Summary
Recent developments in materials, surface modifications, separation schemes, detection systems, and associated instrumentation have allowed significant advances in the performance of lab-on-a-chip devices. These devices, also referred to as micro total analysis systems (µTAS), offer great versatility, high throughput, short analysis time, low cost, and more importantly, performance that is comparable to standard bench-top instrumentation. To date, µTAS have demonstrated advantages in a significant number of fields including biochemical, pharmaceutical, military, and environmental. Perhaps most importantly, µTAS represent excellent platforms to introduce students to microfabrication and nanotechnology, bridging chemistry with other fields such as engineering and biology, enabling the integration of various skills and curricular concepts. Considering the advantages of the technology and the potential impact to society, our research program aims to address the need for simpler, more affordable, faster, and portable devices to measure biologically-active compounds. Specifically, the program is focused on the development and characterization of a series of novel strategies towards the realization of integrated microanalytical devices. One key aspect of our research projects is that the developed analytical strategies must be compatible with each other, therefore enabling their use in integrated devices. The program combines spectroscopy, surface chemistry, capillary electrophoresis, electrochemical detection, and nanomaterials. This article discusses some of the most recent results obtained in two main areas of emphasis:
-
▪
Capillary Electrophoresis, Microchip-CE, Electrochemical Detection, and
-
▪
Interaction of Proteins with Nanomaterials
Key Terms: Capillary electrophoresis, Microfluidics, Spectroscopic ellipsometry, Adsorption kinetics, Electrochemical detection
Capillary Electrophoresis, Microchip-CE, and Electrochemical Detection
Since its development in the early 80s, capillary electrophoresis (CE) has become one of the most powerful and versatile analytical techniques available. CE encompasses a family of separation techniques driven by an electric potential difference applied across a capillary (< 100 µm ID) filled with an electrolyte solution. When a potential difference is applied across the capillary, bulk flow of the solution is generated by a process referred to as electro-osmotic flow (EOF) and analytes can be electrophoretically separated based on their charge/size ratio, the magnitude of the applied potential, and the solution conditions.
CE offers several advantages when compared to other analytical techniques such as gas chromatography (GC) and high performance liquid chromatography (HPLC). First, a wide variety of compounds ranging from small inorganic ions to large organic molecules can be analyzed with the same instrument and even the same capillary by simply adjusting the composition of the electrolyte solution. Second, the flat flow profile generated inside the capillary (due to the EOF) produces very narrow peaks and highly efficient separations. Third, only nanoliters of sample and microliters of buffer are used in each CE analysis, reducing cost and waste. Fourth, the instrumentation required for CE is relatively simple [1] and does not require the use of mechanical pumps, therefore enabling the development of miniaturized systems. In this way, CE and microchip-CE provide high-speed, high-throughput, highly efficient, and reliable separations that can be performed at the point of interest.
Many detection systems have been integrated to CE and microchip-CE devices. Among them, electrochemical detection (ECD) offers remarkable mass sensitivity (approaching that of fluorescence), inherent miniaturization of both the detector and control instrumentation [1], low cost, minimal power demands, high compatibility with microfabrication technologies, and independence from sample turbidity, optical path length, or substrate clarity. In addition, ECD does not scale linearly with decreasing electrode size, a limitation to most optical methods. Therefore, it is easier to miniaturize the detection elements and still maintain a significant signal level. Furthermore, with the use of microfabrication techniques it is possible to integrate multiple electrodes into a single microchip, thus improving the ability to perform more selective and/or more sensitive detection modes. Finally, electrodes for detection can be fabricated at the same time as electrodes for driving the electrophoresis, making the overall microfabrication process very efficient [2]. In order to evaluate the efficacy of the strategies developed in the lab, a series of phenolic compounds were used as model analytes. Advantageously, phenolic compounds can ionized at moderate pH values (<10), display strong hydrophobic interactions with capillary walls, are both electrochemically and optically active, are commercially available, and are important to a variety of biological processes.
