Abstract
Neutralizing antibodies directed against measles virus (MV) surface glycoproteins prevent viral attachment and entry through the natural receptors. H protein specific IgG can enhance MV infectivity in macrophages via Fcγ receptor (FcγR)-dependent mechanism. H-specific IgM, anti-F antibodies and complement cascade activation are protective against antibody-mediated enhancement of MV infection. However, protective role of anti-H IgG against antibody-enhanced infection is not well understood. Here we designed a set of experiments to test the protective effect of H-specific IgG against FcγR-mediated infection in microglial cells. Microglial cells are also potential target of the antibody-mediated enhancement and spread of MV infection in the central nervous system. A partially neutralizing IgG monoclonal antibody (MAb) CL55, specific for MV H protein, at 10 μg/ml enhanced MV infection in mouse microglial cells by 13-14-fold. Infection-enhancing antibody concentrations induced large multinucleated syncytia formation 48-72 h post inoculation. We generated anti-H IgG MAb 20H6 with a strong neutralization capacity >1:80,000 at 1 mg/ml concentration in MV plaque-reduction neutralization assay. In contrast to the partially protective MAb CL55, enhancement of MV infectivity by MAb 20H6 required dilutions below the 1:120 serum titer considered protective against measles infection in humans. At a concentration of 10 μg/ml MAb 20H6 exhibited a dominant protective effect and prevented MAb CL55-mediated enhancement of MV infection and virus-mediated fusion. These results indicate that neutralization capacity of the H-specific IgG determines the balance between antibody enhancement and protection against MV infection in microglial cells.
Keywords: measles virus, microglial cells, antibody-enhanced infection
1. Introduction
Measles is considered the most contagious human infectious disease with millions of cases and more than 150,000 deaths reported annually (Centers for Disease Control and Prevention, 2012). Measles virus (MV) is a lymphotropic virus with activated lymphocytes, dendritic cells and monocyte/macrophages being the main infection target (Griffin, 2001). Massive infection of immune cells results in transient immune suppression and complications by secondary bacterial infections. Replication in respiratory epithelial cells is an important part of the virus life cycle and epidemic spread. MV is a paramyxovirus with lipoprotein envelope and negative RNA genome, consisting of six genes encoding eight viral proteins (Griffin, 2001). The two MV surface glycoproteins – hemagglutin (H) and fusion (F) protein are responsible for binding to viral receptors on the surface of host cells and subsequent entry by cell membrane fusion. Natural infection or vaccination with the live attenuated MV strains induces life-long immunity against re-infection. Introduction of the live MV vaccine and massive immunization program have drastically reduced measles morbidity and mortality. Vaccination is not 100% efficient in measles prevention, however. Genetic polymorphism in HLA, cytokine receptors and anti-viral effector proteins are some of the individual factors contributing to the poor response and low antibody titers (Dhiman et al., 2007; Haralambieva et al., 2011b; Ovsyannikova et al., 2004). Identification of strongly protective B-cell-restricted H and F epitopes is critical in the development of more efficient, highly immunogenic vaccines against measles. Humoral immune response is complex and includes protective envelope glycoprotein specific antibodies and non-protective antibodies directed to the other MV proteins (Griffin, 2001). H and F specific antibodies prevent viral attachment and fusion. Neutralization capacity of these antibodies depends on their epitope specificity, affinity and isotype.
Viruses can utilize antibodies or complement components for attachment and entry via Fcγ receptors (FcγRs) or complement receptors (Halstead et al., 2010; Huisman et al., 2009; Robinson et al., 1990; Takada and Kawaoka, 2003). Antibody-enhanced infectivity is responsible for severe forms of dengue virus infection – hemorrhagic fever and shock syndrome with high fatality rate (Dejnirattisai et al., 2010). Antibody and complement increased infection has been observed for HIV, flavi- and filoviruses (Dowd and Pierson, 2011; June at al., 1991; Meyer et al., 2008; Takada et al., 2003; Takeda et al., 1988). In addition, ligation of Fcγ receptor (FcγR) by immune complexes triggers signaling mechanisms that facilitate pathogen propagation by modulation of the innate anti-viral response (Halstead et al., 2010; Suhrbier and La Linn, 2003). Formation of the virus-antibody immune complexes requires previous sensitization to the pathogen antigens and IgG response. Pre-existing IgG can retarget and increase viral replication in macrophages and mature dendritic cells.
Previously, we have reported the existence of an antibody-mediated mechanism of enhanced MV infectivity (Iankov et al., 2006). Partially neutralizing IgG monoclonal antibody (MAb) against the H protein significantly increased MV infection in macrophageal cells. Human and mouse polyclonal anti-MV antibodies were also infection-enhancing at higher dilutions. In contrast, anti-F antibodies and complement did not increase viral infectivity and were protective preventing anti-H antibody-mediated enhancement of MV infection. H-specific IgM and classical complement activation also prevented IgG-mediated infection enhancement. Passive immunization of animals with serum in corresponding to antibody-enhancing infectivity concentrations increased viral replication and reporter gene expression following intraperitoneal (i.p.) administration of MV (Iankov et al., 2006). These data suggest that H-specific IgG-mediated entry can facilitate MV replication at certain anatomical locations in the presence of strong antiviral immunity, possibly being invoked in the immunopathology of atypical measles. However, the effect of strongly neutralizing H-specific antibodies of IgG isotype on antibody-mediated enhancement of MV infection has not been investigated.
Here, we demonstrate that partially neutralizing IgG antibodies specific for MV hemagglutinin can enhance not only individual cell infection but also can trigger cell-to cell fusion and giant syncytia formation in central nervous system (CNS) microglial cells. Antibodies with strong neutralization activity against infection in epithelial cells at relevant protective levels prevent the antibody enhancement and syncytia formation induced by non-neutralizing IgG. However, at high subprotective dilutions they can also trigger infection in FcγR expressing cells suggesting that neutralization capacity of anti-H IgG determines its ability to increase MV infectivity.
