Abstract
The inability to sialylate recombinant glycoproteins is a critical limitation of the baculovirus-insect cell expression system. This limitation is due, at least in part, to the absence of detectable sialyltransferase activities and CMP-sialic acids in the insect cell lines routinely used as hosts in this system. SfSWT-1 is a transgenic insect cell line encoding five mammalian glycosyltransferases, including sialyltransferases, which can contribute to sialylation of recombinant glycoproteins expressed by baculovirus vectors. However, sialylation of recombinant glycoproteins requires culturing SfSWT-1 cells in the presence of fetal bovine serum or another exogenous source of sialic acid. To eliminate this requirement and extend the utility of SfSWT-1 cells, we have isolated a new baculovirus vector, AcSWT-7B, designed to express two mammalian enzymes that can convert N-acetylmannosamine to CMP-sialic acid during the early phase of infection. AcSWT-7B was also designed to express a model recombinant glycoprotein during the very late phase of infection. Characterization of this new baculovirus vector showed that it induced high levels of intracellular CMP-sialic acid and sialylation of the recombinant N-glycoprotein upon infection of SfSWT-1 cells cultured in serum-free medium supplemented with N-acetylmannosamine. In addition, co-infection of SfSWT-1 cells with AcSWT-7B plus a conventional baculovirus vector encoding human tissue plasminogen activator resulted in sialylation of this recombinant N-glycoprotein under the same culture conditions. These results demonstrate that AcSWT-7B can be used in two different ways to support recombinant N-glycoprotein sialylation by SfSWT-1 cells in serum-free medium. Thus, AcSWT-7B can be used to extend the utility of this previously described transgenic insect cell line for recombinant sialoglycoprotein production.
Keywords: insect cell, baculovirus, glycoproteins, glycosylation, metabolic, engineering
INTRODUCTION
Baculovirus-infected insect cell lines and larvae are widely used to produce recombinant mammalian glycoproteins for various biomedical applications (Jarvis, 1997; O’Reilly et al., 1992; Summers and Smith, 1987). The benefits of this system include its simplicity, safety, and ability to glycosylate recombinant proteins. Protein N-glycosylation is mediated by an elaborate pathway that begins with the co-translational transfer of a pre-assembled oligosaccharide, Glc3Man9GlcNAc2, from a lipid donor to an asparagine residue in the growing polypeptide chain (Kornfeld and Kornfeld, 1985; Varki et al., 1999). In higher eukaryotic cells, this N-glycan precursor is subsequently trimmed and elongated in the endoplasmic reticulum and Golgi apparatus. Elongation of the N-glycans on many glycoproteins culminates with the addition of terminal sialic acid residues. This is a functionally significant event, as these negatively charged sugars can directly or indirectly influence glycoprotein behavior in various ways (Dall’Olio, 2000; Jenkins and Curling, 1994; Raju et al., 1996; Takahashi et al., 2004; Weigel, 1994). In contrast to higher eukaryotes, however, baculovirus-infected lepidopteran insect cells generally fail to sialylate recombinant N-glycoproteins (Altmann et al., 1999; Jarvis, 1997; Marchal et al., 2001; Marz et al., 1995). This is due, at least in part, to the absence of detectable sialyltransferase activities (Butters et al., 1981; Stollar et al., 1976) and CMP-sialic acids (Hooker et al., 1999; Tomiya et al., 2001) in these cells.
Recently, it has been demonstrated that the addition of mammalian protein N-glycosylation functions enables baculovirus-infected lepidopteran cells to produce CMP-sialic acid and/or to sialylate recombinant N-glycoproteins (Jarvis, 2003; Kost et al., 2005; Tomiya et al., 2004). Two general approaches have been utilized. One approach has been to use conventional baculovirus vectors to express genes encoding these functions under the transcriptional control of the polyhedrin (polh) promoter during the very late phase of infection. This approach has been used to introduce mammalian enzymes involved in CMP-sialic acid biosynthesis and to show that this can induce the accumulation of high levels of CMP-sialic acid in the virus-infected insect cells (Lawrence et al., 2000, 2001; Viswanathan et al., 2003, 2005). This approach also has been used to express genes encoding mammalian glycosyltransferases and to obtain evidence that introduction of these enzymes can lead to sialylation of a co-expressed recombinant N-glycoprotein (Chang et al., 2003). The other approach has been to use immediate early expression plasmids to transfer genes encoding protein N-glycosylation functions into the genomes of lepidopteran insect cell lines. This approach has been used to introduce mammalian glycosyltransferase genes and create transgenic lines that constitutively express these new functions (Breitbach and Jarvis, 2001; Hollister and Jarvis, 2001; Hollister et al., 1998, 2002). Upon infection with baculovirus expression vectors, transgenic lepidopteran insect cell lines encoding mammalian glycosyltransferases produced sialylated recombinant N-glycoproteins. However, because these cells contain no detectable CMP-sialic acid (Hooker et al., 1999; Tomiya et al., 2001), their ability to sialylate recombinant glycoproteins depended upon an exogenous source of sialic acid, such as fetal bovine serum, purified sialoglycoproteins, or purified oligosaccharides (Hollister et al., 2003). To overcome this requirement, Aumiller et al. (2003) transformed a transgenic insect cell line designated SfSWT-1, which encodes mammalian N-acetylglucosaminyltransferase I, N-acetylglucosaminyltransferase II, β1,4-galactosyltransferase, α-2,6-sialyltransferase, and α-2,3-sialyltransferase, with a dual immediate early expression plasmid encoding mammalian N-acetylneuraminic acid 9-phosphate synthase (SAS) and cytidine 5′-monophosphate sialic acid synthetase (CMP-SAS). This produced a daughter insect cell line, designated SfSWT-3, which expressed the glycosyltransferases in conjunction with the mammalian SAS and CMP-SAS genes. The addition of these latter enzymes enabled SfSWT-3 cells to convert the sialic acid precursor, N-acetylmannosamine, to CMP-sialic acid and to sialylate a recombinant glycoprotein when cultured in the absence of any other exogenous source of sialic acid.
An intermediate approach that has been used to extend the N-glycosylation pathway in the baculovirus-insect cell system is to use baculovirus vectors encoding mammalian glycosylation functions under the transcriptional control of the ie1 promoter and a glycoprotein of interest under the transcriptional control of a later (e.g., polh) promoter (Jarvis et al., 2001; Seo et al., 2001; Tomiya et al., 2003). The advantage of this approach, as compared to simply using conventional baculovirus expression vectors, is that the mammalian functions are produced early in infection, while the glycoprotein of interest is expressed later. Thus, the mammalian functions are available well before they are needed to process N-glycans on the newly synthesized glycoprotein of interest. It has been shown that infection of insect cell lines with this type of baculovirus vector, encoding one or more mammalian glycosyltransferases and an N-glycoprotein in this temporally regulated fashion, yields products with mammalianized N-glycans.
In the present study, we have extended the intermediate approach described above by creating a novel immediate early baculovirus vector, AcSWT-7B, which encodes murine SAS and CMP-SAS genes under ie1 control and a recombinant N-glycoprotein under polh control. Infection of SfSWT-1 cells with AcSWT-7B enabled these cells to sialylate this recombinant N-glycoprotein in serum-free medium containing N-acetylmannosamine, but no other exogenous source of sialic acid. In addition, co-infection of SfSWT-1 cells with this new baculovirus vector plus a conventional baculovirus encoding human tissue plasminogen activator (t-PA) under polh control enabled these cells to sialylate recombinant human t-PA under these same culture conditions. The ability of AcSWT-7B to provide the biosynthetic machinery needed for CMP-sialic acid production significantly extends the utility of SfSWT-1 cells, as it eliminates the need to cultivate these cells in media containing serum or other additives as sources of sialic acid. This will greatly simplify the purification of secreted recombinant proteins and decrease potential biohazards associated with the use of these complex, animal-derived additives.
