Abstract
This work evaluates the influence of FcγR on the pharmacokinetics and pharmacodynamics of a rat anti–integrin-αIIb IgG1 monoclonal antibody, MWReg30, in mice. The pharmacokinetics and pharmacodynamics of MWReg30 were investigated in C57BL/6 control mice, FcγRI/RIII knockout mice, and FcγRIIb knockout mice, following intravenous doses of 0.04 – 0.4 mg/kg. MWReg30 treatment resulted in a dose-dependent induction of thrombocytopenia in all strains, but sensitivity to MWReg30 was increased in FcγRIIb knockout mice and decreased in FcγRI/RIII knockout mice, relative to results found in control mice. Expressed as a percentage of pre-treatment platelet counts, nadir platelet counts were 28.6±5.0, 88.7±16.6 and 25.3±6.1% at 0.05 mg/kg, 28.4±13.7, 56.7±5.1, and 20.6±9.5% at 0.2 mg/kg, and 24.9±7.2, 38.7±7.5, and 7.4±2.2% at 0.4 mg/kg in in control, FcγRI/RIII(−/−) and FcγRIIb(−/−) mice. However, knocking out FcγR did not affect MWReg30 pharmacokinetics. Plasma areas under the concentration vs. time curves (AUC0–10days)±SD for MWReg30 were: 5.24±0.68, 5.51±0.24, and 5.39±1.05 nM×d at 0.04 mg/kg, and 12.7±0.5, 13.6±1.1, and 14.5±2.0 nM×d at 0.1 mg/kg in control, FcγRI/RIII(−/−) and FcγRIIb(−/−) mice. The findings further emphasize the role of activating vs. inhibitory FcγR in processing immune complexes (i.e., MWReg30-platelets), while also providing an example where monoclonal antibody pharmacokinetics are not substantially influenced by FcγR expression.
Keywords: Anti-platelet antibody, IgG, Fcγ-receptors, pharmacokinetics, pharmacodynamics, tissue disposition
1. Introduction
Several classes of FcγR have been identified in man and in mouse, including receptors that activate effector functions (i.e., FcγRI, FcγRIIA, and FcγRIIIA) and receptors that inhibit effector functions (FcγRIIb) (Nimmerjahn et al., 2005; Nimmerjahn and Ravetch, 2008; Siberil et al., 2007; Tarasenko et al., 2007). Immunoglobulin G (IgG) binds with high affinity (10−8 –10−9 M) to FcγRI, and with lower affinity to FcγRIIA, FcγRIIb, and FcγRIII (10−7–10−8 M). Activating and inhibitory FcγR are expressed on cells of hematopoietic origin (i.e., beta cells, basophils, T-lymphocytes, monocytes, and mast cells), and in antigen-presenting cells that include dendritic cells and macrophages (Amigorena and Bonnerot, 1999a, b; Cassel et al., 1993; Nimmerjahn and Ravetch, 2006; Ravetch and Bolland, 2001). Large populations of cells expressing FcγR are found in the bone marrow, thymus, lung, liver, and spleen (Bhatia et al., 1998; Bordessoule et al., 1993; Ivan and Colovai, 2006; Tuijnman et al., 1993; Van de Winkel and Capel, 1993).
The binding of the Fc domain of IgG antibodies to activating FcγR leads to several immune responses, such as antibody-dependent cell cytotoxicity, the release of inflammatory molecules (such as cytokines), enhancement of antigen presentation, and phagocytosis of immune complexes (Nimmerjahn and Ravetch, 2006, 2008; Siberil et al., 2007; Tarasenko et al., 2007). IgG immune complexes or IgG opsonized particles are rapidly engulfed upon binding to FcγR on macrophages and dendritic cells, leading to the elimination of associated particles or antigens.
Immune thrombocytopenic purpura (ITP) is an autoimmune condition where patients develop autoantibodies with affinity for platelet membrane glycoproteins (e.g., GPIIb/IIIa and GPIb/IX) (Van Leeuwen et al., 1982). Antibody-opsonized platelets are eliminated by phagocytic cells of the reticuloendothelial system, in tissues such as the spleen and liver (Cines and Blanchette, 2002). Due to the well-known role of FcγR in the elimination of immune complexes and opsonized particles, and due to the moderate-to-high affinity of monomeric IgG for FcγR, it has been assumed that FcγR play an important role in the elimination and tissue distribution of all IgG antibodies (McDonagh et al., 2008; Mould and Green, 2010; Nishio et al., 2009; Tabrizi et al., 2010; Tabrizi et al., 2006; Zuckier et al., 1994). Although there has been wide acceptance of the hypothesis that FcγR are key determinants of IgG pharmacokinetics (PK), this supposition has not been thoroughly examined within the published literature.
Recently, we evaluated the effect of FcγR on the elimination and tissue distribution of 8C2, a model monoclonal IgG1 antibody with high affinity for a soluble ligand (topotecan). Our results demonstrated virtually identical 8C2 plasma and tissue disposition in wild-type mice and in FcγR knock-out mice (Abuqayyas and Balthasar, 2012, in press). To evaluate the role of Fcγ receptors as determinants of the disposition and pharmacodynamics (PD) of a model monoclonal antibody with specific affinity for cell surface proteins (i.e., forming opsonized cells), we have now assessed the PK and PD of MWReg30 in control (wild-type) mice and FcγR-knockout mice. MWReg30, which is a rat IgG1 mAb with high affinity for mouse intergrin αIIb (GPIIb), has been shown to induce thrombocytopenia in mice, and has been used to develop mouse models of ITP (Deng and Balthasar, 2007).