2. Materials and Methods
2.1. Cell lines, plasmids, MV strains and culture conditions
African green monkey Vero cell line, mouse J774A.1 macrophageal cells and human embryonic kidney 293 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD). Mouse microglial cell lines N13 (Righi et al., 1989) and BV2 (Blasi et al., 1990) were kindly provided by Dr. J.P. Godbout, Ohio State University, Columbus OH (Wynne et al., 2010). The cells were maintained in DMEM (from ATCC or HyClone) supplemented with 10% fetal bovine serum (FBS) and antibiotics (Invitrogen). Hybridoma clone CL55 expressing IgG2b MAb against MV H protein (Giraudon and Wild, 1985) was kindly provided by T. F. Wild, Institut Pasteur de Lyon, France. MV expressing green fluorescent protein (MV-GFP) (Duprex et al., 1999) and recently characterized MV expressing H. pylori NAP protein (MV-s-NAP) (Iankov et al., 2011) were amplified on Vero cells. Viral stocks were prepared using repeated freezing-thawing procedure and virus titer was determined in both plaque-forming units (PFU) or tissue culture infectious doses 50% (TCID50) per ml (Iankov et al., 2011). MV encoding human sodium iodide symporter (MV-NIS) (Dingli et al., 2004) was purified as previously described (Langfield et al., 2011).
Plasmids PCG-H and PCG-F encoding the H or F protein (Cathomen et al., 1995; Leonard et al., 2008) of MV were kindly provided by Dr. R. Cattaneo, Mayo Clinic, Rochester MN. PCG-H(Edm) and PCG-H(wt-323) encode H protein from Edmonston vaccine strain or wild type MV strain IC-323 respectively.
2.2. Production of MV neutralizing monoclonal antibodies (MAbs)
Hybridomas were generated after immunization of MV infection permissive interferon type I receptor knockout and human CD46 transgenic (Ifnarko-CD46Ge) mice (Mrkic et al., 1998). The animals were immunized with 106 TCID50 of live MV-s-NAP by an i.p. route. Spleen cells were collected and fused with myeloma line Sp2/0-Ag14 (ATCC) as described previously (Köhler and Milstein, 1975; Campbell, 1991). Hybridoma culture supernatants were tested by immunoblotting, virus neutralization (VN) and antigen-mediated ELISA. Hybridomas producing MAbs against MV antigens were cloned from a single cell and grown in DMEM (ATCC) supplemented with 10% FBS, antibiotics (Invitrogen) and 2 ng/ml recombinant IL-6 (Novus Biologicals). MAb isotype was determined using an IsoStrip Monoclonal Antibody Isotyping kit (Santa Cruz Biotechnology).
2.3. MAb characterization, purification and conjugation
MAb reactivity against MV antigens was characterized by VN test, ELISA and flow cytometry. Highly neutralizing H protein specific MAb 20H6 (IgG2a isotype) was selected for further characterization. Neutralization capacity of the clone was determined by VN test. Hybridoma 20H6 cells were cultured in serum-free medium (Invitrogen) supplemented with IL-6 and MAb was purified on Protein G column (Pierce). Protein concentration was determined using a BCA kit (Pierce). Purified antibody was conjugated to horse-radish peroxidase (HRP) or biotin using Lightning-Link conjugation kits (Innova Biosciences, UK). MAb CL55 was purified as described previously (Iankov et al., 2006).
2.4. Virus neutralization (VN) test
Neutralizing titer against MV of MAbs was measured by plaque-reduction microneutralization assay and plaque reduction neutralization titer 50% (PNT50) was calculated as described previously (Haralambieva et al., 2008). In vitro MV neutralization capacity of MAbs was determine based on the antibody concentration required for 50% neutralization of the viral particles (defined as 1 PNT50 activity).
2.5. Human serum antibodies
Serum samples used in the study was collected from AB(+) blood group healthy donors following Institutional Review Board approval and their protective titers against measles have been analyzed and reported previously (Iankov et al., 2010). VN activity of the frozen human serum samples was confirmed before the antibody-mediated infection enhancement tests.
2.6. Antigen-mediated ELISA
ELISA plates (Nunc) were coated with 2×104 TCID50 per well of purified and heat-inactivated (60°C for 1 h) MV-NIS virus particles resuspended in carbonate-bicarbonate buffer (CBB), pH 9.6. After overnight incubation plates were blocked with 1% bovine serum albumin (BSA) for 2 h at room temperature. Hybridoma culture supernatants or purified MAbs were added and the plates were incubated at room temperature for 1 h. After three washes with phosphate-buffered saline (PBS) plus 0.05% Tween 20 (PBS/T), anti-mouse polyvalent immunoglobulin (G, A, M) HRP-conjugated secondary antibody (Sigma) was added to the wells and plates were incubated for 1 h. Plates were washed in PBS/T and reaction was developed using TMB substrate (Bethyl Laboratories).
The degree of cross-reactivity and antigen-binding interference between H-specific MAbs 20H6 and CL55 was determined in a concurrent antigen-mediated ELISA. Briefly, purified MAbs were mixed and added at 1 μg/ml final concentration to a MV-NIS-coated plate (coated and blocked as described above). Antigen-binding of each of the MAbs was detected using isotype specific HRP-conjugated secondary antibodies (Santa Cruz Biotechnology): anti-mouse IgG2a (for MAb 20H6) and anti-mouse IgG2b (for CL55 detection). Pre-incubation with MAb 20H6 or CL55 alone served as control.
2.7. H-specific MAb avidity test
MAb avidity against MV H protein was measured in antigen-mediated ELISA using urea as dissociating reagent (Song et al., 2005). ELISA plates were coated with 2×104 TCID50 or 5×104 TCID50 of MV-NIS as described above. Dilutions of the purified MAb 20H6 and MAb CL55 (from 1,024 ng/ml to 0.5 ng/ml) were added to the wells and incubated for 1 h at room temperature. Plates were washed in PBS/T and incubated in the presence of 6M urea (Sigma) in PBS or PBS alone (for control wells) for different time periods (from 1 to 10 min). Plates were washed in PBS/T and incubated with the secondary antibody and TMB substrate as described above. The assay was also performed using different concentrations of the dissociating reagent − 2M, 4M and 6M urea in PBS.
