Summary
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and is often diagnosed at an advanced stage. We have investigated α-fetoprotein (AFP) as a tumor-associated antigen for HCC. We identified major histocompatibility complex class I-restricted peptide epitopes derived from AFP and studied CD8+ T-cell responses in vivo and in vitro in ongoing immunotherapy studies. Helper T cells are of critical importance in shaping the immune response; therefore, we investigated the frequency and function of AFP-specific CD4+ T cells in the general population and among HCC patients. CD4+ T-cell responses were assessed by direct ex vivo multicytokine enzyme-linked immunospot assay and by measurement of cytokine levels using a multicytokine assay. Our analysis indicates that healthy donors have very low frequencies of AFP-specific CD4+ T-cell responses, which are of TH1 type, detectable ex vivo. In contrast, these T cells were either reduced or eliminated in HCC patients at advanced stages of disease. To better activate these cells, we compared the stimulatory capacity of both AFP protein-fed and AdVhAFP-engineered dendritic cells (DC). Healthy donors have CD4+ T-cell responses, which were activated in response to AFP protein-fed DC whereas HCC patients do not demonstrate significant responses to AFP protein. AdVhAFP-transduced DC were capable of activating higher frequency TH1 CD4+ responses to AFP in both healthy donors and AFP-positive HCC patients. Importantly, CD4+ T-cell cytokine expression profiles were skewed towards interleukin-2 and interferon-γ production when activated by adenovirally engineered DC, which has therapeutic implications for vaccination efforts.
Keywords: dendritic cells, T cells, antigen presentation, vaccination
Hepatocellular carcinoma (HCC) has a very poor prognosis. Small tumors are potentially curable with ablative or surgical approaches, including liver transplantation. 1–3 However, the majority of cases are detected at advanced stages, which, even if adequately treated locally, relapse systemically, and HCC recurs in 75% to 100% of patients at 5 years.4 There are no effective systemic therapies for this disease.5,6 This leads to a 9% 5-year survival rate after diagnosis in the United States, the second lowest survival rate for any type of cancer.7
α-fetoprotein (AFP) is the most common serum protein during embryonic development, and is a physiologic counterpart of adult serum albumin. Suppression of AFP synthesis occurs shortly after birth. However, AFP mRNA can be detected in human liver at low, but consistent levels8,9 and AFP expression can be also increased after liver injury.10 In addition, serum AFP is detected in 10% to 51% of subjects with viral hepatitis, in 25% with liver cirrhosis and 18.8% with benign diffuse liver disease.11–16 Lastly, 90% to 95% of embryonic cancers (embryonic carcinomas, yolk sac tumors, hepatoblastoma) and 50% to 80% HCC show AFP reexpression during tumor development at levels from 10 ng/mL to 1 mg/mL.10,17–19 Thus, all individuals have been exposed to high levels of this antigen during fetal development and those with liver diseases and ultimately HCC are exposed to this secreted antigen, often at high concentrations.
Although the exact function of AFP remains speculative, it seems to be involved in cell differentiation, growth regulation, and carcinogenesis. There are putative AFP cell surface receptors on cancer cell lines (including HCC), and also on normal hepatocytes, placental and immune cells.20–22 Several studies have shown that peripheral blood mononuclear cells (PBMC) with impaired AFP receptor expression show decreased proliferative responses and reduced CD4+ T-cell mitogen responses.23 HCC cell lines also show inhibition of proliferation if AFP receptors are blocked by specific antibodies.24 In addition, the effect of AFP on immune cells is controversial with reports finding both stimulatory and inhibitory effects.25–36
We have demonstrated that human and murine CD8+ T cells can recognize peptide epitopes derived from AFP.37–44 We have also tested major histocompatibility complex (MHC) class I peptide-based vaccines in AFP+HCC subjects. Using MHC tetramer and interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assays, we found that immunization with immunodominant peptides in Montanide adjuvant or pulsed onto dendritic cells (DC) was capable of increasing the frequency and function of AFP-specific CD8+ T cells in the peripheral blood45,46 (Butterfield et al, submitted, 2006). These data indicate that these cells have not been deleted in patients despite high levels of circulating antigen.
AFP-specific helper T cells have also been detected in HCC patients.33,47,48 The frequencies of spontaneous AFP-specific T cells are higher in cirrhotic patients without HCC and in those with lower serum AFP level.48 Similar results were obtained in a study investigating responses to an AFP-derived HLA-DR13–restricted peptide.47 These 3 published reports have focused on IFN-γ production by these helper T cells. In our ongoing efforts to target AFP as a tumor rejection antigen for immunotherapy, we wished to determine the nature of CD4+ helper T-cell immunity to AFP, given the importance of CD4+ cells in shaping the quality of the cellular immune responses, and allowing full function and proliferative response of CD8+ CTL.49–51 Our current study has investigated the baseline frequency and function of spontaneous AFP-specific CD4+ T cells in the general population and among HCC patients. We have investigated potential sexual dimorphism, and the influence of different modes of antigen presentation by DC for amplification of AFP-specific helper responses. We find that healthy donors have detectable preexisting AFP-specific CD4+ T-cell responses whether AFP was presented by protein-pulsed autologous DC or by adenovirally engineered DC. In HCC patients, AFP-specific helper responses were only detected when DC were engineered to express AFP, indicating that genetically modified DC have a significantly improved ability to present this antigen in a stimulatory fashion to CD4+ cells. Importantly, we found that the cytokine profile of the AFP-specific CD4+ T cells depended on the mode of antigen presentation.
MATERIALS AND METHODS
Patient Samples, Cells and Cell Lines
PBMC were obtained from 26 healthy donors (15 males and 11 females) from the Central Blood Bank (Pittsburgh, PA; University of Pittsburgh IRB no. 04-001) and 6 male HCC patients previously enrolled in an AFP peptide-pulsed DC vaccination clinical trial45 (UCLA IRB no. 00-01-026, FDA BB IND no. 9395).
PBMC were separated by Ficoll-Plaque Plus (Amersham Biosciences, Uppsala, Sweden) and stored in 90% human AB serum (Omega Scientific, Inc, Tarzana, CA)/10% dimethyl sulfoxide in liquid nitrogen. All cell culture was performed in culture media containing AFP-free human AB serum (Omega Scientific, Inc, Tarzana, CA) or serum-free X-vivo media (Life Technologies, Inc).
Viruses and Proteins
Recombinant adenoviruses AdVhAFP and Ad-VlacZ are Ad type 5 E1a/E1b-deleted first generation adenoviruses previously described.52,53 AdVhAFP encodes a synthetic form of AFP matching the Genbank reference sequence NM_001134, driven by the CMV promoter. Adenoviral vectors were amplified on 293 cells (ATCC, Manassas, VA) and purifications were performed according to the manufacturer’s instructions (Adeno-X, BD Biosciences, San Diego, CA). Soluble cord blood AFP (CALBIOCHEM, San Diego, CA) was resuspended in phosphate-buffered saline (PBS)/0.1% bovine serum albumin (BSA) at 1 mg/mL.
Cell Isolation
DC
DC were obtained from loosely adherent mononuclear cells after 7 days of culture with interleukin-4 (IL-4) (500 U/mL, Schering-Plough, Kenilworth, NJ) and granulocyte macrophage-colony stimulating factor (GMCSF) (800 U/mL, Immunex, Seattle, WA). Adenoviral transduction of DC was performed in RPMI 1640/2% human AB serum with AdV vectors at multiplicity of infection=1000:1 pfu/DC (moi=1000) on day 7 of DC culture by incubation at 37°C for 2 hours.38,54 This moi routinely results in >90% transduction efficiency. Cells were washed with excess media and resuspended at 1 × 106 cells/mL. AFP protein pulsing of DC was performed in serum-free IMDM media (Life Technologies, Inc) with soluble serum AFP at 10 µg/mL on day 7 of DC culture at room temperature for 2 hours. Cells were washed and resuspended at 1 × 106 cells/mL.
CD4+ T-lymphocytes
CD4+ cells were isolated by first, removal of adherent cells by plastic adherence, second, removal of CD8+ T cells by positive magnetic bead isolation, and finally by negative magnetic cell sorting using a CD4+ T Cell Isolation Kit which specifically removes any CD8+, CD14+, CD16+, CD19+, CD36+, CD56+, CD123+, TCR-γδ+, and CD235a+ cells (Miltenyi Biotec).