MATERIALS AND METHODS
Construction of Baculovirus Transfer Plasmids
One of the starting materials used to isolate AcSWT-7B was a baculovirus transfer plasmid called p64KTV1, which includes a fragment of the Autographa californica multiple nucleopolyhedrovirus (AcMNPV) genome encoding the ChiA, v-cath, gp64, gp16, and pp34 genes (Jarvis and Finn, 1995). To delete some extraneous restriction sites from p64KTV1, we digested it with KpnI and NheI, blunted and re-ligated the ends, and isolated the resulting plasmid. Subsequently, we deleted some additional restriction sites by digesting this intermediate plasmid with SacII and PstI and then blunting and re-ligating the ends to produce a plasmid called p64KTV1ΔK/NΔS/P. This plasmid had a unique EcoRI site within the v-cath open reading frame, which was used to insert an in-frame E. coli LacZ fragment encoding a functional β-galactosidase protein and produce a new baculovirus transfer plasmid, pAcVcath-LacRI. In parallel, we excised a KpnI fragment encoding murine SAS (Nakata et al., 2000) and CMP-SAS (Munster et al., 1998) under the transcriptional control of dual AcMNPV ie1 promoters and the AcMNPV hr5 enhancer element from p64KDIE1TV1/SAS/CMP-SAS (Aumiller et al., 2003) and then blunted the ends and subcloned it into the unique EcoRI site (blunted) of p64KTV1ΔK/NΔS/P to produce another baculovirus transfer plasmid, pAcSWT-7. Both of these new transfer plasmids were isolated from large-scale bacterial cultures by standard alkaline lysis and CsCl-ethidium bromide density gradient ultracentrifugation methods, as described previously (Sambrook et al., 1989), and their genetic structures were confirmed by restriction mapping. These plasmids were then used to isolateAcSWT-7B through two sequential rounds of homologous recombination with a previously described recombinant baculovirus, AcGST-SfManI (Kawar et al., 2000), as described below.
Isolation and Genetic Characterization of Recombinant Baculoviruses
A standard calcium phosphate method (Summers and Smith, 1987) was used to co-transfect Sf9 cells with a mixture of pAcVcath-LacRI and viral genomic DNA from AcGST-SfManI. This cross was designed to introduce the LacZ marker into the v-cath locus of the recombinant baculovirus, which already had a polh-driven Spodoptera frugiperda class I α-mannosidase (GST-SfManI) gene within the polh locus. The anticipated daughter virus, which was designated AcSWT-6B, was identified by its blue-plaque phenotype, purified through two rounds of plaque purification, and amplified in Sf9 cells. Viral genomic DNA was isolated from AcSWT-6B and analyzed by polymerase chain reaction (PCR) with primers directed against viral sequences located inside and outside of the transfer plasmid. The results of this analysis confirmed that the virus had acquired the LacZ gene in the appropriate genomic location (data not shown). Subsequently, the AcSWT-6B genomic DNA was mixed with the other transfer plasmid, pAcSWT-7, and the mixture was used to co-transfect Sf9 cells. The purpose of this second cross was to replace the LacZ gene in the v-cath locus of AcSWT-6B with the ie1/hr5-driven SAS/CMP-SAS genetic cassette (see Fig. 1). Recombinant plaques were identified by their white-plaque phenotypes, picked, and samples of the plaque eluants were used to infect Sf9 cells to produce crude viral DNAs for dot blotting analyses with DNA probes specific for the GST-SfManI, CMP-SAS, and SAS genes, as described previously (Summers and Smith, 1987). Several putative AcSWT-7B clones identified using this method (data not shown) were subjected to a second round of plaque purification, and amplified in Sf9 cells. Samples of the first passage stocks were used to infect fresh Sf9 cell cultures to produce total RNAs for dot blotting analyses with DNA probes specific for the GST-SfManI, CMP-SAS, and SAS genes, as described below. All but one of the putative AcSWT-7B clones expressed RNAs that reacted with all three probes (data not shown). One of these (#177) was chosen as the representative AcSWT-7B clone and large working stocks were prepared by amplification in Sf9 cells, titered, and used for the remainder of the experiments described in this study. PCR analysis of a sample of AcSWT-7B viral DNA with primers directed against viral DNA sequences located inside and outside of the transfer plasmid confirmed that AcSWT-7B had acquired the SAS and CMP-SAS genes in the appropriate genomic location (data not shown).
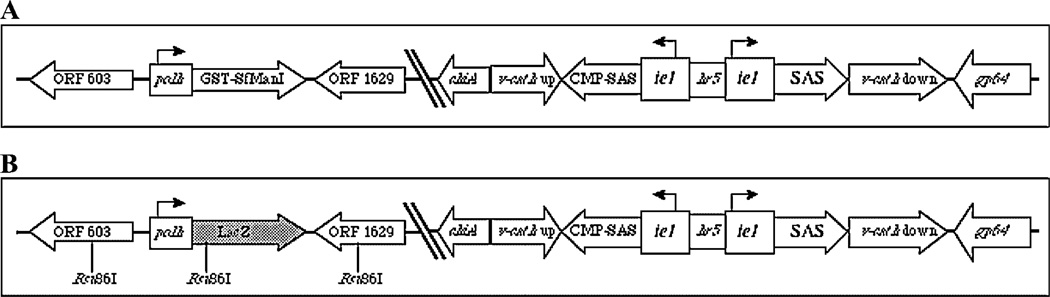
Figure 1.
Genetic structures of baculovirus expression vectors. The relevant genetic features of AcSWT-7B include a GST-SfManI gene under the control of the polh promoter in place of the wild-type AcMNPV polh gene and murine SAS and CMP-SAS genes under the control of the hr5 enhancer and ie1 promoters in the middle of the AcMNPV v-cath gene (A). Simple allelic replacement of the polh-driven GST-SfManI gene using a transfer plasmid containing a polh-driven E. coli LacZ gene (stippled box) flanked by viral DNA sequences modified to contain the indicated Bsu36I sites would produce a downstream recombinant baculovirus vector (B), which could be used to produce daughter viruses encoding any glycoprotein of interest, as described previously (Kitts and Possee, 1993).
Cells and Viruses
Sf9 (Summers and Smith, 1987), SfSWT-1 (Hollister et al., 2002), and SfSWT-3 (Aumiller et al., 2003) cells were routinely maintained as shake-flask cultures at densities from about (0.3 to 3.0) × 106 cells/mL in serum-free medium or complete TNM-FH medium. The serum-free medium used in this study was ESF921 (Expression Systems, Woodland, CA). For experiments requiring the use of serum-free medium containing N-acetylmannosamine, ESF-921 was supplemented with a filter sterilized, concentrated stock solution of this sugar (Sigma-Aldrich Co., St. Louis, MO) at a final concentration of 10 mM. Complete TNM-FH medium was prepared from powdered chemicals and supplemented with 10% fetal bovine serum (HyClone, Logan, UT) and 1% (w/v) Pluronic F68, as described previously (Murhammer and Goochee, 1988; Summers and Smith, 1987). CHO-KI (ATCCCCL 61) cells were obtained from the American Type Culture Collection (Manassas, VA) and routinely maintained as an adherent culture in Alpha minimum essential medium (α-MEM) containing l-glutamine, ribonucleosides, and deoxyribonucleosides (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% (v/v) fetal bovine serum (HyClone) and 0.22% (w/v) sodium bicarbonate, as described previously (Aumiller and Jarvis, 2002). The E2 strain of AcMNPV (Smith and Summers, 1978) was used as wild-type baculovirus. AcGST-SfManI is a previously described recombinant baculovirus encoding a GST-tagged form of the soluble domain of the GST-SfManI protein under the control of the polh promoter (Kawar et al., 2000). NTPA is a previously described recombinant baculovirus encoding the full length, unmodified human t-PA protein under the control of the polh promoter (Jarvis et al., 1993). AcSWT-6B and AcSWT-7B are new recombinant baculoviruses isolated for this study, as described above. Low passage working stocks of each virus were produced by infecting Sf9 cells at a low (0.01–0.1 plaque-forming units/cell) multiplicity of infection, harvesting the cell-free medium by centrifugation for 5 min at 500g, and titering the virus by plaque assays, as described previously (Summers and Smith, 1987).