2. Material and methods
2.1. Materials
MWReg30 was purchased from BD Pharmingen™ (San Diego, CA). Sodium iodide (Na-125I) was obtained from Perkin Elmer Inc. (Waltham, MA). Chloramine-T, sodium metabisulfite, calcium sulphate (CaSO4), and carboxymethyl cellulose (CMC) were from Sigma Life Science (St Louis, MO). Potassium iodide (KI) and sodium iodide (Na-127I) were from Fischer Scientific (Pittsburgh, PA). MWReg30 was labeled with 125I via the Chloramine-T method, as described previously (Garg and Balthasar, 2007). The purity of the iodinated IgG was assessed using instant thin layer chromatography (PE SiL-G, Whatman Ltd, Kent, England), as described previously (Garg and Balthasar, 2007). For all experiments, the purity of the iodinated preparation was higher than 99%.
2.2. Animals
B6.129P2-Fcer1gtm1Rav N12 mice, deficient in the gamma chain subunit of the FcγRI and FcγRIII receptors (FcγRI/RIII(−/−)), B6.129S4-Fcgr2btm1TtK N12, a mouse knockout for the inhibitory receptor, FcγRIIb (FcγRIIb(−/−)), and control C57BL/6 wild type (WT) strains were purchased from Taconic Laboratories (Hudson, NY). Swiss Webster mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed under a standard artificial light/dark cycle, with free access to food and water, and under controlled temperature and humidity. Mice were allowed to acclimate to the animal unit for at least a week prior to investigation. Mice were also kept on autoclaved KI-water (0.2 g/L) to block the thyroidal uptake of free iodine, beginning 2 days prior to injection of 125I-MWReg30. All animal protocols were conducted with approval from the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
2.3. Methods
2.3.1. Assessment of MWReg30-mediated thrombocytopenia in C57BL/6, FcγRI/III(−/−), and FcγRIIb(−/−) mice
MWReg30 was administered intravenously to groups of C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice, at doses of 0.05, 0.2 and 0.4 mg/kg, via penile vein injection (n=5–7 mice / dose / strain). Blood samples were collected from the retro-orbital plexus prior to dosing for determination of baseline platelet measurements. Additional samples were obtained at several time points up to 3 days post dosing. Blood samples were collected using ethylenediaminetetraacetic acid coated capillary pipette tubes. Platelet counts were determined using a Cell-Dyn 1700 multi-parameter hematology analyzer (Abbott Laboratories, Abbott Park, IL), normalized by the baseline platelet counts, and reported as a percentage of pretreatment values.
2.3.2. Effect of iodination on MWReg30-mediated thrombocytopenia
MWReg30 was iodinated with 127I (non-radioactive iodine) using the Chloramine-T modified method (Garg and Balthasar, 2007). MWReg30 or 127I- MWReg30, at a dose of 0.2 mg/kg, was injected intravenously via the penile vein into two groups of Swiss Webster mice (6–7 weeks old, n=3/group). Blood samples were collected before treatment and at 1, 3, 6, 9, 24 and 72 h after treatment. Ten μL blood samples were collected from the retro-orbital plexus and/or the submandibular vein using ethylenediaminetetraacetic acid pre-coated capillary pipette tubes. Platelet counts were determined using a Cell-Dyn Emerald (Abbott Laboratories, Abbott Park, IL).
2.3.3. Assessment of MWReg30 plasma pharmacokinetics in C57BL/6, FcγRI/III(−/−), and FcγRIIb(−/−) mice
The pharmacokinetics of MWReg30 mAb were evaluated at 0.04, 0.1, and 0.4 mg/kg in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice (20–22 g). MWReg30 was administered as a mixture of the indicated MWReg30 dose plus a tracer amount (<10% of total dose) of 125I-MWReg30 (~10 μCi/mouse). The mAb was administered intravenously via the penile vein (n= 3–5 mice per dose per strain). Blood samples, ~20–40 μL, were collected from the retro-orbital plexus or from the sub-mandibular vein at 1 h, 3 h, 8 h, and at 1, 2, 4, 7 and 10 days. Plasma was separated, and counted for radioactivity using a gamma counter (LKB Wallac 1272, Wallac, Turku, Finland). Radioactive counts were corrected for decay and background, and MWReg30 plasma concentrations were determined. Of note, in prior work with intravenous administration of 125I-labeled monoclonal antibodies to mice, we have found that more than 95% of plasma and tissue radioactivity is trichloroacetic acid (TCA) precipitable, up to 10 days post injection, supporting the use of 125I-labeling for evaluating mAb pharmacokinetics in mice (Garg and Balthasar, 2007). In the current study, the efficiency of TCA precipitation was evaluated in samples collected on day 14.
2.3.4. Assessment of MWReg30 tissue distribution
Fourteen days following injection of 0.1 mg/kg 125I-MWReg30, 3 mice from each strain were sacrificed. Blood, spleen, kidney, liver, heart, lung, thymus, GI, muscle, bone, fat and skin samples were harvested, and radioactivity was counted. MWReg30 concentrations in excised tissues were determined following correction for background and decay.
2.3.5. Non-compartmental data analysis
Non-compartmental pharmacokinetic analysis (NCA) (WinNonlin 6.1, Phoenix, Pharsight Corporation, Palo Alto, CA) was used to analyze MWReg30 plasma concentration vs. time data. MWReg30 plasma clearance (CL), mean areas under the concentration vs. time curves (AUC0–10days), and volume of distribution at steady-state (Vss) were obtained. For each dose, 3–5 mice per strain were used. The pharmacokinetic parameters were reported as mean ± standard deviation (SD). MWReg30 tissue to blood concentration ratios were determined, and the mean of the ratios and SD were reported.
