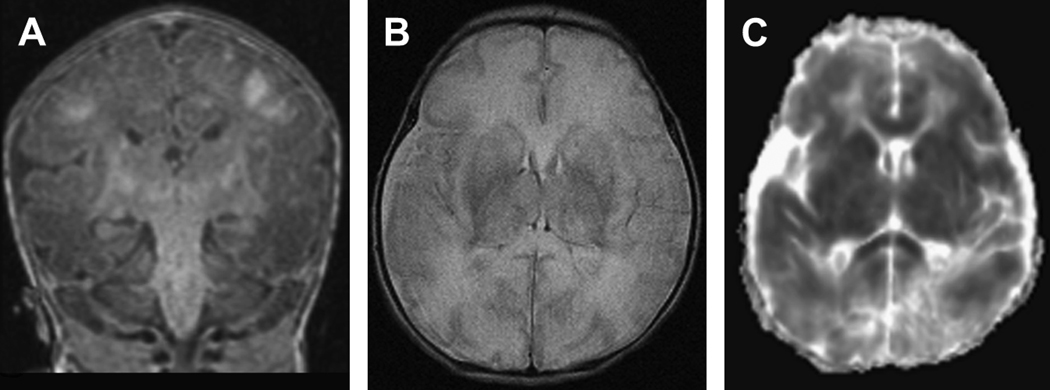
Figure 1.
Diffuse neuronal injury in a term infant with a history of severe hypoxic-ischemic injury. Images were acquired at 7 days of life. A) Coronal T1-weighted image shows interruption of the posterior limbs bilaterally with diffuse hyperintesities in the watershed regions of the gyri in a para-sagittal distribution. In addition, one can visualize the high signal in the deep nuclear gray matter. B) Axial T2-weighted image shows injury throughout the deep nuclear gray matter with diffuse white matter hyper intensity and loss of differentiation between white matter and the cortical ribbon. C) Axial diffusion-weighted image where restricted diffusion from injury (decreased ADC) is represented by dark lesions. This image shows diffuse diffusion restriction in the deep nuclear gray matter, white matter, and cortex bilaterally still apparent at day 7.
The second form of selective neuronal injury is the cerebral-deep nuclear neuronal injury which combines neuronal damage in the deep nuclear gray matter with injury in the cerebral cortex, usually the parasagittal areas of the peri-rolandic cortex (Figure 2). Affected areas of the deep nuclear gray matter that are more vulnerable include the putamen and the ventrolateral thalamus. This pattern accounts for 35–65% of term infants following hypoxic-ischemic insults (18–20). Insults are described as a “prolonged, partial insult” meaning that a moderate to severe vascular insult evolved in a more gradual manner. Like diffuse neuronal injury, these lesions carry with them a poor long term prognosis.
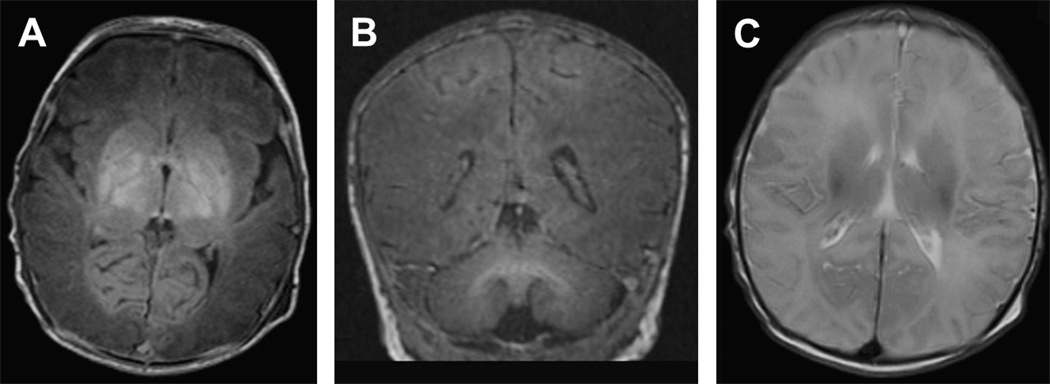
Figure 2.
Deep nuclear gray matter with cortical injury in a term infant with a history of severe hypoxic-ischemic injury. A) Axial T1-weighted image shows hyper intense severe injury throughout the deep nuclear gray matter. B) Coronal T1-weighted image shows the extension of this hyper intense injury from the deep nuclear gray matter up into the parasagittal region of the cortex. C) Axial T2-weighted image demonstrates low intensity extending from the DNGM into the parasagittal cortex bilaterally.
The third form is deep nuclear gray matter-brain stem which often results from an “abrupt- severe” insult. This pattern of neuronal injury only seems to affect the deep gray matter without cerebral involvement. Affected areas include the basal ganglia, the thalamus, and the tegmentum of the brainstem. Only with the increased use of MRI has the frequency of this pattern been appreciated. Long term prognosis like the other forms of deep nuclear grey matter injury is poor, although the severity of the brainstem lesion will influence mortality.
The final form of selective neuronal necrosis is pontosubicular (PSN) necrosis with injury to the neurons of the ventral pons and the subiculum of the hippocampi (21). Etiologies for this injury have included hypoxia, acute ischemia, hypocapnia, hyperoxemia, and hypoglycemia (22–25). The least common form of neuronal necrosis in the term infant, this pattern is more recognized in the premature infant, often associated with periventricular leukomalacia. Recent studies of by Bruck, et al., indicate that in PSN neuronal cell death is apoptotic in nature on pathological exam (26).
Parasagittal Cerebral Injury
Parasagittal cerebral injury is unique to the term infant, occurring over the cerebral cortex and the underlying sub cortical white matter of the parasagittal regions. Parietal-occipital regions are the most commonly affected. Injury is usually bilateral in distribution and results from mild to moderate hypotension in this “watershed” region between the anterior, middle, and posterior cerebral circulation. This lesion is often referred to as a “watershed injury” in many MRI publications. The neuropathology is not well established in the human, as most affected infants survive. As isolated lesions, parasagittal cerebral injury in the term neonate progresses to mild to moderate neurodevelopmental delay (both motor and cognitive). This pattern results in a significantly better prognosis than deep nuclear gray matter injury. In a recent MRI study of 78 term HIE infants, 45% (the largest subgroup) had watershed injury as the predominant pattern and had better neurodevelopmental outcome than those with deep nuclear gray matter involvement (27).