RNA Dot Blotting Analyses
RNA dot blotting analyses were performed by seeding Sf9 cells into 6-well plates (Corning Glass Co., Corning, NY) at a density of 1.0 × 106 cells/well, allowing the cells to attach for at least 1 h, and then infecting the cells with the relevant baculoviruses at a multiplicity of infection of about five plaque-forming units per cell. At various times after infection, total RNA was isolated using the Tri-Reagent (Molecular Research Center, Cincinnati, OH) according to the manufacturer’s recommendations. The RNA preparations were quantified by spectrophotometry and then various amounts were spotted onto nitrocellulose or nylon filters and hybridized with DNA probes specific for the GST-SfManI, CMP-SAS, and SAS genes, which had been radiolabeled by the random primer method, as described previously (Feinberg and Vogelstein, 1983; Jarvis et al., 1996; Luckow and Summers, 1988).
Immunoblotting Analyses
Sf9 cells were infected with the relevant baculoviruses at a multiplicity of infection of about five plaque-forming units per cell, samples of the culture media were removed at various times after infection, and the cells were removed by centrifugation for 5 min at 500g. Equal volumes of the cell-free media were then analyzed by immunoblotting analysis, as described previously (Kawar et al., 2000), except the primary antisera used in this study were polyclonal rabbit antisera against GST (Sigma) or human t-PA (Oxford Biomedical, Oxford, MI).
Sialic Acid and CMP-Sialic Acid Determinations
SfSWT-1 or SfSWT-3 cell cultures were maintained for 12 h in ESF921 with or without 10 mM N-acetylmannosamine, infected for 24 h with AcMNPV or AcSWT-7B at a multiplicity of infection of about two plaque forming units/cell, and then split into 25 cm2 culture flasks (Corning) at a density of 2 × 106 cells/flask. CHO cells were maintained for 36 h in α-MEM with or without 10 mM N-acetylmannosamine as controls. At the end of these incubation periods, the medium was removed from each cell culture, the monolayers were washed with Tris-buffered saline (0.01 M Tris-HCl, 0.14 M NaCl, pH 7.4), and the cells were lysed by treatment for 10 min on ice with thiobarbituric acid (TBA) assay buffer [0.2 M Tris-HCl, pH 9.0; 0.02 M MgCl2, 0.2 mM dithiothreitol, and 1% (v/v) Triton-X-100]. The lysates were clarified for 15 min at full speed in a microcentrifuge and the supernatants were transferred to fresh tubes. Finally, total protein concentrations were determined using a commercial bicinchoninic assay (Pierce Biotechnology, Inc., Rockford, IL) and sialic acid and CMP-sialic acid concentrations were measured using a previously described TBA assay method (Warren, 1959) with modifications described by others (Chen et al., 2002; Vann et al., 1987).
Lectin Blotting Analysis of GST-SfManI
SfSWT-1 or SfSWT-3 cells were cultured for one passage in ESF921 medium with or without 10 mM N-acetylmannosamine, the cells were infected for 72 h with AcSWT-7B or AcGST-SfManI at a multiplicity of infection of about five plaque-forming units/cell, and the cell-free media were isolated by centrifugation for 5 min at 500g. The GST-SfManI from each culture was then affinity-purified and samples containing about equal amounts of GST-SfManI were used for immunoblotting analyses in parallel with Sambucus nigra agglutinin (SNA) blotting analyses, as described previously (Aumiller et al., 2003). The biotinylated SNA was pre-incubated overnight in buffer alone or buffer containing excess competing sugar prior to use in these assays to demonstrate the specificity of the analysis. Further evidence for the specificity of the SNA blotting analyses was obtained by pre-treating GST-SfManI samples with buffer alone, 10,000 U/mL of PNGase-F (New England Biolabs, Beverly, MA), or 100 U/mL of Arthrobacter ureafaciens neuraminidase (Calbiochem, La Jolla, CA) for 16 h at 37°C.
Analysis of Human t-PA Glycosylation
SfSWT-1 cells were cultured for 12 h in ESF921 with or without 10 mM N-acetylmannosamine, split into 25 cm2 culture flasks (Corning) at a density of 2 × 106 cells/flask, and infected for 48 h with AcSWT-7B plus AcMNPVor AcSWT-7B plus NTPA at a multiplicity of infection of about 2 plaque forming units of each virus/cell. After 48 h, the cell-free media were isolated from each culture by centrifugation for 5 min at 500g and then immunoprecipitated with polyclonal rabbit anti-human t-PA, essentially as described previously (Hollister et al., 1998). The immunoprecipitates were then analyzed by immunoblotting in parallel with SNA blotting, as described above. One modification of the immunoblotting procedure for these experiments was that only the top half of the filters were used for the analysis to prevent loss of the secondary goat anti-rabbit IgG by binding to the excess amounts of IgG heavy chain incorporated during the immunoprecipitation.
RESULTS
Isolation of a Recombinant Baculovirus Encoding Murine CMP-SAS and SAS Genes
AcSWT-7B encodes two murine enzymes, SAS (Nakata et al., 2000) and CMP-SAS (Munster et al., 1998), which can convert N-acetylmannosamine through a sialic acid intermediate to the nucleotide sugar, CMP-sialic acid (Fig. 1). Each of these genes is positioned under the transcriptional control of the AcMNPV ie1 promoter and hr5 enhancer, which can induce foreign gene expression during the immediate early phase of baculovirus infection (Jarvis et al., 1996). The immediate early SAS/CMP-SAS gene cassette is inserted into the open reading frame of the AcMNPV cathepsin-like protease (v-cath) gene (Slack et al., 1995). Thus, this viral gene is insertionally inactivated and, as a result, AcSWT-7B cannot produce a functional form of this protease, which could degrade recombinant protein products. AcSWT-7B also has a new gene in the polh locus, which encodes a glutathione-S-transferase-tagged version of the soluble domain of a GST-SfManI (Kawar et al., 2000) under the transcriptional control of the polh promoter (Fig. 1A). We routinely use GST-SfManI as a model recombinant glyco-protein to assess our attempts to genetically modify the lepidopteran insect protein N-glycosylation pathway because it is produced in large amounts, it has a single N-glycan that is efficiently processed, it is easy to purify, and it can be efficiently deglycosylated. However, we recognize that GST-SfManI is not a biotechnologically relevant recombinant glycoprotein. Thus, it should be noted that AcSWT-7B could be easily converted to a linearizable baculovirus vector of the type widely used in the field by simply crossing it with an appropriate LacZ-based transfer plasmid (Fig. 1B; (Kitts and Possee, 1993). The resulting virus could then be used to efficiently produce daughter recombinants capable of expressing any glycoprotein of interest in conjunction with the early-expressed SAS and CMP-SAS genes.
Potential AcSWT-7B clones were isolated as described in Materials and Methods and screened after one round of plaque purification to identify clones containing each of the three new genes of interest. This was accomplished by analyzing samples of viral DNA isolated from each clone by genomic dot blotting with DNA probes derived from the SAS, CMP-SAS, and GST-SfManI genes. Viral DNAs from several clones hybridized with all three probes (data not shown) and the absence of any detectable hybridization with control DNA isolated from wild type AcMNPV indicated that the results obtained with each probe were specific (data not shown). Four positive clones were subjected to a second round of plaque purification and screened for expression of the SAS and CMP-SAS genes by dot blotting analysis with the relevant DNA probes and total RNAs isolated from cells infected for 48 h with one of the clones of interest or with AcMNPV. The results showed that all four viral clones specifically expressed both genes (data not shown). In addition, immunoblotting analysis of the extracellular media isolated from cells infected with these clones showed that three of the four expressed GST-SfManI at about the expected levels (data not shown). One (#177) was chosen as the representative AcSWT-7B clone and used for the remainder of this study.