2.3.6. Analysis of effect of dose on MWReg30-mediated thrombocytopenia
To characterize the induction of thrombocytopenia across the strains, the relationship between the % change in platelet counts at the nadir vs. MWReg30 dose was fitted to the Hill equation (equation 1), with use of mixed-effect modeling with NONMEM (ICON Development Solutions Ellicott City, Maryland). Due to the sparseness of the data, the first-order estimation method was employed. The magnitude of unexplained inter-animal variability in model parameters, as well as the magnitude of unexplained residual variability, were estimated. A log-normal distribution was assumed for inter-animal variability of model parameters, and an additive error model was used to describe unexplained residual variability.
| (1) |
In this equation, E represents the % change in the platelet counts at the nadir, E0 is initial change in % platelet counts when no drug was administered, Emax is the maximal achieved effect, and ED50 is the dose associated with 50% change in the platelet counts. Data from all strains were modeled simultaneously. E0 was fixed to zero, assuming no changes when no drug was administered. The effect of strain as a predictor of model parameters was explored. A likelihood ratio test was used to assess statistical significance of each covariate effect within NONMEM® using stepwise forward selection (α = 0.05), followed by stepwise backward elimination (α = 0.001) procedures.
2.3.7. Analysis of MWReg30 plasma pharmacokinetics
One-compartment and two-compartment mammillary models were used to characterize MWReg30 plasma pharmacokinetics in control, FcγRI/III(−/−), and FcγRIIb (−/−) mice. The model with optimal fitting criteria was selected for more data analysis. The structure of the two compartment model is presented in Figure 1. The model consists of a central compartment and a peripheral distribution compartment. MWReg30 elimination is assumed to occur only from the central compartment. Linear distribution to and from the peripheral distribution compartment were assumed. The data from all strains at all dose levels were fit simultaneously using equations 2 and 3.
| (2) |
| (3) |
Fig. 1. Schematic representation of the pharmacokinetic model structure for MWReg30 disposition.
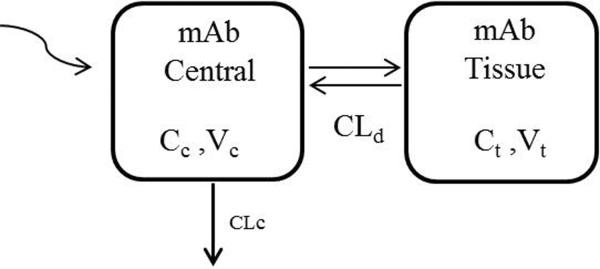
CLc represents linear clearance from the central compartment, and CLd is the distribution clearance between the central and peripheral compartments. Vc and Vt are the volumes of distribution of the central and peripheral compartments.
MWReg30 plasma and tissue concentrations are symbolized as Cc and Ct. CLc represents the elimination clearance, and CLd represents the distribution clearance between the central and peripheral compartments. Vc and Vt are the volumes of distribution for the central and tissue compartments. Model fitting was performed using a population nonlinear mixed effect modeling approach in NONMEM 7. The first order conditional estimation method, ADVAN3, and TRANS4 were used. Structural model parameters, the magnitude of inter-animal variability in these parameters, and the magnitude of residual variability were estimated. Incorporation of inter-animal variability was evaluated for all model parameters. Inter-animal variability was described using an exponential variance model:
| (4) |
Pj and Ppop represent parameters for the jth animal and the typical animal value, ηj is the inter-animal variability in the jth animal, with a normal distribution around 0 and variance of ω2. The exponential model assumes a log-normal distribution of the parameters. Residual variability was described using a constant coefficient of variation (proportional) error model:
| (5) |
Cij represents the measured plasma concentration at the ith time-point in the jth animal; is the model predicted MWReg30 plasma concentration at the ith time-point in the jth animal, and Eij is a random variable representing discrepancy between the ith measurement in the jth animal and the predicted value. Eij is assumed to be normally distributed with a mean of 0 and variance of σ2prop. The precision of the parameter estimates were expressed as the standard error of the mean (SEM). Covariate analyses were performed on all model parameters using forward selection and backward elimination methods. An additive shift was used to describe the relationship between strain and the relevant pharmacokinetics parameter. A 3.84 drop in the mean value of objective function (MVOF) was required to compare two nested models. This value corresponds to a p-value of 0.05, for the addition of a single parameter, based on a χ2–distribution. In the forward selection, the covariate that was associated with the most significant change in the MVOF was included to form the new base covariate model. This process was repeated until there were no further covariates that produced significant changes in the MVOF. Visual predictive checks (VPC) were used to evaluate whether the fixed and random effects of the final model adequately described the observed pharmacokinetic data. VPCs are based on simulations of model predictions, conditional on fixed and random effect final estimates, including significant covariates, if any. Identical dosing and sampling schemes for all dose groups were used for simulations. 500 replicates of the analysis dataset design and dosing schemes were simulated. The simulated population median and the 5th and 95th percentiles (90% prediction interval) were calculated. The observed concentrations and the population prediction interval were overlaid to allow visual comparisons of the central tendencies for observed and predicted data. Simulation-based plots were stratified by dose.
2.3.8. Statistical analysis
The NCA-calculated pharmacokinetic parameters and concentration ratios in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice were statistically compared using one-way Analysis of Variance (ANOVA). A significance level of α = 0.05 was assumed (Graph Pad Prism 5, Graph Pad, San Diego, CA). Bonferroni's correction was used to allow multiple comparisons of different groups (C57BL/6 vs. /RIII(−/−) and C57BL/6 vs. FcγRIIb(−/−)). Student's t-test was also used with α = 0.05.