ELISPOT
The ELISPOT assay was performed according to Herr et al55 with minor modifications. Ninety-six-well plates with nitrocellulose membranes (Millipore, Bedford, MA) were coated with primary antibodies (IL-2; IFN-γ, TNF-α, IL-5, IL-10) (BD Biosciences Pharmingen) in PBS at 4 µg/mL and incubated overnight at 4°C. Plates were washed with PBS, and then blocked with PBS/1% BSA for an hour at 37°C. Plates were washed with PBS and cells were plated in 200 µL of serum-free X-Vivo-10 media (Life Technologies, Inc). Negative controls included DC alone (1 × 105 per well), DC without antigen with CD4+ T cells (2 × 105, 1 × 105, and 5 × 104 per well), DC transduced with control vector (an empty AdV vector or with AdVlacZ) plus CD4+ T cells (as above), CD4+ T cells alone (105). CD4+ T cell stimulated with 10 to 100µg/mL of PHA (Sigma) served as a positive control. Experimental conditions included DC transduced with AdVhAFP, or pulsed with soluble serum AFP plus CD4+ T cells. Each condition was plated in duplicate (each of 3 conditions, 6 wells total). Cells were incubated 24 hours (IL-2, IFN-γ, and TNF-α) or 48 hours (IL-5, IL-10). Cell-free supernatants from individual wells were frozen for subsequent Luminex assay analysis. Plates were washed with PBS and with PBS/0.05% Tween-20. Corresponding secondary antibody in PBS/0.05% Tween-20/1% BSA was added and incubated overnight at 4°C. Plates were washed 2 times with PBS, and then with PBS/0.05% Tween-20 at room temperature. Avidin-horseradish peroxidase (Vector Laboratories) was added at 1:2000 and incubated in the dark 2 hours. Plates were washed with PBS/Tween-20 and developed with AEC buffer [3-amino-9-ethylcarbozole (Sigma) in formamide/0.05M NaOAc buffer, pH5.0] with H2O2. The reaction was stopped in tap water. Spots were counted with an ImmunoSpot Analyzer Series 3A (Cellular Technology Ltd, Cleveland, OH).
Luminex
The Luminex assay was performed by the Luminex Core Facility of University of Pittsburgh (A. Lokshin, Director), the limit of detection depended on cytokine, and ranged between 3 and 40 pg/mL. The LabMAP technology (Luminex) combines the principle of a sandwich immunoassay with the fluorescent-bead–based technology allowing individual and multiplex analysis of up to 100 different analytes in a single microtiter well. The LabMAP media supernatant assays for IL-2, IFN-γ, TNFα, IL-5, IL-10, IL-4, GM-CSF were performed in 96-well microplate format according to the protocol by Biosource International (Camarillo, CA). A filter-bottom, 96-well microplate (Millipore, Billerica, MA) was blocked for 10 minutes with PBS/BSA. To generate a standard curve, 5-fold dilutions of appropriate standards were prepared in serum diluent. Standards and samples were pipetted at 50 µL per well in duplicate and mixed with 50 µL of the bead mixture. The microplate was incubated for 1 hour at room temperature on a microtiter shaker. Wells were then washed thrice with washing buffer using a vacuum manifold. Phycoerythrin-conjugated secondary antibody was added to the appropriate wells and the wells were incubated for 45 minutes in the dark with constant shaking. Wells were washed twice, assay buffer was added to each well, and samples were analyzed using the Bio-Plex suspension array system (Bio-Rad Laboratories, Hercules, CA). Analysis of experimental data was performed using 5-parametric-curve fitting, all as described in.56
Statistical Analysis
Statistical analyses assessing the relationship between cytokine levels in a treated group and those in a control group were performed using a stratified Wilcoxon rank sum test. Two-sided P values were computed under the null hypothesis, that within each stratum, the ranked data are random, and do not depend on group. For analyses of individual cytokines, strata consisted of the 3 dilutions. TH1 response was also assessed in an analysis that combined IL-2, IFN-γ, and TNF-α data; in this case, there were 9 strata: 3 dilutions for each of the 3 cytokines. The Wilcoxon test relies on the assumption that the strata are independent. Spearman test was performed to examine relationships between clinical data and the level of CD4+ immune responses in HCC patients. Two-sided P values less or equal to 0.05 were accepted as significant.
RESULTS
To optimally target AFP-expressing HCC for immunotherapy, CD4+ and CD8+ T cells should be activated for TH1 and cytotoxic responses. Because of the fetal expression of AFP and its reactivation in disease states, the ability to develop potent AFP-specific CD4+ T-cell responses to this complex self-antigen has been investigated.
CD4+ T-cell Responses to AFP Protein in Healthy Donors
To establish a baseline for AFP CD4+ immunity, we wished to determine whether AFP-specific CD4+ T cells could be detected in the peripheral blood of healthy donors and, if so, to characterize the frequencies and cytokine profile of these cells. We initially examined this by stimulating cells overnight with autologous immature DC-fed AFP protein, which would approximate the expected mode of presentation when serum AFP is present. AFP-specific CD4+ T-cell responses were determined by direct ex vivo multicytokine ELISPOT assay and by measurement of cytokine levels in cell-free supernatants from the ELISPOT assay using Luminex array technology.
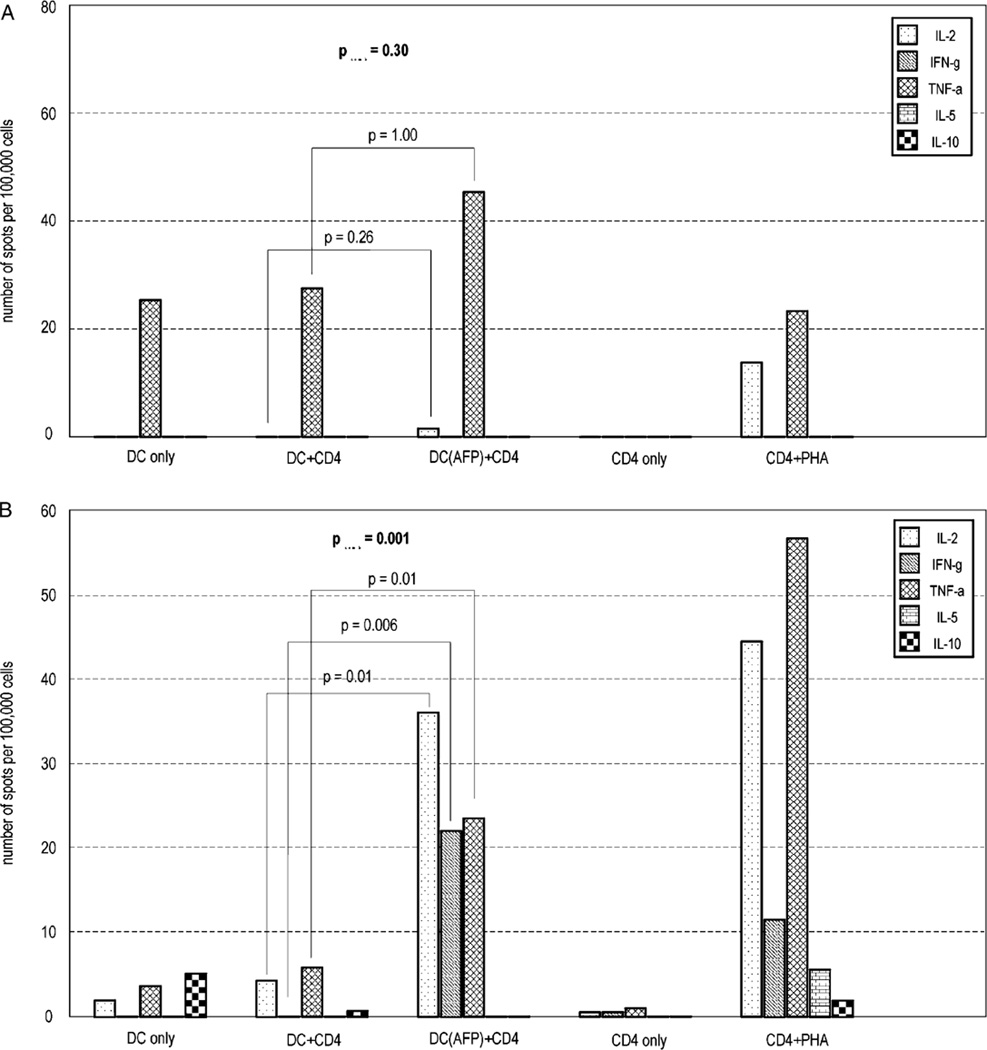
Eight healthy donors of 26 (30%) studied showed statistically significant preexisting CD4+ T-cell responses to soluble AFP protein, by at least 1 cytokine by ELISPOT assay (Table 1). Frequencies of helper T-cell responses were very low, at 2/1,000,000 to 47/1,000,000 CD4+ T cells (with a limit of detection of 1/1,000,000 cells). All 8 of the healthy donors had a TH1 type of response: donor 11 had significant responses for IL-2, IFN-γ and TNF-α; donor 24 had a significant IL-2 response, and donors 02, 03, 13, 14, and 30 demonstrated significant TNF-α responses. Donor 18 had a TH1 (TNF-α) and IL-10 response. By combining the trends in positivity in single cytokines, the pooled TH1 cytokine data identified 3 additional positive donors (donors 6, 15, and 32) for a total of 11. Together, we observed that 10 of the 11 AFP-responsive healthy donors had purely TH1 antigen-specific responses by ELISPOT assay. We considered that pregnancy might have an impact on responses, but did not have such data from female donors. Regardless, among the responders, 5 were females and 6 were males, indicating a lack of sexual dimorphism. Figure 1 shows 2 examples of cytokine profiles detected in responding and nonresponding healthy donors. Figure 1A shows a cytokine profile in an AFP protein “negative” donor (no. 08), and Figure 1B shows the cytokine profile in an AFP protein “positive” donor (no. 11).