Characterization of AcSWT-7B
AcSWT-7B was amplified, titered, and its ability to induce SAS and CMP-SAS expression during infection was re-examined by RNA dot blot analysis with DNA probes derived from the SAS and CMP-SAS genes, as described in Materials and Methods. The results showed that both probes hybridized with total RNA isolated from insect cells infected for 24, 48, and 72 h with AcSWT-7B (Fig. 2, even-numbered lanes). The signals were specific, as neither probe hybridized with any of the total RNA preparations isolated from insect cells infected with AcMNPV (Fig. 2, odd-numbered lanes). The hybridization signals intensified between 24 and 48 h post-infection, but there was little or no additional change between 48 and 72 h post-infection. These results demonstrated that AcSWT-7B encodes the murine SAS and CMP-SAS genes and begins to express them during the first 24 h of infection. Thus, AcSWT-7B expresses two mammalian functions that should allow the insect cell host to convert N-acetylmannosamine to CMP-sialic acid at relatively early times of infection.
Figure 2.
SAS and CMP-SAS gene expression by AcSWT-7B. Total RNA was isolated from AcMNPV-(lanes 1, 3, and 5) or AcSWT-7B-(lanes 2, 4, and 6) infected insect cells at 24, 48, and 72 h post-infection, quantified, and 1 µg (top rows), 3 µg (middle rows), and 8 µg (bottom rows) samples were spotted onto nitrocellulose membranes. The membranes were then hybridized with murine SAS (A) and CMP-SAS (B) probes, washed, and used for autoradiography.
The ability of AcSWT-7B to produce the model recombinant glycoprotein, GST-SfManI, was examined by immunoblotting analysis using equivalent samples of the extracellular media from insect cells infected for 24, 48, or 72 h with AcMNPV, AcGST-SfManI, or AcSWT-7B. The results showed that GST-SfManI was detectable in samples harvested from cells infected for 48 and 72 h with AcGST-SfManI and AcSWT-7B (Fig. 3, lanes 5, 6 and 8, 9). The specificity of the anti-GST antiserum used for this analysis was indicated by the absence of any immunoreactivity with samples isolated from AcMNPV-infected cells (Fig. 3, lanes 1, 4, and 7). There was little or no difference in the amounts of GST-SfManI that accumulated by 48 or 72 h post-infection. Importantly, AcSWT-7B- and AcGST-SfManI-infected cells produced about the same amounts of this model glycoprotein at both time points, indicating that insertion and expression of the SAS and CMP-SAS genes did not adversely influence the ability of AcSWT-7B to induce production of this recombinant glycoprotein.
Figure 3.
GST-SfManI expression by AcSWT-7B. Extracellular media were isolated from AcMNPV-(lanes 1, 4, and 7), AcGST-SfManI-(lanes 2, 5, and 8), and AcSWT-7B-(lanes 3, 6, and 9) infected insect cells at 24, 48, and 72 h post-infection. Equivalent samples were then resolved by SDS–PAGE, transferred to an Immobilon filter, and probed with a polyclonal antibody against GST. The arrow shows the position of the 97.2-kDa marker.
Together, the results of these functional analyses indicated that we had successfully isolated a new baculovirus vector, AcSWT-7B, which expressed SAS and CMP-SAS and then GST-SfManI, in the desired temporally regulated fashion. These results indicated that this new vector had the potential to support recombinant glycoprotein sialylation by SfSWT-1 cells cultured in serum-free medium containing N-acetylmannosamine, but no other exogenous source of sialic acids.
Production of CMP-Sialic Acid in AcSWT-7B-Infected SfSWT-1 Cells
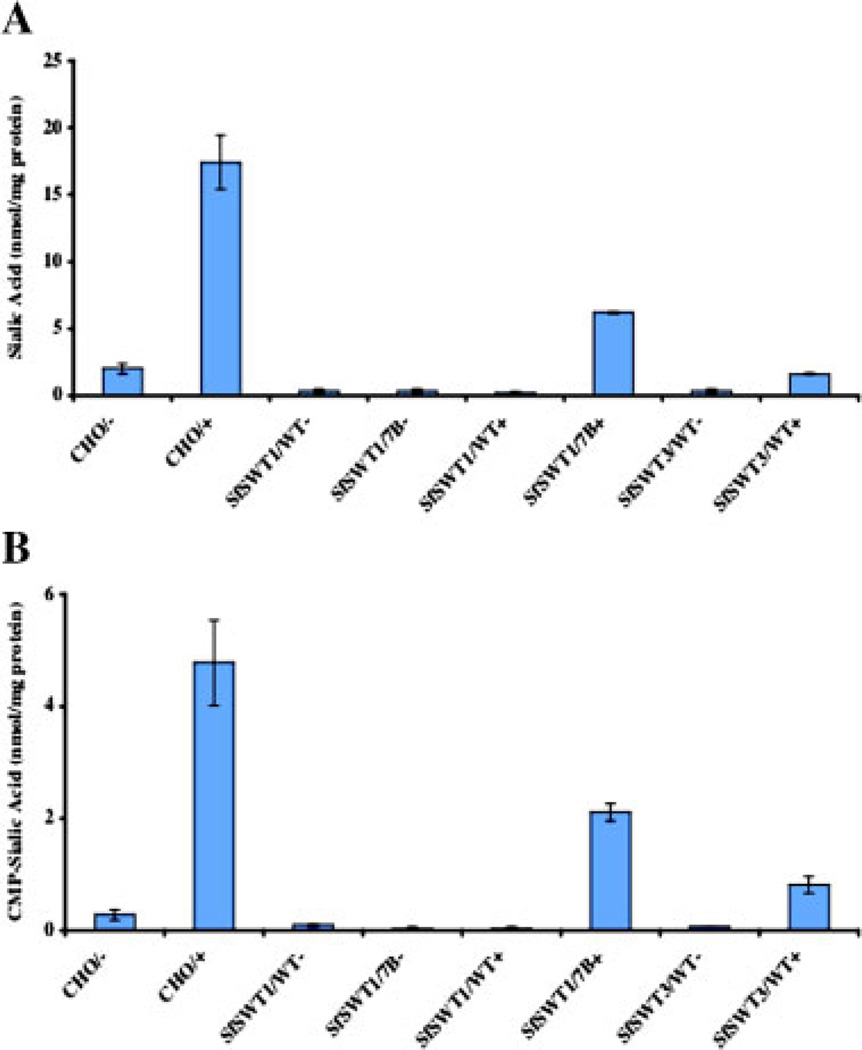
Accordingly, our next goal was to determine if AcSWT-7B could actually induce CMP-sialic acid production in SfSWT-1 cells cultured under these conditions. The cells were passaged once in serum-free medium with or without N-acetylmannosamine and then infected with AcMNPVor AcSWT-7B. Intracellular extracts were prepared at 24 h post-infection and sialic acid and CMP-sialic acid contents were measured in samples containing equal amounts of total protein, as described in Materials and Methods. Extracts prepared from Chinese hamster ovary (CHO) or AcMNPV-infected SfSWT-3 cells cultured in the presence or absence of N-acetylmannosamine were used as controls. The results of these assays showed that AcSWT-7B-infected SfSWT-1 cells cultured in serum-free medium with N-acetylmannosamine contained substantial amounts of both sialic acid (Fig. 4A; SWT1/7B+) and CMP-sialic acid (Fig. 4B; SWT1/7B+). In contrast, SfSWT-1 and SfSWT-3 cells cultured in serum-free medium without N-acetylmannosamine and then infected with either baculovirus contained little or no sialic acid or CMP-sialic acid. One might take the data obtained with these negative controls as an indication that they actually contain very low levels of sialic acid and/or CMP-sialic acid. However, the actual absorbance values obtained with these samples were well below the linear range of the assay and were, therefore, inconclusive. The intracellular levels of sialic acid and CMP-sialic acid in the AcSWT-7B-infected SfSWT-1 cells cultured in the presence of N-acetylmannosamine were about 36% and 43% of the corresponding levels in CHO cells. AcSWT-7B-infected SfSWT-1 cells cultured in the presence of N-acetylmannosamine contained about fourfold and threefold higher levels of sialic acid and CMP-sialic acid, respectively, than AcMNPV-infected SfSWT-3 cells cultured in the presence of N-acetylmannosamine. These results demonstrated that AcSWT-7B can convert N-acetylmannosamine to CMP-sialic acid through a sialic acid intermediate and, therefore, can provide the donor substrate needed for recombinant glycoprotein sialylation by SfSWT-1 cells cultured in serum-free medium.