3. Results
3.1. MWReg30-mediated thrombocytopenia
Treatment of wild-type and FcγR knockout strains with MWReg30 (0.05–0.4 mg/kg) resulted in dose-dependent thrombocytopenia in all strains. The maximal reduction of platelet counts was reached within 3–12 h post dosing. Platelet counts returned to baseline levels within 3 days post dosing (Figure 2). The % reduction in platelet counts at nadir, relative to baseline, differed between the stains and across the dose levels. The reduction in platelet counts at 0.4 mg/kg was ~93%, 75%, and 61% for FcγRIIb(−/−) mice, control mice, and FcγRI/RIII(−/−) mice. The extent of induced thrombocytopenia was much less severe for the FcγRI/RIII(−/−) mice relative to the other strains, at all doses (Figure 2). Nadir % platelet counts were 28.6±5.0, 88.7±16.6, and 25.3±6.1 % (p<0.0001) at 0.05 mg/kg, 28.4±13.7, 56.7±5.1, and 20.6±9.5% (p<0.0001) at 0.2 mg/kg, and 24.9±7.2, 38.7±7.5, and 7.4±2.2% (p<0.0001) at 0.4 mg/kg. A significant difference in the magnitude of thrombocytopenia between C57BL6 and FcγRIIb(−/−) mice was observed at the 0.4 mg/kg dose, but not at 0.5 or 0.1 mg/kg doses.
Fig. 2. MWReg30-mediated thrombocytopenia in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice.
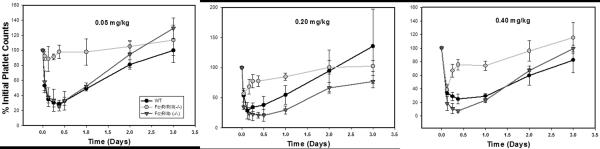
Platelet count data are presented following MWReg30 doses of 0.05 mg/kg, 0.2 mg/kg, and 0.4 mg/kg, administered to C57BL/6 wild-type mice (WT), FcγRI/RIII(−/−) mice, and FcγRIIb(−/−) mice. Platelet counts were determined at several time points up to 3 days, and normalized by the baseline platelet counts. Symbols represent the mean % platelet counts, relative to pretreatment values, and error bars represent the standard deviation about the mean (n=5–7 mice per strain per dose). Relative to wild-type mice, FcγRI/RIII(−/−) mice demonstrated reduced sensitivity to MWReg30 and FcγRIIb(−/−) mice demonstrated increased sensitivity to MWReg30.
At the end of forward covariate selection, strain was a significant predictor of both ED50 and Emax (p-value <0.00001). However, at the end of backward elimination (α = 0.001), only ED50 was shown to exhibit a significant relationship with strain (p = 0.0043). The final multivariable model-fitted Emax and ED50 values are summarized in Table 1. Emax and ED50 parameters were estimated with good precision. The FcγRIIb(−/−) strain was most sensitive to MWReg30 treatment (ED50 =0.00232 mg/kg), followed by the wild type strain (ED50 =0.00854 mg/kg), and then the FcγRI/RIII(−/−) strain (ED50 =0.156 mg/kg).
Table 1.
Parameter estimates for analysis of relationships between MWReg30 dose and nadir platelet counts
| Parameter (unit) | Definition | Estimate | %SEM |
|---|---|---|---|
| Emax (%) | Maximal achieved effect | 81.0 | 3.3 |
| ED50 (mg/kg) (wild type) | The dose associated with 50% change in effect | 0.00854 | 39.4 |
| Additive shift in ED50 for FcγRIRIII(−/−) | +0.147 | 17.2 | |
| Additive shift in ED50 for FcγRIIb(−/−) | −0.00622 | 51.0 | |
| Inter-animal Variability | |||
| ! 2Emax | Variance of inter-animal variability for Emax | 0.008 (8.9 %CV) | 32.6 |
| ! 2ED50 | Variance of inter-animal variability for ED50 | 0.212 (46.0 %CV) | 39.9 |
| Residual variability | |||
| σ | Variance of residual error | 0.0062 (7.7 % CV) | 31.1 |
Minimum value of the objective function= 258.229
3.2. Effect of iodination on MWReg30-mediated thrombocytopenia
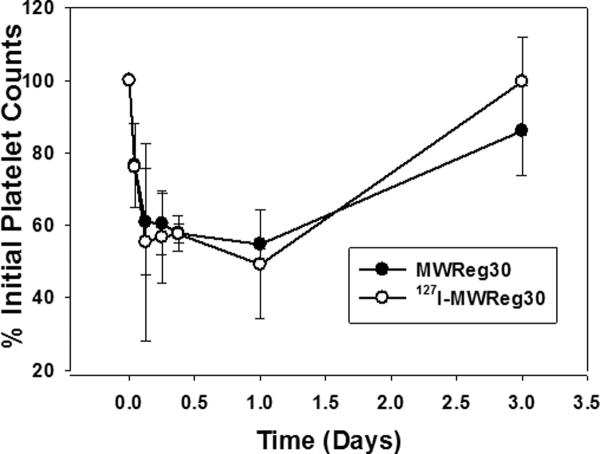
Iodination of MWReg30 did not affect MWReg30-induced thrombocytopenia in mice. As shown in Figure 3, treating groups of Swiss Webster mice with 0.2 mg/kg 127I-MWReg30 or 0.2 mg/kg MWReg30 resulted in very similar effects on platelet counts. Nadir % initial platelet counts were 49.2±14.9% vs. 54.7±1.4% (p=0.56) and raw platelet counts were 554±221 k/μL vs. 591±29 k/μL, p=0.79), for 127I-MWReg30 vs. MWReg30. Platelet counts returned to baseline within 3 days post dosing. The areas of thrombocytopenia were 94.6±12.5 vs. 99.5±9.99 (% × day) (p=0.62) for the 127I-MWReg30 and MWReg30 treated groups. This validation that iodination does not influence MWReg30-mediated thrombocytopenia enabled confident use of 125I-MWReg30 for characterizing of MWReg30 pharmacokinetics and tissue distribution, minimizing concerns of the effect of iodination on MWReg30 binding or function.