2.8. Quantitative inhibition ELISA
The assay was developed in order to quantify the levels of antibodies in human serum samples that react directly with MAb 20H6 epitope or with neighboring epitopes in the same region of H protein. ELISA plates coated with MV-NIS antigen (as described above) were pre-incubated with serially diluted human sera (1:10-1:320) for 1-2 h at room temperature. Dilutions of purified MAb 20H6 were used as standard for binding inhibition. After washing in PBS/T 1 μg/ml of HRP-conjugated MAb 20H6 was added for 1-h incubation. The reaction was developed using TMB as HRP substrate. The level of MAb 20H6 epitope-reacting antibodies in human serum samples was determined as an equivalent to MAb 20H6 self-inhibitory concentration based on the assay standard curve.
2.9. Flow cytometry
Expression of macrophage surface markers and Fc receptors by microglial cells and J774 A.1 macrophages was determined using fluorescein isothiocyanate (FITC) and phycoerythrin (PE) labeled antibodies against mouse CD45, CD11b, FcγRIII/FcγRII (CD16/32) and FcγRI (CD64) (BioLegend). Control samples were incubated with corresponding FITC or PE conjugated isotype control antibodies (BioLegend). For characterization of MAb 20H6 specificity 293 cells were transfected with PCG-H or PCG-F plasmids using Lipofectamine 2000 (Invitrogen). After 48-72-h cells were collected and incubated with 10 μg/ml purified MAb 20H6 diluted in PBS with 1% BSA for 30 min on ice. Then the cells were washed in PBS and incubated with anti-mouse IgG PE-labeled secondary antibody for 30 min on ice (BioLegend). Expression of functional H and F proteins on the cell surface was confirmed by co-transfection of the cells with both PCG-H(Edm) and PCG-F and monitoring syncytia formation 48-72 h post transfection. Flow cytometry analysis was performed on a Becton-Dickinson FACScan cytometer using CellQuest software (Becton-Dickinson).
2.10. Antibody-mediated enhancement of MV infection
Dilutions of purified MAbs and serum antibodies were mixed MV-GFP in serum-free culture medium and incubated for 30 min. J774A.1, N13 and BV2 cells were harvested, washed in serum-free DMEM and mixed with the opsonized MV (at a final multiplicity of infection (MOI) of 0.2) for 30-60 min of incubation as described previously (Iankov et al., 2006). DMEM with 10% FBS was added 1:1 vol./vol. without removal of the viral inoculum. The number of GFP positive individual cells or syncytia was counted 36-72 h post infection using fluorescent microscopy. The samples were run in 5-6 wells in 24- or 96- well plates (Becton Dickinson).
2.11. Statistical analyses
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego CA).
3. Results
3.1. Characterization of highly neutralizing IgG MAb against MV
Hybridomas were screened by VN test, immunoblotting and ELISA for production of MV-specific antibodies. Although multiple MAbs reacting with protein bands corresponding viral nucleoprotein (N) or phosphoprotein (P) were identified, none of the VN test positive hybridomas reacted in micro-immunoblotting initial screening (not shown). These data confirmed previous observations that MV neutralizing antibodies are directed predominantly against conformational epitopes (Muller et al., 1993). Hybridoma clone 20H6 producing a strongly neutralizing IgG2a MAb against MV was selected and characterized. The antibody was purified by affinity chromatography, dialyzed against PBS and aliquots with a concentration of 1 mg/ml were stored frozen at -20°C. Flow cytometry analysis using 293 cells transfected to express MV surface glycoproteins confirmed H protein specificity of the antibody (Fig.1). Purified MAb 20H6 reacted with both Edmonston vaccine strain derivative and wild type MV hemagglutinin (H) but not with F protein. Expression of functional H and F was confirmed by co-transfection with both H and F encoding plasmids and subsequent syncytia formation in transfected cell monolayers (not shown).
Fig.1.
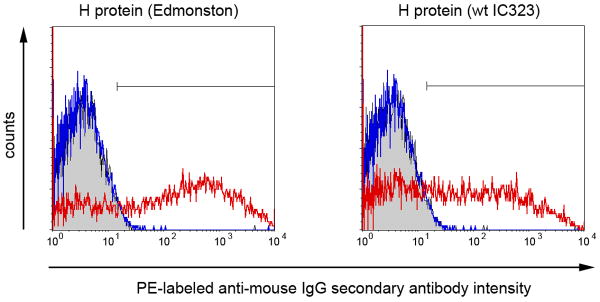
MAb 20 H6 reacts with H protein of both attenuated (Edmonston vaccine strain) and wild type (strain IC323) MV but not with F protein. 293 cells were transfected either with H protein or F protein using PCGH or PCGF plasmids using Lipofectamine 2000. 48-72 h later cells were harvested using Versene solution and incubated with 10 μg/ml 20H6 for 1h. The cells were washed in PBS and incubated with PE-conjugated anti-mouse IgG secondary antibody for 30 min and analyzed by flow cytometry. Histograms show MAb 20 H6 reactivity with H protein transfected cells (red line) compared to F protein transfected cell (blue line) and control (Lipofectamine 2000 without plasmid transfection) cells (in grey).
MAb 20H6 possesses very strong neutralization capacity against MV. In virus microneutralization assays the PNT50 titer of purified antibody at a concentration of 1 mg/ml was 1:83,082. Complete MV neutralization titer was 1:1,600. Based on these data 1 PNT50 neutralization capacity of MAb 20H6 (the concentration that reduced MV infectivity in Vero cells with 50%) was 12 ng/ml.