Figure 4.
Induction of sialic acid and CMP-sialic acid biosynthesis by AcSWT-7B. Intracellular extracts were isolated from AcMNPV-(WT) or AcSWT-7B-(7B) infected SfSWT-1 or SfSWT-3 cells cultured in serum-free medium with (+) or without (−) N-acetylmannosamine at 24 h post-infection. Total protein was quantified and then triplicate samples containing equivalent amounts of total protein were assayed for sialic acid (A) and CMP-sialic acid (B) contents using a modified thiobarbituric acid method, as described in Materials and Methods. Control extracts were prepared from CHO cells cultured in the presence (+) or absence (−) of N-acetylmannosamine. The bars indicate the average values obtained using triplicate samples and the error bars show the standard deviations. [Color figure can be seen in the online version of this article, available at www.interscience.wiley.com.]
Sialylation of a Recombinant Glycoprotein in AcSWT-7B-Infected SfSWT-1 Cells
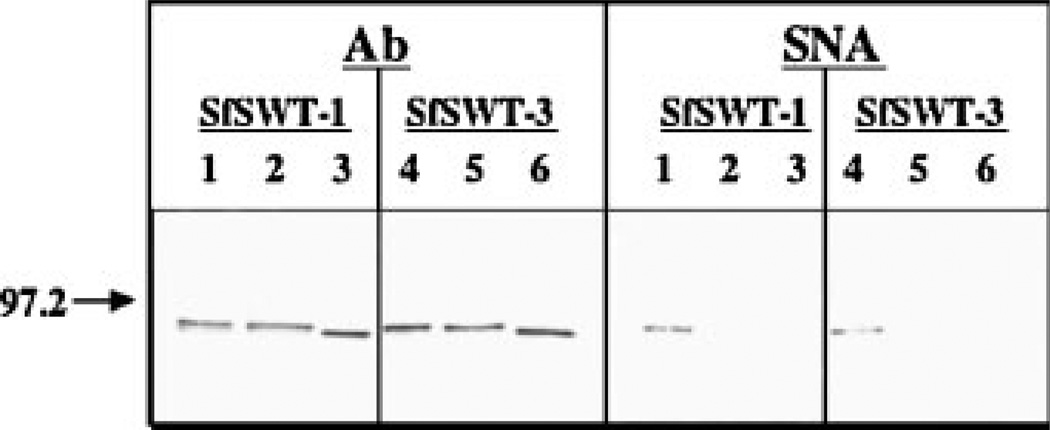
To examine the ability of AcSWT-7B to support recombinant glycoprotein sialylation, lectin blotting was used to examine the glycosylation of GST-SfManI. The recombinant glycoprotein product was affinity-purified from AcSWT-7B-infected SfSWT-1 cells cultured in serum-free medium with or without N-acetylmannosamine. The controls were equivalent samples of GST-SfManI purified from AcGST-SfManI-infected SfSWT-3 cells cultured under the same conditions. The GST-SfManI produced by AcSWT-7B-infected SfSWT-1 cells cultured in serum-free medium plus N-acetylmannosamine reacted with SNA, which is a lectin that binds preferentially to terminal, α2,6-linked sialic acids (Fig. 5B). In contrast, the GST-SfManI produced by AcSWT-7B-infected SfSWT-1 cells cultured in serum-free medium without N-acetylmannosamine did not react with SNA. The control samples yielded the expected results, as the GST-SfManI produced by AcGST-SfManI-infected SWT-3 cells cultured in serum-free medium failed to react with SNA, but it reacted with the GST-SfManI produced by the same cells cultured in serum-free medium plus N-acetylmannosamine. In this control virus-host combination, the SfSWT-3 cells encodes the glycosyltransferase, SAS, and CMP-SAS genes, while the virus encodes the recombinant glycoprotein. The absence of any detectable SNA reactivity with the GST-SfManI produced in either cell line when cultured in the absence of N-acetylmannosamine indicated that this lectin bound specifically to terminal sialic acids on the recombinant glycoprotein. This interpretation was supported by the absence of any detectable SNA reactivity with the GST-SfManI produced under any conditions when the lectin was pre-incubated with a competing sugar (Fig. 5C). The immunoblotting control panel shows that approximately the same amounts of GST-SfManI were loaded into each lane, irrespective of its source (Fig. 5A).
Figure 5.
Sialylation of GST-SfManI by AcSWT-7B-infected SfSWT-1 cells. Extracellular media were isolated from AcSWT-7B-infected SfSWT-1 cells that had been cultured in serum-free medium with (+) or without (−) N-acetylmannosamine at 72 h post-infection and GST-SfManI was purified by affinity chromatography. Sialylated and unsialylated GST-SfManI were purified from AcGST-SfManI-infected SfSWT-3 cells cultured in the same fashion and used as controls. Equivalent amounts of the purified products were resolved by SDS–PAGE, transferred to an Immobilon filter, and probed with either a polyclonal antibody against GST (A) or with SNA (B). One set of reactions was performed using SNA that had been pre-incubated with a competing sugar (C). The arrows to the left of each panel show the positions of the 97.2-kDa markers.
Further evidence in support of the specificity of the lectin blotting analysis was obtained by pre-treating the target glycoprotein with various glycosidases (Fig. 6). SNA reactivity was unaffected by pre-treating GST-SfManI with buffer alone (lanes 1 and 4), but abolished when the relevant forms of this glycoprotein were pre-treated with either neuraminidase (lanes 2 and 5) or peptide N-glycosidase F (PNGase-F; lanes 3 and 6). Together, these data demonstrated that AcSWT-7B supports sialylation of GST-SfManI by SfSWT-1 cells cultured in serum-free medium containing N-acetylmannosamine.
Figure 6.
Specificity of SNA lectin blotting analysis. GST-SfManI affinity-purified from AcSWT-7B-infected SfSWT-1 (lanes 1–3) or AcGST-SfManI-infected SfSWT-3 (lanes 4–6) cells cultured in the presence of N-acetylmannosamine were pre-treated with buffer alone (lanes 1 and 4), neuraminidase (lanes 2 and 5), or PNGase-F (lanes 3 and 6). The treated glycoproteins were then resolved by SDS–PAGE, transferred to Immobilon filters, and probed with either anti-GST (Ab) or SNA (SNA). The arrow on the left shows the position of the 97.2-kDa marker.