Periventricular Leukomalacia
Necrotic damage to the white matter dorsal and lateral to the external angles of the lateral ventricle (PVL) has been well described in the premature infant, but is also seen (less commonly) in the term infant following hypoxic-ischemic injury (Figure 3). Diffuse and cystic PVL, especially in the preterm infant, relates to an increased risk of adverse neurodevelopmental outcome(28).
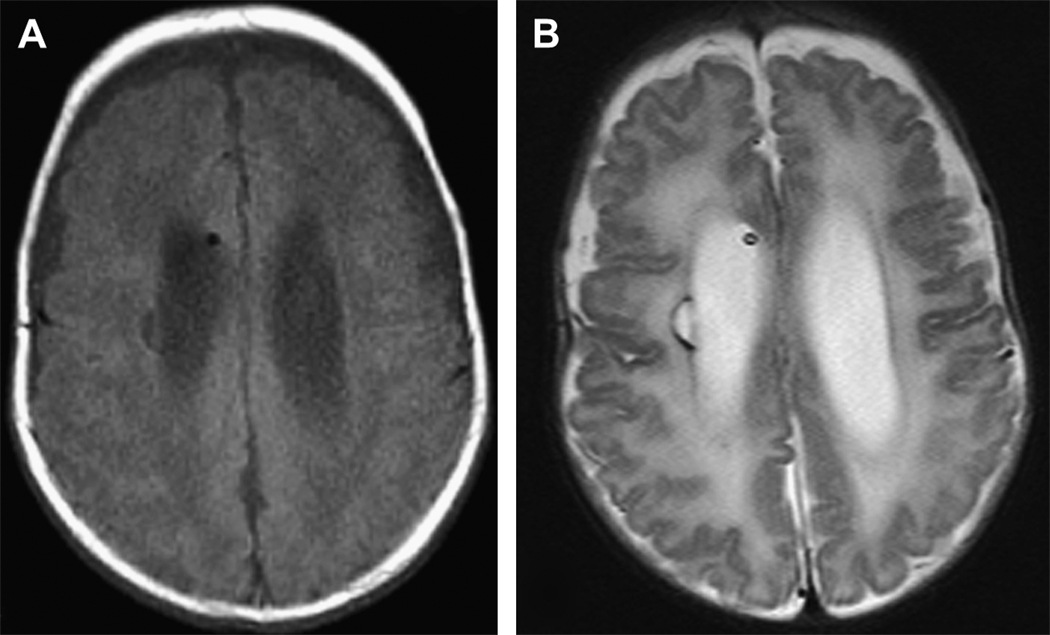
Figure 3.
Periventricular leukomalacia in a former 26 week male with grade III IVH and post hemorrhagic hydrocephalus requiring a shunt. Images were acquired at term equivalent. A) Axial T1-weighted image shows a focal cystic hypo intensity along the right periventricular region. B) Axial T2-weighted image shows this injury as a hyper intense lesion again along the right periventricular area. Furthermore, there is diffuse hyper intensity of the white matter bilaterally consistent with diffuse as well as focal periventricular leukomalacia.
Ischemic Perinatal Stroke (IPS)
The incidence of IPS ranges from 1/2300 – 1/5000 live births (29–32) and is now recognized as the second commonest etiology for term encephalopathy. Arterial lesions, usually unilateral, most commonly involve the middle cerebral artery, with the left cerebral hemisphere being more frequently affected than the right. Venous thrombosis most commonly affects the superior sagittal sinus.
MRI Methods
The diagnosis of the nature and timing of cerebral injury in encephalopathic infants requires the optimal application of MRI. In this section the most common MRI modalities including conventional images (T1 and T2W), diffusion-weighted imaging, diffusion-tensor imaging, MR spectroscopy, and MR angiogram and venogram are discussed.
Conventional Images
Conventional images include T1- weighted (short repetition and short echo times) and T-2 weighted images (long repetition times and long echo times). These sequences offer superiority in differentiating cortical gray matter from cerebral white matter and myelinated from unmyelinated white matter when compared to ultrasound and CT. Injury will appear hypointense on T1-W and hyperintense in T2-W in the acute phase, then hyperintense on T1-W and hypointense on T2-W later within the first week. While incredibly useful in the clinical setting, conventional images alone can overlook damage in the acute phase (< 4 days).
Diffusion weighted imaging (DWI)
Diffusion weighted MR imaging measures the random self-diffusion of water through the brain tissue. This self-diffusion of water in tissue is referred to as apparent diffusion, thus the term apparent diffusion coefficient (ADC), the quantitative measure of tissue diffusivity. Brain tissue following ischemic injury will experience a decrease in water diffusion compared to healthy adjacent tissue. Following neonatal ischemic brain injury, ADC values can decrease slowly as compared to adult stroke, with up to 30% of infants having “normal appearing DWI in the first 12–24 hours(33) (Figure 4). DWI imaging progresses with “pseudonormalization” of the ADC occurring by 7–10 days, a time when injury should be apparent on conventional MRI. Secondary alterations in cerebral regions, such as Wallerian degeneration in axonal pathways following a primary neuronal injury, can frame shift these “acute” changes. For example, restriction in the posterior limb of the internal capsule or corpus callosum may become noticeable on day 6–10 (34). Thus, one must take care in the interpretation of the timing of the insult based solely on restriction in ADC but consider the pattern of restriction.