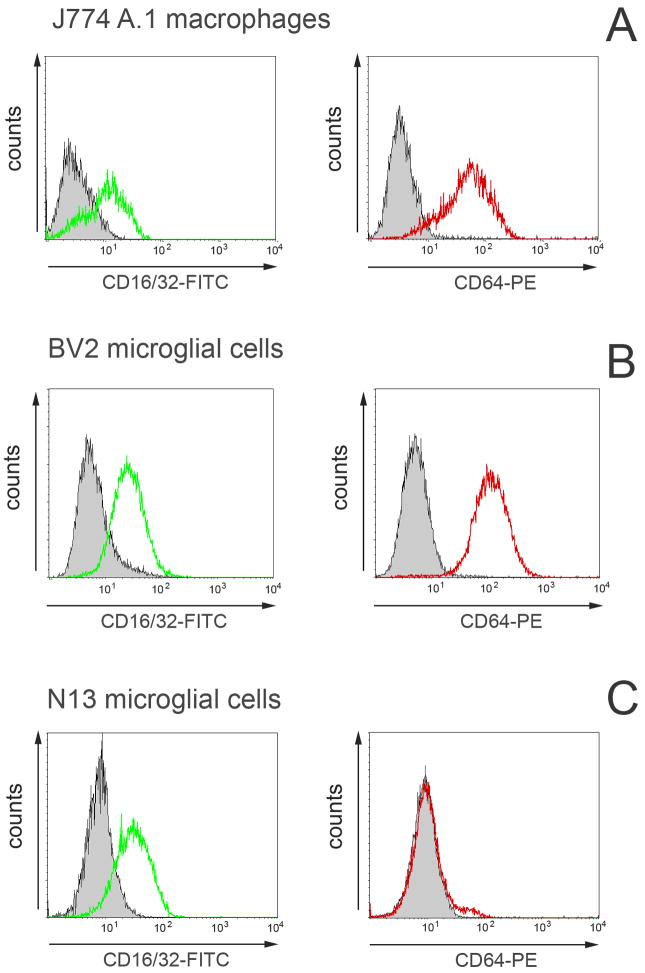
3.2. Expression of FcγRs by mouse microglial cells
N13 and BV2 microglial cells lines were positive for surface CD45 and monocyte/macrophage marker CD11b (Suppl.fig.1A). Both markers were expressed in lower level compared to J774A.1 macrophages (histograms in Suppl.fig.1B).
Flow cytometry analysis confirmed that both high and low affinity FcγRs were expressed at a high level in J774A.1 macrophages and BV2 microglia line (Fig.2A,B). The combined CD16/32-FITC and CD64-PE staining showed that virtually all cells were double positive. In contrast, the high affinity FcγRI (CD64) was not present on the surface of N13 cells. Only CD16/32 markers (low affinity FcγRIII and FcγRII respectively) were expressed by N13 microglial cells. (Fig.2C).
Fig.2.
Expression of FcγR by microglial cell lines. FITC or PE labeled antibodies against mouse FcγRIII and FcγRII low affinity receptors CD16/32 and high affinity FcγRI (CD64) were used in flow cytometry analysis. Control J774 A.1 macrophages and BV2 microglial cells express all of the FcγR (A and B). In contrast, N13 cells were negative for CD64 marker and express only CD16/32 receptors on the cell surface. Histograms show fluorescence marker intensity compared to that of the isotype control antibody.
3.3. MAbs of IgG isotype significantly enhance MV infection in microglial cells
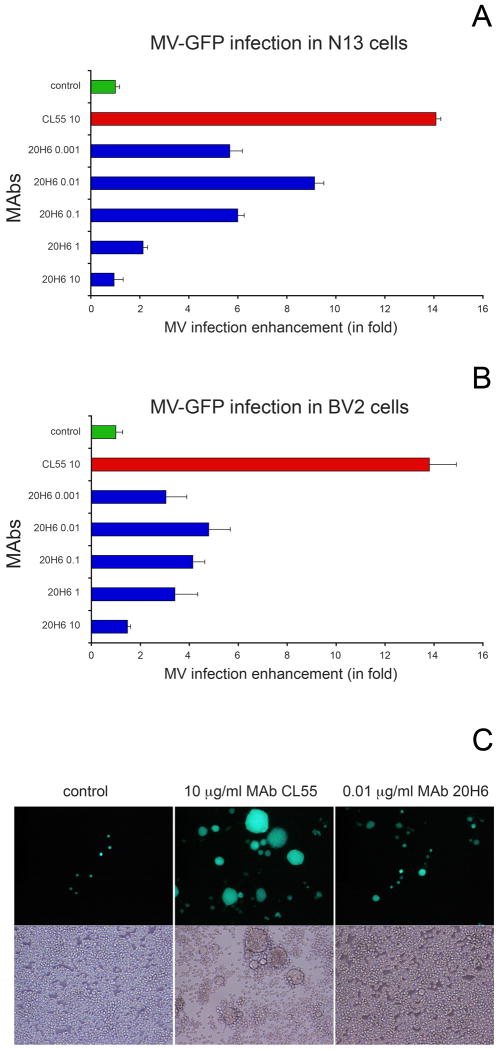
Mouse cells do not express the natural receptors for MV (CD46 and SLAM), thus they are ideal to isolate the effect of antibody-mediated enhancement of MV infectivity (Iankov et al., 2006). FcγR bearing mouse cells were incubated with MAb-opsonized MV-GFP and infection rate was compared to that of control cells incubated with virus alone. H-specific MAb CL55 (IgG2b) is not completely neutralizing for MV in vitro and partially protective in vivo (Giraudon and Wild, 1985; Iankov et al., 2006). The optimal MAb CL55 opsonizing concentration for enhanced MV infection in macrophages was determined to be 5-10 μg/ml (Iankov et al., 2006). At these concentrations MAb CL55 neutralized 60-80% of the MV infectivity in Vero and higher concentrations of the antibody did not increase virus neutralization (Iankov et al., 2006). Opsonization with 10 μg/ml of MAb CL55 increased MV infectivity in microglial cells by 13-14-fold compared to the no-antibody controls (Fig.3A,B). The presence of 5 μg/ml MAb CL55 during the 48-72-h MV-GFP infection in microglial cells triggered cell-to-cell fusion and large syncytia formation in microglial cells (Fig.3C).
Fig.3.
Antibody-mediated enhancement of MV infection in microglial N13 (A) and BV2 cells (B). MV-GFP was mixed with different concentrations of MAb 20H6 or 10 μg/ml of MAb CL55 and incubated for 30 min. Opsonized virions were then incubated with cells and infection number of infected GFP-positive cells and syncytia were counted following 36-72 h of incubation. Samples are run in 6 repeated wells. MV-GFP was added to the cells at a final MOI=0.2. In contrast to partially neutralizing antibody CL55, MAb 20H6 at concentration of 10 μg/ml did not enhance MV infectivity. The peak of MAb 20H6 enhanced infection was at 10 ng/ml or approximately the concentration corresponding to 1 PNT50 (12 ng/ml).