AcSWT-7B Supports Sialylation of a Relevant Human Glycoprotein
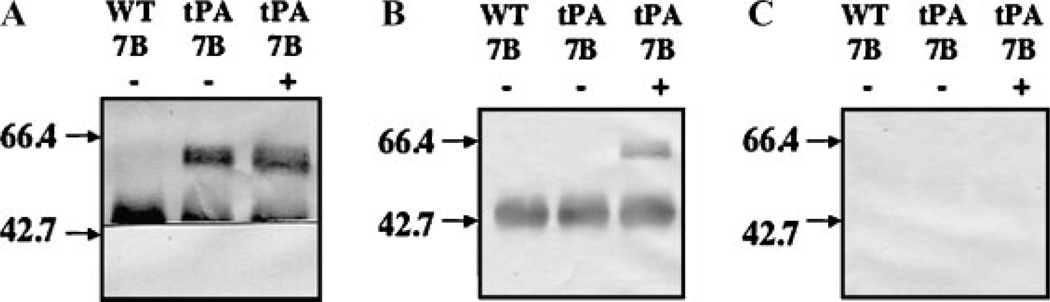
It also was of interest to determine if AcSWT-7B could support sialylation of a biotechnologically relevant human glycoprotein expressed by SfSWT-1 cells cultured under these conditions. This issue was addressed by passaging SfSWT-1 cells once in serum-free medium with or withoutN-acetylmannosamine and then co-infecting the cells with a mixture of AcSWT-7B plus either AcMNPV or NTPA, a recombinant baculovirus that encodes human t-PA under the control of the polh promoter (Jarvis et al., 1993). Extracellular fractions were harvested at 48 h post-infection, immunoprecipitated with anti-t-PA, and then the immunoprecipitates were resolved by SDS–PAGE, transferred to membranes, and subjected to immunoblotting or lectin blotting analyses. The immunoblotting results established the identity of t-PA, which was only immunoprecipitated from the NTPA co-infected cells, and also showed that the relevant lanes contained approximately the same amount of this recombinant protein (Fig. 7A). The lectin blotting results showed that SNA only reacted with the t-PA produced by AcSWT-7B/NTPA co-infected SfSWT-1 cells when those cells were cultured in serum-free medium containing N-acetylmannosamine (Fig. 7B). SNA did not react with the t-PA produced by this virus-host combination when the cells were cultured in the absence of N-acetylmannosamine, indicating that the SNA reacted specifically with sialic acid residues on this recombinant glycoprotein. This interpretation was supported by the lack of SNA reactivity with t-PA when the lectin was pre-incubated with a competing sugar (Fig. 7C).
Figure 7.
Human t-PA sialylation by AcSWT-7B-infected SfSWT-1 cells. Serum-free media were isolated from SfSWT-1 cells cultured in the presence (+) or absence (−) of N-acetylmannosamine at 48 h after being co-infected with a mixture of AcSWT-7B (7B) plus either AcMNPV (WT) or NTPA (tPA), as indicated by the labels above each lane. The media were treated with a rabbit antiserum against t-PA and then immune complexes were washed, disrupted, and split into equivalent samples for SDS–PAGE. Resolved proteins were transferred to Immobilon filters and replicate filters were then probed with either anti-t-PA (A) or SNA (B). One filter was probed with SNA that had been pre-incubated with a competing sugar (C). The arrows to the left of each panel show the positions of 66.4 and 42.7-kDa markers.
DISCUSSION
The baculovirus-insect cell protein expression system is generally considered to be an excellent system to use for recombinant glycoprotein production. However, in contrast to mammalian cells, baculovirus-infected lepidopteran insect cells typically fail to produce glycoproteins with terminally sialylated N-glycans. This limitation reflects a fundamental difference between the N-glycan processing pathways of lepidopteran insect and mammalian cells, as most evidence indicates that the former lack functional levels of sialyltransferases (Butters et al., 1981; Stollar et al., 1976) and CMP-sialic acids (Hooker et al., 1999; Tomiya et al., 2001). Clearly, there are some published results that raise questions about the overall generality of this conclusion as it applies to lepidopteran (Davidson et al., 1990; Joosten et al., 2003; Joosten and Shuler, 2003a, 2003b; Joshi et al., 2001; Palomares et al., 2003; Watanabe et al., 2001) and other (Kim et al., 2002; Koles et al., 2004) insect systems. However, the fact remains that most recombinant N-glycoproteins produced by the most commonly used baculovirus-insect cell combinations are not terminally sialylated. Coupled with the functional significance of this terminal N-glycan processing event, this fact has led to efforts to modify the N-glycan processing pathway in this system (Jarvis, 2003; Kost et al., 2005; Tomiya et al., 2004).
As detailed in the Introduction, the approaches that have been used for this purpose have involved introducing mammalian genes encoding late-acting glycosyltransferases or enzymes involved in CMP-sialic acid biosynthesis into insect cells using conventional baculovirus vectors or immediate early expression plasmids. The former approach provides transient, high-level expression of these functions during the very late phase of baculovirus infection, while the latter yields transgenic insect cell lines that encode and constitutively express these functions in the absence of baculovirus infection. An intermediate approach has been to produce hybrid baculovirus expression vectors that encode mammalian protein glycosylation functions and a recombinant glycoprotein of interest under the control of immediate early and very late promoters, respectively. These vectors can induce expression of the modifying functions and the glycoprotein of interest in a sequential, temporally regulated fashion. Thus, this approach can be used to extend the N-glycan processing functions of the host in advance of the time when they are needed to more appropriately modify the recombinant glycoprotein of interest.
The purpose of the present study was to use this intermediate approach to create a new recombinant baculovirus vector that encodes murine SAS and CMP-SAS under ie1 control and a recombinant glycoprotein, GST-SfManI, under polh control. Additional engineering of this virus can be performed to create downstream recombinants capable of expressing any recombinant glycoprotein of interest, as illustrated in Figure 1B. Characterization of this hybrid recombinant baculovirus, which was designated AcSWT-7B, demonstrated that it expressed the SAS and CMP-SAS genes earlier and the GST-SfManI gene later in infection, as expected. The former gene products allowed an insect cell host to produce large amounts of sialic acid and CMP-sialic acid when cultured in serum-free medium containing N-acetylmannosamine. The production of intracellular CMP-sialic acid supported sialylation of GST-SfManI by an existing transgenic insect cell line, SfSWT-1, infected under these culture conditions. Thus, the ability of AcSWT-7B to induce CMP-sialic acid production is important because it extends the utility of SfSWT-1 cells, which encode five mammalian glycosyltransferases that are needed to support recombinant sialoglycoprotein production by baculovirus vectors, but fail to do so when cultured in serum-free medium (Hollister et al., 2002, 2003). The ability of AcSWT-7B to support recombinant sialoglycoprotein production by SfSWT-1 cells cultured in serum-free medium containing N-acetylmannosamine will simplify the purification of secreted recombinant glycoprotein products and reduce potential biohazards by eliminating the need to use fetal bovine serum or other complex additives in the culture medium. It is notable that this can be accomplished by infecting SfSWT-1 cells with a single virus encoding both the functions needed to support recombinant glycoprotein sialylation and the recombinant glycoprotein, itself, as this circumvents problems associated with infecting insect cell lines with multiple baculovirus vectors providing individual functions (Berger et al., 2004). Finally, it is useful to reiterate that the SAS/CMP-SAS cassette insertionally inactivates the v-cath gene in AcSWT-7B, as this virus no longer encodes a functional form of this protease, which could contribute to proteolysis of recombinant proteins produced in the baculovirus-insect cell system (Kaba et al., 2004).