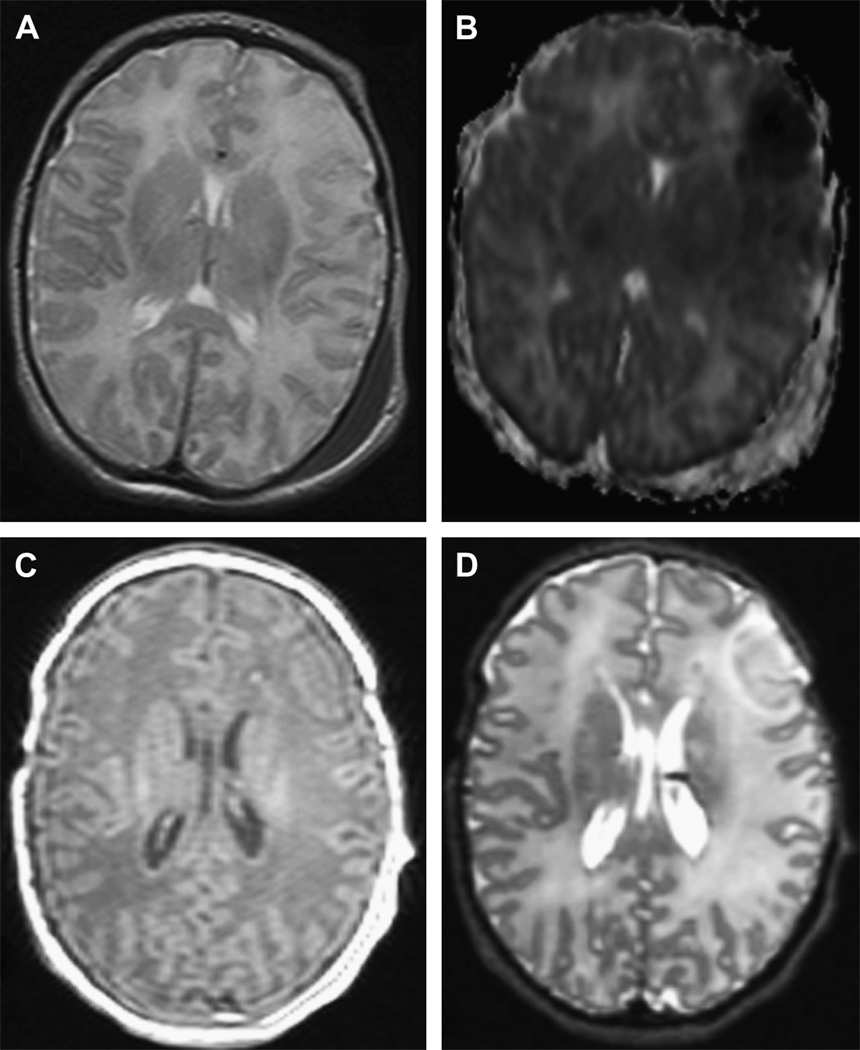
Figure 4.
Left frontal cortical stroke in a term infant who was imaged twice during the first week of life. The first two images (A,B) were obtained at 48–72 hrs of life and images (C,D) were done at 7 days of life. Axial T2-weighted image (A) shows no obvious cortical injury while in the diffusion-weighted image (B) there is restricted diffusion over the left frontal cortex. At 7 days, axial T1-weighted (C) and T2-weighted(D) sow evidence of injury to the right frontal cortex.
MR Spectroscopy (MRS)
MR spectroscopy is based on the ability of the same nuclei in various molecules to demonstrate different resonant frequencies due to different electron densities. The most commonly applied nucleus for this use is the 1H proton. The signal is expressed in parts per million (ppm) and can detect N-acetylasparate (NAA), creatine+phosphocreatine (Cr), choline (Cho), myoinositol (mI), glutamine (Gln), glutamate (Glu), glucose (G), taurine (Tau), and lactate (Lac). NAA (a free amino acid) is present in large quantities in the developing brain both in neuronal tissue and developing oligodendrocytes making it a great indicator of intact central nervous tissue (35). Multiple studies have used NAA ratios (NAA/Cr and NAA/Cho) to assess the degree of brain injury both in animal models and human infants (36–38). 1H-MRS also detects this presence of lactate, a doublet peak at 1.3 ppm that is upright at 288s echo time and inverted at 144s echo time (Figure 5), and is a useful marker for tissue injury.
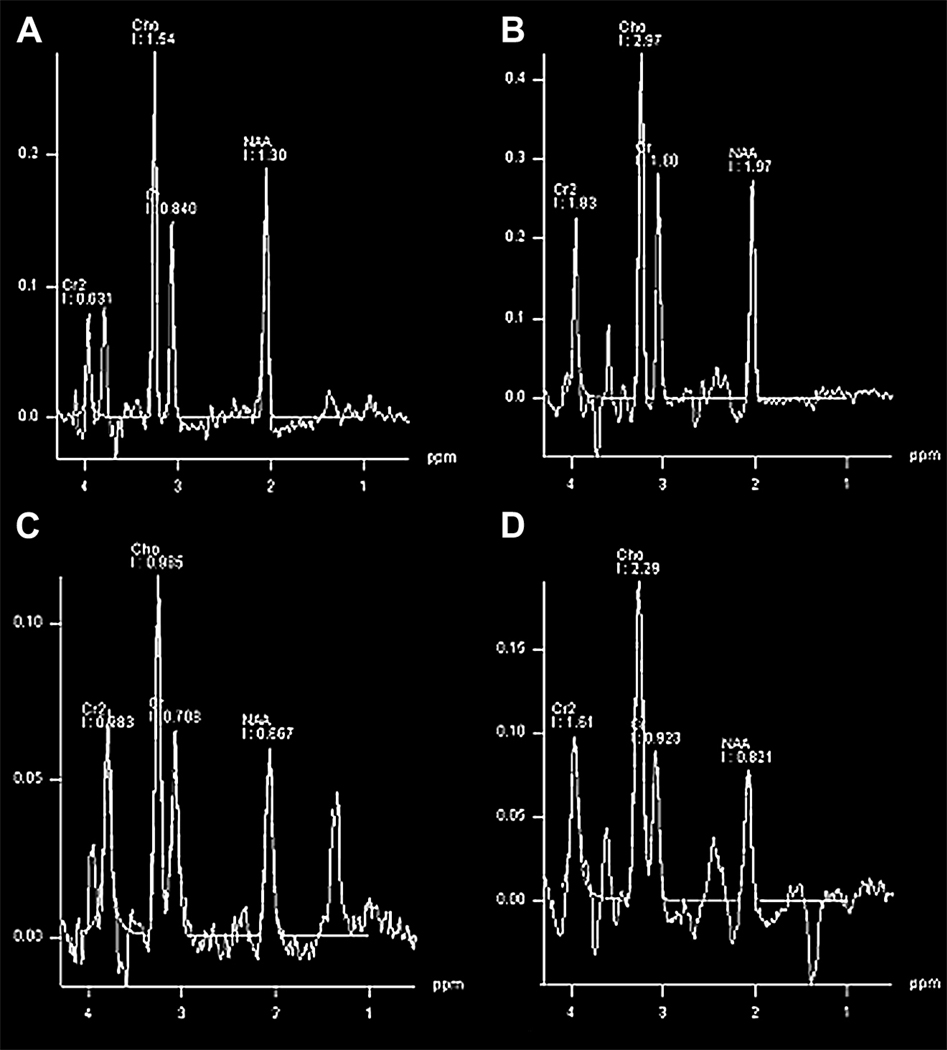
Figure 5.