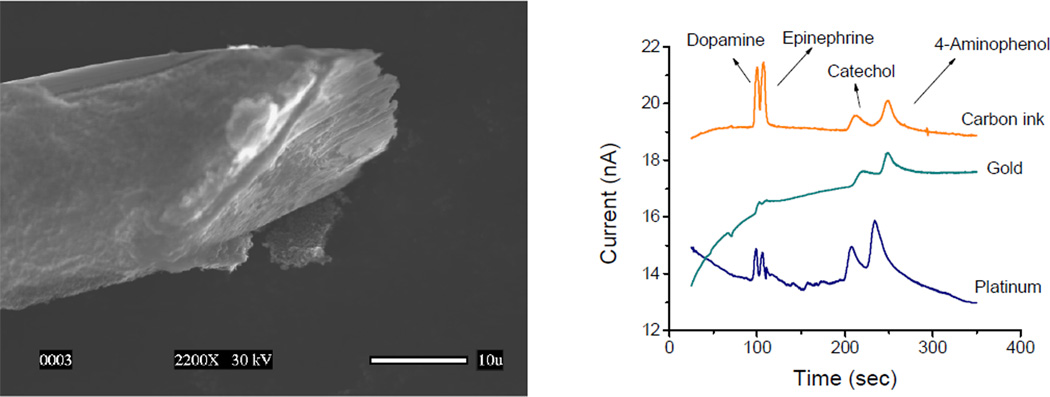
Considering the aforementioned advantages, the first priority was to demonstrate the possibility of analyzing different phenolic compounds using capillary electrophoresis, microchips, and electrochemical detection. This was initially achieved by analyzing three priority pollutants [3], as defined by the US – Environmental Protection Agency. Those studies also allowed to demonstrate that electrochemical detection can be effectively used for the analysis of phenolic compounds, avoiding electrode fouling [4–7]. Similar conditions were used to perform the analysis of other phenolic compounds including acids [8], antioxidants [9], and pharmaceuticals [10]. It is also worth highlighting that the use of carbon-ink electrodes (see Figure 1) enabled the analysis of catecholamines down to nM concentrations, without preconcentration, and using only 1.5 nL of sample [11].
Figure 1.
A) SEM picture of a cylindrical gold electrode coated with 7 layers of carbon ink. B) Electropherograms obtained with carbon-coated, gold, and platinum electrodes for dopamine, epinephrine, catechol, and 4-aminophenol. Adapted from Ref [11].
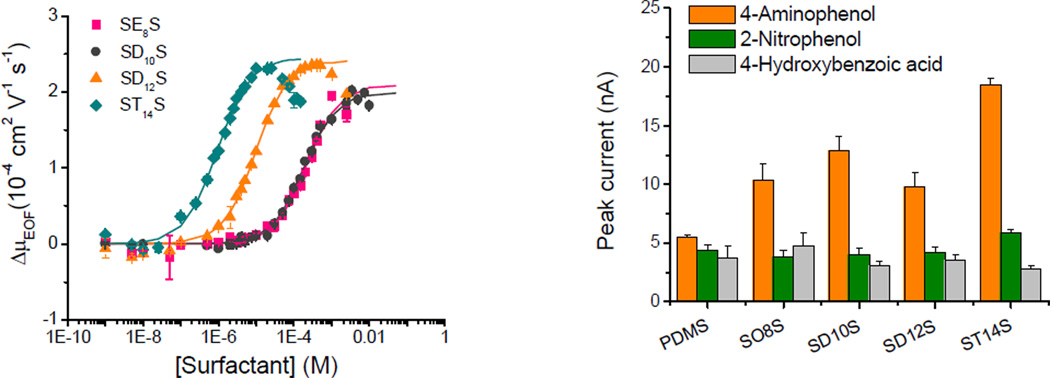
Although highly efficient separations were obtained, decreasing the analyte-wall interactions and enhancing the stability of the EOF was critical to further improve CE and microchip-CE separations. Considering this, our group developed a simple and inexpensive method to coat silica capillaries with montmorillonite, a natural clay [12]. In addition, we studied the adsorption of alkyl surfactants with different chain lengths and head groups to poly(dimethylsiloxane) (PDMS), one of the most commonly used materials to fabricate microchip-CE devices. Based on EOF measurements [13], the affinity constant of different surfactants was derived and related to the corresponding critical micellar concentration. Besides enabling control of the EOF, decreasing the analysis time, and increasing the separation efficiency, the addition of surfactants significantly improved the performance of the electrochemical detection step (Figure 2).
