Abstract
CD4+ T helper (Th) cells exist in a variety of epigenetic states that determine their function, phenotype, and capacity for persistence. These polarization states include Th1, Th2, Th17, and Foxp3+ T regulatory cells, as well as the more recently described T follicular helper, Th9, and Th22 cells. Th17 cells express the master transcriptional regulator retinoic acid–related orphan receptor γ thymus and produce canonical interleukin (IL)-17A and IL-17F cytokines. Th17 cells display a great degree of context-dependent plasticity, as they are capable of acquiring functional characteristics of Th1 cells. This late plasticity may contribute to the protection against microbes, plays a role in the development of autoimmunity, and is necessary for antitumor activity of Th17 cells in adoptive cell transfer therapy models. Moreover, plasticity of this subset is associated with higher in vivo survival and self-renewal capacity and less senescence than Th1 polarized cells, which have less plasticity and more phenotypic stability. New findings indicate that subset polarization of CD4+ T cells not only induces characteristic patterns of surface markers and cytokine production but also has a maturational aspect that affects a cell’s ability to survive, respond to secondary stimulation, and form long-term immune memory.
Introduction
CD4+ T helper (Th) cells are central to the normal functioning of the entire immune system,1 coordinating the expansion and regulation of CD8+ T cells, facilitating B-cell responses, and recruiting and modulating multiple components of innate immunity.2-5 The initial antigenic encounter of naive CD4+ T cells varies depending on the anatomical site, pathogen type, and presence of assorted cytokines and costimulatory molecules, and these variations cause Th cell differentiation into antigen-experienced effectors with distinct functional characteristics termed polarization states.
In their early descriptions of the Th1/Th2 paradigm, Mossman, Coffman and colleagues6-8 attempted to explain responses observed in many experimental models of infection, autoimmunity, and allergy.9 Th1 cells were considered essential for antiviral immunity and for providing help to CD8+ cytotoxic T cells and were viewed as the main perpetrators of autoimmunity.10,11 Th1 cells are promoted by interleukin (IL)-12 (via signal transducer and activator of transcription [Stat]4) signaling and interferon (IFN)-γ (via Stat1), which induce the expression of master transcription factor T-box 21 (tbx21, T-bet) and secretion of the hallmark cytokine IFN-γ.12-14 Th2 cells, induced by IL-4 via Stat6 signaling that up-regulates the transcription factor GATA3,15,16 were linked to humoral responses to extracellular organisms or parasites and development of atopy and allergic reactions,17-20 producing cytokines that include IL-4, IL-5, and IL-13. It seemed that Th1 and Th2 cells represented mutually exclusive binary epigenetic states because IL-12, IFN-γ, and the expression of T-bet inhibited Th2 differentiation, whereas IL-4 and Gata3 expression antagonized Th1 polarization.21-25 The discovery of FoxP3-expressing regulatory CD4+ T cells (Treg) that restrained immune responses complement these early views of Th differentiation.26 However, not all CD4+ T-cell activity could easily be classified as either type 1 or type 2. Finally, Th17 cells producing the canonical cytokine IL-17A emerged as the most prominent addition to the old paradigm of two-mode Th polarization.27,28
Generation of Th17 cells
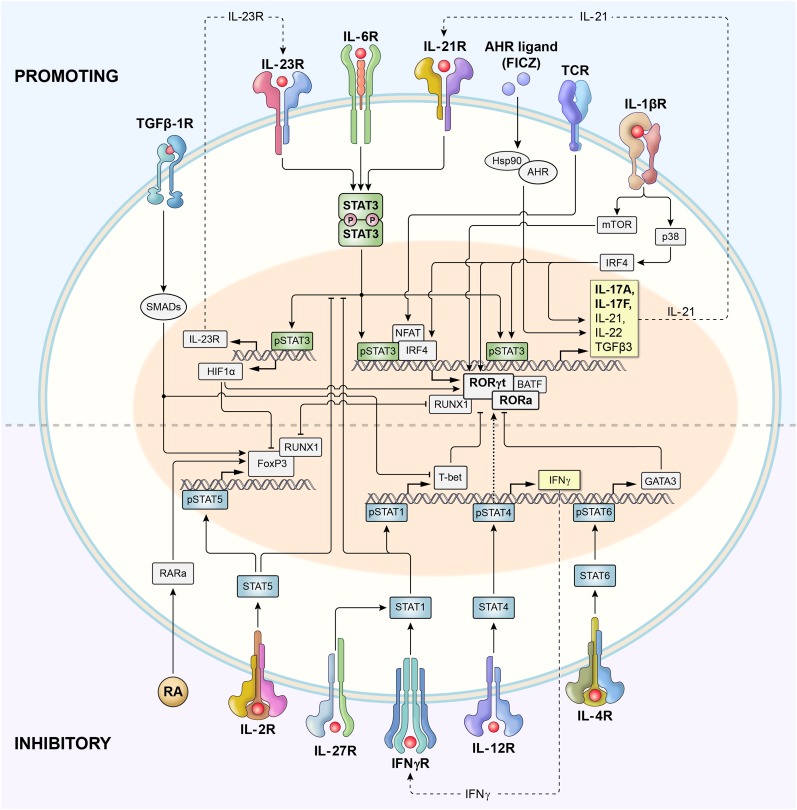
Harrington et al29 and Park et al30 established that Th17 cells were a true distinct lineage whose in vitro generation was enhanced when IL-4 and IFN-γ were blocked and was independent of Th1- or Th2-related transcription factors (T-bet, Stat1, Stat4, and Stat6). Induction of the Th17 polarization program was initially attributed to the effect of IL-23 (a heterodimer of p40 shared with IL-12 and p19 subunit) signaling via its distinct IL-23 receptor (IL-23R), triggering the Stat3 pathway31; however, this process produced cultures containing only small fractions of IL-17A–secreting Th cells.32 Subsequently, Th17 cells were efficiently generated in the presence of transforming growth factor (TGF)-β1 and IL-6, which signaled via Smad family proteins (Smads) and Stat3, respectively (Figure 1).33
Figure 1.
Schematic representation of signaling and transcriptional regulation of Th17 polarization. Th17 cells are induced upon T-cell receptor activation in the presence of cytokines that activate Stat3, including IL-6, IL-21, and IL-23. IL-12 and IFN-γ, which signal via Stat4 and Stat1, respectively, promote type 1 differentiation and inhibit Th17 polarization. However, IL-23 also activates Stat4 (not shown), and it remains perplexing that animals deficient in Stat4 have impaired functionality of Th17 cells. Similarly, IL-4 signaling via Stat6 inhibits Th17 polarization and promotes type 2 differentiation. Phosphorylated Stat3 (pStat3) binds to the promoter regions and activates transcription of genes encoding master regulators of Th17 polarization: rorc (encoding Rorγt) and rora (encoding Rora) transcription factors. Batf and Irf4 form a functional complex that plays a central role in Rorγt-mediated activation of the type 17 molecular signature.41 Together with pStat3, Rorγt and Rorα activate the expression of genes encoding canonical Th17-associated cytokines IL-17A and IL-17F, as well as IL-21, IL-22, and others. IL-6–induced activation of Stat3 also augments the expression of the IL-23 receptor (IL-23R), thus increasing the sensitivity of early Th17 cells to the polarizing effects of IL-23. pStat3 also induces expression of Hif1α, which inhibits FoxP3 and promotes Th17 differentiation. IL-21 secreted by early Th17 cells acts in a self-amplified autocrine loop via the IL-21 receptor. IL-1 promotes Th17 polarization via activities of p38 mitogen-activated protein kinase (MAPK) and the Akt/mTOR pathway. IL-1 also induces Irf4, which directly augments IL-21 secretion. Th17 polarization is also increased by activation of aryl hydrocarbon receptors (Ahr). TGF-β1 signals via Smads that most likely limit expression of genes encoding T-bet, Gata3, and other Th1- and Th2-associated factors, thus increasing Th17 differentiation. TGF-β1 signaling in conjunction with retinoic acid (RA) and IL-2–induced pStat5 promotes FoxP3 expression and Treg differentiation. RA has been shown to either limit or (in lower concentrations) augment Th17 polarization. pStat5 directly inhibits pStat3 binding to IL-17 promoter (not shown). Both FoxP3 and Rorγt form complexes with Runx1 and reciprocally regulate each other.