MR spectroscopy in 2 different term infants. The first (A,B) are from a term infant with seizures on day of life four without evidence of hypoxic-ischemic encephalopathy. The second data (C, D) are in a 1 day old term infant with severe hypoxic-ischemic encephalopathy. A,B) Both 288 and 144 echo times shows a normal NAA peak without evidence of a lactate doublet peak at 1.3 ppm. C) 288 echo time shows a decreased NAA peak along with a doublet peak at 1.3 ppm suspicious for lactate. D) The 144 echo time helps confirm the doublet as a lactate peak and not artifact from lipid. Lactate, unlike lipid, will invert downward during the shorter echo time. These (C,D) are consistent with severe neuronal injury and poor prognosis.
Magnetic Resonance Angiography/ Magnetic Resonance Venography (MRA/MRV)
MR angiography and venography (MRA/MRV) are noninvasive techniques used to delineate arterial and venous supply and topography while avoiding catheterization and contrast administration (39–41). However, in the newborn there are limitations with small caliber vessels and slow cerebral flow compared to children and adults making flow voids more frequent (42). Care in interpretation is critical.
The clinical interpretation of MR imaging in the term encephalopathic infant The aim in undertaking neurologic imaging of a sick term infant with suspected brain injury is to aid the history and examination in answering - What is the nature and extent of brain injury? What was the likely etiology of the injury? When did the injury occur? Are there ways to intervene other than supportive care that could impact on outcome? What is the prognosis for this patient? The final section of this review will try to address these questions utilizing MR imaging.
Conventional Imaging
In the first 48 hours following injury there may be no visible changes on conventional MR imaging. If MRI abnormalities are present at this time, T1-W images will appear hypointense, while T2-W images will be hyperintense. The hyperintensity in affected areas on T2-W images evolves into hypointensity by 6–10 days of life (16). Acutely affected infants may only demonstrate diffuse edema of the cortical tissue on conventional images. This increase in water content of the brain tissue causes the cortex to become isointense with adjacent white matter, making differentiation of the border between the two tissues difficult. Areas of involvement vary according to the nature and severity of the insult. Mild hypoxic-ischemic injuries commonly involve the bilateral putamen, the ventro-lateral nucleus of the thalamus, the parasagittal cortex, and the underlying subcortical white matter overlying the vascular boundary zone. More severe injuries will also include diffuse areas of the deep and superficial gray matter along with diffuse white matter involvement. Chronically, this pattern will evolve into a picture of multicystic encephalopathy (Figure 6).
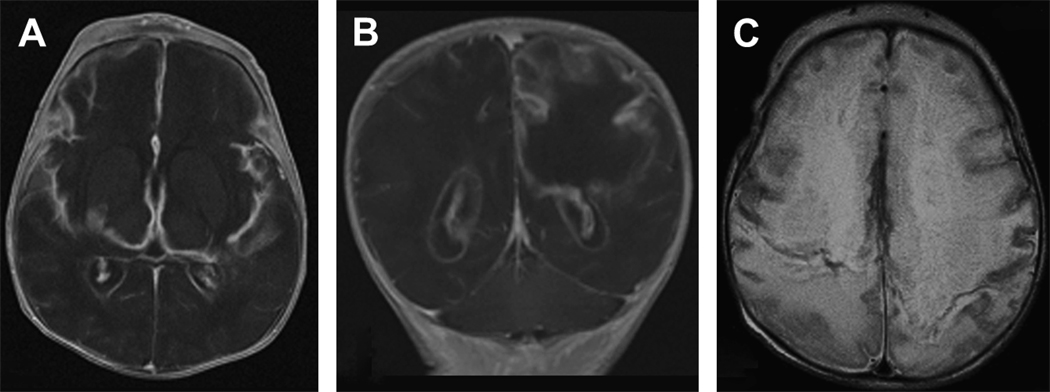
Figure 6.
Severe multi-cystic encephalopathy in a 12 day old term infant with proteus meningitis. The first group (A,B) are T1-weighted axial (A) and coronal (B) images showing severe white and gray matter loss in the frontal, parietal, and temporal lobes. This tissue loss is replaced largely by fluid. (C) T2-weighted axial image also shows diffuse injury with necrosis of both white and gray matter.
An area to pay particular attention to is the posterior limb of the internal capsule (PLIC). In images of healthy neonates, the internal capsule appears hyperintense on T1-W imaging and hypointense on T2-W images compared to adjacent structures. If injured, the PLIC will appear hypointense on T1 images relative to the thalamus and putamen by 5 days after injury. Abnormal signal intensity in the posterior limb is a powerful prognostic indicator for poor neurodevelopmental outcome (15), correctly predicting outcome in 92% of infants with stage II HIE (5).
The pattern of injury on conventional imaging carries important prognostic value. Several studies have divided patterns of injury into either watershed predominant or deep nuclear gray matter predominant with long term follow up. Deep nuclear gray matter (basal ganglia, thalamus) predominance is associated with more intensive need for resuscitation, more severe encephalopathy, increased seizure burden, and worse neurodevelopmental outcome as far out as 5 years of age(19, 27, 43).