TABLE 1.
Distribution of CD4+ T-cell Responses to Soluble AFP Protein-fed DC in Healthy Donors
| Donor ID* | Sex† | Combined TH1 Cytokine‡ | IL-2§ | IFN-γ§ | TNF-α§ | IL-5§ | IL-10§ |
|---|---|---|---|---|---|---|---|
| 00 | Female | 0.433 | 0.452 | 1.000 | 1.000 | 1.000 | 1.000 |
| 02 | Female | 0.027 | 0.328 | 0.978 | 0.009 | 1.000 | 1.000 |
| 03 | Male | 0.001 | 0.327 | 1.000 | 0.006 | 1.000 | 1.000 |
| 04 | Male | 0.561 | 0.231 | 0.995 | 0.817 | 1.000 | 1.000 |
| 05 | Male | 0.585 | 0.920 | 1.000 | 0.094 | 1.000 | 0.658 |
| 06 | Male | <0.001 | 0.058 | 0.141 | 0.160 | 1.000 | 0.987 |
| 07 | Male | 0.788 | 0.879 | 0.965 | 0.982 | 1.000 | 1.000 |
| 08 | Male | 0.295 | 0.257 | 1.000 | 1.000 | 1.000 | 1.000 |
| 09 | Male | 0.743 | 0.997 | 1.000 | 0.326 | 1.000 | 1.000 |
| 10 | Male | 0.779 | 1.000 | 1.000 | 0.830 | 0.419 | 0.076 |
| 11 | Male | <0.001 | 0.010 | 0.006 | 0.010 | 0.514 | 0.947 |
| 13 | Male | 0.092 | 0.866 | 0.993 | 0.007 | 1.000 | 0.518 |
| 14 | Female | 0.979 | 0.197 | 0.335 | 0.007 | 1.000 | 1.000 |
| 15 | Male | 0.007 | 0.150 | 1.000 | 0.051 | 1.000 | 0.992 |
| 17 | Male | 0.159 | 0.969 | 1.000 | 0.163 | 1.000 | 0.489 |
| 18 | Male | 0.014 | 0.581 | 1.000 | 0.007 | 1.000 | 0.032 |
| 19 | Male | 1.000 | 0.561 | 1.000 | 0.750 | 1.000 | 1.000 |
| 21 | Female | 0.669 | 1.000 | 1.000 | 0.673 | 0.164 | 1.000 |
| 22 | Female | 0.363 | 1.000 | 1.000 | 0.265 | 1.000 | 0.997 |
| 23 | Female | 0.438 | 0.491 | 1.000 | 0.995 | 1.000 | 1.000 |
| 24 | Female | <0.001 | 0.027 | 0.054 | 0.122 | 1.000 | 1.000 |
| 28 | Female | 0.093 | 0.488 | 1.000 | 0.295 | 1.000 | 1.000 |
| 29 | Female | 0.865 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| 30 | Female | 0.034 | 0.733 | 1.000 | 0.021 | 1.000 | 1.000 |
| 32 | Female | 0.039 | 0.253 | 1.000 | 0.254 | 1.000 | 0.251 |
| 33 | Male | 0.391 | 0.708 | 1.000 | 0.275 | 1.000 | 1.000 |
Results are presented as P values for combined TH1 cytokine responses and for individual cytokine responses. Significant P values (P<0.05) are shown in bold font. Underlined font is used to show full TH1 cytokine profile (IL-2/IFN-γ/TNF-α) responders. Italic font is used to show samples with mixed TH1/IL-10 regulatory CD4 T-cell responses.
Identification number of healthy donor.
Sex of donor.
Combined TH1 cytokine is statistically significant TH1 type responses calculated by combining trends in positivity responses for IL-2, IFN-γ and TNF-α.
Significance of responses of individual cytokines.
FIGURE 1.
Examples of CD4+ T-cell responses to AFP protein presented by autologous DC by direct ex vivo multicytokine ELISPOT assay. A, Cytokine profile in AFP protein nonresponsive donor 08. The difference between AFP protein-fed DC versus DC only was not significant with P = 0.29 for combined positivity in TH1 cytokine responses, and P = 1.000 for TNF-α. B, Cytokine profile in AFP protein reactive donor 11. Results are expressed in average spot distribution between wells. "DC+CD4" is DC without antigen+CD4+ T cells; "DC (AFP)+CD4" is AFP protein-fed DC+CD4+ T cells.
To expand the number of cytokines examined, ELISPOT supernatants were tested by Luminex methodology for the 5 cytokines tested by ELISPOT as well as IL-4 and GM-CSF. Unfortunately, the Luminex array results for AFP protein-specific helper T-cell responses were below the level of detection and were not significant. Therefore, there was no evidence for additional cytokines produced (other than to PHA positive mitogen control, not shown).
CD4+ T-cell Responses to AdVhAFP and Their Comparison With Responses to AFP Protein in Healthy Donors
We next wished to determine whether a more potent and immunogenic method of antigen presentation would allow detection of AFP-specific CD4+ T cells. We have previously shown that AdV transduction is an efficient method of engineering DC to express a transgene for at least 10 days.54,57 Because AdV-transduced DC also process and present AdV epitopes,52 which would be recall antigens for most subjects, responses to AdVhAFP-transduced DC (AdVhAFP/DC) were compared with responses to AdVlacZ-transduced DC. AdVlacZ uses the same backbone as AdVhAFP and should process and present the same AdV epitopes as AdVhAFP/DC. All analyses were performed by 2 assays: ELISPOT and Luminex array, in parallel.
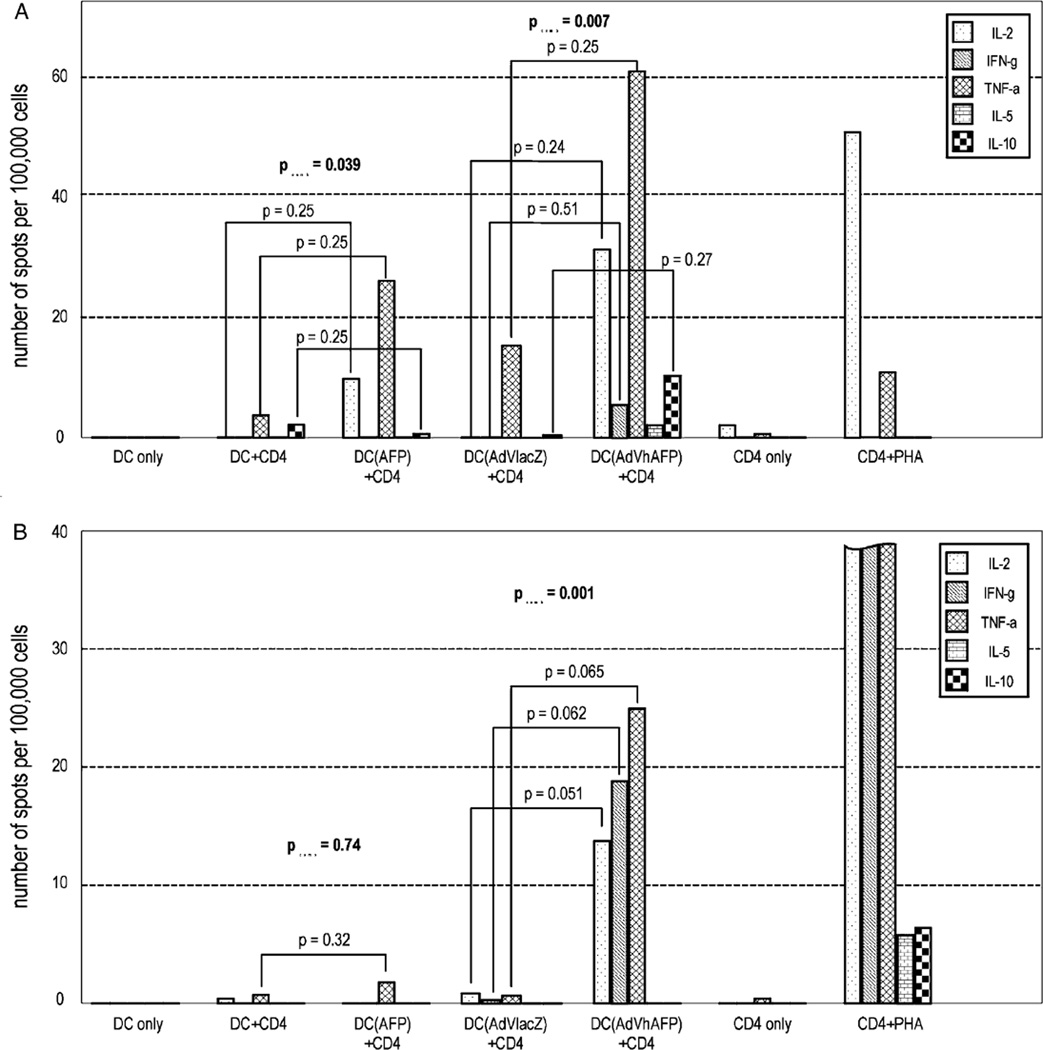
Fifteen healthy donors (57%) had statistically significant responses to at least 1 individual cytokine and when TH1 cytokines were combined, 22 healthy donors (84%) demonstrated significant CD4+ T-cell responses to AdVhAFP/DC by ELISPOT assay (Table 2), from 54 to 2050 positive cells/106. Twenty donors had exclusively TH1 responses and 2 donors (donors 18 and 24) showed mixed TH1/TH2/IL-10 regulatory CD4+ helper responses. We were able to detect AFP-specific CD4+ responses in a much higher percentage of donors by presenting the antigen via AdVhAFP/DC, compared with AFP protein-fed DC. Donor 18, who had a mixed TH1/regulatory response to AFP protein (Table 1), reproduced this type of response with AdVhAFP/DC, adding significant production of IL-2 and IFN-γ. Surprisingly, 1 sample (donor 02) positive for an AFP-specific response to AFP protein was negative for response to AdVhAFP. All other donors who had detectable AFP protein responses were also positive with AdVhAFP/DC. Two examples are shown in Figure 2 (donors 32 and 09).