Retinoic acid (RA)–related orphan receptor γ thymus (Rorγt), encoded by the gene Rorc, was identified as the master transcription factor defining Th17 cells as a distinct lineage.34 RA-related orphan receptor α (Rorα) was recognized as the additional transcription factor critical for Th17 differentiation.35 The genetic deletion of Stat3 abrogates Th17 differentiation, and genomewide chromatin-immunoprecipitation sequencing (Chip-seq) revealed a broad and pleiotropic function of Stat3 as a promoter of Rorc, Il17, Il17f, and multiple other genes associated with Th17 polarization and survival.36 The transcriptional network controlling type 17 differentiation also includes Batf/Irf4 complexes that mediate chromatin accessibility, cMaf, Runx1, and hypoxia inducible factor 1 alpha (Hif1α), among others.37-41 IL-2 signaling via Stat5 attenuates Th17 polarization, and Stat5 has a direct negative effect on IL-17A gene expression.42,43 IFN-γ and IL-27 (via Stat1) and IL-12 (via Stat4) clearly block Th17 differentiation, but the underlying mechanism remains unclear and possibly related to T-bet repressor activity.44,45 Perplexingly, Stat4 deficiency also impairs type 17 polarization, possibly because of lower IL-23R expression.46
Naïve CD4+ T cells express very low levels of IL-23R, but its expression is induced on exposure to TGF-β1 and IL-6.47 Naïve CD4+ T cells cultured in only IL-6 and TGF-β and not in IL-23 secrete IL-17A but also produce high levels of IL-10 and fail to induce experimental autoimmune encephalitis (EAE) on adoptive cell transfer.48 Furthermore, in vivo IL-23R–deficient CD4+ Th17 cells fail to maintain their phenotype and demonstrate reduced persistence.49 Thus, IL-23 is not required for the initial Th17 polarization but instead stabilizes and expands pathogenic Th17-polarized cells.
Many additional cytokines and other molecules have been identified as factors regulating Th17 induction and expansion. Notably IL-21 (via Stat3 signaling) and TGF-β are sufficient to induce Rorγt and IL-23R expression and drive robust Th17 polarization in the absence of IL-6.50 In the presence of IL-6, stimulated CD4+ T cells produce IL-21 themselves, which further augments its secretion in an autocrine self-amplifying loop.51,52 IL-1β has emerged as another crucial factor driving Th17 polarization in a variety of inflammatory conditions.53 IL-1 serves as a prototypic inflammasome-related cytokine produced by cells of myeloid origin.54 IL-1 signaling promotes proliferation and survival of antigenically stimulated Th cells. This process involves activation of the serine-threonine protein kinase Akt-mammalian target of rapamycin (Akt-mTOR) pathway via induction of Ikbke inhibitor of kappaB kinase epsilon (IKKi) that inhibits glycogen synthtase kinase 3 (GSK3)α.55 IL-1 also induces Irf4, a regulator of the IL-21 autocrine signaling loop.56-58 Irf4-deficient animals have increased numbers of Foxp3+ Tregs and a diminished ability to form Th17 responses despite intact Stat3 signaling.39
The contribution of TGF-β1 to Th17 polarization remains a matter of debate, perhaps in part because it can be found in biologically significant concentrations in culture media containing animal or human sera.59,60 Animals deficient in TGF-β signaling have Th17 cells in their gut mucosa,53 and Th17 cells generated using serum-free (thus, TGF-β–free) media in the presence of IL-1, IL-6, and IL-23 coexpress RORγt and T-bet and cause aggressive EAE.53 TGF-β1 inhibits IL-2–mediated STAT5 signaling and diminishes the expression of the Th1- and Th2-defining transcription factors T-bet and Gata3, respectively.61 Thus, at low concentrations, TGF-β1 contributes indirectly to the initial development of Th17 responses but is not critical to the inflammatory milieu that drives type 17 responses.62 At high concentrations, TGF-β signaling inhibits IL-23R expression and converts naïve Th cells into induced FoxP3+ regulatory cells (iTregs) by antagonizing Rorγt.63 This interaction involves the Runx1 transcription factor that binds either Rorγt or FoxP3.40 Thus, there is an active balance between the development of either iTregs or Th17 cells and even plasticity with FoxP3+ T cells capable of converting into FoxP3− Th17 progeny.64 All-trans retinoic acid (ATRA), a vitamin A metabolite produced in the gut mucosa, antagonizes the expression of rorc and promotes in vitro foxp3 expression via its nuclear receptors.65 Akin to the inhibitory function of TGF-β, ATRA also has been reported to negatively regulate Th1 and Th2 polarization. Both TGF-β and ATRA induce expression of Mir10a that might stabilize iTreg phenotype and limit Th17 differentiation.66 The same report indicated that physiological concentrations of ATRA might promote, rather than impair, Th17 polarization.66 Indeed, in vivo ATRA has a proinflammatory effect and is required for the efficient Th17 responses against mucosal pathogens.67
Aryl hydrocarbon receptors (AHRs) are the cytosolic receptors with transcription factor activity that have been reported to promote Th17 polarization.68 AHRs sense a variety of small natural and man-made compounds including products of tryptophan metabolism and highly toxic dioxins, providing an intriguing link between the environment and autoimmunity.69 Early Th17 polarization induces high expression of AHRs, and AHR-deficient animals are partly protected from the induction of EAE.68
Gut-residing commensal microbiota can profoundly affect Th17-mediated immunity and autoimmunity. Germ-free animals have fewer intraepithelial intestinal Th17 cells and an attenuated form of autoimmune arthritis but develop severe arthritis after exposure to segmented filamentous bacteria.70,71 Conversely, infected nonobese diabetic (NOD) mice were protected from the progression of diabetes.72
Th17 cells as mediators of host defense against infection
Under physiological conditions, IL-17–producing CD4+ T cells reside mainly in the lamina propria of the small intestine but are readily induced at other mucosal sites during infection or vaccination.34,71,73,74 Consequently, Th17 cells and type 17–associated cytokines contribute to the protection against a variety of predominantly extracellular bacterial and fungal pathogens in the gastrointestinal tract, airway, lungs, and skin.75,76
Th17 cells secrete IL-17 (IL-17A), IL-17F, and IL-17A/F heterodimers, as well as IL-21, IL-22, granulocyte macrophage–colony-stimulating factor (GM-CSF), and many other factors.77 The proinflammatory effects of IL-17 are mediated through the IL-17 receptor (IL-17R), composed of IL-17RA and IL-17RC subunits.78 IL-17R is widely expressed by immune system cells (T and B lymphocytes and neutrophils), and by many other tissues (epithelium, endothelium, fibroblasts, mesenchymal stromal cells, and keratinocytes).78 Thus, IL-17 is highly pleiotropic, causing a variety of inflammatory effects that link adaptive and innate immunity.75 Other rich sources of IL-17 include γσ T cells and innate lymphocytes.