CL55 10 = 10 μg/ml MAb CL55, 20H6 0.001 = MAb 20H6 0.001 μg/ml; 20H6 0.01 = MAb 20H6 0.01 μg/ml; 20H6 1 = MAb 20H6 1 μg/ml; 20H6 10 = MAb 20H6 10 μg/ml. MV incubated without MAbs was used as control (A,B).
Antibody-enhanced MV infection and large syncytia formation in N13 microglial cells (C). The presence of MAb CL55 significantly enhanced MV infection and induced large syncytia formation as compared to control MV without antibodies. Incubation in the presence of MAb 20H6 also resulted in increased MV infectivity (at a 1,000-fold lower concentration) demonstrated mainly by GFP-positive small syncytia or individual cells.
In the same experiment a highly neutralizing MAb 20H6 was run in 10-fold dilutions starting from 10 μg/ml. Enhancement of MV infection was not observed at 10 μg/ml of MAb 20H6. Although at this concentration (10 μg/ml) MAb 20H6 completely blocks infection in epithelial cells, it did not prevent the background level of infection in mouse microglial cells (the number of infected control cells incubated with non-opsonized by antibodies MV). MAb 20H6 did enhance infection at much lower concentrations. The peak of antibody-enhanced MV infection by MAb 20H6 was observed at an antibody concentration of 10 ng/ml or approximately 1 PNT50 neutralization activity. MV infectivity was increased over 8-fold in N13 and more than 4-fold in BV2 microglial cells (Fig.3A,B) MAb 20H6 induced infection enhancement in control J774A.1 macrophages was approximately 6-fold (not shown). In contrast to the results with MAb CL55, MAb 20H6 did not promote efficient cell-to-cell fusion even at the peak infection-enhancing concentration (10 ng/ml).
3.4. A balance between the IgG-mediated enhancement and virus neutralization
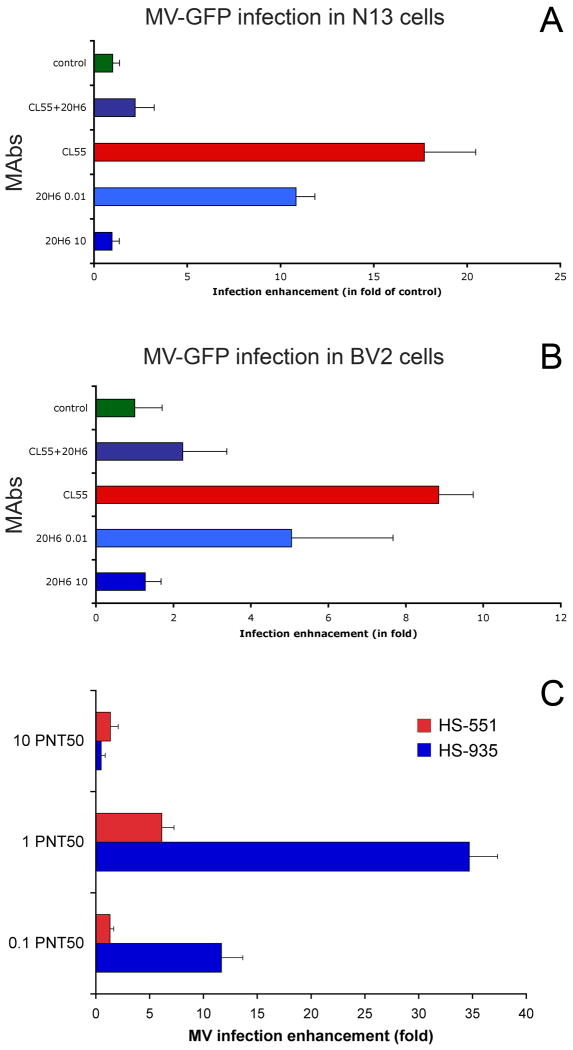
In further experiments we evaluated the efficacy of the highly neutralizing MAb in protective concentrations. For human serum, anti-MV titers >1:120 in PNT50 assay are considered protective (Haralambieva et al., 2008). A titer of 1:120 corresponds to a concentration of MAb 20H6 of 1.44 μg/ml. When 10 μg/ml MAb 20H6 was incubated together with equivalent concentration of 10 μg/ml MAb CL55, the antibody-mediated enhancement of MV infection and synscytia formation triggered by MAb CL55 in microglial cells were completely abolished (Fig.4A,B). Concurrent-inhibition ELISA demonstrated a partial cross-reactivity between the two MAbs. MAbs interfered equally for antigen-binding showing that the dominant protective effect of MAb 20H6 over CL55-mediated infection enhancement was not due blockade of the MAb CL55 interaction with MV H protein (Suppl.fig.2). However, MAb 20H6 showed stronger avidity to H antigen as compared to MAb CL55. Following incubation with 6M urea for 5 min, the binding of MAb 20H6 at 128 ng/ml concentration was reduced to 31% (Suppl.fig.3). In contrast, MAb CL55 binding was almost completely abolished (4% residual binding). Antibody-binding recovered after 2.5-min urea incubation was 56% for MAb 20H6 and 37% for MAb CL55 respectively. The urea-based avidity assay is used to evaluated immune response and vaccination efficacy against measles. High avidity anti-MV antibodies correlate with the serum VN capacity, induction of B-cell memory and long-term protective immunity (Song et al., 2005). Concentration of 10 μg/ml of MAb 20H6 corresponds to a titer >1:800 that can confer sterile immunity against measles (>1:800-1,052) in vaccinated individuals (Chen et al., 1990; Ward et al., 1999). These data indicate that at relevant concentrations strongly neutralizing IgG antibodies are protective against MV entry in the host cells through both natural receptors and FcγRs by antibody enhancement mechanism.
Fig.4.