Conventional images provide a robust measure of the nature and severity of injury when done after a week from the initial insult, correlating well with neurodevelopmental outcome (4, 5, 15, 44). However, conventional MRI in the first 4 days is often limited in delineating injury.
Diffusion Weighted Imaging/ Diffusion Tensor Imaging
In contrast to conventional MRI, diffusion imaging is sensitive to acute cerebral injury. By 2–3 days restriction in the ADC is clearly visible with brain injury, although it may underestimate the full extent (13) or be difficult to visualize with moderate injury (14). The evolution in diffusion over time in HIE infants has been described with basal ganglia injury in the early postnatal period (45). In the first 12–24 hours there may be no visible changes in diffusion imaging (33). The pattern of the early predominance of the ventro-lateral nucleus of the thalami (1st 48 hours) becomes more evident in the putamen, corticospinal tracts, and the perirolandic cortex by 3–5 days of life. Subcortical white matter and white matter pathways such as the corpus callosum and the cingulum show involvement by 6–7 days after injury. Clues to the timing and extent of brain injury can therefore be elucidated by DWI within the first 4 days of life. While changes in diffusion can be detected as early as 6 hours after injury, the most significant change in diffusion occurs between 2–4 days after the insult (33, 46). These changes in diffusion not only correlate with later conventional imaging, but also with neurodevelopmental outcome (47). If DWI shows involvement of the posterior limb of the internal capsule with an ADC of 0.74 or less, poor neurodevelopmental outcome is highly likely (48). The presence of Wallerian degeneration in the PLIC on DWI is also a strong predictor for hemiplegia (49).
Measuring anisotropy (relative (RA) or fractional anisotropy (FA) with diffusion tensor imaging gives additional information. Anisotropy decreases with both severe and moderate WM and DNGM injury during the first week of life, while ADC decreases only with severe injury. Anisotropy (FA) values continue to decrease during the 2nd week of life and do not undergo pseudonormalization like ADC values. Thus, the pairing of FA values with ADC can add information on severity and timing of injury. In the mild and moderate HIE population FA correlated with short term neurodevelopmental outcome (50).
Magnetic Resonance Spectroscopy
The in-vivo assessment of metabolites was first described in neonates by Groendaal who observed higher lactate and lower NAA values in the basal ganglia in infants with poor outcome at 3 months following postasphyxial encephalopathy (36). Since this observation, studies have looked at both metabolite ratios (Lac/Cho, Lac/Cr, NAA/Cho) and absolute levels (Lac, NAA) and correlated them with outcome. The abnormal rise in lactate and fall in NAA are most significant within the first week after injury, with lactate being detected within 24 hours following injury while NAA starts to decrease after 48 hours (38) ((45). An elevated lactate/Cho ratio has been shown to be most predictive for neurodevelopmental outcome (51–57). These metabolite levels can remain abnormal for long periods of time. Elevated lactate levels are seen for months after injury, and thus do not indicate acute injury. Persistence of lactate signifies a worse prognosis (53). When MRS of the basal ganglia and parasagittal cortex (watershed area) has been compared to DWI in the acute postnatal period, MRS appeared superior in predicting poor outcome (58).
Putting it all together
Modern MRI imaging techniques offer ways to answer the fundamental questions on etiology, timing, and prognosis in the term encephalopathic infant. The information garnered from the MRI must always be placed into the context of the clinical picture of the infant. The combination of imaging done around 48 hours, followed by a second scan between 7–10 days, appears to offer the most robust amount of information. For example, an acute perinatal injury may reveal no injury on conventional images on the first scan, but show obvious changes on diffusion, anisotropy, or abnormalities on spectroscopy. A second scan would then confirm the injury with visible changes on conventional images by day 7–10. On the other hand, if an early scan at 2 days demonstrates clear changes on conventional imaging, this may indicate that the initial injury occurred in-utero. Spectroscopy, and diffusion images can help support or contradict this assumption based on their appearance. If ADC values on the first MRI scan in the primary area of involvement have already begun to pseudonormalize or increase in value, this would support that the injury occurred greater than 5 days ago. Anisotropy measures, as mentioned above, remain decreased for a longer period of time than diffusion, and are more sensitive to moderate injury. Caution should be taken when attempting to interpret scans done between 4–5 days of age. If the injury is perinatal, diffusion changes may be commencing pseudonormalization, while the conventional imaging may not yet display clear changes. This “normal scan” can lead to false reassurance for both the family and physicians.
Etiologies may be teased out once the pattern and the timing of the initial insult has been established. Combining this observation with the clinical history will provide clues to possible mechanisms. For example, the pattern of diffuse neuronal injury with acute diffuse abnormalities on day 2 along with elevated lactate (MRS) and minimal conventional changes would be consistent with an acute perinatal event such as placental abruption, cord prolapse, or maternal shock. Of note, such sentinel acute perinatal events occur in < 25% of term encephalopathic infants (VON registry data personal communication). In contrast, a pattern of abnormal T1//T2 imaging with less pronounced diffusion findings and reduced NAA (MRS) on day 2 may be found with a history of IUGR or maternal diabetes, suggesting a much earlier timing that labor/delivery with fetal vulnerability. Again, there can be overlap with chronic and acute changes co-existing.
Interventions that can alter the course of hypoxic-ischemic injury in the neonate are still mainly hypothetical and not yet fully integrated into clinical practice. One exception is hypothermia (either systemic or selective head cooling) which is gaining acceptance among the neonatal community and is discussed elsewhere in this volume. Several large prospective randomized studies have shown promising results (59, 60). How hypothermia affects pattern, severity, and evolution of injury by MRI are not yet well established. Preliminary observations, all on small groups of infants, have found that mild hypothermia reduced the severity of basal ganglia, thalamic, and cortical lesions, likely mirroring improved clinical outcome on neurodevelopmental follow-up (61–63). Concern has been raised about the potential of hypothermia to alter the extent and timing of injury but more systematic data is required.