IL-17A promotes granulopoiesis by triggering the secretion of granulocyte–colony-stimulating factor in bone marrow stroma,79 and IL-17R–deficient mice show a deeply impaired ability to reconstitute following irradiation.80 In the gut, IL-17 maintains mucosal homeostasis and integrity.81 IL-17 induces GM-CSF, tumor necrosis factor (TNF)α, IL-1β, IL-6, and multiple chemokines and chemokine receptors that act as powerful chemo-attractants for granulocytes. IL-17 also mediates formation of inducible secondary lymphoid tissues following local infection.82 IL-17 and Th17 cells play an important role in immunity against Klebsiella pneumoniae73 and many other bacteria including Staphylococcus aureus, Citrobacter rodentium, Salmonella and Shigella sp., and Bordetella pertusis.76,83 Th17 cells may augment Th1 responses against some intracellular pathogens (Mycobacteria and Francisella tularensis).84,85 Th17 cells are also involved in protection against mucocutanoeous candidiasis via the stimulation of pattern recognition elements (Dectin 1 and Dectin 2) in macrophages and dendritic cells.86 The role for Th17 cells and IL-17 in immunity against Asperillus fumigatus and some other fungi is more controversial and possibly detrimental.75,87
IL-22 and IL-17 synergistically trigger production of many antimicrobial peptides and proteins (β-defensins, S100 proteins, regenerating islet-derived protein 3 γ, and lipocalin 2).88 The IL-22 receptor (IL-22R) is predominantly expressed by cells of nonhematopoietic origin (keratinocytes, hepatocytes, and enterocytes) and enables tissue-protective effects against invasion by Toxoplasma gondii and various gram-negative bacteria.89 IL-21 regulates CD8-mediated responses and facilitates B-cell responses and memory formation.90
The importance of the Th17 subset in protecting humans is vividly demonstrated in patients with an autosomal-dominant form of hyper-IgE syndrome (Job’s syndrome) that have an inactivating STAT3 mutation; thus, they are incapable of generating Th17 cells.91,92 Patients with hyper-IgE syndrome suffer from recurrent staphylococcal soft tissue infections, pneumonias and bronchoalveolar infections, pneumatoceles, eczema, and mucocutaneous candidiasis. They also show impaired immunity against varicella zoster (VZV), Epstein Barr (EBV), and defective proliferative responses and reduced central memory formation caused by STAT3 dysfunction.93 Similar sequelae are seen in HIV infection, which is associated with early preferential loss of Th17-mediated mucosal immunity in the gut.92 Th17 depletion in the gastrointestinal tract leads to rapid disease progression in simian immunodeficiency virus–infected macaques, whereas sooty mangabeys that retain Th17 cells in the gastrointestinal mucosa are protected.94
Th17 cells as mediators of tissue damage
Early discoveries of IL-23 as an inducer of Th17 cells revealed their capacity to induce EAE, a disease initially attributed to Th1 cells and induced in the presence of IL-12 (a heterodimer of IL-12 p40 and p35 subunits). However, animals deficient in the IL-12 p35 subunit developed EAE despite diminished Th1 responses95 because they retained IL-23, which is a heterodimer of IL-12 p40 and a distinct p19 subunit.31 These experimental data were complemented by studies linking genetic polymorphisms of IL-23R and its downstream signaling pathways with susceptibility to human autoimmune diseases including inflammatory bowel disease, psoriasis, and aplastic anemia.96-98
Tissue damage by Th17 cells might be caused by direct recognition of the antigen-specific target, or it can result from the recruitment of neutrophils and macrophages into the microenvironment.99 In EAE, IL-17 induces reactive oxygen species production in the endothelium, leading to a disruption of the blood–brain barrier.100 IL-17 promotes inflammatory cytokines (IL-1 and IL-6) and CCL20 (MIP3α), a potent chemotactic factor for myeloid cells, and additional Th17 cells expressing high levels of its receptor CCR6.101 IL-9 produced by Th17 cells exacerbates EAE via its effect on T cells and IL-6–secreting macrophages.102 Th17 cells also produce GM-CSF,103,104 which acts on myeloid cells by induction of IL-1, IL-6, and IL-23 and major histocompatibility complex class II expression.103 Blockade of GM-CSF signaling effectively protected mice from autoimmunity.104 Nevertheless, despite the latest emphasis on Th17 cells, both Th1 and Th17 cells can cause EAE and murine uveitis manifestations, albeit with distinct histopathological features.105-107 Furthermore, both type 1– and type 17–related factors can be produced by pathogenic Th17 cells, but both subset-defining cytokines, IL-17A and IFN-γ, are dispensable, whereas T-bet, thought of as the master transcription factor for Th1 cells, may be essential for pathogenicity of Th17 cells in EAE.108 Recently, TGF-β3 was identified as a critical IL-23– and T-bet–dependent factor produced by the highly pathogenic Th17 population.109 Moreover, the presence of TGF-β3 during initial Th17 polarization induced highly encephalitogenic T-bet–independent type 17 cells.
In patients with multiple sclerosis, elevated frequencies of Th17 cells were found in peripheral blood, and double producers of IL-17A and IFN-γ have been reported in central nervous system (CNS) lesions, mimicking the findings observed in mice with EAE. However, depletion of IL-23 in patients with multiple sclerosis caused only minimal improvement in disease control, possibly because of poor CNS penetration of anti–IL-23 antibody formulation used clinically or deficiencies in the mouse model.110
Th17 responses are associated with psoriasis, where IL-23, IL-17, and IL-22 seem to contribute to the development of the human disease.111,112 In mice, IL-22 is crucial in inducing psoriatic-like lesions with acanthocytosis in kerationocytes.113 Targeting the p40 subunit of IL-12/IL-23 with the monoclonal antibody ustekinumab appears to be highly effective in patients with psoriasis.114,115 Recently, a phase 2 clinical trial of the anti–IL-17 receptor monoclonal antibody brodalumab in patients with psoriasis demonstrated significant improvement in a majority of patients.116
IL-23 directly drives pathogenic Th cells in a mouse model of colitis.117 Whereas IFN-γ is required for tissue damage, the role of IL-17 remains disputed.118 IL-17 might protect the colon via inhibition of colitogenic Th1 differentiation.119 Likewise, IL-22 from Th17 and natural killer cells might attenuate the development of intestinal pathology via Stat3-mediated effects on epithelial cells.120,121 In contrast, IL-21 significantly potentiates pathogenic effects of Th17 cells in the gut.122,123 Increased frequency of IL-17–secreting CD4+ T cells can be found in the gut of patients with Crohn disease,124 and these cells express CD161 in addition to the previously described CCR6 and CCR4 chemokine receptors.101,125 In the clinical randomized proof of concept trial, the human anti–IL-17A monoclonal antibody failed to control the symptoms of Crohn disease and was associated with increased adverse events in comparison with the placebo,126 whereas depletion of IL-23 with ustekinumab was more effective.127 This might underscore the role of IL-23 not only in Th17 responses but also as a driver of inflammation mediated by the innate lymphoid cells that are abundant in the colonic mucosa.128
Multiple other autoimmune diseases including rheumatoid arthritis, uveitis, atherosclerosis, emphysema, and allergies have been associated with Th17 responses in murine models and in patients.129,130 Currently, various agents modulating the type 17 axis of inflammation are under development. Recently, digoxin has been shown to inhibit Th17 development, and novel small molecules that modulate Rora and Rorγt activity have been described.131,132 Nevertheless, IL-23 rather than IL-17 plays a central role in Th17-related autoimmunity, and pathogenic Th17 cells usually evolve and display Th1-like characteristics.
Th17 cells in GVHD
Graft-versus-host disease (GVHD) is a complex immune syndrome that develops in organ-specific sites under influences of a variety of inflammatory conditions.133 The resemblance of GVHD to some autoimmune diseases has shed light on Th17 biology.134 IL-23R polymorphism might be a prognostic factor in patients undergoing allogeneic stem cell transplantation,135 and highly polarized allogeneic Th17 cells can induce severe forms of GVHD in mice.136 However, Th17 responses are frequently described as “sufficient but not necessary” for the development of GVHD, as evidenced by pathogenicity of IL-17A or Rorγt-deficient Th cells.137,138 Concurrent elimination of T-bet and Rorγt was reported as protective against GVHD development in fully mismatched settings (C57/BL6 into BALB/c), whereas the graft-versus-leukemia effect was spared.139 Perplexingly, a recent report demonstrated reduced numbers of Th17 cells within the lamina propria of animals with GVHD, but the development of GVHD colitis was attributed to the critical role of STAT3 signaling that promoted instability and plasticity of the nTreg compartment and blockade of iTreg generation.140 In a more clinically relevant haploidentical transplant model, rorc-deficient CD25-depleted CD4+ T cells induced only a mild form of GVHD.141 This protective effect correlated with diminished systemic levels of TNF-α and IL-17A, but colonic levels of IL-17A were preserved in mice treated with rorc-deficient CD4+ T cells. In the same model, tbx21−/− cells caused marked GVHD.