TABLE 2.
Distribution of CD4+ T-cell Responses to AdVhAFP in Healthy Donors
| Donor ID* | Sex† | Combined TH1 Responses‡ | IL-2§ | IFN-γ§ | TNF-α§ | IL-5§ | IL-10§ |
|---|---|---|---|---|---|---|---|
| 00 | Female | 0.877 | 0.245 | 0.466 | 1.000 | 0.972 | 0.927 |
| 02 | Female | 1.000 | 1.000 | 0.133 | 1.000 | 1.000 | 1.000 |
| 03 | Male | 0.015 | 0.158 | 0.157 | 0.435 | 0.717 | 1.000 |
| 04 | Male | 0.002 | 0.049 | 0.057 | 0.544 | 1.000 | 0.961 |
| 05 | Male | 0.047 | 0.681 | 0.062 | 0.569 | 1.000 | 0.677 |
| 06 | Male | <0.001 | 0.012 | 0.033 | 0.049 | 1.000 | 1.000 |
| 07 | Male | <0.001 | 0.053 | 0.060 | 0.057 | 1.000 | 1.000 |
| 08 | Male | 0.280 | 0.591 | 1.000 | 0.527 | 1.000 | 1.000 |
| 09 | Male | <0.001 | 0.051 | 0.062 | 0.065 | 1.000 | 1.000 |
| 10 | Male | <0.001 | 0.195 | 0.009 | 0.012 | 0.187 | 0.259 |
| 11 | Male | <0.001 | 0.008 | 0.032 | 0.007 | 1.000 | 1.000 |
| 13 | Male | <0.001 | 0.009 | 0.012 | 0.009 | 0.507 | 0.970 |
| 14 | Female | <0.001 | 0.009 | 0.007 | 0.039 | 1.000 | 0.058 |
| 15 | Male | <0.001 | 0.090 | 0.010 | 0.010 | 0.981 | 0.091 |
| 17 | Male | <0.001 | 0.057 | 0.158 | 0.012 | 1.000 | 0.111 |
| 18 | Male | <0.001 | 0.008 | 0.027 | 0.008 | 1.000 | 0.041 |
| 19 | Male | <0.001 | 0.013 | 0.066 | 0.008 | 1.000 | 1.000 |
| 21 | Female | 0.345 | 1.000 | 1.000 | 0.313 | 0.326 | 1.000 |
| 22 | Female | 0.012 | 0.241 | 0.507 | 0.277 | 1.000 | 1.000 |
| 23 | Female | <0.001 | 0.005 | 0.332 | 0.013 | 1.000 | 1.000 |
| 24 | Female | 0.001 | 0.146 | 0.292 | 0.011 | 0.047 | 0.008 |
| 28 | Female | <0.001 | 0.009 | 0.049 | 0.035 | 1.000 | 1.000 |
| 29 | Female | 0.002 | 0.056 | 0.665 | 0.051 | 1.000 | 0.984 |
| 30 | Female | <0.001 | 0.015 | 1.000 | 0.005 | 1.000 | 1.000 |
| 32 | Female | 0.007 | 0.245 | 0.518 | 0.251 | 0.997 | 0.274 |
| 33 | Male | <0.001 | 0.009 | 0.006 | 0.012 | 1.000 | 1.000 |
Results are presented as P values for combined TH1 cytokine responses and for individual cytokine responses. Significant P values (P<0.05) are shown in bold font. Underlined font is used to show full TH1 cytokine profile (IL-2/IFN-γ/TNF-α) responders. Italic font is used to show samples with mixed TH1/IL-10 regulatory CD4 T-cell responses.
Identification number of healthy donor.
Sex of donor.
Combined TH1 cytokine is statistically significant TH1 type responses calculated by combining trends in positivity responses for IL-2, IFN-γ and TNF-α.
Significance of responses of individual cytokines.
FIGURE 2.
Example of differences in cytokine profiles between AFP protein/DC-specific and AdVhAFP/DC-specific CD4+ T-cell responses in healthy donors. A, Cytokine profile in donor 32, responsive for AdVhAFP/DC and for AFP protein/DC. B, Cytokine profile in donor 09, nonresponsive for AFP protein/DC but positive to AdVhAFP/DC. Results are expressed in average spot distribution between wells. DC+CD4 is DC without antigen+CD4+ T cells; DC (AFP)+CD4 is AFP protein-fed DC+CD4+ T cells; "DC (AdVlacZ)+CD4" is AdVlacZ engineered DC+CD4+ T cells; "DC (AdVhAFP)+CD4" is AdVhAFP-engineered DC+CD4+ T cells.
The cytokine profiles from AdVhAFP/DC-stimulated cells were significantly different from AFP protein/DC-stimulated cells, with more cells producing IL-2 and IFN-γ and lower frequencies of cells exclusively producing TNF-α. In fact, significant responses to TNF-α alone were reduced from 5/8 (AFP protein) to 1/15 (donor 17, AdVhAFP) among responders. The significant frequencies of IFN-γ producing cells strongly increased from 1/8 (donor 11) (AFP protein) to 8/15 (AdVhAFP). A similar tendency was observed for IL-2, from 2/8 (AFP protein) to 11/15 (AdVhAFP). Spot sizes and their intensity in the ELISPOT assay were also strongly increased when antigen was presented by AdVhAFP/DC (data not shown).
Using a Luminex assay, many samples had insufficient levels of cytokine to be in range. However, a majority of the analyzable samples showed similar results for AdVhAFP-specific responses in healthy donors between Luminex and ELISPOT assays for pooled TH1 cytokine data (18/19 concordant, Table 3).
TABLE 3.
Comparison of ELISPOT and Luminex Assays Results for CD4+ T-cell Responses to AdVhAFP in Healthy Donors
| Combined TH1 Cytokine‡ | |||
|---|---|---|---|
| Donor ID* | Sex† | ELISPOT | Luminex |
| 00 | Female | 0.877 | 1.00 |
| 04 | Male | 0.002 | 0.044 |
| 05 | Male | 0.047 | 0.352 |
| 10 | Male | 0.001 | 0.008 |
| 11 | Male | 0.001 | 0.010 |
| 13 | Male | 0.001 | 0.004 |
| 14 | Female | 0.001 | 0.014 |
| 15 | Male | 0.001 | 0.010 |
| 17 | Male | 0.001 | 0.004 |
| 18 | Male | 0.001 | 0.026 |
| 19 | Male | 0.001 | 0.008 |
| 21 | Female | 0.345 | 0.282 |
| 22 | Female | 0.012 | 0.006 |
| 23 | Female | 0.001 | 0.006 |
| 24 | Female | 0.001 | 0.008 |
| 29 | Female | 0.002 | 0.044 |
| 30 | Female | 0.001 | 0.008 |
| 32 | Female | 0.007 | 0.026 |
| 33 | Male | 0.001 | 0.010 |
Results are presented as P values for combined TH1 cytokine responses and for individual cytokine responses. Combined TH1 cytokine—statistically significant TH1 type responses calculated by combining trends in positivity responses for IL-2, IFN-γ and TNF-α. Significant P values (P < 0.05) are shown in bold font.
Identification number of healthy donor.
Sex of donor.
Combined TH1 cytokine is statistically significant TH1 type responses calculated by combining trends in positivity responses for IL-2, IFN-γ and TNF-α.
CD4+ T-cell Responses to Soluble AFP in HCC Patients
We next wished to assess the state of CD4+ immunity in subjects with AFP-positive HCC. The characteristics of these patients are shown in Table 4. They were all male, most had stage IV disease, they had different HCC risk factors and a wide range of serum AFP concentrations. They are listed in order of serum AFP levels. We tested the same modes of antigen presentation (protein-fed and AdVhAFP-transduced DC) and the same ex vivo multicytokine ELISPOT and Luminex assays. Unfortunately, there were insufficient cells to study IL-5 and IL-10 cytokine responses by ELISPOT.
TABLE 4.