MAb 20H6 at a high concentration is protective against MAb CL55-mediated enhancement of MV infection in N13 (A) and BV2 (B) microglial cells. 10 μg/ml CL55 (shown as CL55 in the figure) and 0.01 μg/ml 20H6 (shown as 20H6 0.01) increased MV infection, while at a highly protective for epithelial cells concentration of 10 μg/ml MAb 20H6 (shown as 20H6 10) did not increased MV infectivity. At equivalent concentration of 10 μg/ml MAb 20H6 act as protective antibody abolishing CL55 infection enhancement (shown as CL55+20H6).
Human serum antibodies enhanced MV infection in microglial cells in concentration dependent manner (C). N13 cells were inoculated at MOI=0.2 of MV-GFP opsonized with human AB+ blood group serum antibodies. Two sera (HS-551 and HS-935) with the highest virus neutralization titers were used in dilutions corresponding to 10, 1 or 0.1 PNT50 neutralization activity. Peak of MV enhanced infectivity was determined to be at 1 PNT50 neutralization activity for both serum samples.
3.5. Human antibody-mediated enhancement of MV infection in microglial cells
To confirm the relevance of the results observed using MAbs we tested human serum antibodies in similar experimental conditions. All nine human sera were measured to have anti-measles titers above the protective level of 1:120 in PNT50 assay. Samples were collected from blood group AB(+) donors (Iankov et al., 2010) to avoid potential interference with blood group specific antibodies. Neutralization activity of the frozen serum samples was re-tested to verify the PNT50 titers against MV (Tab.1). We designed a specific inhibition ELISA in order to quantify the antibodies directed against the highly protective MAb 20H6 epitope or neighboring epitopes on H protein that could sterically interfere with MAb 20H6 binding. The antibody levels that blocked MAb 20H6 binding were detected in all nine samples and corresponded to equivalent concentration of MAb 20H6 in the range between 113 ng/ml and 14 μg/ml (Tab.1). Two of the sera with the highest anti-measles titers (HS-551 and HS-935) had also the highest concentration of cross-reactive antibodies against MAb 20H6 epitope. Concentration-dependent enhancement of MV infection by HS-551 and HS-935 serum antibodies, similarly to MAb 20H6, peaked at the dilutions corresponding to 1 PNT50 virus neutralization activity (Fig.4C). Eight of nine human sera at dilution of 1 PNT50 induced enhancement of MV infection in N13 microglial cells from 4 to more than 40-fold as compared to control infection using non-opsonized viruses (see Tab.1).
Table 1.
Concentration of antibodies of MAb 20H6 cross-reacting antibodies in human sera and enhancement of MV infectivity in N13 microglial cells.
| Human AB+ serum | VN activity (PNT50 titer) | MAb 20H6 equivalent antibody concentration (in μg/ml)a | Antibody-enhancement of MV infection (fold)b |
|---|---|---|---|
| HS-055 | 1:715 | 1.193 | 6.89 |
| HS-193 | 1:1,179 | 4.729 | 11.53 |
| HS-283 | 1:890 | 1.024 | 35.72 |
| HS-415 | 1:568 | 0.113 | 0.97 |
| HS-475 | 1:679 | 3.620 | 40.97 |
| HS-551 | 1:4,686 | 9.975 | 6.14 |
| HS-853 | 1:478 | 2.176 | 11.94 |
| HS-935 | 1:2,771 | 14.125 | 34.70 |
| HS-1313-MM | 1:321 | 0.322 | 4.33 |
cross-reactive antibody concentration was measured in inhibition ELISA and is presented in ng/ml equivalent to MAb 20H6 standard concentration
antibody-mediated increase (in fold) of MV infection in N13 cells as compared to control cells incubated with non-opsonized virus
4. Discussion
Neutralizing antibodies are critical for specific protection against pathogenic viruses. Natural infection or immunizations with live attenuated MV vaccine elicit strong life-long immunity against measles (Griffin, 2001). However, the failure to develop long-term protective antibody titers (>1:120 PNT50 assays) may exceed 8% of the vaccinated population (Haralambieva et al., 2011a). In this study, we generated a MAb of IgG2a class with very strong MV neutralization capacity, by immunization of MV permissive Ifnarko-CD46Ge mice. MAb 20H6 reacts with a highly protective epitope expressed on the viral H glycoprotein. Since species-specific antibody repertoire could influence development of humoral immunity, it was important to confirm an antibody response to the MAb 20H6 epitope in humans. We designed an inhibition ELISA in order to quantify MAb 20H6 epitope-specific human serum antibodies. The sera were collected from AB(+) blood group donors in order to avoid blood group specific antibody reactions to MV antigens. High levels of antibodies reacting directly or interfering with MAb 20H6 epitope were found in all human serum samples. Identification of the “ultimate” virus neutralization B-cell epitopes is a key design issue in the development of live attenuated vaccines against measles. The inhibition ELISA using MAb 20H6 as a standard could be a useful test for assessment of protective immunity following MMR vaccination. Previously, we demonstrated that H-specific IgG antibodies that partially neutralized the virus (such as MAb CL55) were poor protectors and augmented the MV infection in macrophages (Iankov et al., 2006). Human sera at low concentrations (high dilutions) also increased the viral infectivity. In contrast, H protein specific IgM, anti-F antibodies and activation of the classical complement cascade by anti-H antibodies blocked antibody-mediated MV infection enhancement. Antibody-dependent enhancement allowed MV replication within peritoneal macrophages in measles-immune Ifnarko-CD46Ge mice (Iankov et al., 2006). These results suggested that MV could use FcγR-mediated entry as immuno-evasive mechanism in the absence of anti-F antibodies or in anatomical locations where the complement cascade components are not present at functionally active levels.