Undoubtedly, the functional plasticity of the Th17 subset that can produce Th1-like progeny is important for a better understanding of their complex role in mediating GVHD.134 Reminiscent of some autoimmune conditions, the role of IL-17 in GVHD is unclear, and IL-22 might be protective to colonic mucosa. IL-21, IL-6, IL-23, STAT3, and RA receptors have been reported as potentially useful targets for therapeutic intervention.142-145
Th17 cells and cancer
Carcinogenesis as a consequence of chronic inflammation was described in the 19th century by Rudolph Virchow. There is a strong correlation between cancer and autoimmunity (eg, in inflammatory bowel disease) or prolonged inflammation caused by cigarette smoke or chronic infection, such as Helicobacter pylori or hepatitis.146,147 Inflammation, especially involving Stat3 signaling pathways, plays an important role in the induction, progression, and metastasis of tumors either directly or by modulation of the various components of tumor stroma, recruitment of myeloid cells, and impairment of T-cell responses.148,149 IL-1, IL-6, IL-21, and IL-23 are frequently induced in the proinflammatory tumorigenic microenvironment, and Th17 cells are commonly found in precancerous and cancerous lesions.150,151
The role of Th17 cells in protection or progression of tumors is controversial.152,153 IL-17 promoted tumor neovascularization in nude mice,154 supported neoplastic growth directly or via induction of IL-6,155 and induced myeloid suppressor cells,156 but could be also protective in immunocompetent animals.157,158 Th17 cells can suppress CD8-mediated immunosurvillance.159 Genetic Rorγt deficiency results in a slower growth of transplantable melanoma, and elimination of IL-23 or IL-21 inhibits carcinogenesis in some models.160-162 On the other hand, exogenous inflammatory cytokines can activate immune responses against tumors.163,164 In humans with cancer, the presence of Th17 cells is a poor prognostic indicator in some studies and a favorable indicator in other reports.153,165
The unknown antigenic specificity of the Th populations in question undermines many animal and clinical studies. Furthermore, Th17 cells are often viewed as static and incapable of plasticity. We investigated the relative antitumor activity of Th1 and Th17 cells in the setting of adoptive immunotherapy of murine B16.10 melanoma using T-cell receptor transgenic CD4+ T cells recognizing melanocyte differentiation antigen tyrosinase related protein 1 (TRP-1).166 Th17-polarized TRP-1 cells swiftly eradicated large (>1 cm2) B16.10 melanomas on transfer into tumor-bearing C57/B6 animals, whereas Th1-polarized TRP-1 cells were significantly less effective. TRP-1 Th17 cells recruited endogenous cytotoxic CD8+ T cells into pulmonary metastases.167 The potency of tumor-specific type 17 responses has also been demonstrated using murine CD8+ T cells (Tc17) and genetically engineered human CD4+ T cells.168,169
Fate of Th17 cells: plasticity with constraints
The Th1/Th2 paradigm implied that polarized lineages were mutually exclusive and stable, because of the self-enforcing nature of the signals involved in the acquisition of each lineage. This view of fixed phenotype and function was also initially applied to the newly defined Th17 subset. However, IL-17A and IFN-γ double-producing cells are found in vivo, suggesting that Th17 cells could at least in part function like Th1 cells.77 In the setting of immunotherapy of melanoma with Th17-polarized TRP-1 cells, in vivo elimination of IFN-γ or T-bet abrogated the antitumor effect of adoptively transferred Th17 cells, suggesting evolution of a transferred population into a Th1-like subset.166,170 This late developmental plasticity of the Th17 lineage was examined in detail using the IL-17F reporter system by Lee et al,171 who demonstrated that type 17 cells could be maintained as such in the presence of TGF-β and IL-23 but were readily converted into the IFN-γ–secreting population on restimulation in the presence of IL-12 or IL-23 and in the absence of TGF-β. An analogous switch occurs in vivo where transferred IL-17F–expressing CD4+ T cells give rise to IFN-γ–producing colitogenic effectors.171 In a model of diabetes mellitus, Th17 cells converted into pathogenic IFN-γ–producing daughter cells in lymphopenic conditions, but in the intact (lymphoreplete) recipients, the same Th17 cells caused only benign insulitis and maintained a stable type 17 phenotype.172
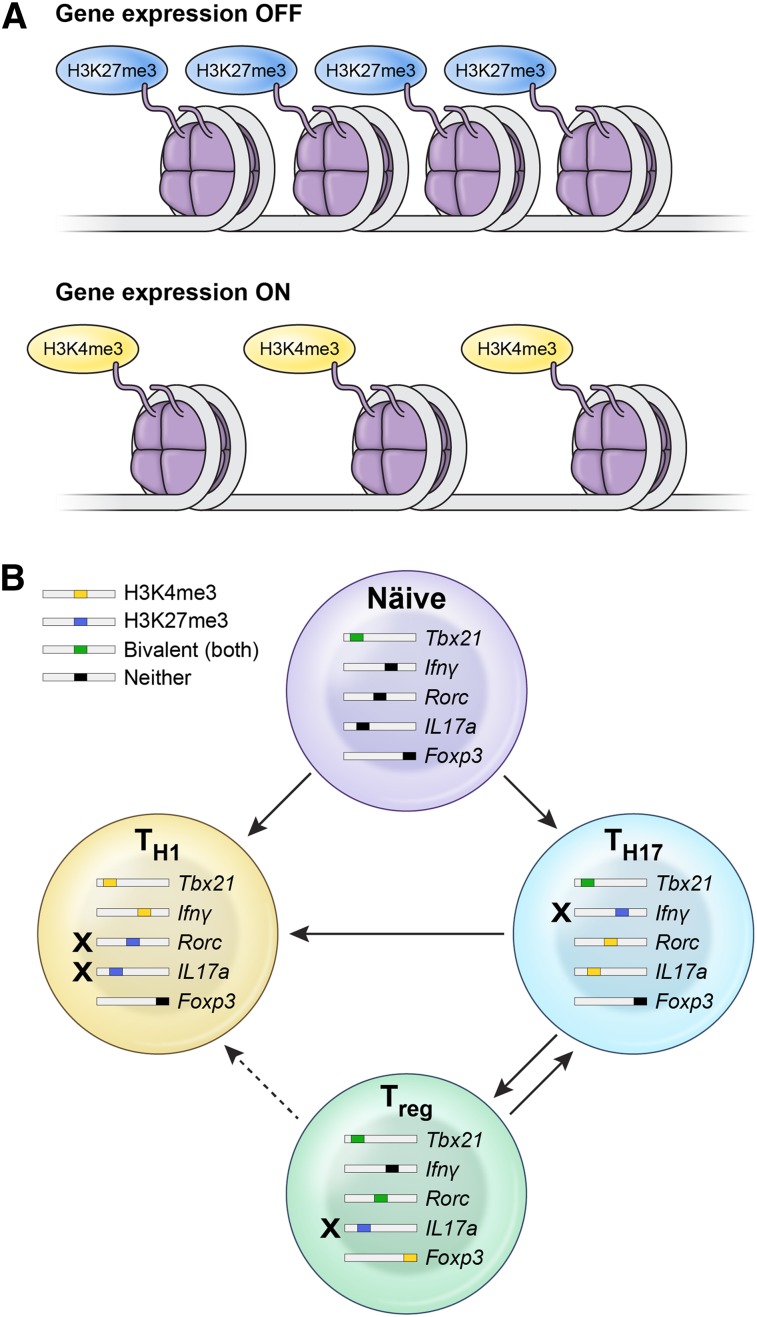
The observed stability or plasticity of Th subsets is governed by the epigenetic regulation of the key transcription factors and cytokines determining the polarization status.173 Whether a particular gene is poised for expression or not is determined by the chromatin structure, as well as histone and DNA methylation states. Wei et al174 evaluated histone 3H methylation status over the entire genome in a variety of CD4+ Th subsets. Predictably, gene promoters for tbx21 in Th1 cells and rorc in Th17 cells displayed a permissive methylation state (H3K4me3) associated with full expression of these master regulators in each lineage. However, gene promoters for tbx21 in Th17 cells had a bivalent status characterized by H3K4me3/H3K27me3 dual positivity, thus substantiating the relative instability and propensity of these cells to acquire type 1 features in the presence of IL-12 (Figure 2).
Figure 2.
Epigenetic mechanisms explain the lineage relationship between Th17, Th1, and iTreg subsets. (A) Whether a particular gene is poised for expression or not is determined by the chromatin structure, as well as histone and DNA methylation states. Trimethylation of histone 3H on lysine 4 (H3K4me3) is considered permissive for gene expression, whereas trimethylation of lysine 27 on histone 3H (H3K27me3) is a marker of gene silencing. In some cases, both states can be found in a gene locus, thus making it susceptible for either expression or negative regulation. (B) Plasticity of Th1, Th17, and iTreg cells is constrained by the epigenetic status of genes encoding for the master transcriptional regulators of polarization and canonical cytokines. Th17 cells display permissive H3K4me3 histone modification over the rorc and il17 genes and bivalent, poised status over loci encoding for tbx21 and foxp3, thus substantiating the relative instability of this subset and its propensity to evolve into Th1 progeny. In contrast, Th1 cells display only repressive H3K27me3 methylation status over gene loci encoding for rorc and Il17, rendering them much more stable. iTregs are another relatively unstable subset that can acquire Th1 or Th17 properties, based on the poised bivalent status of type 1– or type 17–associated genes.