Figure 2.
Effect of sodium 2-ethylhexyl sulfate (–■–), sodium decyl sulfate (–●–), sodium dodecyl sulfate (–▲–), and sodium tetradecyl sulfate (–◆–) on the µEOF and the peak current of different phenolic compounds. Conditions described in Ref.[13,14].
Collaborations with other faculty members have enabled the application of microchips to the analysis of chemical warfare agents [15], the development of the first lab-on-a-robot (Integrated Microchip – Capillary Electrophoresis, Power Supply, Electrochemical Detector, Wireless Unit, and Mobile Platform) [16] and the multivariate evaluation of the separation conditions for five bisphenols [17].
Interaction of Proteins with Nanomaterials
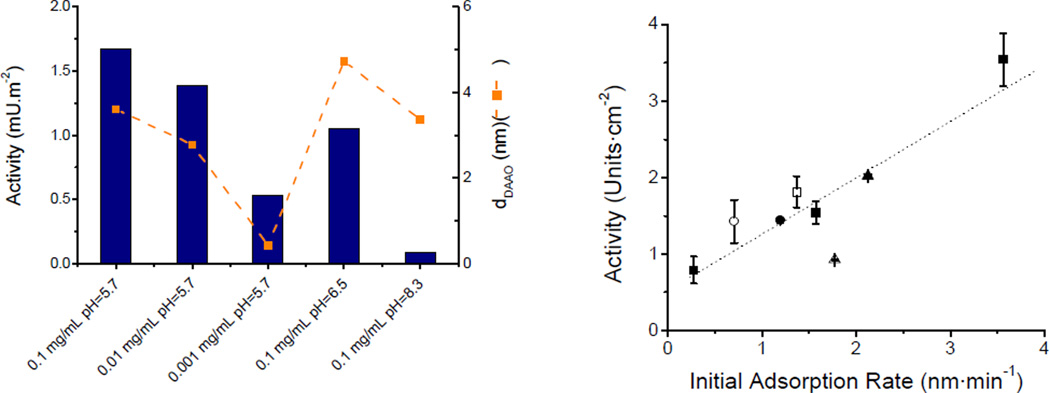
Aiming to improve the selectivity of µchip-CE devices, our group investigated the possibility of integrating an immobilized enzyme reactor (IMER) at the end of the separation channel [18]. Although this modification improved the selectivity of the device, the peak widths were significantly compromised. For that reason, and considering the potential advantages of carbon nanotubes (CNT) [19], our group decided to investigate the possibility of integrating biosensors. The hypothesis of the project was that a clear understanding of the driving forces and consequences of the adsorption process would lead to a more rational way to develop CNT-based sensors. In this regard, laser reflectometry was used to perform the first kinetic study (followed in real time) of the interaction of albumin with transparent films of carbon nanotubes [20]. In order to obtain information related not only to the adsorption process but also the structure, thickness [21], optical constants, and microstructure of films [22], our lab is now focused on the use of variable angle spectroscopic ellipsometry (VASE) [23,24]. VASE enabled the possibility of extending the scope of the project to DNA [25] and other proteins such as albumin [26], D-amino acid oxidase [27], catalase [28], and glucose oxidase [29]. In general, our results demonstrated that the activity of enzymes adsorbed to CNT is not only exclusively proportional to the adsorbed amount of protein (which is the only variable optimized in most systems) but also to the initial adsorption rate (Figure 4). These observations are critical to develop efficient biosensors.
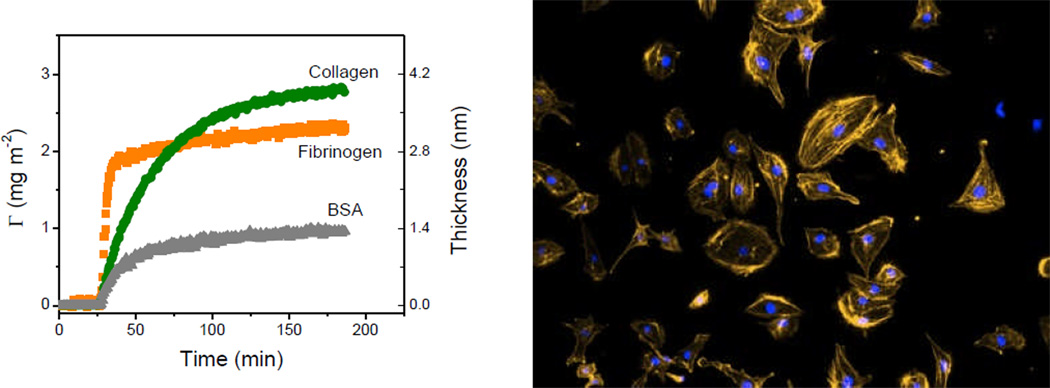
Figure 4.