The capacity of MRI to aid in prognosis based on the pattern of injury is well established. While conventional imaging, spectroscopy, diffusion-weighted, and diffusion-tensor imaging have all shown prognostic value on their own, using them in combination holds the most potential (64). Injury documented by conventional changes or abnormalities of diffusion or spectroscopy, involving the deep nuclear gray matter, carries a poor prognosis(4, 19, 20, 27, 51, 58, 65). Most significant of the structures in the deep nuclear gray matter is the internal capsule, carrying crucial motor and sensory pathways from the cortex to the spinal cord and thalamus. Injury involving the white matter without deep nuclear gray matter or cerebral involvement has an overall more favorable prognosis. However, focal and diffuse white matter injury in the absence of gray matter involvement predicts long term morbidity, many times presenting in the early school years as learning and behavioral issues(43). Stage I–II HIE will often present with normal conventional imaging, and only with measures of diffusion and anisotropy can these abnormalities of white matter be detected (50). More data on long term neurodevelopmental outcomes in school age from milder forms of injury is required.
Summary
Magnetic Resonance Imaging offers a powerful tool in assessing the encephalopathic term infant. Understanding the classical patterns of brain injury and the clinical scenarios can aid the physician in the interpretation of the imaging findings for answering crucial questions such as timing, possible etiology, and long term prognosis of the infant. Finally, as future neuroprotective strategies become standard of care in the neonatal intensive care, MRI will provide a powerful tool for evaluating the impact of such interventions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Volpe J, editor. Neurology of the newborn. 4th edn. Philadelphia: Saunders; 2001. [Google Scholar]
- 2.Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P, Himmelmann K, et al. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatrica. 2005;94(3):287–294. doi: 10.1111/j.1651-2227.2005.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 3.Gaffney G, Flavell V, Johnson A, Squier M, Sellers S. Cerebral palsy and neonatal encephalopathy. Archives of Disease in Childhood Fetal & Neonatal Edition. 1994;70(3):F195–F200. doi: 10.1136/fn.70.3.f195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biagioni E, Mercuri E, Rutherford M, Cowan F, Azzopardi D, Frisone MF, et al. Combined use of electroencephalogram and magnetic resonance imaging in full-term neonates with acute encephalopathy.[see comment] Pediatrics. 2001;107(3):461–468. doi: 10.1542/peds.107.3.461. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford MPJ, Counsell SJ, et al. Abnormal Magnetic Resonance Signal in the Internal Capsule Predicts Poor Neurodevelopmental Outcome in Infants with Hypoxic-Ischemic Encephalopahty. Pediatrics. 1998;102(2):323–328. doi: 10.1542/peds.102.2.323. [DOI] [PubMed] [Google Scholar]
- 6.Nelson KB, Leviton A. How much of neonatal encephalopathy is due to birth asphyxia? American Journal of Diseases of Children. 1991;145(11):1325–1331. doi: 10.1001/archpedi.1991.02160110117034. [DOI] [PubMed] [Google Scholar]
- 7.Blair E, Stanley FJ. Intrapartum asphyxia: a rare cause of cerebral palsy.[see comment][erratum appears in J Pediatr 1988 Aug;113(2):420] Journal of Pediatrics. 1988;112(4):515–519. doi: 10.1016/s0022-3476(88)80161-6. [DOI] [PubMed] [Google Scholar]
- 8.Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy.[see comment] Lancet. 2003;361(9359):736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- 9.Golomb MR, Dick PT, MacGregor DL, Armstrong DC, DeVeber GA. Cranial ultrasonography has a low sensitivity for detecting arterial ischemic stroke in term neonates. J Child Neurol. 2003;18(2):98–103. doi: 10.1177/08830738030180021401. [DOI] [PubMed] [Google Scholar]
- 10.Hall P, Adami HO, Trichopoulos D, Pedersen NL, Lagiou P, Ekbom A, et al. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. Bmj. 2004;328(7430):19. doi: 10.1136/bmj.328.7430.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt F, Grosu D, Mohr C, Purdy D, Salem K, Scott KT, et al. [3 Tesla MRI: successful results with higher field strengths] Radiologe. 2004;44(1):31–47. doi: 10.1007/s00117-003-1000-x. [DOI] [PubMed] [Google Scholar]
- 12.Frayne R, Goodyear BG, Dickhoff P, Lauzon ML, Sevick RJ. Magnetic resonance imaging at 3.0 Tesla: challenges and advantages in clinical neurological imaging. Investigative Radiology. 2003;38(7):385–402. doi: 10.1097/01.rli.0000073442.88269.c9. [DOI] [PubMed] [Google Scholar]
- 13.Robertson RL, Ben-Sira L, Barnes PD, Mulkern RV, Robson CD, Maier SE, et al. MR line-scan diffusion-weighted imaging of term neonates with perinatal brain ischemia.[see comment] Ajnr: American Journal of Neuroradiology. 1999;20(9):1658–1670. [PMC free article] [PubMed] [Google Scholar]
- 14.Rutherford M, Counsell S, Allsop J, Boardman J, Kapellou O, Larkman D, et al. Diffusion-weighted magnetic resonance imaging in term perinatal brain injury: a comparison with site of lesion and time from birth. Pediatrics. 2004;114(4):1004–1014. doi: 10.1542/peds.2004-0222. [DOI] [PubMed] [Google Scholar]
- 15.Jyoti R, O'Neil R, Jyoti R, O'Neil R. Predicting outcome in term neonates with hypoxic-ischaemic encephalopathy using simplified MR criteria. Pediatric Radiology. 2006;36(1):38–42. doi: 10.1007/s00247-005-0024-y. [DOI] [PubMed] [Google Scholar]