Deeper insight into the evolution of Th17 responses in vivo was gained by Hirota et al175 using a reporter system permanently labeling Th cells that express the il17a gene, thus allowing for fate mapping of all “ex-Th17” cells in vivo by yellow fluorescent protein. In vaccination-induced EAE almost all myelin-specific CD4+ T cells infiltrating CNS were yellow fluorescent proteinhi and thus of Th17 origin, although they ceased secretion of IL-17A and switched to production of IFN-γ or other cytokines. This in vivo conversion was critically dependent on IL-23. In contrast, in acute cutaneous candidiasis, responding Th17 cells remained firmly committed to IL-17 production, possibly because of low local levels of IL-23. Thus, in vivo–induced murine Th17 cells display context-dependent stability or flexibility, just like Th17 cells generated in vitro. If the same phenomenon is true in the case of human Th17 cells remains to be determined; however, T-cell receptor clonotypes present in the Th17 cell compartment and Th1 compartment found in joint fluid recovered from patients with juvenile rheumatoid arthritis were remarkably similar, suggesting in vivo conversion.176 In another report, Th17 cells specific for the cancer testis antigen MAGE-A3 isolated from a patient with lung cancer were readily converted into IFN-γ–secreting Th1-like effectors.177
Overall, the Th17 cells generated in vitro or in vivo can maintain the pure type 17 phenotype or can acquire certain type 1 characteristics on secondary stimulation. This plasticity, while significant, is clearly restrained by the epigenetic status of the genes encoding for the master transcriptional regulators and cytokines. As a result, the observed plasticity is asymmetrical because committed Th1 cells cannot easily acquire Th17 features. Thus, the plasticity of Th cell subsets is constrained and in this regard is similar to other self-renewing tissues with stem cell functionality as postulated by Hal Waddington almost 60 years ago; differentiation is strictly controlled in its directionality, and once differentiated, cells rarely if ever revert to the plastic progenitor state, in a way analogous to biochemical or physical process of entropy.178
Maturational aspect of Th plasticity
Development of long-term memory is one of the key features of adaptive immunity and is crucial for the ability to mount effective protection on the subsequent antigenic encounter. The formation of CD4+ T-cell memory is complicated by the existence of multiple polarized Th subsets, which can evolve and experience plasticity. It is thought that CD4-mediated memory responses might be less robust in contrast to more stable persistence of CD8+ memory T cells.179
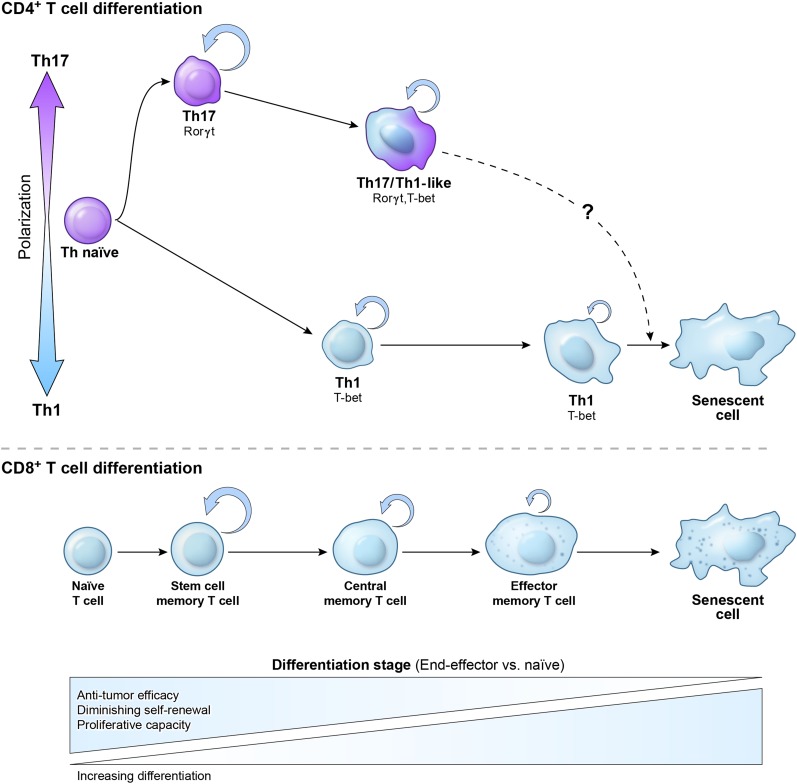
T-cell memory has been extensively studied in the CD8+ compartment using phenotypic markers wherein naïve CD44loCD62Lhi CD8+ T cells differentiate on stimulation into CD62LhiCD44hi central memory (TCM) cells or CD62LloCD44hi effector memory (TEM) cells. Recently a novel, less-differentiated, and very early memory cell subset termed memory stem cells (TSCM) has been described in the setting of GVHD and adoptive immunotherapy. TSCM displays stem cell–like features such as longevity, a capacity for self-renewal, and the ability to give rise to more differentiated (TCM and TEM) memory subsets.180,181 These cells display a naïve-like phenotype (CD62Lhi, CCR7hi, and CD44lo) but can be identified by the presence of some activation markers (Sca1 in mice and CD95 in humans).181
TSCM also shares certain molecular traits common to other self-renewing populations including the activity of the Wnt/β-catenin pathway that contributes to the maintenance of normal and transformed stem cells and the development of thymocytes.182 Inhibition of GSK3β during antigenic stimulation of naïve CD8+ T cells stabilizes β-catenin and enhances TSCM formation.181 Stabilized β-catenin induces Tcf7 gene expression that encodes T-cell factor 1 (Tcf1). Tcf1 participates in maintenance of memory T cells and Tregs in vivo. Its forced overexpression prevents secretion of type 1 and type 17 effector molecules (IFN-γ and IL-17A, respectively) and promotes Th2 differentiation.183,184 CD8+ TEM cells are highly cytotoxic in vitro but display very poor persistence and antitumor effect in vivo; thus, they behave in a senescent manner.179,185-186 TCM and even more dramatically TSCM cells that do not achieve full terminal differentiation in vitro are far more effective in eradicating the tumor in vivo, and this is associated with superior persistence.181,185 Therefore, therapeutic activity and self-renewal potential can be used as surrogate measurements of the maturational stage of the particular effector population.179,185
When judged by some common phenotypic markers, Th17 cells appear to resemble terminally differentiated TEM cells, with low expression of CD62L, CD45RA, and CD27, a costimulatory molecule important for survival of T cells that is down-regulated with advanced maturational stage.170 In contrast, Th1 cells maintain a high expression of CD27, and on in vitro polarization, they retain a higher frequency of CD62Lhi cells. Because of the terminally differentiated phenotype (CD27lo), Th17 cells were described as short lived and unable to form long-term memory.74 However, type 17 responses can be clearly protective against rechallenge in immunization models.73 This assertion was also at odds with observations from adoptive cell transfer experiments, where even highly purified Th17 cells can survive, persist, and retain functionality, as evidenced by protracted tissue damage in some models of autoimmunity or GVHD.136,187 The persistence of Th17 cells is robustly observed in the TRP-1 model of cancer immunotherapy, where Th17 cells are highly effective in eradicating the tumor, whereas Th1 cells are less efficient.166,170 Furthermore, TRP-1 Th17 cells purified for the lowest expression of CD27 showed the highest in vivo activity and survival. This finding is consistent with a recent report that CD27 signaling reduced the incidence and severity of disease in an EAE model.188 Therefore, we hypothesized that despite certain phenotypic features of advanced maturation, considerable multipotency and plasticity demonstrated by Th17 cells indicated that these cells are not terminally differentiated, at least when induced in vitro. Despite their phenotype, Th1 cells might represent a more differentiated subset with less capacity to expand, persist, and eliminate tumors in vivo (Figure 3).
Figure 3.
Relationship between Th1 and Th17 polarization and maturational stage of Th memory cells. The initial polarization of naïve CD4+ T cells into the Th1 or Th17 subset not only induces a canonical set of transcription factors and cytokines but also affects CD4+ T-cell plasticity and fate. Th17 cells are relatively more plastic and less terminally differentiated than their Th1 cell counterparts. This is reflected by the higher ability of Th17 cells to self-renew, generate a distinctive highly functional Th1-like Th17 progeny, and form long-term memory following the secondary antigen experience. Th1 cells rapidly acquire a senescent phenotype and molecular signature, are less functional, and display less ability for self-renewal and memory formation. The lower panel demonstrates a proposed relationship between polarized CD4+ Th cells and a model of linear CD8+ T-cell memory formation. Upon antigen stimulation CD8+, T cells progress from naïve via self-renewing early memory stem cells (TSCM), to central memory (TCM and TEM) cells, and to the senescent terminally differentiated cells with no self-renewal potential. In vivo antitumor efficacy of adoptively transferred cells used for immunotherapy of cancer inversely correlates with the maturational stage of T cells.