Clinical HCC Patient’s Data and Distribution of AFP – /AdVhAFP-specific CD4+ T-cell Responses
| ID* | Age† | Sex‡ | Race§ | Risk Factors‖ | Stage¶ | AFP Level (ng/ml)# | AFP-specific Responses** | AdVhAFP-specific Responses†† |
|---|---|---|---|---|---|---|---|---|
| B12 | 52 | M | White | Unknown | III | 74.3 | 0.095 | 0.013 |
| A3 | 69 | M | White | Alcohol | IVa | 3080 | 0.293 | 0.981 |
| B11 | 60 | M | White | Porphypia | IVa | 4340 | 0.461 | 0.031 |
| A4 | 62 | M | Asian | HCV | IVb | 10,800 | 0.987 | 0.033 |
| B1 | 67 | M | White | HBV | IVb | 60,800 | 0.083 | 0.090 |
| B10 | 59 | M | White | HCV | IVa | 463,040 | 0.327 | 1.000 |
Results are presented as P values for combined TH1 cytokine responses.
Combined TH1 cytokine—statistically significant TH1 type responses calculated by combining trends in positivity responses for IL-2, IFN-γ, and TNF-α. Significant P values (P<0.05) are shown in bold font.
HCC patient identification.
Age of patient.
M—male sex.
Race of patient.
Risk factor identified for HCC, HCV, hepatitis C virus; HBV, hepatitis B virus.
Stage of HCC disease.
AFP level—level AFP in the blood in time of leukapheresis.
AFP-specific responses: CD4+ T-cell responses to AFP protein-fed DC.
AdVhAFP-specific responses: CD4+ T-cell responses to AFP activated by AdVhAFP-engineered DC.
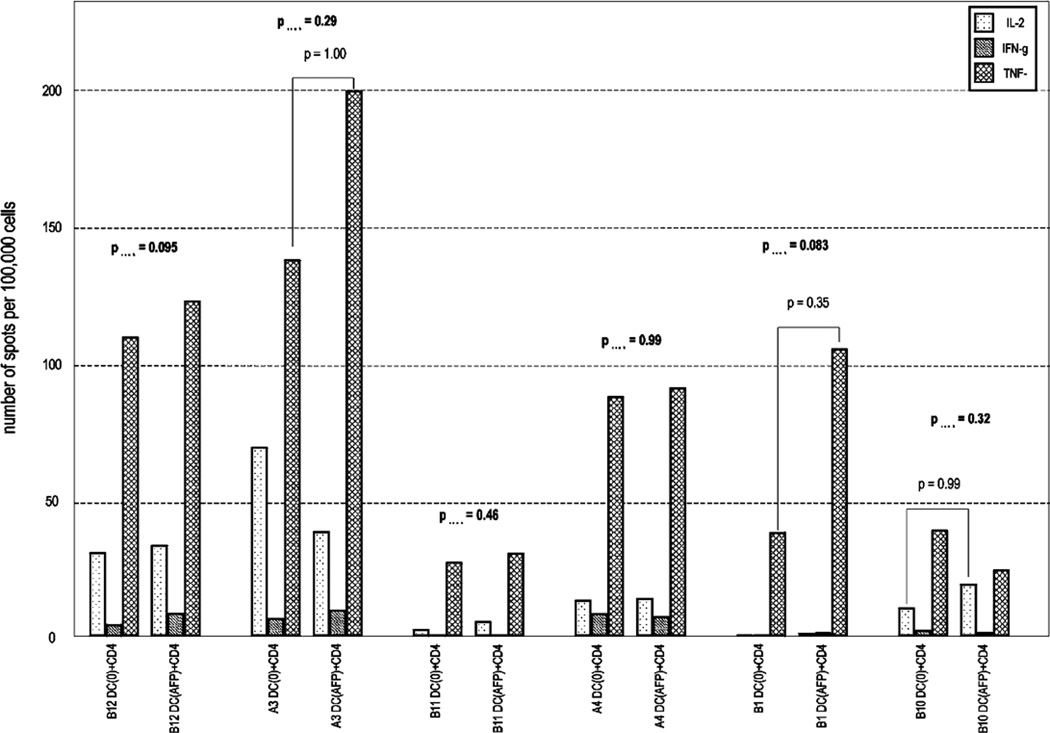
We did not detect any statistically significant CD4+ T-cell responses to AFP protein/DC by single or by combination of TH1 cytokines among these HCC patients. A summary of the cytokine expression profiles in HCC obtained by ex vivo ELISPOT assay to AFP protein-fed DC is shown in Figure 3. Three of the 6 HCC patients appeared to have a trend toward AFP-specific CD4+ responses (B12, A3, B1) but these were not significant. Patients A3 and B1 demonstrated slight but not significant TNF-α increases. PHA stimulation of CD4+ T cells stimulated high frequencies of IL-2, IFN-γ, and TNF-α producing T cells, demonstrating that the cells were capable of strong, multicytokine responses. The only exception was patient B10 [who had the highest AFP level (463,040 ng/mL)] with viable cells but very low PHA responses.
FIGURE 3.
Summary of TH1 cytokine profiles of CD4+ helper cells activated by AFP-fed DC versus empty DC among HCC patients. Patients are listed in order of increasing serum AFP level. Results are expressed as average spot counts. DC+CD4 is DC without antigen+CD4+ T cells; DC (AFP)+CD4 is AFP protein-fed DC+CD4+ T cells.
CD4+ T-cell Responses to AdVhAFP and Clinical Data in HCC Patients
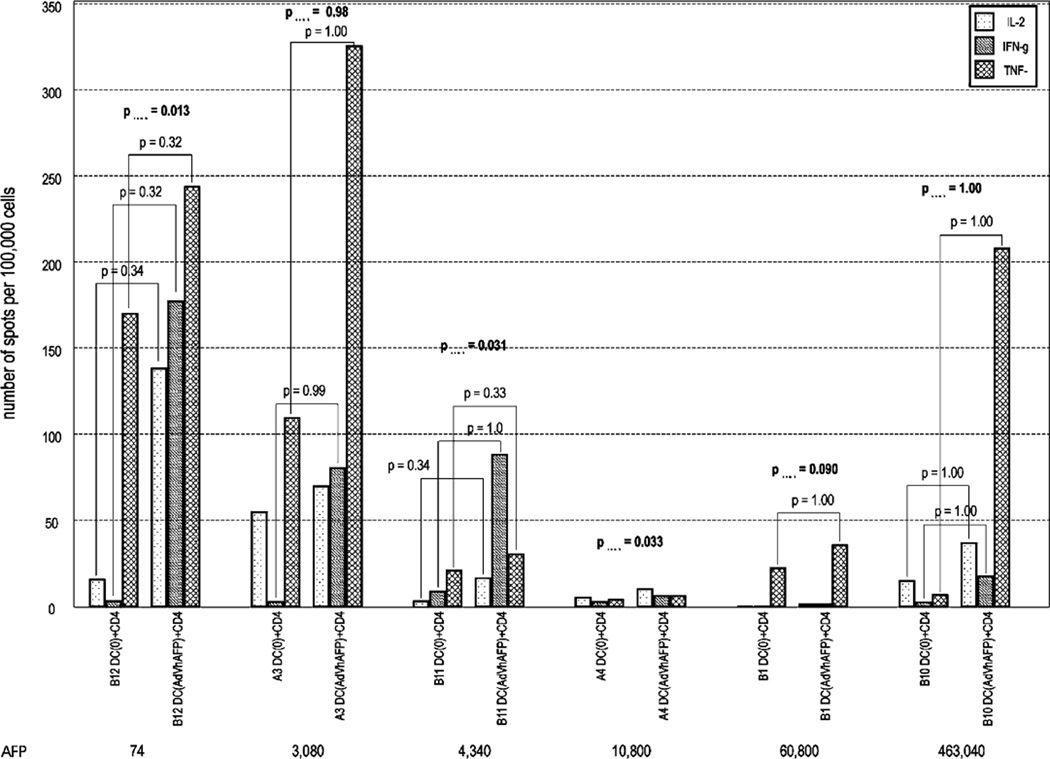
When HCC patient cells were stimulated by AFP in the form of AdVhAFP/DC, the responses were much stronger, as we observed with the healthy donors. However, single cytokine responses were still not statistically significant (Fig. 4). By combined TH1 cytokine analysis, 3 HCC patients (50%) had significant responses to AdVhAFP/DC (B12, B11, A3). There has been a report that high level AFP (10 µg/mL) is inhibitory to DC function, 36 and half of the HCC patients in our study (A4, B1, B10) were above this level. Notably, most of the pooled TH1 cytokine responders had lower serum AFP levels than nonresponders, and also earlier stages of disease (Table 4), in agreement with Um et al.36 In addition, patient B12 (stage III, lowest serum AFP) had a detectable [but not statistically significant (P=0.095)] increase in combined TH1 cytokine (IL-2, IFN-γ, and TNF-α) response to AFP protein/DC and significant responses to AdVhAFP engineered DC (Figs. 3, 4; Table 4).
FIGURE 4.