- 16.Triulzi F, Parazzini C, Righini A, Triulzi F, Parazzini C, Righini A. Patterns of damage in the mature neonatal brain. Pediatric Radiology. 2006;36(7):608–620. doi: 10.1007/s00247-006-0203-5. [DOI] [PubMed] [Google Scholar]
- 17.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 18.Martin E, Barkovich AJ. Magnetic resonance imaging in perinatal asphyxia. Archives of Disease in Childhood Fetal & Neonatal Edition. 1995;72(1):F62–F70. doi: 10.1136/fn.72.1.f62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuenzle C, Baenziger O, Martin E, Thun-Hohenstein L, Steinlin M, Good M, et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics. 1994;25(4):191–200. doi: 10.1055/s-2008-1073021. [DOI] [PubMed] [Google Scholar]
- 20.Rutherford M, Pennock J, Schwieso J, Cowan F, Dubowitz L. Hypoxic-ischaemic encephalopathy: early and late magnetic resonance imaging findings in relation to outcome.[see comment] Archives of Disease in Childhood Fetal & Neonatal Edition. 1996;75(3):F145–F151. doi: 10.1136/fn.75.3.f145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friede R, editor. Developmental Neuropathology. 2nd ed. Berlin: Springer Verlag; 1989. [Google Scholar]
- 22.Mito T, Kamei A, Takashima S, Becker LE. Clinicopathological study of pontosubicular necrosis. Neuropediatrics. 1993;24(4):204–207. doi: 10.1055/s-2008-1071540. [DOI] [PubMed] [Google Scholar]
- 23.Friede RL. Ponto-subicular lesions in perinatal anoxia. Archives of Pathology & Laboratory Medicine. 1972;94(4):343–354. [PubMed] [Google Scholar]
- 24.Hashimoto K, Takeuchi Y, Takashima S. Hypocarbia as a pathogenic factor in pontosubicular necrosis. Brain & Development. 1991;13(3):155–157. doi: 10.1016/s0387-7604(12)80021-0. [DOI] [PubMed] [Google Scholar]
- 25.Ahdab-Barmada M, Moossy J, Painter M. Pontosubicular necrosis and hyperoxemia. Pediatrics. 1980;66(6):840–847. [PubMed] [Google Scholar]
- 26.Bruck Y, Bruck W, Kretzschmar HA, Lassmann H. Evidence for neuronal apoptosis in pontosubicular neuron necrosis. Neuropathology & Applied Neurobiology. 1996;22(1):23–29. [PubMed] [Google Scholar]
- 27.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, et al. Patterns of brain injury in term neonatal encephalopathy. Journal of Pediatrics. 2005;146(4):453–460. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 29.Schulzke S, Weber P, Luetschg J, Fahnenstich H. Incidence and diagnosis of unilateral arterial cerebral infarction in newborn infants. Journal of Perinatal Medicine. 2005;33(2):170–175. doi: 10.1515/JPM.2005.032. [DOI] [PubMed] [Google Scholar]
- 30.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109(1):116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 31.Hunt RW, Inder TE. Perinatal and neonatal ischaemic stroke: a review. Thrombosis Research. 2006;118(1):39–48. doi: 10.1016/j.thromres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3(3):150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- 33.McKinstry RC, Miller JH, Snyder AZ, Mathur A, Schefft GL, Almli CR, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns.[see comment] Neurology. 2002;59(6):824–833. doi: 10.1212/wnl.59.6.824. [DOI] [PubMed] [Google Scholar]
- 34.Neil JJ, Inder TE. Detection of wallerian degeneration in a newborn by diffusion magnetic resonance imaging (MRI) Journal of Child Neurology. 2006;21(2):115–118. doi: 10.1177/08830738060210021501. [DOI] [PubMed] [Google Scholar]
- 35.Bhakoo KK, Pearce D. In vitro expression of N-acetyl aspartate by oligodendrocytes: implications for proton magnetic resonance spectroscopy signal in vivo. Journal of Neurochemistry. 2000;74(1):254–262. doi: 10.1046/j.1471-4159.2000.0740254.x. [DOI] [PubMed] [Google Scholar]
- 36.Groenendaal F, Veenhoven RH, van der Grond J, Jansen GH, Witkamp TD, de Vries LS. Cerebral lactate and N-acetyl-aspartate/choline ratios in asphyxiated full-term neonates demonstrated in vivo using proton magnetic resonance spectroscopy. Pediatric Research. 1994;35(2):148–151. doi: 10.1203/00006450-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Penrice J, Lorek A, Cady EB, Amess PN, Wylezinska M, Cooper CE, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatric Research. 1997;41(6):795–802. doi: 10.1203/00006450-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, et al. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. Ajnr: American Journal of Neuroradiology. 1999;20(8):1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 39.Koelfen W, Wentz U, Freund M, Schultze C. Magnetic resonance angiography in 140 neuropediatric patients. Pediatr Neurol. 1995;12(1):31–38. doi: 10.1016/0887-8994(94)00096-k. [DOI] [PubMed] [Google Scholar]
- 40.Koelfen W, Freund M, Konig S, Varnholt V, Rohr H, Schultze C. Results of parenchymal and angiographic magnetic resonance imaging and neuropsychological testing of children after stroke as neonates. Eur J Pediatr. 1993;152(12):1030–1035. doi: 10.1007/BF01957231. [DOI] [PubMed] [Google Scholar]
- 41.Ayanzen RH, Bird CR, Keller PJ, McCully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol. 2000;21(1):74–78. [PMC free article] [PubMed] [Google Scholar]
- 42.Widjaja E, Shroff M, Blaser S, Laughlin S, Raybaud C. 2D time-of-flight MR venography in neonates: anatomy and pitfalls. AJNR Am J Neuroradiol. 2006;27(9):1913–1918. [PMC free article] [PubMed] [Google Scholar]
- 43.Barnett A, Mercuri E, Rutherford M, Haataja L, Frisone MF, Henderson S, et al. Neurological and perceptual-motor outcome at 5 – 6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics. 2002;33(5):242–248. doi: 10.1055/s-2002-36737. [DOI] [PubMed] [Google Scholar]