A)Dynamic adsorption of BSA, fibrinogen, and collagen onto the nanostructured PDMS films. B) Photograph of human endothelial cells on a substrate coated with collagen, under optimum conditions (as selected by adsorption studies). Adapted from Ref [31].
Besides all the well-known advantages of CNT, our lab demonstrated that three-dimensional scaffolds of CNT can significantly increase the amount of enzymes adsorbed per unit area, preserve the catalytic activity of the adsorbed molecules, and provide significant improvements (300%) in the sensitivity of the resulting biosensors [29]. Furthermore, the conclusions of these studies allowed the rational selection of the experimental conditions required to develop a CNT-based biosensor for glutamate with a wide linear range (0.01–10 µM), low detection limit (10 nM, S/N≥3), fast response time (≤5 s), and good stability [30]. More recently, and aiming to provide rational guidelines to improve the biocompatibility of PDMS substrates, the adsorption kinetics of fibrinogen, collagen type-I, and bovine serum albumin to PDMS was investigated [31]. As can be observed in Figure 4, although the three model proteins can be adsorbed to PDMS, different adsorption rates and saturation amounts were obtained indicating that specific conditions are required for the adsorption of each protein. Most importantly, these results demonstrated that the adsorption kinetics can have a major effect on the subsequent adhesion and morphology of cells.
Figure 3.
A) Effect of experimental conditions and B) initial adsorption rate on the enzymatic activity of Damino acid oxidase and catalase, respectively. Conditions as described in references [27] and [28].
Future Perspective.
Clearly, the combination of instrumentation, microfluidics, nanomaterials, and proteins has the potential to provide significant improvements in the analysis of biological molecules. We will continue working in these main areas of research, aiming to provide rational guidelines to select the conditions to perform more efficient sample preparation, separation, and detection steps. Because we ultimately aim to develop microfluidic devices that combine the advantages of different approaches in the lab, one critical aspect of our research projects is that they should be compatible with each other. In addition to these projects, we are starting to develop computational tools to understand the interactions of proteins with solid surfaces at the molecular level. We envision this approach to become one of the most efficient ways to complement the development of analytical devices. Although the current use of nanomaterials and nanostructured films in analytical chemistry is a fairly young approach that mixes art, intuition, and science, many researchers around the world have recognized their utility.
Executive Summary.
-
▪
Research efforts in the µ-Analytical Chemistry group at UTSA have focused on development of novel analytical strategies that can be integrated to microfluidic devices.
-
▪
The final goal is to significantly improve the performance of capillary electrophoresis and microchip-CE devices, particularly when applied to the analysis of biological molecules.
-
▪
The integration of electrochemical methods has allowed reaching nM limits of detection, enabling the application of this technology to target a series of real samples.
-
▪
Complementary information obtained by variable angle spectroscopic ellipsometry has significantly improved the understanding of the driving forces and consequences of the interaction between proteins and nanomaterials.
-
▪
The implications of these results have wide applicability to the development of devices where the activity of a biorecognition element determines the overall performance.
Acknowledgements
The author would like to express the deepest appreciation to all the colleagues who participated in the projects described in this review.
Financial Disclosure
Financial support for these projects has been provided in part by the University of Texas at San Antonio, the National Institutes of Health through the National Institute of General Medical Sciences (1SC3GM081085, 2SC3 GM081085) and the Research Centers at Minority Institutions (G12MD007591). Financial support for an additional project, aimed at providing international training opportunities in microfluidics, has been provided by the National Science Foundation (OISE-0965814).