The developmental program associated with Th17 plasticity has been elucidated using global gene expression profiles of transferred Th1 and Th17 cells. Indeed, molecular signatures of persisting Th1 and Th17 cells underwent rapid convergence, and Th17 cells readily down-regulated expression of type 17–defining cytokines (IL-17A, IL-17F, IL-21, and IL-22) and up-regulated transcripts associated with type 1 polarization (tbx21 and Ifng). Recent reports describe a role of T-bet expression gradient as a regulator of CD4+ T-cell memory formation, with highly Th1-polarized T-bethi cells displaying end-effector features and a short lifespan, whereas Th cells with a lower expression of T-bet formed a long-lived stable TCM population.189,190 This to some degree mimicked our observations, as long-lived ex-Th17 cells acquired T-bet expression, albeit at levels lower than their Th1 counterparts.
Multiple other transcripts remained differentially expressed. This difference between persisting Th1- and Th17-derived cells was analyzed by gene set enrichment analysis that revealed the core molecular program of long-lived Th17 cells resembled that of CD8+ T cells at an early stage of differentiation, even after they converted to a Th1-like subset in vivo.191 Reciprocally, the Th1 population acquired a global signature of terminal differentiation and senescence, with high expression of molecular and phenotypic markers of an advanced maturational stage, including cytotoxic effector molecules (granzymes, perforin), klrg1 and other killer-like lectin receptors, and prdm1 (a gene encoding a molecular marker of terminal Th1 differentiation, Blimp1).170 Furthermore, Th17 cells overexpressed tcf7 and some other downstream molecules of the Wnt/β-catenin signaling pathway. In CD8+ T cells, the level of tcf7 expression closely correlates with the maturational status of T cells: it is highly expressed in naïve T cells and is gradually lost following antigenic stimulation and acquisition of the TCM phenotype.180 TEM cells have the lowest tcf7 expression. Thus, tcf7 expression might be used as a possible surrogate marker of maturational state and self-renewal capacity. Strikingly, Th17 polarization in vitro induced tcf7 expression higher than in a naïve starting population. This was associated with a massive accumulation of stable β-catenin in Th17 cells, therefore mimicking a molecular signature found in naïve and TSCM cells induced by activation of Wnt signaling or pharmacological inhibition of GSK3β,179 but in type 17 cells, it most likely resulted from downstream effects of IL-6 or IL-1 mediated by phosphatidylinositol 3-kinase/Akt activity. As indicated by Luckey and Weaver,192 many questions regarding the CD4+ memory and self-renewal remain unanswered, but functionally, Th17 cells displayed traits similar to TSCM, as they were long lived, demonstrated plasticity, and gave rise to more differentiated Th1-like progeny, but also retained the ability to self-renew as IL-17A producers.
The findings from the murine model were substantiated by the description of stem cell–like behavior and a self-renewal molecular program in human Th17 cells that express high levels of HIF1α and antiapoptotic molecule BCL2.193 Intriguingly, the Stat3 signaling pathway that induces Th17 differentiation is critical for survival of long-lived memory T cells but also plays an important role in maintenance of normal and malignant self-renewing populations with attributes of stemness.
Beyond Th17: expanding spectrum of Th subsets
The discovery of Th17 cells has been followed by the realization that Th effectors can produce various other cytokines alone or in combination in patterns not fitting the preconceived definitions of Th1/Th2 or Th17 subsets. These findings have led to the description of additional Th cell lineages, including Th22, Th9, and, most notably, follicular T helper cells (Tfh). The plasticity, stability, and potential for memory formation by each of these subsets remain yet to be elucidated.
Tfh cells express CXCR5 and predominantly traffic to lymph nodes where they provide help to B cells and produce IL-21 (just like Th17 cells).194 Tfh cell induction is governed by Stat3 and the expression of Bcl6, a molecule important for the maintenance of CD8+ memory. Bcl6 is a prosurvival transcriptional repressor that antagonizes Blimp1, a molecule that drives terminal differentiation of T cells; thus, the balance between these two factors might have maturational implications.195 Likewise, Tfh cells have a high capacity to form long-term memory.196 The relationship of Tfh with other Th cell subsets such as Th1, Th2, and Th17 cells remains unclear, and cells have been investigated mostly in the context of infection in vivo and humoral immunity.196,197 When observing the in vivo responses to infection, it is difficult to ascertain the sequence of maturational events with long-lived cells emerging on contraction of the massively expanded effectors induced by the presence of pathogen. Thus, it is conceivable that Tfh cells might represent a self-renewing, plastic, and non–terminally differentiated early memory subset.
Th22 cells producing only IL-22 but neither IFN-γ nor IL-17A have been identified in humans.198 They are induced in the presence of TNF-α and IL-6 and require ligation of Ahr. Phenotypically, they can be identified by expression of CCR10 and the presence of some typical markers of human Th17 cells (CCR4, CCR6).200 Th22 cells via IL-22 influence the function of mesenchymal and epithelial cells and have been implicated in the dermatopathology of psoriasis and atopic dermatitis.199-201
Th9 cells are induced in the presence of IL-4 and TGF-β1, but their master transcription factor has not been identified, and the functional roles of Th9 cells and IL-9 are ill defined. Recent findings demonstrated that IL-9 is expressed only transiently and is predominantly produced in vivo not by Th9 cells but by a novel subset of innate lymphoid cells termed ILC2.202 Willhelm et al203 theorized that IL-9 might have a regulatory and prosurvival function for many lymphoid and myeloid cells. A recent report described the development of endogenous antitumor Th9 responses in Rorγt-deficient animals and proposed a protective role for IL-9 in tumor immunity.162,204
Conclusions
Recent years have brought a new level of appreciation of the complexities involved in the function of effector CD4+ Th cells. The discovery of Th17 cells has filled the gaps and deficiencies existing in the previous simplistic Th1/Th2 paradigm and has revolutionized our understanding of immune responses against certain pathogens and pathophysiology associated with development and progression of some autoimmune diseases or genetic defects of host defense, progression of HIV and development of AIDS, solid organ transplantation rejection, and GVHD. In addition, Th17 cells have been described as mediators of carcinogenesis, by facilitating early progression of solid and hematologic malignancy through the direct effects of inflammatory cytokines on some cancer cells, but more frequently by impairing immune surveillance or promoting neovascularization and supporting stroma.
The investigation of Th17 cells led to a new appreciation of the flexibility and plasticity of Th cell–mediated immunity. In contrast to the previously postulated dichotomous Th1/Th2 division of labor, it is now clear that naïve Th cells can achieve a multiplicity of polarization states and experience remarkably different cell fates that are highly dependent on the context of their encounter with antigen. Th17 cells can provide an initial inflammatory response via their canonical type 17 cytokines and recruit various myeloid populations to the site of the immune response before transitioning into type 1–like cells that can also target the intracellular pathogens. This developmental plasticity has significant implications for the rational design of new treatments of the type 17–linked autoimmune syndromes such as multiple sclerosis or GVHD. It can also be harnessed for the design of novel adoptive cell transfer therapies, as evidenced by the antitumor efficacy of Th17-polarized tumor-specific cells, where superior engraftment and persistence of highly active transferred effectors are desired.
The initial differentiation toward the Th17 subset prevents the effector cells from a premature acquisition of terminal differentiation that is associated with highly polarized Th1 cells. Instead, these cells acquire a molecular program reminiscent of the recently described TSCM subset that is functionally characterized by self-renewal, persistence, and the ability to efficiently form memory and at the same time generate more differentiated progeny critical for in vivo effector function. Thus, polarization of Th cells conveys not only characteristic phenotypic and cytokine profiles but also has a maturational aspect in the spectrum from early self-renewing subsets to those that are senescent.
Acknowledgments
This work was supported by the Center for Cancer Research, National Cancer Institute–National Institutes of Health Intramural Research Program, the National Heart, Lung, and Blood Institute Intramural Program, and the National Institutes of Health Center for Regenerative Medicine.