Fig. 3. Effects of iodination on MWReg30-mediated thrombocytopenia.
Swiss Webster mice were treated with 0.2 mg/kg MWReg30 or 127I-MWReg30. Platelet counts were normalized by the baseline platelet counts. Symbols represent the mean % platelet counts, relative to pretreatment values, and error bars represent the standard deviation about the mean (n=3 mice per group). The two profiles are essentially superimposed. Iodination did not affect MWReg30 induced thrombocytopenia.
3.3. MWReg30 pharmacokinetics and tissue disposition
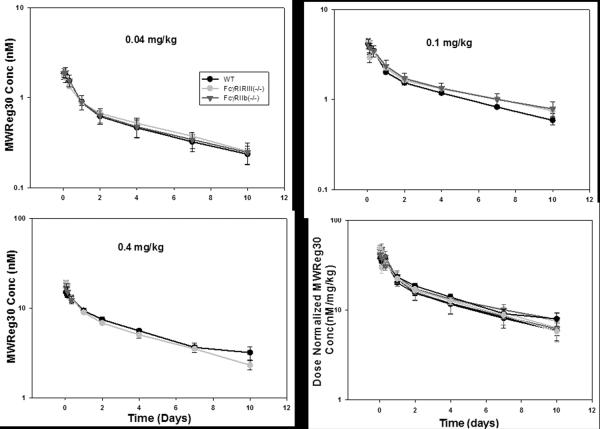
MWReg30 plasma concentration vs. time profiles are illustrated in Figure 4. Biexponential disposition of MWReg30 was observed in all strains, at each dose level. MWReg30 plasma concentration increased in direct proportion with dose, indicating linear pharmacokinetics (Figure 4). No significant differences in dose-normalized AUC and NCA-estimated pharmacokinetics parameters were observed (Table 2). Plasma AUC0–10days ±SD (nM×d) for MWReg30 were: 5.24±0.68, 5.51±0.24, and 5.39±1.05 at 0.04 mg/kg and 12.7±0.5, 13.6±1.1, and 14.5±2.0 at 0.1 mg/kg in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice. MWReg30 plasma clearance values (mL/day/kg) were 31.4±6.0, 29.7±1.5, 30.5±6.4 at 0.04 mg/kg and 37.5±3.4, 36.0±12.0, 28.9±5.4 at 0.1 mg/kg in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice. All mice from the FcγRIIb(−/−) 0.4 mg/kg group died within 24h post injection, consistent with hyper-sensitivity to MWReg30 in this strain. Therefore, no pharmacokinetic parameters were reported for this strain at the 0.4 mg/kg dose.
Fig. 4. MWReg30 plasma pharmacokinetics.
125I-MWReg30was injected intravenously at 0.04, 0.1, and 0.4 mg/kg into C57BL/6 wild type (WT), FcγRI/RIII(−/−), and FcγRIIb(−/−) mice. MWReg30 mean plasma concentrations (nM) following doses of 0.04, 0.1, and 0.4 mg/kg are represented by symbols. Error bars indicate the standard deviation associated with each mean concentration (n=3–5/dose/strain). The plot depicts the dose-normalized MWReg30 concentration (nM/mg/kg) vs. time profiles at 0.04, 0.1, and 0.4 mg/kg in all mouse strains at all doses. The symbols represent mean dose normalized MWReg30 concentrations. Error bars represent the standard deviation associated with mean concentration/dose. Relative to results found in control mice, MWReg30 pharmacokinetics were not altered in FcγRI/RIII(−/−) mice or in FcγRIIb(−/−) mice.
Table 2.
Non-compartmental analysis of MWReg30 plasma concentration-time data in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice
| Parametera | Group | 0.04 (mg/kg) | 0.1 (mg/kg) | 0.4(mg/kg) |
|---|---|---|---|---|
| CL (mL/day/kg) | C57BL/6 | 31.4 ± 6.0 | 37.5 ± 3.4 | 26.0 ± 4.0 |
| FcγRI/RIII (−/−) | 29.7± 1.5 | 36.0 ± 12.0 | 31.5 ± 2.0 | |
| FcγRIIb (−/−) | 30.5 ± 6.4 | 28.9 ±5.4 | NAb | |
| P-value | 0.884 | 0.308 | 0.104 | |
| AUC0–10days (nM×day) | C57BL/6 | 5.24 ± 0.68 | 12.7 ± 0.5 | 57.5 ± 3.0 |
| FcγRI/RIII (−/−) | 5.51 ± 0.24 | 13.6 ± 1.1 | 53.9 ±1.3 | |
| FcγRIIb (−/−) | 5.39 ± 1.05 | 14.5 ± 2.0 | NA | |
| P-value | 0.86 | 0.235 | 0.132 | |
| Vss (mL/kg) | C57BL/6 | 243 ± 25 | 291 ± 11 | 275 ± 23 |
| FcγRI/RIII (−/−) | 229 ± 28 | 266 ± 71 | 197 ± 50 | |
| FcγRIIb (−/−) | 244 ± 43 | 301 ± 42 | NA | |
| P-value | 0.755 | 0.594 | 0.054 |
Parameter values are listed as mean ± standard deviation (n=3–5). % Extrapolated AUC values were less than 25% for all strains at all dose level. P-value of <0.05 was considered significant.
Mice died within 24 h post MWReg30 injection.