Antibodies neutralize viruses by blocking viral attachment to virus-specific receptors and entry into the host cells. Binding of antigen-antibody complexes to Fc receptors on immune cells triggers effector immune mechanisms of pathogen elimination and clearance of virus-infected cells including phagocytosis, antibody-dependent cellular cytotoxicity, oxidative burst and cytokine release. In brain, Fc receptors are detected on astrocytes, oligodendrocytes and microglial cells (Nakahara and Aiso, 2006; Nitta et al., 1992; Quan et al., 2009). Microglial cells are the resident phagocytic cells in CNS involved in inflammatory response to invading pathogens, antigen-presentation and pathogenesis of autoimmune neurological disorders. Microglial cells express FcγRs and significantly upregulate their expression upon activation in the course of inflammation and neurodegenerative diseases (Lunnon et al., 2011; Quan et al., 2009). Here, we demonstrated that at a high concentration (10 μg/ml) partially neutralizing IgG MAb CL55 directed against MV H protein efficiently mediated MV infection and cell-to-cell fusion among FcγR-expressing microglial cells. Strongly neutralizing H-specific MAb 20H6 and human serum antibodies also were able to enhance MV infectivity but at high dilutions, well below the titer levels considered protective against measles (Chen et al., 1990; Haralambieva et al., 2008; Ward et al., 1999). The presence of high concentration of infection-enhancing MAb CL55 promoted MV-mediated cell-to-cell fusion. Opsonization of MV with mAb 20H6 or human serum resulted in an increased number of individual infected FcyR expressing cells, but, in contrast to the results with Mab CL55, did not facilitate large syncytia formation. The optimal antibody-enhancing level was determined to correspond to a titer of 1 PNT50. At higher concentrations corresponding to the relevant protective levels determined in human sera (>1:120 PNT50 titer), MAb 20H6 did not enhance MV infection and was protective against MAb CL55-mediated infection. The lack of efficient cell-to-cell MV spread in the presence of low (infection-enhancing) concentrations of strongly protective IgG suggests that high antibody level and engagement of more surface FcγR molecules are possibly required to trigger fusion between infected and non-infected neighboring cells. Inhibition of cell-to-cell fusion by H-specific antibodies could be simply explained by the blockade of H protein binding to MV receptor CD46 or SLAM. However, this simple mechanism cannot explain anti-H IgG protection against infection and fusion triggered via FcγRs. Our findings suggest that the mechanism of MV neutralization by strongly protective anti-H antibodies is not only through direct blocking of virus attachment. One possible explanation is that anti-H antibodies interfere with the conformational changes and activation of F protein triggered by H protein interaction with the virus receptors.
Persistent MV infection in the face of strong antibody response has been observed in the CNS of patients with subacute sclerotic panencephalitis (SSPE) (Griffin, 2001). Our data suggest a mechnism of IgG-mediated enhancement of MV infection and cell-to-cell fusion within microglial cells in the absence of adequate protection by anti-F or fusion-blocking anti-H antibodies. The proposed mechanism could be involved in pathogenesis of MV infection persistence in CNS tissue. Further studies using tissue specimens and sera collected from SSPE patients as well as development of relevant animal model are required to test this hypothesis.
5. Conclusions
In this study we demonstrate that CNS resident cells expressing FcγRs are targets of H antigen specific antibody-increased MV infectivity. IgG/FcR complex formation can serve as an alternative receptor enhancing not only viral entry but also promoting fusion and cell-to-cell spread of infection among microglial cells. Our data indicate that neutralization capacity determines the balance between virus neutralization and enhanced MV infectivity by IgG antibodies directed against H protein. Highly neutralizing MAb or human serum antibodies at a relevant concentrations are strong protectors providing an adequate immune defense against non-neutralizing IgG-mediated enhancement of MV infection. Selective boost of highly protective IgG antibodies is an ultimate goal of MV vaccine strategy. The inhibition ELISA developed in this study may serve as a tool to evaluate anti-measles immune response in humans and facilitate the development of MV vaccines with improved immunogenicity.
Supplementary Material
We characterized infection-enhancing activity of IgG monoclonal antibodies (MAbs) against measles virus (MV) H protein.
Partially neutralizing MAb CL55 enhanced MV infection in Fc receptor-expressing microglial cells.
Strongly neutralizing MAb 20H6 enhanced MV infectivity in microglial cells only at sub-protective titers.
At relevant protective titers, MAb 20H6 had a dominant blocking effect against antibody-enhanced MV infection.
The antibody neutralization capacity determined the balance between protection and IgG-enhanced MV infectivity.
Acknowledgments
We wish to thank Dr. J.P. Godbout, Ohio State University, Columbus OH for providing mouse microglial cell lines N13 and BV2, Dr. T. F. Wild, Institut Pasteur de Lyon, France, for the hybridoma clone CL55 and Dr. R. Cattaneo from Mayo Clinic, Rochester MN for providing the PCG-H and PCG-F plasmids encoding MV H and F proteins. This work is supported by P50 CA 116201 grant, Paul Leibson Memorial Fund, and Atwater grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2-3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Campbell AM. Laboratory Techniques in Biochemistry and Molecular Biology. Vol. 23 Elsevier; Amsterdam: 1991. Monoclonal antibody and immunosensor technology. [Google Scholar]
- Cathomen T, Buchholz CJ, Spielhofer P, Cattaneo R. Preferential initiation at the second AUG of the measles virus F mRNA: a role for the long untranslatedregion. Virology. 1995;214(2):628–632. doi: 10.1006/viro.1995.0075. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Progress in global measles control, 2000-2010. MMWR Morb Mortal Wkly Rep. 2012;61(4):73–78. [PubMed] [Google Scholar]
- Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- Griffin D. Measles virus. In: Knipe DM, Howley PM, editors. Fields Virology. 4th. Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. pp. 1401–1441. [Google Scholar]
- Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328(5979):745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman N, Ovsyannikova IG, Cunningham JM, Vierkant RA, Kennedy RB, Pankratz VS, Poland GA, Jacobson RM. Associations between measles vaccine immunity and single-nucleotide polymorphisms in cytokine and cytokine receptor genes. J Infect Dis. 2007;195(1):21–29. doi: 10.1086/510596. [DOI] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, Morris JC, Russell SJ. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103(5):1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411(2):306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73(11):9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudon P, Wild TF. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination inhibiting activity. Virology. 1985;144(1):46–58. doi: 10.1016/0042-6822(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10(10):712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, O'Byrne M, Pankratz VS, Jacobson RM, Poland GA. A large observational study to concurrently assess persistence of measles specific B-cell and T-cell immunity in individuals following two doses of MMR vaccine. Vaccine. 2011a;29(27):4485–4491. doi: 10.1016/j.vaccine.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, Umlauf BJ, Vierkant RA, Shane Pankratz V, Jacobson RM, Poland GA. Genetic polymorphisms in host antiviral genes: associations with humoral and cellular immunity to measles vaccine. Vaccine. 2011b;29(48):8988–8997. doi: 10.1016/j.vaccine.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clin Vaccine Immunol. 2008;15(7):1054–1059. doi: 10.1128/CVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman W, Martina BE, Rimmelzwaan GF, Gruters RA, Osterhaus AD. Vaccine-induced enhancement of viral infections. Vaccine. 2009;27(4):505–512. doi: 10.1016/j.vaccine.2008.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankov ID, Haralambieva IH, Galanis E. Immunogenicity of attenuated measles virus engineered to express Helicobacter pylori neutrophil-activating protein. Vaccine. 2011;29(8):1710–1720. doi: 10.1016/j.vaccine.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankov ID, Msaouel P, Allen C, Federspiel MJ, Bulur PA, Dietz AB, Gastineau D, Ikeda Y, Ingle JN, Russell SJ, Galanis E. Demonstration of anti-tumor activity of oncolytic measles virus strains in a malignant pleural effusion breast cancer model. Breast Cancer Res Treat. 2010;122(3):745–754. doi: 10.1007/s10549-009-0602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iankov ID, Pandey M, Harvey M, Griesmann GE, Federspiel MJ, Russell SJ. Immunoglobulin g antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J Virol. 2006;80(17):8530–8540. doi: 10.1128/JVI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June RA, Schade SZ, Bankowski MJ, Kuhns M, McNamara A, Lint TF, Landay AL, Spear GT. Complement and antibody mediate enhancement of HIV infection by increasing virus binding and provirus formation. AIDS. 1991;5(3):269–274. doi: 10.1097/00002030-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Langfield KK, Walker HJ, Gregory LC, Federspiel MJ. Manufacture of measles viruses. Methods Mol Biol. 2011;737:345–366. doi: 10.1007/978-1-61779-095-9_14. [DOI] [PubMed] [Google Scholar]
- Leonard VH, Sinn PL, Hodge G, Miest T, Devaux P, Oezguen N, Braun W, McCray PB, Jr, McChesney MB, Cattaneo R. Measles virus blind to its epithelial cell receptorremains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J Clin Invest. 2008;118(7):2448–2458. doi: 10.1172/JCI35454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunnon K, Teeling JL, Tutt AL, Cragg MS, Glennie MJ, Perry VH. Systemic inflammation modulates Fc receptor expression on microglia during chronic neurodegeneration. J Immunol. 2011;186(12):7215–7224. doi: 10.4049/jimmunol.0903833. [DOI] [PubMed] [Google Scholar]
- Meyer K, Ait-Goughoulte M, Keck ZY, Foung S, Ray R. Antibody-dependent enhancement of hepatitis C virus infection. J Virol. 2008;82(5):2140–2149. doi: 10.1128/JVI.01867-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rülicke T, Volpe P, Buchholz CJ, Hourcade D, Atkinson JP, Aguzzi A, Cattaneo R. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72(9):7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CP, Schroeder T, Tu R, Brons NH, Jung G, Schneider F, Wiesmüller KH. Analysis of the neutralizing antibody response to the measles virus using synthetic peptides of the haemagglutinin protein. Scand J Immunol. 1993;38(5):463–471. doi: 10.1111/j.1365-3083.1993.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Nakahara J, Aiso S. Fc receptor-positive cells in remyelinating multiple sclerosis lesions. J Neuropathol Exp Neurol. 2006;65(6):582–591. doi: 10.1097/00005072-200606000-00006. [DOI] [PubMed] [Google Scholar]
- Nitta T, Yagita H, Sato K, Okumura K. Expression of Fc gamma receptors on astroglial cell lines and their role in the central nervous system. Neurosurgery. 1992;31(1):83–87. doi: 10.1227/00006123-199207000-00012. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Jacobson RM, Poland GA. Variation in vaccine response in normal populations. Pharmacogenomics. 2004;5(4):417–427. doi: 10.1517/14622416.5.4.417. [DOI] [PubMed] [Google Scholar]
- Quan Y, Möller T, Weinstein JR. Regulation of Fcγ receptors and immunoglobulin G-mediated phagocytosis in mouse microglia. Neurosci Lett. 2009;464(1):29–33. doi: 10.1016/j.neulet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19(8):1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Robinson WE, Jr, Montefiori DC, Mitchell WM. Complement-mediated antibody-dependent enhancement of HIV-1 infection requires CD4 and complement receptors. Virology. 1990;175(2):600–604. doi: 10.1016/0042-6822(90)90449-2. [DOI] [PubMed] [Google Scholar]
- Song MK, Vindurampulle CJ, Capozzo AV, Ulmer J, Polo JM, Pasetti MF, Barry EM, Levine MM. Characterization of immune responses induced by intramuscular vaccination with DNA vaccines encoding measles virus hemagglutinin and/or fusion proteins. J Virol. 2005;79(15):9854–9861. doi: 10.1128/JVI.79.15.9854-9861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhrbier A, La Linn M. Suppression of antiviral responses by antibody-dependent enhancement of macrophage infection. Trends Immunol. 2003;24(4):165–168. doi: 10.1016/s1471-4906(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Takada A, Feldmann H, Ksiazek TG, Kawaoka Y. Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77(13):7539–7544. doi: 10.1128/JVI.77.13.7539-7544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13(6):387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tuazon CU, Ennis FA. Antibody-enhanced infection by HIV-1 via Fc receptor-mediated entry. Science. 1988;242(4878):580–583. doi: 10.1126/science.2972065. [DOI] [PubMed] [Google Scholar]
- Ward BJ, Aouchiche S, Martel N, Bertley FM, Bautista-Lopez N, Serhir B, Ratnam S. Measurement of measles virus-specific neutralizing antibodies: evaluation of the syncytium inhibition assay in comparison with the plaque reduction neutralization test. Diagn Microbiol Infect Dis. 1999;33(3):147–152. doi: 10.1016/s0732-8893(98)00069-8. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010;24(7):1190–1201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.