As part of this Discussion, it is perhaps important to emphasize that we cannot and do not claim that AcSWT-7B-infected SfSWT-1 cells can support sialylation of every recombinant glycoprotein of interest. To our knowledge, it has been shown to date that SfSWT-1 cells can support baculovirus-mediated production and sialylation of four different foreign glycoproteins, including GST-SfManI, human t-PA, AcMNPV gp64, and human β-trace protein (Hollister et al., 2002). However, SfSWT-1 cells are commercially available from Invitrogen under the trade name, MIMIC™, and a recent study suggested that MIMIC™ cells cultured in the presence of fetal bovine serum failed to support sialylation of equine luteinizing hormone/chorionic gonadotropin (Legardinier et al., 2005). Thus, it is clear that it will be necessary to survey the ability of this transgenic insect cell line to support sialylation of a much larger and broader array of recombinant glycoprotein products before drawing any sweeping conclusions. In context of this discussion, it is interesting to note that many of the studies reporting recombinant glycoprotein sialylation with the baculovirus-insect cell system have used human secreted alkaline phosphatase as the model glycoprotein (Joosten and Shuler, 2003a, b; Joosten et al., 2003, 2001; Palomares et al., 2003). Perhaps this particular product has some biophysical properties that make its N-glycans particularly amenable to elongation or resistant to deglycosylation. This idea has been previously suggested as one possible explanation for the ability of the baculovirus-insect cell system to sialylate human plasminogen (Davidson et al., 1990; Jarvis and Finn, 1995). In the end, it will likely come as no surprise to glycobiologists if additional research shows that the ability of unmodified or genetically modified lower eukaryotic expression systems to produce recombinant glycoproteins with mammalianized N-glycans depends upon the properties of specific glycoproteins of interest. The availability of the AcSWT-7B/SfSWT-1 vector host system will facilitate the wider use of engineered baculovirus-insect cell systems for recombinant glycoprotein production and characterization and a broader analysis of the capabilities of these systems.
Acknowledgments
We thank Jenny Loveridge and Jeremiah Hensley for technical assistance during the course of this study.
Contract grant sponsors: National Institutes of Health; National Institutes of Standards and Technology Advance Technology Program
Contract grant numbers: GM49374; 70NANB3H3042
NOMENCLATURE
- AcMNPV
Autographa californica multiple nucleopolyhedrovirus
- CHO
Chinese hamster ovary
- CMP-SAS
cytidine 5′-monophosphate sialic acid synthetase
- GST-SfManI
glutathione-S-transferase-tagged soluble domain of the Spodoptera frugiperda class I α-mannosidase
- PCR
polymerase chain reaction
- PNGase-F
peptide:N-glycosidase F
- polh
polyhedrin
- SAS
N-acetylneuraminic acid 9-phosphate synthase
- SDS–PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SNA
Sambucus nigra agglutinin
- TBA
thiobarbituric acid
- t-PA
human tissue plasminogen activator
- v-cath
viral cathepsin-like gene.
References
- Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- Aumiller JJ, Jarvis DL. Expression and functional characterization of a nucleotide sugar transporter from Drosophila melanogaster: Relevance to protein glycosylation in insect cell expression systems. Prot Expr Purif. 2002;26:438–448. doi: 10.1016/s1046-5928(02)00550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller JJ, Hollister JR, Jarvis DL. A transgenic lepidopteran insect cell line engineered to produce CMP-sialic acid and sialoglycoproteins. Glycobiology. 2003;13:497–507. doi: 10.1093/glycob/cwg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I, Fitzgerald DJ, Richmond TJ. Baculovirus expression system for heterologous multiprotein complexes. Nat Biotechnol. 2004;22:1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- Breitbach K, Jarvis DL. Improved glycosylation of a foreign protein by Tn-5B1-4 cells engineered to express mammalian glycosyltransferases. Biotechnol Bioengr. 2001;74:230–239. doi: 10.1002/bit.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters TD, Hughes RC, Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim Biophys Acta. 1981;640:672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Chang GD, Chen CJ, Lin CY, Chen HC, Chen H. Improvement of glycosylation in insect cells with mammalian glycosyltransferases. J Biotechnol. 2003;102:61–71. doi: 10.1016/s0168-1656(02)00364-4. [DOI] [PubMed] [Google Scholar]
- Chen H, Blume A, Zimmermann-Kordmann M, Reutter W, Hinderlich S. Purification and characterization of N-acetylneuraminic acid-9-phosphate synthase from rat liver. Glycobiology. 2002;12:65–71. doi: 10.1093/glycob/12.2.65. [DOI] [PubMed] [Google Scholar]
- Dall’Olio F. The sialyl-alpha2,6-lactosaminyl-structure: Biosynthesis and functional role. Glycoconj J. 2000;17:669–676. doi: 10.1023/a:1011077000164. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Fraser MJ, Castellino FJ. Oligosaccharide processing in the expression of human plasminogen cDNA by lepidopteran insect (Spodoptera frugiperda) cells. Biochemistry. 1990;29:5584–5590. doi: 10.1021/bi00475a024. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Analyt Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hollister J, Jarvis DL. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian β1,4-galactosyltransferase and alpha 2,6-sialyltransferase genes. Glycobiology. 2001;11:1–9. doi: 10.1093/glycob/11.1.1. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Shaper JH, Jarvis DL. Stable expression of mammalian beta 1,4-galactosyltransferase extends the N-glycosylation pathway in insect cells. Glycobiology. 1998;8:473–480. doi: 10.1093/glycob/8.5.473. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Grabenhorst E, Nimtz M, Conradt HO, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister JR, Conradt HO, Jarvis DL. Evidence for a sialic acid salvaging pathway in lepidopteran insect cell lines. Glycobiology. 2003;13:487–495. doi: 10.1093/glycob/cwg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker AD, Green NH, Baines AJ, Bull AT, Jenkins N, Strange PG, James DC. Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotechnol Bioengr. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Jarvis DL. Baculovirus expression vectors. In: Miller LK, editor. The baculoviruses. New York: Plenum Press; 1997. pp. 389–431. [Google Scholar]
- Jarvis DL. Humanizing recombinant glycoprotein production in the baculovirus-insect cell expression system. Virology. 2003;310:1–7. doi: 10.1016/s0042-6822(03)00120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis DL, Finn EE. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology. 1995;212:500–511. doi: 10.1006/viro.1995.1508. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Summers MD, Garcia A, Jr, Bohlmeyer DA. Influence of different signal peptides and prosequences on expression and secretion of human tissue plasminogen activator in the baculovirus system. J Biol Chem. 1993;268:16754–16762. [PubMed] [Google Scholar]
- Jarvis DL, Weinkauf C, Guarino LA. Immediate early baculovirus vectors for foreign gene expression in transformed or infected insect cells. Prot Expr Purif. 1996;8:191–203. doi: 10.1006/prep.1996.0092. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Howe D, Aumiller JJ. Novel baculovirus expression vectors that provide sialylation of recombinant glycoproteins in lepidopteran insect cells. J Virol. 2001;75:6223–6227. doi: 10.1128/JVI.75.13.6223-6227.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N, Curling EMA. Glycosylation of recombinant proteins: Problems and prospects. Enz Microb Technol. 1994;16:354–364. doi: 10.1016/0141-0229(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Shuler ML. Effect of culture conditions on the degree of sialylation of a recombinant glycoprotein expressed in insect cells. Biotechnol Prog. 2003a;19:739–749. doi: 10.1021/bp0201049. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Shuler ML. Production of a sialylated N-linked glycoprotein in insect cells: Role of glycosidases and effect of harvest time on glycosylation. Biotechnol Progr. 2003b;19:193–201. doi: 10.1021/bp025695h. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Park TH, Shuler ML. Effect of silkworm hemolymph on N-linked glycosylation in two Trichoplusia ni insect cell lines. Biotechnol Bioeng. 2003;83:695–705. doi: 10.1002/bit.10696. [DOI] [PubMed] [Google Scholar]