Comparison of tissue to blood concentration ratios did not show any significant differences in the tissue distribution of MWReg30 between C57BL/6, FcγRI/RIII(−/−), and FcγRIIb(−/−) mice (Figure 5). Higher concentrations of MWReg30 were observed in highly perfused organs and organs associated with “leaky” vasculature (i.e., spleen, lung); lower concentrations were found for organs with low rates of perfusion (muscle, fat, and thymus). In organs associated with cell-types expressing FcγR, no significant differences in MWReg30 concentrations were observed. For example, tissue:blood concentration ratios were 0.541±0.086, 0.56±0.0839, and 0.4391±0.0371 (p= 0.169) for the spleen, 0.203±0.049, 0.531±0.281, and 0.146±0.0186 (p=0.054) for the liver, 0.369±0.0182, 0.381±0.0145, and 0.438±0.068 (p= 0.411) for the lung, 0.155±0.05, 0.143±0.006, and 0.144±0.007 (p= 0.868) for the bone and 0.129±0.014, 0.166±0.03, and 0.138±0.053(p= 0.411) for the thymus, in C57BL/6, FcγRI/RIII(−/−), and FcγRIIb (−/−) mice. More than 95% of the radioactivity associated with collected blood samples on day 14 was precipitated with treatment of plasma with TCA, thus indicating that the plasma samples were free from significant quantities of low-molecular weight radiolabeled catabolites.
Fig. 5. MWReg30 tissue distribution.
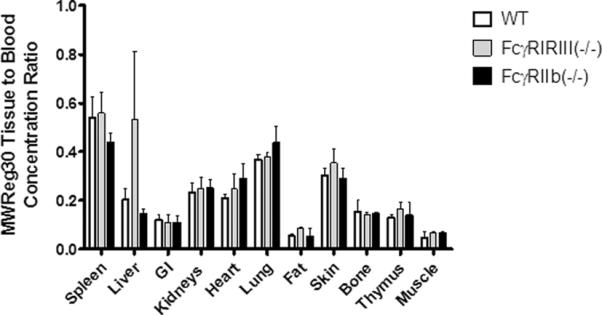
MWReg30 tissue to blood concentration ratios were determined following necropsy, 14 days after administration of 0.1 mg/kg MWReg30 to C57BL/6 mice (WT), FcγRI/RIII(−/−) mice, and FcγRIIb(−/−) mice. Bars represent mean concentration ratios and the error bars indicate the standard deviation of the ratios. Ratios were compared statistically using one way ANOVA with Bonferroni's multiple comparison. Relative to results found in control mice, MWReg30 tissue concentrations were not altered in FcγRI/RIII(−/−) mice or in FcγRIIb(−/−) mice.
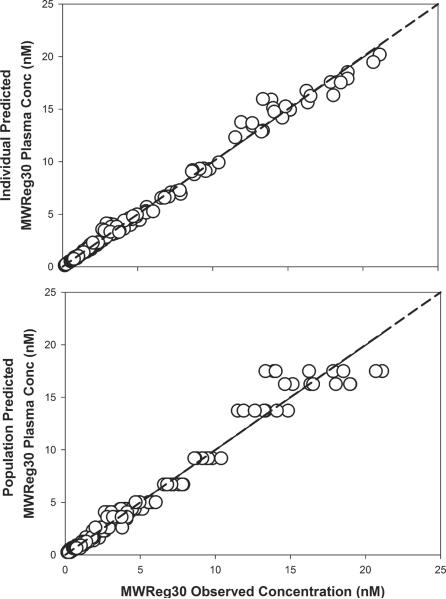
MWReg30 plasma data were characterized using the base model structure shown in Figure 1. The final model parameter estimates are reported in Table 3. All model parameters were estimated with moderate to high precision. Based on the covariate forward selection analysis, strain was not a significant predictor of any of the tested pharmacokinetic parameters. Hence, one estimate for each parameter was considered for all strains in the final model. Goodness-of-fit plots for MWReg30 plasma data are shown in Figure 6. As shown in the figure, the model described the data reasonably well. The model predicted and observed data were along the line of unity. Visual predictive check plots are shown in Figure 7. For all dose levels, the observed data were well captured by the simulated prediction interval. The observed data were in line with the population predicted median concentrations at all doses, in all strains.
Table 3.
Parameter estimates for the final pharmacokinetic model
| Parameter (unit) | Definition | Estimate | %SEM |
|---|---|---|---|
| Vc (L/kg) | Central volume of distribution | 0.147 | 2.8 |
| Vt (L/kg) | Volume of tissue compartment | 0.143 | 2.0 |
| CLd (L/day/kg) | Distribution clearance | 0.101 | 3.9 |
| CLc (L/day/kg) | Apparent clearance | 0.0343 | 5.6 |
| Inter-animal Variability | |||
| ! 2Vc | Variance of inter-animal variability for Vc | 0.0218 (14.8 %CV) | 39.9 |
| ! 2CLd | Variance of inter-animal variability for CLd | 0.0114 (10.7 %CV) | 28.2 |
| ! 2CLd | Variance of inter-animal variability for CLc | 0.208 (38.0 %CV) | 76.0 |
| Proportional residual variability | |||
| σprop | Variance of proportional residual error | 0.0068 | 14.6 |
Minimum value of the objective function=−586.012
Fig. 6. Goodness-of-fit plots for MWReg30 plasma concentrations.
Scatter plots and regression lines (solid) for MWReg30 observed and predicted concentrations in plasma. Shown are MWReg30 plasma individual predictions vs. observed values (r2=0.99), and MWReg30 plasma population predictions vs. observed values (r2=0.97). The dotted lines represent the line of identity.
Fig. 7. Visual predictive check.
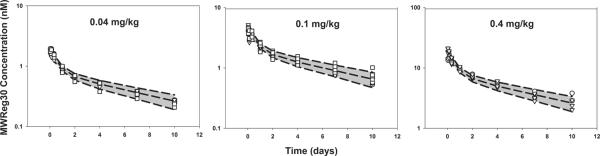
Visual predictive check were performed using data simulated with the final pharmacokinetic model. The shaded area represents the confidence interval (CI) for the 5th,50th, and 95th percentiles of the simulated data. Observed data are shown for (∘) wild type mice, (!) FcγRI/RIII(−/−) mice, and (□) FcγRIIb(−/−) mice, following doses of 0.04, 0.1, 0.4 mg/kg.