- 44.Barkovich AJ, Hajnal BL, Vigneron D, Sola A, Partridge JC, Allen F, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. Ajnr: American Journal of Neuroradiology. 1998;19(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- 45.Barkovich AJ, Miller SP, Bartha A, Newton N, Hamrick SE, Mukherjee P, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. Ajnr: American Journal of Neuroradiology. 2006;27(3):533–547. [PMC free article] [PubMed] [Google Scholar]
- 46.Soul JS, Robertson RL, Tzika AA, du Plessis AJ, Volpe JJ. Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics. 2001;108(5):1211–1214. doi: 10.1542/peds.108.5.1211. [DOI] [PubMed] [Google Scholar]
- 47.Johnson AJ, Lee BC, Lin W. Echoplanar diffusion-weighted imaging in neonates and infants with suspected hypoxic-ischemic injury: correlation with patient outcome. AJR 1999;American Journal of Roentgenology. 172(1):219–226. doi: 10.2214/ajr.172.1.9888771. [DOI] [PubMed] [Google Scholar]
- 48.Hunt RW, Neil JJ, Coleman LT, Kean MJ, Inder TE. Apparent diffusion coefficient in the posterior limb of the internal capsule predicts outcome after perinatal asphyxia. Pediatrics. 2004;114(4):999–1003. doi: 10.1542/peds.2003-0935-L. [DOI] [PubMed] [Google Scholar]
- 49.De Vries LS, Van der Grond J, Van Haastert IC, Groenendaal F. Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics. 2005;36(1):12–20. doi: 10.1055/s-2005-837544. [DOI] [PubMed] [Google Scholar]
- 50.Malik GK, Trivedi R, Gupta RK, Hasan KM, Hasan M, Gupta A, et al. Serial quantitative diffusion tensor MRI of the term neonates with hypoxic-ischemic encephalopathy (HIE) Neuropediatrics. 2006;37(6):337–343. doi: 10.1055/s-2007-964869. [DOI] [PubMed] [Google Scholar]
- 51.Boichot C, Walker PM, Durand C, Grimaldi M, Chapuis S, Gouyon JB, et al. Term neonate prognoses after perinatal asphyxia: contributions of MR imaging, MR spectroscopy, relaxation times, and apparent diffusion coefficients. Radiology. 2006;239(3):839–848. doi: 10.1148/radiol.2393050027. [DOI] [PubMed] [Google Scholar]
- 52.Miller SP, Newton N, Ferriero DM, Partridge JC, Glidden DV, Barnwell A, et al. Predictors of 30-month outcome after perinatal depression: role of proton MRS and socioeconomic factors. Pediatric Research. 2002;52(1):71–77. doi: 10.1203/00006450-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Hanrahan JD, Cox IJ, Edwards AD, Cowan FM, Sargentoni J, Bell JD, et al. Persistent increases in cerebral lactate concentration after birth asphyxia. Pediatric Research. 1998;44(3):304–311. doi: 10.1203/00006450-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Hanrahan JD, Cox IJ, Azzopardi D, Cowan FM, Sargentoni J, Bell JD, et al. Relation between proton magnetic resonance spectroscopy within 18 hours of birth asphyxia and neurodevelopment at 1 year of age. Developmental Medicine & Child Neurology. 1999;41(2):76–82. doi: 10.1017/s0012162299000171. [DOI] [PubMed] [Google Scholar]
- 55.Malik GK, Pandey M, Kumar R, Chawla S, Rathi B, Gupta RK. MR imaging and in vivo proton spectroscopy of the brain in neonates with hypoxic ischemic encephalopathy. European Journal of Radiology. 2002;43(1):6–13. doi: 10.1016/s0720-048x(01)00435-1. [DOI] [PubMed] [Google Scholar]
- 56.Amess PN, Penrice J, Wylezinska M, Lorek A, Townsend J, Wyatt JS, et al. Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Developmental Medicine & Child Neurology. 1999;41(7):436–445. [PubMed] [Google Scholar]
- 57.Kadri M, Shu S, Holshouser B, Deming D, Hopper A, Peverini R, et al. Proton magnetic resonance spectroscopy improves outcome prediction in perinatal CNS insults. J Perinatol. 2003;23(3):181–185. doi: 10.1038/sj.jp.7210913. [DOI] [PubMed] [Google Scholar]
- 58.Zarifi MK, Astrakas LG, Poussaint TY, Plessis Ad Ad, Zurakowski D, Tzika AA. Prediction of adverse outcome with cerebral lactate level and apparent diffusion coefficient in infants with perinatal asphyxia. Radiology. 2002;225(3):859–870. doi: 10.1148/radiol.2253011797. [DOI] [PubMed] [Google Scholar]
- 59.Gluckman PDWJ, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopahty: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 60.Shankaran SLA, Ehrenkranz RA, et al. Whole-Body Hypothermia for Neonates with Hypoxic-Ischemic Encephalopathy. New England Journal of Medicineq. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 61.Inder TE, Hunt RW, Morley CJ, Coleman L, Stewart M, Doyle LW, et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J Pediatr. 2004;145(6):835–837. doi: 10.1016/j.jpeds.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 62.Rutherford MA, Azzopardi D, Whitelaw A, Cowan F, Renowden S, Edwards AD, et al. Mild hypothermia and the distribution of cerebral lesions in neonates with hypoxic-ischemic encephalopathy. Pediatrics. 2005;116(4):1001–1006. doi: 10.1542/peds.2005-0328. [DOI] [PubMed] [Google Scholar]
- 63.Compagnoni G, Pogliani L, Lista G, Castoldi F, Fontana P, Mosca F. Hypothermia reduces neurological damage in asphyxiated newborn infants. Biol Neonate. 2002;82(4):222–227. doi: 10.1159/000065890. [DOI] [PubMed] [Google Scholar]
- 64.L'Abee CdVL, van der Grond J, et al. Early DIffusion-Weighted MRI and H-Magnetic Resonance Spectroscopy in Asphyxiated Full-Term Neonates. Biology of the Neonate. 2005;88:306–312. doi: 10.1159/000087628. [DOI] [PubMed] [Google Scholar]
- 65.Mercuri ERM, Cowan F, et al. Early Prognostic Indicators of Outcome in Infants With Neonatal Cerebral Infarction: A Clinical, Electroencephalogram, and Magnetic Resonance Imaging Study. Pediatrics. 1999;103:39–46. doi: 10.1542/peds.103.1.39. [DOI] [PubMed] [Google Scholar]