- Joshi L, Shuler ML, Wood HA. Production of a sialylated N-linked glycoprotein in insect cells. Biotechnol Progr. 2001;17:822–827. doi: 10.1021/bp010071h. [DOI] [PubMed] [Google Scholar]
- Kaba SA, Salcedo AM, Wafula PO, Vlak JM, van Oers MM. Development of a chitinase and v-cathepsin negative bacmid for improved integrity of secreted recombinant proteins. J Virol Meth. 2004;122:113–118. doi: 10.1016/j.jviromet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Kawar Z, Romero PA, Herscovics A, Jarvis DL. N-Glycan processing by a lepidopteran insect alpha1,2-mannosidase. Glycobiology. 2000;10:347–355. doi: 10.1093/glycob/10.4.347. [DOI] [PubMed] [Google Scholar]
- Kim K, Lawrence SM, Park J, Pitts L, Vann WF, Betenbaugh MJ, Palter KB. Expression of a functional Drosophila melanogaster N-acetylneuraminic acid (Neu5Ac) phosphate synthase gene: Evidence for endogenous sialic acid biosynthetic ability in insects. Glycobiology. 2002;12:73–83. doi: 10.1093/glycob/12.2.73. [DOI] [PubMed] [Google Scholar]
- Kitts PA, Possee RD. A method for producing recombinant baculovirus expression vectors at high frequency. Biotechniques. 1993;14:810–817. [PubMed] [Google Scholar]
- Koles K, Irvine KD, Panin VM. Functional characterization of a drosophila sialyltransferase. J Biol Chem. 2004;279:4346–4357. doi: 10.1074/jbc.M309912200. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Ann Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SM, Huddleston KA, Pitts LR, Nguyen N, Lee YC, Vann WF, Coleman TA, Betenbaugh MJ. Cloning and expression of the human N-acetylneuraminic acid phosphate synthase gene with 2-keto-3-deoxy-D-glycero- D-galacto-nononic acid biosynthetic ability. J Biol Chem. 2000;275:17869–17877. doi: 10.1074/jbc.M000217200. [DOI] [PubMed] [Google Scholar]
- Lawrence SM, Huddleston KA, Tomiya N, Nguyen N, Lee YC, Vann WF, Coleman TA, Betenbaugh MJ. Cloning and expression of human sialic acid pathway genes to generate CMP-sialic acids in insect cells. Glycoconj J. 2001;18:205–213. doi: 10.1023/a:1012452705349. [DOI] [PubMed] [Google Scholar]
- Legardinier S, Klett D, Poirier JC, Combarnous Y, Cahoreau C. Mammalian-like nonsialyl complex-type N-glycosylation of equine gonadotropins in Mimic insect cells. Glycobiology. 2005;15:776–790. doi: 10.1093/glycob/cwi060. [DOI] [PubMed] [Google Scholar]
- Luckow VA, Summers MD. Signals important for high-level expression of foreign genes in Autographa californicanuclear polyhedrosis virus expression vectors. Virology. 1988;167:56–71. doi: 10.1016/0042-6822(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: sialylated or not? Biol Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins. Amsterdam: Elsevier; 1995. pp. 543–563. [Google Scholar]
- Munster AK, Eckhardt M, Potvin B, Muhlenhoff M, Stanley P, Gerardy-Schahn R. Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: A nuclear protein with evolutionarily conserved structural motifs. Proc Natl Acad Sci USA. 1998;95:9140–9145. doi: 10.1073/pnas.95.16.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhammer DW, Goochee CF. Scaleup of insect cell cultures: Protective effects of pluronic F-68. Bio/Technology. 1988;6:1411–1418. [Google Scholar]
- Nakata D, Close BE, Colley KJ, Matsuda T, Kitajima K. Molecular cloning and expression of the mouse N-acetylneuraminic acid 9-phosphate synthase, which does not have deaminoneuraminic acid (KDN) 9-phosphate synthase activity. Biochem Biophys Res Comm. 2000;273:642–648. doi: 10.1006/bbrc.2000.2983. [DOI] [PubMed] [Google Scholar]
- O’Reilly DR, Miller LK, Luckow VA. Baculovirus expression. New York: W.H. Freeman and Company; 1992. [Google Scholar]
- Palomares L, Joosten CE, Hughes PR, Granados RR, Shuler ML. Novel insect cell line capable of complex N-glycosylation and sialylation of recombinant proteins. Biotechnol Progr. 2003;19:185–192. doi: 10.1021/bp025598o. [DOI] [PubMed] [Google Scholar]
- Raju TS, Lerner L, O’Connor JV. Glycopinion: Biological significance and methods for the analysis of complex carbohydrates of recombinant glycoproteins. Biotechnol Appl Biochem. 1996;24:191–194. [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Press; 1989. [Google Scholar]
- Seo NS, Hollister JR, Jarvis DL. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Prot Expr Purif. 2001;22:234–241. doi: 10.1006/prep.2001.1432. [DOI] [PubMed] [Google Scholar]
- Slack JM, Kuzio J, Faulkner P. Characterization of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J Gen Virol. 1995;76:1091–1098. doi: 10.1099/0022-1317-76-5-1091. [DOI] [PubMed] [Google Scholar]
- Smith GE, Summers MD. Analysis of baculovirus genomes with restriction endonucleases. Virology. 1978;89:517–527. doi: 10.1016/0042-6822(78)90193-9. [DOI] [PubMed] [Google Scholar]
- Stollar V, Stollar BD, Koo R, Harrap KA, Schlesinger RW. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976;69:104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Summers MD, Smith GE. Tx Ag Expt Stn Bull No. 1555. College Station, TX: 1987. A manual of methods for baculovirus vectors and insect cell culture procedures. [Google Scholar]
- Takahashi M, Tsuda T, Ikeda Y, Honke K, Taniguchi N. Role of N-glycans in growth factor signaling. Glycoconj J. 2004;20:207–212. doi: 10.1023/B:GLYC.0000024252.63695.5c. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Ailor E, Lawrence SM, Betenbaugh MJ, Lee YC. Determination of nucleotides and sugar nucleotides involved in protein glycosylation by high-performance anion-exchange chromatography: Sugar nucleotide contents in cultured insect cells and mammalian cells. Analyt Biochem. 2001;293:129–137. doi: 10.1006/abio.2001.5091. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Howe D, Aumiller JJ, Pathak M, Park J, Palter K, Jarvis DL, Betenbaugh MJ, Lee YC. Complex-type biantennary N-glycans of recombinant human transferrin from Trichoplusia ni insect cells expressing mammalian β1,4-galactosyltransferase and β1,2-N-acetyl-glucosaminyltransferase II. Glycobiology. 2003;13:23–34. doi: 10.1093/glycob/cwg012. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Narang S, Lee YC, Betenbaugh MJ. Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj J. 2004;21:343–360. doi: 10.1023/B:GLYC.0000046275.28315.87. [DOI] [PubMed] [Google Scholar]
- Vann WF, Silver RP, Abeijon C, Chang K, Aaronson W, Sutton A, Finn CW, Lindner W, Kotsatos M. Purification, properties, and genetic location of Escherichia coli cytidine 5′-monophosphate N-acetylneuraminic acid synthetase. J Biol Chem. 1987;262:17556–17562. [PubMed] [Google Scholar]
- Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth J. Essentials of glycobiology. Cold Spring Harbor, New York: Cold Spring Harbor Press; 1999. [PubMed] [Google Scholar]
- Viswanathan K, Lawrence S, Hinderlich S, Yarema KJ, Lee YC, Betenbaugh MJ. Engineering sialic acid synthetic ability into insect cells: Identifying metabolic bottlenecks and devising strategies to overcome them. Biochemistry. 2003;42:15215–15225. doi: 10.1021/bi034994s. [DOI] [PubMed] [Google Scholar]
- Viswanathan K, Narang S, Hinderlich S, Lee YC, Betenbaugh MJ. Engineering intracellular CMP-sialic acid metabolism into insect cells and methods to enhance its generation. Biochemistry. 2005;44:7526–7534. doi: 10.1021/bi047477y. [DOI] [PubMed] [Google Scholar]
- Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- Watanabe S, Kokuho T, Takahashi H, Takahashi M, Kubota T, Inumaru S. Sialylation of N-glycans on the recombinant proteins expressed by a baculovirus-insect cell system under β-N-acetylglucosaminidase inhibition. J Biol Chem. 2001;277:5090–5093. doi: 10.1074/jbc.M110548200. [DOI] [PubMed] [Google Scholar]
- Weigel PH. Galactosyl and N-acetylgalactosaminyl homeostasis: A function for mammalian asialoglycoprotein receptors. Bioessays. 1994;16:519–524. doi: 10.1002/bies.950160713. [DOI] [PubMed] [Google Scholar]