Authorship
Contribution: P.M. and N.P.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pawel Muranski, Hematology Branch, NHLBI/NIH, 10 Center Dr, 3E-5288, Bethesda, MD 20892; e-mail: muranskp@mail.nih.gov.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557-1569. [DOI] [PMC free article] [PubMed]
- 2.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science (New York, N.Y.) 2003;300(5617):339-342. [DOI] [PMC free article] [PubMed]
- 3.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 4.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21(2):200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 7.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 8.Heinzel FP, Sadick MD, Holaday BJ, et al. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18(6):263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 10.Parronchi P, Romagnani P, Annunziato F, et al. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol. 1997;150(3):823–832. [PMC free article] [PubMed] [Google Scholar]
- 11.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3(6):549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 13.Szabo SJ, Kim ST, Costa GL, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 14.Oestreich KJ, Weinmann AS. Transcriptional mechanisms that regulate T helper 1 cell differentiation. Curr Opin Immunol. 2012;24(2):191–195. doi: 10.1016/j.coi.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan MH, Schindler U, Smiley ST, et al. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4(3):313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 16.Zheng WP, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda K, van Deursen J, Sangster MY, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380(6575):630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 18.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht BN, De Veerman M, Coyle AJ, et al. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106(4):551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce EJ, Caspar P, Grzych JM, et al. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo SJ, Jacobson NG, Dighe AS, et al. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2(6):665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang W, Ranganath SH, Weindel K, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9(5):745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 23.Usui T, Preiss JC, Kanno Y, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203(3):755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science (New York, N.Y.) 2005;307(5708):430-433. [DOI] [PubMed]
- 25.Oestreich KJ, Weinmann AS. T-bet employs diverse regulatory mechanisms to repress transcription. Trends Immunol. 2012;33(2):78–83. doi: 10.1016/j.it.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (New York, N.Y.) 2003; 299(5609):1057-1061. [DOI] [PubMed]
- 27.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 28.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Li ZX, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13(5):715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 32.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirahara K, Ghoreschi K, Laurence A, et al. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21(6):425–434. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durant L, Watford WT, Ramos HL, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32(5):605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schraml BU, Hildner K, Ise W, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460(7253):405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi LZ, Wang R, Huang G, et al. HIF1α–dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367-1376. [DOI] [PMC free article] [PubMed]
- 39.Brüstle A, Heink S, Huber M, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 40.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat Immunol. 2008;9(11):1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciofani M, Madar A, Galan C, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151(2):289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Lazarevic V, Chen X, Shim JH, et al. T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol. 2011;12(1):96–104. doi: 10.1038/ni.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diveu C, McGeachy MJ, Boniface K, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182(9):5748-5756. [DOI] [PubMed]
- 46.Mathur AN, Chang HC, Zisoulis DG, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 47.Yang XO, Panopoulos AD, Nurieva R, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 48.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 49.McGeachy MJ, Chen Y, Tato CM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19(6):400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 52.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghoreschi K, Laurence A, Yang XP, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009:27:519-550. [DOI] [PubMed]
- 55.Gulen MF, Bulek K, Xiao H, et al. Inactivation of the enzyme GSK3α by the kinase IKKi promotes AKT-mTOR signaling pathway that mediates interleukin-1-induced Th17 cell maintenance. Immunity. 2012;37(5):800–812. doi: 10.1016/j.immuni.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gulen MF, Kang Z, Bulek K, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32(1):54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen Q, Yang W, Gupta S, et al. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29(6):899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oida T, Weiner HL. Depletion of TGF-β from fetal bovine serum. J Immunol Methods. 2010;362(1-2):195–198. doi: 10.1016/j.jim.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gutcher I, Donkor MK, Ma Q, et al. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34(3):396–408. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin H, Wang L, Feng T, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol. 2009;183(1):97–105. doi: 10.4049/jimmunol.0801986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L, Lopes JE, Chong MM, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Valmori D, Raffin C, Raimbaud I, et al. Human RORγt+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci USA. 2010;107(45):19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mucida D, Park Y, Kim G, et al. Reciprocal T(H)17 and regulatory T cell differentiation mediated by retinoic acid. Science (New York, N.Y.) 2007;317(5835):256-260. [DOI] [PubMed]
- 66.Takahashi H, Kanno T, Nakayamada S, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012;13(6):587–595. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall JA, Cannons JL, Grainger JR, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34(3):435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453(7191):106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 69.Stockinger B, Hirota K, Duarte J, et al. External influences on the immune system via activation of the aryl hydrocarbon receptor. Semin Immunol. 2011;23(2):99–105. doi: 10.1016/j.smim.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Wu H-J, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kriegel MA, Sefik E, Hill JA, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U.S.A. 2011;108(28):11548-11553. [DOI] [PMC free article] [PubMed]
- 73.Chen K, McAleer JP, Lin Y, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35(6):997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pepper M, Linehan JL, Pagán AJ, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ouyang WJ, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2(5):403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Korn T, Bettelli E, Oukka M, et al. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27(1):485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 78.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593-2603. [DOI] [PMC free article] [PubMed]
- 80.Tan W, Huang W, Zhong Q, et al. IL-17 receptor knockout mice have enhanced myelotoxicity and impaired hemopoietic recovery following gamma irradiation. J Immunol. 2006;176(10):6186–6193. doi: 10.4049/jimmunol.176.10.6186. [DOI] [PubMed] [Google Scholar]
- 81.Kinugasa T, Sakaguchi T, Gu XB, et al. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118(6):1001–1011. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 82.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 84.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8(4):369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 85.Lin YY, Ritchea S, Logar A, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31(5):799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LeibundGut-Landmann S, Gross O, Robinson MJ, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8(6):630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 87.Wuthrich M, Deepe GS, Klein B. Adaptive immunity to fungi. In: Paul WE, editor. Annual Review of Immunology. Vol 30. Palo Alto, CA: Annual Reviews; 2012:; pp. 115–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang SC, Tan X-Y, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203(10):2271-2279. [DOI] [PMC free article] [PubMed]
- 89.Aujla SJ, Chan YR, Zheng MQ, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57-79. [DOI] [PubMed]
- 91.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milner JD, Sandler NG, Douek DC. Th17 cells, Job’s syndrome and HIV: opportunities for bacterial and fungal infections. Curr Opin HIV AIDS. 2010;5(2):179–183. doi: 10.1097/COH.0b013e328335ed3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Siegel AM, Heimall J, Freeman AF, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35(5):806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112(7):2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110(4):493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80(2):273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takaku T, Calado RT, Kajigaya S, et al. Interleukin-23 receptor (IL-23R) gene polymorphisms in acquired aplastic anemia. Ann Hematol. 2009;88(7):653–657. doi: 10.1007/s00277-008-0666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zwiers A, Kraal L, van de Pouw Kraan TCTM, Wurdinger T, Bouma G, Kraal G. Cutting edge: A variant of the IL-23R gene associated with inflammatory bowel disease induces loss of microRNA regulation and enhanced protein production. J Immunol. 2012;188(4):1573-1577. [DOI] [PubMed]
- 99.Spolski R, Wang L, Wan CK, et al. IL-21 promotes the pathologic immune response to pneumovirus infection. J Immunol. 2012;188(4):1924–1932. doi: 10.4049/jimmunol.1100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huppert J, Closhen D, Croxford A, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 101.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 102.Nowak EC, Weaver CT, Turner H, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206(8):1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12(6):568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Codarri L, Gyülvészi G, Tosevski V, et al. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12(6):560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 105.Luger D, Silver PB, Tang J, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kroenke MA, Carlson TJ, Andjelkovic AV, et al. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205(7):1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmer MT, Weaver CT. Autoimmunity: increasing suspects in the CD4+ T cell lineup. Nat Immunol. 2010;11(1):36–40. doi: 10.1038/ni.1802. [DOI] [PubMed] [Google Scholar]
- 108.Yang Y, Weiner J, Liu Y, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J Exp Med. 2009;206(7):1549–1564. doi: 10.1084/jem.20082584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13(10):991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Segal BM, Constantinescu CS, Raychaudhuri A, et al. Ustekinumab MS Investigators. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7(9):796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 111.Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159(5):1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 113.Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118(2):597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leonardi CL, Kimball AB, Papp KA, et al. PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 115.Benson JM, Sachs CW, Treacy G, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29(7):615–624. doi: 10.1038/nbt.1903. [DOI] [PubMed] [Google Scholar]