Summary of TH1 cytokine profiles of CD4+ helper cells activated by AdVhAFP-engineered DC versus DC transduced with AdVlacZ among HCC patients (B12, A3, B11, A4, B1, and B10). Patients are listed in order of increasing serum AFP level. Results are expressed as average spot counts. DC (AdVlacZ)+CD4 is AdVlacZ engineered DC+CD4+ T cells; DC (AdVhAFP)+CD4 is AdVhAFP-engineered DC+CD4+ T cells.
We found that the frequency of AdVhAFP/DC activated CD4+ helper T cells expressing IL-2 and IFN-γ seemed to be related to level of serum AFP level (Fig. 4); however, Spearman test indicated no significant correlation between the level of serum AFP and the expression of IL-2 (P=0.10), IFN-γ (P=0.24) (or TNF-α). If this nonsignificant trend in IL-2 and IFN-γ (not TNFα) with high AFP level were found significant in a study with a larger sample size, it would suggest that serum AFP concentration may affect CD4+ immunity in HCC patients. There were no trends or significant differences in responses to AdV antigens (AdVlacZ) or AFP protein/DC and serum AFP level.
DISCUSSION
In the present study, we have investigated spontaneous CD4+ T-cell responses to the oncofetal antigen AFP presented by either soluble protein-fed immature DC or AdVhAFP-engineered DC in healthy donors and HCC patients. The majority of reports of AFP-specific T-cell responses have focused on CD8+ T-cell responses to MHC class I peptides.33,38,39,41–46,58 It is clear, however, that activation of fully functional CTL requires helper T cells.51 “Helped” CTL are capable of undergoing secondary expansion and acquiring full effector function. 50 Here, using autologous DC presenting 2 forms of the antigen, we have detected low levels of preexisting TH1 CD4+ T-cell responses by direct ex vivo multicytokine ELISPOT assays in healthy donors (to AFP protein-fed DC) and higher frequency of TH1 helper responses (to AdVhAFP-transduced DC) in both HCC patients and healthy donors, by both ELISPOT and Luminex cytokine assays.
Our analysis indicates that 30% to 57% of healthy donors (8/26-15/26) have significant AFP-specific CD4+ helper cell responses, but that HCC patients at advanced stages of disease no longer have significant responses. This may be due to exhaustion or activation-induced cell death from chronic antigen exposure. It is also possible that these cells are present in HCC patients at a frequency not detectable ex vivo, and that brief in vitro stimulation would reveal that these cells are not completely eliminated. This is under investigation. It is also possible that there are AFP-specific helper cells producing cytokines other than the 7 most commonly described cytokines tested here.
We have also shown that the mode of antigen presentation has an important effect on the ability to detect these T-cell responses. AdVhAFP-engineered DC are clearly more potent APC than DC pulsed with soluble AFP protein. It has been shown that AdV-transduced DC become more mature owing to down-regulation of CD14, up-regulation of CD83, CD86, and HLA-DR, and also decreased production of IL-10, and increased expression of IL-12p70.59 AdV-transduced DC also present antigen for as long as 10 days57; however, in the context of a 24 to 48-hour ex vivo cytokine assay, this is unlikely to be a critical difference. It has also been shown that DC transduced with type 5 AdV vectors produce type I IFN (IFN-α), which can also drive DC maturation.59,60 Here, we find that, for healthy donors, the frequencies of responding CD4+ T cells were 10 to 100 times higher with AdVhAFP/DC-stimulated helper T cells and the amount of expressed cytokines (measured by Luminex array) was often 2 to 10 times higher (data not shown).
Interestingly, the cytokine profiles elicited by these 2 modes of antigen presentation were different. TNF-α was the most commonly expressed cytokine in CD4+ T cells activated by soluble AFP, whereas in AdVhAFP-induced T cells, IL-2, IFN-γ, and TNFα were more equivalently expressed. Only one donor had a significant IFN-γ response when activation was via soluble AFP, and 2 had significant IL-2 responses (one of which was also IFN-γ +), whereas 5 were TNF-α responses exclusively and 1 was both TNF-α and IL-10 (Table 1). Therefore, if we had only investigated IFN-γ, we would have concluded that 1/26 donors had preexisting levels of AFP-specific CD4+ T cells. These data are in sharp contract to those obtained with stimulation of cells with AdVhAFP/DC. Table 2 shows that only 1 donor had exclusively TNF-α responses, whereas 13 donors responded with 2 to 3 different TH1 cytokines. This indicates that the more potent APC mode stimulated a greater frequency and functional breadth in responding cells. The weaker mode of presentation was designed to mimic the mode naturally occurring in vivo when immature DC take up soluble AFP during fetal life and in the presence of tumor. This seems to result in low level activation of cells with a limited range of function (primarily TNF-α secretion). The AdVhAFP/DC were more potent CD4+ T-cell activators, and have also been recently found to activate a broad range of CD8+ T-cell antigenic specificities.44
The ability of CD4+ T cells to produce IFN-γ can positively impact antitumor immunity by inducing expression of the immunoprotoesome in APC, as well as TAP transporter proteins and MHC class I molecules, also making tumors more easily recognized by CD8+ T cells.61 IL-2 acts in several ways, potentially promoting CD8+ T-cell proliferation and death. It is thought that IL-2 produced by helper cells promotes activation and expansion of CTL with low CD8+ precursor frequencies and low affinity of peptide-MHC interactions.62 Importantly, IL-2 is an early signal for establishing long-term T-cell expansion and ability to differentiate into effector cells,63 and anergy of T cells can be reversed by exogenous IL-2.64
B-cell responses to AFP are generally not detected, which may point to deficits in CD4+ T-cell responses. However, it has been shown that AFP-specific CD4+ T-cell responses (to an HLA class II DR-restricted AFP-derived epitope) were only found in HCC and cirrhotic patients (n=40 and n=13, respectively) but not in any healthy donors (n=7).47 We have found that frequencies of AFP-specific responses to soluble AFP protein detected by ELISPOT assay are very low in healthy donors (in agreement with47). Importantly, given that we are detecting all possible CD4+ helper cells responding to the full length AFP protein, the frequencies of cells responding to the single AFP-derived epitope would be lower.
There are several potential scenarios in which healthy individuals might be exposed to AFP transiently, allowing for expansion of AFP-specific CD4+ T cells. First, AFP expression has been identified in normal liver cells, localized in areas of liver blood vessels and sinusoids.8,9,65 Second, reactivation of AFP synthesis in hepatocytes occurs reversibly after liver injuries10 and during acute viral hepatitis A, B, and C infections. 11,12,14,15 Third, it has been shown that some immune cells can develop into memory cells owing to exposure to AFP during early stages of embryonic development.66 Fourth, women are exposed to AFP during their pregnancies. It may be important in future studies to test healthy donor cells and serum for evidence of exposure to hepatitis viruses to determine the potential role of exposure to antigen from viral infection in helper cell activation.
Our data indicate lower frequencies of AFP-specific CD4+ T cells in some of the HCC patients with the highest levels of serum AFP. The trend toward this inverse correlation was found for IL-2 and IFN-γ producing cells, although it did not reach significance (P = 0.10 and 0.24, respectively). Earlier studies have found that high levels of AFP (greater than 100 ng/mL to 10 µg/mL) suppress MHC class II expression on monocyte-derived DCs and on CD4+ T cells.26,34,36 AFP-exposed DC exhibited lower cell viability, inhibition of cell stimulatory capacity, decreased ability to produce cytokines, and increased susceptibility to apoptosis.36 TNF-α did not show any significant relationship with either serum AFP level or other cytokine production, which could be complicated by binding of this cytokine by AFP protein.67–69
There have been 2 clinical trials conducted in which advanced stage HCC patients were immunized with MHC class I-restricted AFP peptides with the goal ofactivating CD8+ T cells.45,46 Although the majority of patients were successfully immunized, there were no objective clinical responses. Several other immunotherapy trials have been tested in HCC. Most recently, Gao et al70 have tested autologous tumor lysate-pulsed DC in 30 postoperative HCC patients. The survival rate at 18 months was improved in the DC-vaccine treated group (vs. the chemotherapy group) and the hepatic recurrence rate in the DC group was 13% (vs. 54% in the chemotherapy group, P < 0.05),70 which suggests that immunotherapy may clinically impact HCC. The complex mixture of protein antigens in tumor lysate would be expected to broadly stimulate multiple CD8+ and CD4+ T cells, which might have played a role in the improved clinical outcomes. We have not observed any AFP vaccine-related toxicities, but as we test improved vaccine strategies, improved T-cell responses to this self-antigen could result in some level of autoimmune toxicity. In previous murine studies, mice immunized with murine AFP were examined pathologically, and there was no evidence of toxicity.43
An important advantage of using the adenovirally engineered DC as the mode of antigen presentation maybe to program a TH1 type of adaptive response to tumor associated antigens. However, the exact role of virus-specific adenoviral responses in promotion of tumor antigen responses has yet to be identified. We have detected these antiadenovirus cytotoxic responses in addition to tumor antigen responses from AdV-transduced DC in vitro.52 Notably, in murine model studies, systemic AdV delivery resulted in neutralizing anti-AdV antibody responses, and multiple AdV-engineered DC immunizations also resulted in AdV antibody stimulation.71 However, transgene-specific T-cell responses induced by AdV/DC were not reduced by the presence of anti-AdV neutralizing antibodies. Potentially higher avidity, TH-1 skewed recall T-cell responses to the foreign adenoviral epitopes may also play a critical role in shaping these CD4+ responses; this is an area of active investigation.