References
Papers of considerable interest have been highlighted as *
- 1.Felhofer JL, Blanes L, Garcia CD. Recent developments in instrumentation for capillary electrophoresis and microchipcapillary electrophoresis. Electrophoresis. 2010;31:2469–2486. doi: 10.1002/elps.201000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia CD, Henry C. Coupling Electrochemical Detection with Microchip Capillary Electrophoresis. In: Wang W, Soper SA, editors. BioMEMS: Technologies and Applications. Boca Raton, FL: Taylor and Francis Group; 2007. pp. 265–298. [Google Scholar]
- 3.Ding Y, Garcia CD. Pulsed amperometric detection with poly(dimethylsiloxane)-fabricated capillary electrophoresis microchips for the determination of EPA priority pollutants. Analyst. 2006;131(2):208–214. doi: 10.1039/b509405d. [DOI] [PubMed] [Google Scholar]
- 4.García CD, De Pauli CP, Ortiz PI. Electrochemical characterization of glassy carbon electrodes modified by resol mixtures. J. Electroanal. Chem. 2001;510(1–2):115–119. [Google Scholar]
- 5.Garcia CD, Ortiz PI. BHA and TBHQ quantification in cosmetic samples. Electroanalysis. 2000;12(13):1074–1076. [Google Scholar]
- 6.García CD, Ortiz PI. Glassy carbon electrodes modified with different electropolymerized resol prepolymer mixtures for phenol and derivatives quantification. Anal. Sci. 1999;15(5):461–465. [Google Scholar]
- 7.García G, García CD, Ortiz PI, De Pauli CP. Reflectometry applied to electrochemically generated phenoxy radical adsorption monitoring. J. Electroanal. Chem. 2002;519(1–2):53–59. [Google Scholar]
- 8.Ding Y, Garcia CD. Application of microchip - capillary electrophoresis to follow the degradation of phenolic acids by aquatic plants. Electrophoresis. 2006;27(24):5119–5127. doi: 10.1002/elps.200600081. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Mora MF, Garcia CD. Analysis of alkyl gallates and nordihydroguaiaretic acid using plastic capillary electrophoresis - microchips. Anal. Chim. Acta. 2006;561(1–2):126–132. [Google Scholar]
- 10.Ding Y, García CD. Determination of Non-Steroidal Anti-Inflammatory Drugs in Serum by Capillary Electrophoresis Microchip and Electrochemical Detection. Electroanalysis. 2006;18(22):2202–2209. [Google Scholar]
- 11.Ding Y, Ayon A, Garcia CD. Electrochemical detection of phenolic compounds using cylindrical carbon-ink electrodes and microchip capillary electrophoresis. Anal. Chim. Acta. 2007;584(2):244–251. doi: 10.1016/j.aca.2006.11.064. [DOI] [PubMed] [Google Scholar]
- 12.Mora MF, Garcia CD. Electrophoretic separation of phenolic contaminants using fused silica capillaries coated with montmorillonite. Electrophoresis. 2007;28(8):1197–1203. doi: 10.1002/elps.200600493. [DOI] [PubMed] [Google Scholar]
- 13.Mora MF, Giacomelli CE, Garcia CD. Electrophoretic Effects of the Adsorption of Anionic Surfactants to Poly(dimethylsiloxane) – Coated Capillaries. Anal. Chem. 2007;79:6675–6681. doi: 10.1021/ac070953g. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Mora MF, Merrill GN, Garcia CD. The Effects of Alkyl Sulfates on the Analysis of Phenolic Compounds by Microchip Capillary Electrophoresis with Pulsed Amperometric Detection. Analyst. 2007;132(10):997–1004. doi: 10.1039/b704364c. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Rogers K, Garcia CD. Poly(dimethylsiloxane) Microchip Electrophoresis with Contactless Conductivity Detection for Measurement of Chemical Warfare Agent Degradation Products. Anal. Lett. 2008;41(2):335–350. [Google Scholar]
- 16.Berg C, Valdez DC, Bergeron P, Mora MF, Garcia CD, Ayon A. Lab-on-a-Robot: Integrated Microchip – Capillary Electrophoresis, Power supply, Potentiostat, Wireless Unit, and Controller Circuitry. Electrophoresis. 2008;(29):4914–4921. doi: 10.1002/elps.200800215. [DOI] [PubMed] [Google Scholar]