4. Discussion
The main objective of the current work was to evaluate the role of FcγR on the pharmacokinetics and pharmacodynamics of MWReg30, an anti-platelet antibody, through the use of FcγR-knockout mice. FcγR knockout mice have been previously employed to characterize the role of FcγR in the initiation or in the inhibition of immunomodulatory effects of antibodies (Nimmerjahn and Ravetch, 2006, 2008; Siberil et al., 2007; Tarasenko et al., 2007). Knocking out FcγR receptors was found to abrogate effector cell functions in vitro and immune responses in vivo (Gessner et al., 1998; Takai et al., 1994). The FcγR-dependent phagocytosis of IgG1 was found to be muted in γ chain and FcγRIII knockout strains.
MWReg30 is a rat IgG1 antibody that binds to mice glycoprotein (GP) IIb (CD41). GPIIb is associated with GPIIIa, forming the GPIIb/IIIa complex in mice. The complex is important in platelet adhesion and aggregation. GPIIb is expressed on platelets, megakaryocytes, hematopoietic progenitors, bone marrow cells, and mast cells (Berlanga et al., 2005; Soligo et al., 1989). The interaction of MWReg30 with GPIIb on platelets led to a dose proportional increase in platelet destruction, with substantial differences observed between the control mice and the knockout strains. Population modeling demonstrated that the FcγRIIb(−/−) mice are more sensitive to MWReg30-induced thrombocytopenia, relative to C57BL/6 or the FcγRI/RIII(−/−) mice. Approximately 67- and 3.6-fold higher MWReg30 doses are required to achieve the 50% inhibition in the FcγRI/RIII(−/−) and C57BL6 mice, compared to FcγRIIb(−/−) mice.
Assessment of the MWReg30 plasma pharmacokinetics and tissue distribution, across all strains, failed to demonstrate a significant influence of FcγR on MWReg30 disposition. MWReg30 demonstrated linear pharmacokinetics, with clear dose-proportionality across the dose-range that was investigated. There were no signs indicating target-mediated mAb elimination, and MWReg30 tissue concentrations were very similar across the mouse strains. Population modeling, with a two compartment model, was employed to characterize the plasma concentration vs. time data. Covariate analysis with forward selection showed that strain was not a significant predictor of inter-animal variability in any of the model parameters. Model parameters were all estimated with high precision (%SEM < 10%).
The profound thrombocytopenia induced by MWReg30, and the lack of target-mediated elimination, indicates that only a small fraction of the MWReg30 dose is eliminated with the destruction of platelets. Other pathways of antibody elimination (e.g., catabolism of unbound MWReg30 in vascular endothelieal cells) (Lobo et al., 2004; Wang et al., 2008) are likely to be much more significant relative to the rate of mAb elimination through FcγR-mediated phagocytosis of opsonized platelets. Of note, it is possible that following phagocytosis of opsonized platelets, MWReg30 dissociates from GPIIb, and is “recycled” to plasma via the IgG protection receptor, FcRn (Chaparro-Riggers et al., 2012; Igawa et al., 2010; Junghans and Anderson, 1996).
The findings from this work showed that FcγR knockout significantly affected the degree of anti-platelet antibody induced thrombocytopenia; however, FcγR knockout did not affect the plasma or tissue disposition of MWReg30. The lack of effect of FcγR knockout on MWReg30 pharmacokinetics is consistent with our earlier report that demonstrated a lack of influence of FcγR knockout on the pharmacokinetics of 8C2, a monoclonal IgG1 antibody with high affinity for a soluble ligand (topotecan) (Abuqayyas and Balthasar, 2012, in press). However, based solely on the results obtained with MWReg30 and 8C2, it is not prudent to conclude that FcγR are unimportant as determinants of mAb pharmacokinetics in all cases (i.e., for every monoclonal antibody). Nonetheless, our results do provide strong support for the converse argument. As illustrated with 8C2 and MWReg30, it is clear that FcγR are not important contributors to the pharmacokinetics of all IgG mAb.
Acknowledgments
This work was supported by funding from the Center for Protein Therapeutics at the State University of New York University at Buffalo, and by NIH grant HL67437.