ACKNOWLEDGMENTS
The authors thank Core Luminex Facility of University of Pittsburgh (A. Lokshin, Director) for multiplex cytokine analyses; and Sean Daley and David D. Zdobinski for technical assistance.
Supported by a Scientist Development Grant from the American Heart Association (No. 0330102N), the Pittsburgh Foundation, the University of Pittsburgh Cancer Institute, and the Henry L. Hillman Foundation.
Footnotes
Financial Disclosure: One of the authors is a coinventor on a recently granted US Patent (L. H. B., US Patent No. 7,098,306, “Method and compositions for treating hepatocellular cancer,” granted August 2006), which covers some of the MHC class I peptides used in a recently published clinical trial testing AFP class I peptides pulsed onto autologous DC. The intellectual property is owned by the University of California, has not been licensed, and there has been no financial interest for the inventors, to date. The HCC patient PBMC samples used in this work were from that clinical trial. The remaining authors have declared there are no financial conflicts of interest related to this work.
REFERENCES
- 1.Levin B, Amos C. Therapy of unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1294–1296. doi: 10.1056/NEJM199505113321910. [editorial; comment] [published erratum appears in N Engl J Med.1995 Sep 7;333(10):675]. [DOI] [PubMed] [Google Scholar]
- 2.Venook AP. Treatment of hepatocellular carcinoma: too many options? J Clin Oncol. 1994;12:1323–1334. doi: 10.1200/JCO.1994.12.6.1323. [DOI] [PubMed] [Google Scholar]
- 3.Geller DA, Tsung A, Marsh JW, et al. Outcome of 1000 liver cancer patients evaluated at the UPMC Liver Cancer Center. J Gastrointest Surg. 2006;10:63–68. doi: 10.1016/j.gassur.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. [PubMed] [Google Scholar]
- 5.Ince N, Wands JR. The increasing incidence of hepatocellular carcinoma. N Engl J Med. 1999;340:798–799. doi: 10.1056/NEJM199903113401009. [editorial; comment]. [DOI] [PubMed] [Google Scholar]
- 6.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 7.Society AC. Cancer Facts and Figures. 2006 [Google Scholar]
- 8.Lemire JM, Fausto N. Multiple alpha-fetoprotein RNAs in adult rat liver: cell type-specific expression and differential regulation. Cancer Res. 1991;51:4656–4664. [PubMed] [Google Scholar]
- 9.Ohguchi S, Nakatsukasa H, Higashi T, et al. Expression of alpha-fetoprotein and albumin genes in human hepatocellular carcinomas: limitations in the application of the genes for targeting human hepatocellular carcinoma in gene therapy. Hepatology. 1998;27:599–607. doi: 10.1002/hep.510270239. [DOI] [PubMed] [Google Scholar]
- 10.Abelev GI, Eraiser TL. Cellular aspects of alpha-fetoprotein reexpression in tumors. Semin Cancer Biol. 1999;9:95–107. doi: 10.1006/scbi.1998.0084. [DOI] [PubMed] [Google Scholar]
- 11.Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis C. Am J Gastroenterol. 1998;93:2452–2456. doi: 10.1111/j.1572-0241.1998.00703.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen DS, Sung JL. Relationship of hepatitis B surface antigen to serum alpha-fetoprotein in nonmalignant diseases of the liver. Cancer. 1979;44:984–992. doi: 10.1002/1097-0142(197909)44:3<984::aid-cncr2820440328>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Collazos J, Genolla J, Ruibal A. Preliminary study of alpha-fetoprotein in nonmalignant liver diseases. A clinico-biochemical evaluation. Int J Biol Markers. 1992;7:97–102. doi: 10.1177/172460089200700205. [DOI] [PubMed] [Google Scholar]
- 14.Harada T, Shigeta K, Noda K, et al. Clinical implications of alpha-fetoprotein in liver cirrhosis: five-year follow-up study. Hepatogastroenterology. 1980;27:169–175. [PubMed] [Google Scholar]
- 15.Hu KQ, Kyulo NL, Lim N, et al. Clinical significance of elevated alpha-fetoprotein (AFP) in patients with chronic hepatitis C, but not hepatocellular carcinoma. Am J Gastroenterol. 2004;99:860–865. doi: 10.1111/j.1572-0241.2004.04152.x. [DOI] [PubMed] [Google Scholar]
- 16.Lazarevich NL. Molecular mechanisms of alpha-fetoprotein gene expression. Biochemistry (Mosc) 2000;65:117–133. [PubMed] [Google Scholar]
- 17.Kubota M, Yagi M, Kanada S, et al. Effect of postoperative chemotherapy on the serum alpha-fetoprotein level in hepatoblastoma. J Pediatr Surg. 2004;39:1775–1778. doi: 10.1016/j.jpedsurg.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawara A, Ikeda K, Tsuneyoshi M, et al. Hepatoblastoma producing both alpha-fetoprotein and human chorionic gonadotropin. Clinicopathologic analysis of four cases and a review of the literature. Cancer. 1985;56:1636–1642. doi: 10.1002/1097-0142(19851001)56:7<1636::aid-cncr2820560729>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Van Tornout JM, Buckley JD, Quinn JJ, et al. Timing and magnitude of decline in alpha-fetoprotein levels in treated children with unresectable or metastatic hepatoblastoma are predictors of outcome: a report from the Children’s Cancer Group. J Clin Oncol. 1997;15:1190–1197. doi: 10.1200/JCO.1997.15.3.1190. [DOI] [PubMed] [Google Scholar]
- 20.Newby D, Dalgliesh G, Lyall F, et al. Alphafetoprotein and alphafetoprotein receptor expression in the normal human placenta at term. Placenta. 2005;26:190–200. doi: 10.1016/j.placenta.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Zeng CQ, Alpert E. Isolation and partial characterization of a specific alpha-fetoprotein receptor on human monocytes. J Clin Invest. 1992;90:1530–1536. doi: 10.1172/JCI116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres CA, Iwasaki A, Barber BH, et al. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 23.Macho A, Aguilar JJ, Naval J, et al. Expression of alpha-fetoprotein and interleukin 2 receptors and impairment of membrane fluidity in peripheral blood mononuclear cells from AIDS and related syndromes. AIDS Res Hum Retroviruses. 1994;10:995–1001. doi: 10.1089/aid.1994.10.995. [DOI] [PubMed] [Google Scholar]
- 24.Severin SE, Rodina AV, Nitsvetov MB, et al. Regulation of anti-tumor activity using monoclonal antibodies to alpha-fetoprotein receptor and after immunization with this protein. Russ J Immunol. 2001;6:249–256. [PubMed] [Google Scholar]
- 25.Cavin LG, Venkatraman M, Factor VM, et al. Regulation of alpha-fetoprotein by nuclear factor-kappaB protects hepatocytes from tumor necrosis factor-alpha cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030–7038. doi: 10.1158/0008-5472.CAN-04-1647. [DOI] [PubMed] [Google Scholar]
- 26.Cohen BL, Orn A, Gronvik KO, et al. Suppression by alpha-fetoprotein of murine natural killer cell activity stimulated in vitro and in vivo by interferon and interleukin 2. Scand J Immunol. 1986;23:211–223. doi: 10.1111/j.1365-3083.1986.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 27.Dattwyler RJ, Tomasi TB. Inhibition of sensitization of T-cells by alpha-fetoprotein. Int J Cancer. 1975;16:942–945. doi: 10.1002/ijc.2910160608. [DOI] [PubMed] [Google Scholar]
- 28.Geissler M, Mohr L, Ali MY, et al. Immunobiology and gene-based immunotherapy of hepatocellular carcinoma. Z Gastroenterol. 2003;41:1101–1110. doi: 10.1055/s-2003-44304. [DOI] [PubMed] [Google Scholar]
- 29.Lu CY, Changelian PS, Unanue ER. Alpha-fetoprotein inhibits macrophage expression of Ia antigens. J Immunol. 1984;132:1722–1727. [PubMed] [Google Scholar]
- 30.Matsuura E, Kang Y, Kitakawa H, et al. Modulation of T cell function by alpha-fetoprotein: an in vivo study on porcine thyroid peroxidase-induced experimental autoimmune thyroiditis in transgenic mice producing human alpha-fetoprotein. Tumour Biol. 1999;20:162–171. doi: 10.1159/000030059. [DOI] [PubMed] [Google Scholar]
- 31.Neumann U. Inhibition of in vitro lymphocyte blastogenesis by chicken alpha-fetoprotein. Comp Immunol Microbiol Infect Dis. 1984;7:43–52. doi: 10.1016/0147-9571(84)90015-8. [DOI] [PubMed] [Google Scholar]
- 32.Peck AB, Murgita RA, Wigzell H. Cellular and genetic restrictions in the immunoregulatory activity of alpha-fetoprotein. III. Role of the MLC-stimulating cell population in alpha-fetoprotein-induced suppression of T cell-mediated cytotoxicity. J Immunol. 1982;128:1134–1140. [PubMed] [Google Scholar]