- 17.Felhofer J, Hanrahan G, García CD. Multivariate versus Univariate Optimization of Separation Conditions in Micellar Electrokinetic Chromatography. Talanta. 2008 doi: 10.1016/j.talanta.2008.08.016. in press. [DOI] [PubMed] [Google Scholar]
- 18.Blanes L, Mora Maria F, do Lago Claudimir L, Ayon A, Garcia Carlos D. Lab-on-a-Chip Biosensor for Glucose Based on a Packed Immobilized Enzyme Reactor. Electroanalysis. 2007;19(23):2451–2456. [Google Scholar]
- 19.Scida K, Stege PW, Haby G, Messina GA, Garcia CD. Recent Applications of Carbon-Based Nanomaterials to Analytical Chemistry: A Critical Review. Anal. Chim. Acta. 2011;691:6–17. doi: 10.1016/j.aca.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valenti LE, Fiorito PA, Garcia CD, Giacomelli CE. The adsorption-desorption process of bovine serum albumin on carbon nanotubes. J. Colloid Interface Sci. 2007;307(2):349–356. doi: 10.1016/j.jcis.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 21.Nejadnik MR, Garcia CD. Staining proteins: A simple method to increase the sensitivity of ellipsometric measurements in adsorption studies. Colloids Surf. B. 2011;82(1):253–257. doi: 10.1016/j.colsurfb.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soetedjo H, Mora MF, Garcia CD. Optical properties of single-wall carbon nanotube films deposited on Si/SiO2 wafers. Thin Solid Films. 2010;518(14):3954–3959. doi: 10.1016/j.tsf.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora MF, Valenti LE, García CD, Giacomelli CE. Advanced Bioceramics in Nanomedicine and Tissue Engineering. Zurich, Switzerland: Trans Tech Publications; 2010. Driving Forces and Consequences of the Adsorption of Proteins to Carbon Nanotubes; pp. 75–94. [Google Scholar]
- 24.Mora MF, Wehmeyer J, Synowicki R, Garcia CD. Investigating the Adsorption of Proteins Via Spectroscopic Ellipsometry. In: Bizios R, Puleo D, editors. Biological Interactions on Material Surfaces: Understanding and Controlling Protein, Cell, and Tissue Responses. 2009. [Google Scholar]
- 25.Carot ML, Torresi RM, Garcia CD, Esplandiu MJ, Giacomelli CE. Electrostatic and Hydrophobic Interactions Involved in CNT Biofunctionalization with Short ss-DNA. J. Phys. Chem. C. 2010;114(10):4459–4465. doi: 10.1021/jp9085359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehmeyer J, Bizios R, Garcia CD. Adsorption of Bovine Serum Albumin to Nanostructured Thin- Films of TiO2. Mat. Sci. Eng. C. 2010;30(2):277–282. doi: 10.1016/j.msec.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mora MF, Giacomelli CE, Garcia CD. Interaction of D-Amino Acid Oxidase to Carbon Nanotubes: Implications in the Design of Biosensors. Anal. Chem. 2009;81:1016–1022. doi: 10.1021/ac802068n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felhofer JL, Caranto J, Garcia CD. Adsorption Kinetics of Catalase to Thin Films of Carbon Nanotubes. Langmuir. 2010;26:17178–17183. doi: 10.1021/la103035n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nejadnik MR, Garcia CD. Nanoscale Scaffolds of Carbon Nanotubes for Immobilization of Glucose Oxidase. Electroanalysis. 2011;23:1462–1469. doi: 10.1002/elan.201000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan R, Gorski W, Garcia CD. Nanomolar Detection of L-Glutamate Using Screen-Printed Electrodes Containing Multi-Wall Carbon Nanotubes. Electroanalysis. 2011;23:2357–2363. doi: 10.1002/elan.201100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chumbimuni-Torres KY, Coronado RE, Mfuh AM, et al. Adsorption of Proteins to Thin-Films of PDMS and Its Effect on the Adhesion of Human Endothelial Cells. RSC Advances. 2011;(4):706–714. doi: 10.1039/C1RA00198A. [DOI] [PMC free article] [PubMed] [Google Scholar]