Abbreviations
- IgG
Immunoglobulin G
- FcγR
Fc receptors for IgG
- ITP
immune thrombocytopenic purpura
- mAbs
monoclonal antibodies
- PK
pharmacokinetics
- PD
pharmacodynamics
- AUC
area under the concentration vs. time profiles
- CL
clearance
- Vss
volume of distribution at steady-state
- NCA
noncompartmental pharmacokinetic analysis
- MVOF
mean value of objective function
- VPC
visual predictive checks
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuqayyas L, Balthasar JP. Application of Knockout Mouse Models to Investigate the Influence of Fc<gamma>R on the Tissue Distribution and Elimination of 8C2, a Murine IgG1 Monoclonal Antibody. International Journal of Pharmaceutics. 2012 doi: 10.1016/j.ijpharm.2012.09.042. in press. [DOI] [PubMed] [Google Scholar]
- Amigorena S, Bonnerot C. Fc receptor signaling and trafficking: a connection for antigen processing. Immunol. Rev. 1999a;172:279–284. doi: 10.1111/j.1600-065x.1999.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Amigorena S, Bonnerot C. Fc receptors for IgG and antigen presentation on MHC class I and class II molecules. Semin. Immunol. 1999b;11:385–390. doi: 10.1006/smim.1999.0196. [DOI] [PubMed] [Google Scholar]
- Berlanga O, Emambokus N, Frampton J. GPIIb (CD41) integrin is expressed on mast cells and influences their adhesion properties. Exp. Hematol. 2005;33:403–412. doi: 10.1016/j.exphem.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Bhatia A, Blades S, Cambridge G, Edwards JC. Differential distribution of Fc gamma RIIIa in normal human tissues and co-localization with DAF and fibrillin-1: implications for immunological microenvironments. Immunology. 1998;94:56–63. doi: 10.1046/j.1365-2567.1998.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordessoule D, Jones M, Gatter KC, Mason DY. Immunohistological patterns of myeloid antigens: tissue distribution of CD13, CD14, CD16, CD31, CD36, CD65, CD66 and CD67. Br. J. Haematol. 1993;83:370–383. doi: 10.1111/j.1365-2141.1993.tb04659.x. [DOI] [PubMed] [Google Scholar]
- Cassel DL, Keller MA, Surrey S, Schwartz E, Schreiber AD, Rappaport EF, McKenzie SE. Differential expression of Fc gamma RIIA, Fc gamma RIIB and Fc gamma RIIC in hematopoietic cells: analysis of transcripts. Mol. Immunol. 1993;30:451–460. doi: 10.1016/0161-5890(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Chaparro-Riggers J, Liang H, Devay RM, Bai L, Sutton JE, Chen W, Geng T, Lindquist K, Casas MG, Boustany LM, Brown CL, Chabot J, Gomes B, Garzone P, Rossi A, Strop P, Shelton D, Pons J, Rajpal A. Increasing Serum Half-life and Extending Cholesterol Lowering in Vivo by Engineering Antibody with pH-sensitive Binding to PCSK9. J Biol Chem. 2012;287:11090–11097. doi: 10.1074/jbc.M111.319764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N. Engl. J. Med. 2002;346:995–1008. doi: 10.1056/NEJMra010501. [DOI] [PubMed] [Google Scholar]
- Deng R, Balthasar JP. Pharmacokinetic/pharmacodynamic modeling of IVIG effects in a murine model of immune thrombocytopenia. J. Pharm. Sci. 2007;96:1625–1637. doi: 10.1002/jps.20828. [DOI] [PubMed] [Google Scholar]
- Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J. Pharmacokinet. Pharmacodyn. 2007;34:687–709. doi: 10.1007/s10928-007-9065-1. [DOI] [PubMed] [Google Scholar]
- Gessner JE, Heiken H, Tamm A, Schmidt RE. The IgG Fc receptor family. Ann. Hematol. 1998;76:231–248. doi: 10.1007/s002770050396. [DOI] [PubMed] [Google Scholar]
- Igawa T, Ishii S, Tachibana T, Maeda A, Higuchi Y, Shimaoka S, Moriyama C, Watanabe T, Takubo R, Doi Y, Wakabayashi T, Hayasaka A, Kadono S, Miyazaki T, Haraya K, Sekimori Y, Kojima T, Nabuchi Y, Aso Y, Kawabe Y, Hattori K. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol. 2010;28:1203–1207. doi: 10.1038/nbt.1691. [DOI] [PubMed] [Google Scholar]
- Ivan E, Colovai AI. Human Fc receptors: critical targets in the treatment of autoimmune diseases and transplant rejections. Hum. Immunol. 2006;67:479–491. doi: 10.1016/j.humimm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. Journal of Pharmaceutical Sciences. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- McDonagh CF, Kim KM, Turcott E, Brown LL, Westendorf L, Feist T, Sussman D, Stone I, Anderson M, Miyamoto J, Lyon R, Alley SC, Gerber HP, Carter PJ. Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index. Mol. Cancer Ther. 2008;7:2913–2923. doi: 10.1158/1535-7163.MCT-08-0295. [DOI] [PubMed] [Google Scholar]
- Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010;24:23–39. doi: 10.2165/11530560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Nishio S, Yamamoto T, Kaneko K, Tanaka-Matsumoto N, Muraoka S, Kaburaki M, Kusunoki Y, Takagi K, Kawai S. Pharmacokinetic study and Fcgamma receptor gene analysis in two patients with rheumatoid arthritis controlled by low-dose infliximab. Mod. Rheumatol. 2009;19:329–333. doi: 10.1007/s10165-009-0158-0. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu. Rev. Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Siberil S, Dutertre CA, Fridman WH, Teillaud JL. FcgammaR: The key to optimize therapeutic antibodies? Crit. Rev. Oncol. Hematol. 2007;62:26–33. doi: 10.1016/j.critrevonc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Soligo D, Cattoretti G, Colombi M, Polli N, Capsoni F, Rilke F, Deliliers GL. Bone marrow and tissue expression of gpIIb/IIIa, LFA-1, Mac-1 and gp150,95 glycoproteins. Eur. J. Haematol. 1989;42:173–181. doi: 10.1111/j.1600-0609.1989.tb01207.x. [DOI] [PubMed] [Google Scholar]
- Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12:33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov. Today. 2006;11:81–88. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Tarasenko T, Dean JA, Bolland S. FcgammaRIIB as a modulator of autoimmune disease susceptibility. Autoimmunity. 2007;40:409–417. doi: 10.1080/08916930701464665. [DOI] [PubMed] [Google Scholar]
- Tuijnman WB, Van Wichen DF, Schuurman HJ. Tissue distribution of human IgG Fc receptors CD16, CD32 and CD64: an immunohistochemical study. APMIS. 1993;101:319–329. doi: 10.1111/j.1699-0463.1993.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol. Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen EF, Van der Ven JT, Engelfriet CP, Von Dem Borne AE. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood. 1982;59:23–26. [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- Zuckier LS, Georgescu L, Chang CJ, Scharff MD, Morrison SL. The use of severe combined immunodeficiency mice to study the metabolism of human immunoglobulin G. Cancer. 1994;73:794–799. doi: 10.1002/1097-0142(19940201)73:3+<794::aid-cncr2820731308>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]