- 33.Ritter M, Ali MY, Grimm CF, et al. Immunoregulation of dendritic and T cells by alpha-fetoprotein in patients with hepatocellular carcinoma. J Hepatol. 2004;41:999–1007. doi: 10.1016/j.jhep.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Semeniuk DJ, Boismenu R, Tam J, et al. Evidence that immuno-suppression is an intrinsic property of the alpha-fetoprotein molecule. Adv Exp Med Biol. 1995;383:255–269. doi: 10.1007/978-1-4615-1891-4_27. [DOI] [PubMed] [Google Scholar]
- 35.Toder V, Blank M, Nebel L. Immunoregulatory mechanisms in pregnancy. 1. Evidence for the alpha-fetoprotein-induced generation of suppressor cells in vitro. Transplantation. 1982;33:41–44. [PubMed] [Google Scholar]
- 36.Um SH, Mulhall C, Alisa A, et al. Alpha-fetoprotein impairs APC function and induces their apoptosis. J Immunol. 2004;173:1772–1778. doi: 10.4049/jimmunol.173.3.1772. [DOI] [PubMed] [Google Scholar]
- 37.Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127:S232–S241. doi: 10.1053/j.gastro.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 38.Butterfield LH, Koh A, Meng W, et al. Generation of human T-cell responses to an HLA-A2.1-restricted peptide epitope derived from alpha-fetoprotein. Cancer Res. 1999;59:3134–3142. [PubMed] [Google Scholar]
- 39.Butterfield LH, Meng WS, Koh A, et al. T cell responses to HLA-A*0201-restricted peptides derived from human alpha fetoprotein. J Immunol. 2001;166:5300–5308. doi: 10.4049/jimmunol.166.8.5300. [DOI] [PubMed] [Google Scholar]
- 40.Butterfield LH, Ribas A. Immunotherapy of hepatocellular carcinoma. Expert Opin Biol Ther. 2002;2:123–133. doi: 10.1517/14712598.2.2.123. [DOI] [PubMed] [Google Scholar]
- 41.Meng WS, Butterfield LH, Ribas A, et al. Alpha-fetoprotein-specific tumor immunity induced by plasmid prime-adenovirus boost genetic vaccination. Cancer Res. 2001;61:8782–8786. [PubMed] [Google Scholar]
- 42.Meng WS, Butterfield LH, Ribas A, et al. Fine specificity analysis of an HLA-A2.1-restricted immunodominant T cell epitope derived from human alpha-fetoprotein. Mol Immunol. 2000;37:943–950. doi: 10.1016/s0161-5890(01)00017-7. [DOI] [PubMed] [Google Scholar]
- 43.Vollmer CM, Jr, Eilber FC, Butterfield LH, et al. Alpha-fetoprotein-specific genetic immunotherapy for hepatocellular carcinoma. Cancer Res. 1999;59:3064–3067. [PubMed] [Google Scholar]
- 44.Liu Y, Daley S, Evdokimova VN, et al. Hierarchy of AFP-specific T cell responses in subjects with AFP-positive hepatocellular cancer. J Immunol. 2006;177:712–721. doi: 10.4049/jimmunol.177.1.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butterfield LH, Ribas A, Dissette VB, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 46.Butterfield LH, Ribas A, Meng WS, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902–5908. [PubMed] [Google Scholar]
- 47.Alisa A, Ives A, Pathan AA, et al. Analysis of CD4+ T-cell responses to a novel alpha-fetoprotein-derived epitope in hepatocellular carcinoma patients. Clin Cancer Res. 2005;11:6686–6694. doi: 10.1158/1078-0432.CCR-05-0382. [DOI] [PubMed] [Google Scholar]
- 48.Hanke P, Rabe C, Serwe M, et al. Cirrhotic patients with or without hepatocellular carcinoma harbour AFP-specific T-lymphocytes that can be activated in vitro by human alpha-fetoprotein. Scand J Gastroenterol. 2002;37:949–955. doi: 10.1080/003655202760230928. [DOI] [PubMed] [Google Scholar]
- 49.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 50.Janssen EM, Lemmens EE, Wolfe T, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 51.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butterfield LH, Jilani SM, Chakraborty NG, et al. Generation of melanoma-specific cytotoxic T lymphocytes by dendritic cells transduced with a MART-1 adenovirus. J Immunol. 1998;161:5607–5613. [PubMed] [Google Scholar]
- 53.Toloza EM, Hunt K, Miller AR, et al. Transduction of murine and human tumors using recombinant adenovirus vectors. Ann Surg Oncol. 1997;4:70–79. doi: 10.1007/BF02316813. [DOI] [PubMed] [Google Scholar]
- 54.Arthur JF, Butterfield LH, Roth MD, et al. A comparison of gene transfer methods in human dendritic cells. Cancer Gene Ther. 1997;4:17–25. [PubMed] [Google Scholar]
- 55.Herr W, Linn B, Leister N, et al. The use of computer-assisted video image analysis for the quantification of CD8+ T lymphocytes producing tumor necrosis factor alpha spots in response to peptide antigens. J Immunol Methods. 1997;203:141–152. doi: 10.1016/s0022-1759(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 56.Gorelik E, Landsittel DP, Marrangoni AM, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 57.Mehrotra S, Chhabra A, Chakraborty A, et al. Antigen presentation by MART-1 adenovirus-transduced interleukin-10-polarized human monocyte-derived dendritic cells. Immunology. 2004;113:472–481. doi: 10.1111/j.1365-2567.2004.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo J, Cai M, Wei D, et al. Immune responses of dendritic cells after loaded with cytotoxicity T lymphocyte epitope based peptide of human alpha-fetoprotein (hAFP) Zhonghua Gan Zang Bing Za Zhi. 2002;10:178–180. [PubMed] [Google Scholar]
- 59.Schumacher L, Ribas A, Dissette VB, et al. Human dendritic cell maturation by adenovirus transduction enhances tumor antigen-specific T-cell responses. J Immunother. 2004;27:191–200. doi: 10.1097/00002371-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Hensley SE, Giles-Davis W, McCoy KC, et al. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol. 2005;175:6032–6041. doi: 10.4049/jimmunol.175.9.6032. [DOI] [PubMed] [Google Scholar]
- 61.Whitmire JK, Benning N, Whitton JL. Cutting edge: early IFN-gamma signaling directly enhances primary antiviral CD4+ T cell responses. J Immunol. 2005;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- 62.Malek TR. T helper cells, IL-2 and the generation of cytotoxic T-cell responses. Trends Immunol. 2002;23:465–467. doi: 10.1016/s1471-4906(02)02308-6. [DOI] [PubMed] [Google Scholar]
- 63.Malek TR, Yu A, Scibelli P, et al. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol. 2001;166:1675–1683. doi: 10.4049/jimmunol.166.3.1675. [DOI] [PubMed] [Google Scholar]
- 64.Tham EL, Shrikant P, Mescher MF. Activation-induced nonresponsiveness: a Th-dependent regulatory checkpoint in the CTL response. J Immunol. 2002;168:1190–1197. doi: 10.4049/jimmunol.168.3.1190. [DOI] [PubMed] [Google Scholar]
- 65.Bisgaard HC, Nagy P, Ton PT, et al. Modulation of keratin 14 and alpha-fetoprotein expression during hepatic oval cell proliferation and liver regeneration. J Cell Physiol. 1994;159:475–484. doi: 10.1002/jcp.1041590312. [DOI] [PubMed] [Google Scholar]
- 66.Kim Y, Nakagawa Y, Sugiyama H, et al. Induction of CD4+ murine natural killer T-like cells by immunization with syngeneic thymoma expressing embryonic alpha-fetoprotein. Cell Immunol. 2003;226:1–10. doi: 10.1016/j.cellimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Mackiewicz A, Speroff T, Ganapathi MK, et al. Effects of cytokine combinations on acute phase protein production in two human hepatoma cell lines. J Immunol. 1991;146:3032–3037. [PubMed] [Google Scholar]
- 68.Semenkova LN, Dudich EI, Dudich IV, et al. Alpha-fetoprotein as a TNF resistance factor for the human hepatocarcinoma cell line HepG2. Tumour Biol. 1997;18:30–40. doi: 10.1159/000218013. [DOI] [PubMed] [Google Scholar]
- 69.Yamashita T, Nakane A, Watanabe T, et al. Evidence that alpha-fetoprotein suppresses the immunological function in transgenic mice. Biochem Biophys Res Commun. 1994;201:1154–1159. doi: 10.1006/bbrc.1994.1826. [DOI] [PubMed] [Google Scholar]
- 70.Gao J, Chen M, Ren H. Clinical effects of dendritic cells pulsed with autologous hepatoma cell lysates on the postoperative recurrence and metastasis of hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi. 2005;13:432–435. [PubMed] [Google Scholar]
- 71.Ribas A, Butterfield LH, Hu B, et al. Immune deviation and Fasmediated deletion limit antitumor activity after multiple dendritic cell vaccinations in mice. Cancer Res. 2000;60:2218–2224. [PubMed] [Google Scholar]