- 116.Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 117.Ahern PP, Schiering C, Buonocore S, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33(2):279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Monteleone I, Sarra M, Pallone F, et al. Th17-related cytokines in inflammatory bowel diseases: friends or foes? Curr Mol Med. 2012;12(5):592–597. doi: 10.2174/156652412800620066. [DOI] [PubMed] [Google Scholar]
- 119.O’Connor W, Jr, Kamanaka M, Booth CJ, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sugimoto K, Ogawa A, Mizoguchi E, et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118(2):534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zenewicz LA, Yancopoulos GD, Valenzuela DM, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fantini MC, Rizzo A, Fina D, et al. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol. 2007;37(11):3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 123.Peluso I, Fantini MC, Fina D, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178(2):732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 124.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8)1849-1861. [DOI] [PMC free article] [PubMed]
- 125.Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205(8):1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab in Crohn’s Disease Study Group. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sandborn WJ, Feagan BG, Fedorak RN, et al. Ustekinumab Crohn’s Disease Study Group. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135(4):1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 128.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tesmer LA, Lundy SK, Sarkar S, et al. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilke CM, Bishop K, Fox D, et al. Deciphering the role of Th17 cells in human disease. Trends Immunol. 2011;32(12):603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Solt LA, Kumar N, Nuhant P, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huh JR, Leung MWL, Huang P, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature. 2011;472(7344):486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Serody JS, Hill GR. The IL-17 differentiation pathway and its role in transplant outcome. Biol Blood Marrow Transplant. 2012;18(1 Suppl):S56–S61. doi: 10.1016/j.bbmt.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carvalho A, Cunha C, Di Ianni M, et al. Prognostic significance of genetic variants in the IL-23/Th17 pathway for the outcome of T cell-depleted allogeneic stem cell transplantation. Bone Marrow Transplant. 2010;45(11):1645–1652. doi: 10.1038/bmt.2010.28. [DOI] [PubMed] [Google Scholar]
- 136.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113(6):1365-1374. [DOI] [PMC free article] [PubMed]
- 137.Kappel LW, Goldberg GL, King CG, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113(4):945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Iclozan C, Yu Y, Liu C, et al. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(2):170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu Y, Wang D, Liu C, et al. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118(18):5011-5020. [DOI] [PMC free article] [PubMed]
- 140.Laurence A, Amarnath S, Mariotti J, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37(2):209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fulton LM, Carlson MJ, Coghill JM, et al. Attenuation of acute graft-versus-host disease in the absence of the transcription factor RORγt. J Immunol. 2012;189(4):1765-1772. [DOI] [PMC free article] [PubMed]
- 142.Bucher C, Koch L, Vogtenhuber C, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114(26):5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen X, Das R, Komorowski R, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114(4):891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Das R, Komorowski R, Hessner MJ, et al. Blockade of interleukin-23 signaling results in targeted protection of the colon and allows for separation of graft-versus-host and graft-versus-leukemia responses. Blood. 2010;115(25):5249–5258. doi: 10.1182/blood-2009-11-255422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nishimori H, Maeda Y, Teshima T, et al. Synthetic retinoid Am80 ameliorates chronic graft-versus-host disease by down-regulating Th1 and Th17. Blood. 2012;119(1):285–295. doi: 10.1182/blood-2011-01-332478. [DOI] [PubMed] [Google Scholar]
- 146.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19(4):429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kujawski M, Kortylewski M, Lee H, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118(10):3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 150.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178(11):6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 151.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141-1149. [DOI] [PMC free article] [PubMed]
- 152.Muranski P, Restifo NP. Does IL-17 promote tumor growth? Blood. 2009;114(2):231–232. doi: 10.1182/blood-2009-04-215541. [DOI] [PubMed] [Google Scholar]
- 153.Zou W, Restifo NPT. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10(4):248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tartour E, Fossiez F, Joyeux I, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59(15):3698–3704. [PubMed] [Google Scholar]
- 155.Wang L, Yi T, Kortylewski M, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.He D, Li H, Yusuf N, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Benchetrit F, Ciree A, Vives V, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99(6):2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 158.Kryczek I, Wei S, Szeliga W, et al. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114(2):357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chalmin F, Mignot G, Bruchard M, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36(3):362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 160.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 161.Stolfi C, Rizzo A, Franzè E, et al. Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J Exp Med. 2011;208(11):2279–2290. doi: 10.1084/jem.20111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Purwar R, Schlapbach C, Xiao S, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18(8):1248–1253. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Overwijk WW, de Visser KE, Tirion FH, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176(9):5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tong Z, Yang XO, Yan H, et al. A protective role by interleukin-17F in colon tumorigenesis. PLoS ONE. 2012;7(4):e34959. doi: 10.1371/journal.pone.0034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cho BS, Lim JY, Yahng SA, et al. Circulating IL-17 levels during the peri-transplant period as a predictor for early leukemia relapse after myeloablative allogeneic stem cell transplantation. Ann Hematol. 2012;91(3):439–448. doi: 10.1007/s00277-011-1318-9. [DOI] [PubMed] [Google Scholar]
- 166.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paulos CM, Carpenito C, Plesa G, et al. The inducible costimulator (ICOS) is critical for the development of human T(H)17 cells. Sci Transl Med. 2010;2(55):55ra78. doi: 10.1126/scitranslmed.3000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hinrichs CS, Kaiser A, Paulos CM, et al. Type 17 CD8+ T cells display enhanced anti-tumor immunity [published online ahead of print May 26, 2009]. Blood. 2009;114(3):596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Muranski P, Borman ZA, Kerkar SP, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35(6):972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martin-Orozco N, Chung Y, Chang SH, et al. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39(1):216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.O’Shea JJ, Lahesmaa R, Vahedi G, et al. Genomic views of STAT function in CD4+ T helper cell differentiation. Nat Rev Immunol. 2011;11(4):239–250. doi: 10.1038/nri2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wei G, Wei L, Zhu J, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hirota K, Duarte JH, Veldhoen M, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cosmi L, Cimaz R, Maggi L, et al. Evidence of the transient nature of the Th17 phenotype of CD4+CD161+ T cells in the synovial fluid of patients with juvenile idiopathic arthritis. Arthritis Rheum. 2011;63(8):2504–2515. doi: 10.1002/art.30332. [DOI] [PubMed] [Google Scholar]
- 177.Hamaï A, Pignon P, Raimbaud I, et al. Human T(H)17 immune cells specific for the tumor antigen MAGE-A3 convert to IFN-γ-secreting cells as they differentiate into effector T cells in vivo. Cancer Res. 2012;72(5):1059–1063. doi: 10.1158/0008-5472.CAN-11-3432. [DOI] [PubMed] [Google Scholar]
- 178.Waddington CH. The Strategy of the Genes. London, United Kingdom: George Allen & Unwin; 1957. [Google Scholar]
- 179.MacLeod MKL, Clambey ET, Kappler JW, Marrack P. CD4 memory T cells: What are they and what can they do? Seminars in Immunology. 2009;21:53–61. doi: 10.1016/j.smim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16(19):4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yu Q, Sharma A, Ghosh A, et al. T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. J Immunol. 2011;186(7):3946–3952. doi: 10.4049/jimmunol.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yu Q, Sharma A, Oh SY, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10(9):992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gattinoni L, Klebanoff CA, Palmer DC, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shi G, Ramaswamy M, Vistica BP, et al. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. J Immunol. 2009;183(11):7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Coquet JM, Middendorp S, van der Horst G, et al. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity. 2013;38(1):53-65. [DOI] [PubMed]
- 189.Pepper M, Pagán AJ, Igyártó BZ, et al. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35(4):583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Marshall HD, Chandele A, Jung YW, et al. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity. 2011;35(4):633–646. doi: 10.1016/j.immuni.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wirth TC, Xue HH, Rai D, et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33(1):128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luckey CJ, Weaver CT. Stem-cell-like qualities of immune memory; CD4+ T cells join the party. Cell Stem Cell. 2012;10(2):107–108. doi: 10.1016/j.stem.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kryczek I, Zhao ED, Liu Y, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3(104):ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ma CS, Deenick EK, Batten M, et al. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209(7):1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11(2):114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu X, Yan X, Zhong B, et al. Bcl6 expression specifies the T follicular helper cell program in vivo. J Exp Med. 2012;209(10):1841-1852. [DOI] [PMC free article] [PubMed]
- 197.Crotty S. Follicular helper CD4 T cells (T-FH). In: Paul WE, Littman DR, Yokoyama WM, editors. Annual Review of Immunology. Vol 29. Palo Alto, CA: Annual Reviews; 2011:; pp. 621–663. [DOI] [PubMed] [Google Scholar]
- 198.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119(12):3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Trifari S, Spits H. IL-22-producing CD4+ T cells: middle-men between the immune system and its environment. Eur J Immunol. 2010;40(9):2369–2371. doi: 10.1002/eji.201040848. [DOI] [PubMed] [Google Scholar]
- 200.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 201.Kagami S, Rizzo HL, Lee JJ, et al. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130(5):1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wilhelm C, Hirota K, Stieglitz B, et al. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12(11):1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wilhelm C, Turner JE, Van Snick J, et al. The many lives of IL-9: a question of survival? Nat Immunol. 2012;13(7):637–641. doi: 10.1038/ni.2303. [DOI] [PubMed] [Google Scholar]
- 204.Zou W, Restifo NP. Nine lives for TH9s? Nat Med. 2012;18(8):1177–1178. doi: 10.1038/nm.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]