Abstract
We have previously engineered transgenic insect cell lines to express mammalian glycosyltransferases and showed that these cells can sialylate N-glycoproteins, despite the fact that they have little intracellular sialic acid and no detectable CMP–sialic acid. In the accompanying study, we presented evidence that these cell lines can salvage sialic acids for de novo glycoprotein sialylation from extracellular sialogly-coproteins, such as fetuin, found in fetal bovine serum. This finding led us to create a new transgenic insect cell line designed to synthesize its own sialic acid and CMP–sialic acid. SfSWT-1 cells, which encode five mammalian glycosyltransferases, were transformed with two additional mammalian genes that encode sialic acid synthase and CMP–sialic acid synthetase. The resulting cell line expressed all seven mammalian genes, produced CMP–sialic acid, and sialylated a recombinant glycoprotein when cultured in a serum-free growth medium supplemented with N-acetylmannosamine. Thus the addition of mammalian genes encoding two enzymes involved in CMP–sialic acid biosynthesis yielded a new transgenic insect cell line, SfSWT-3, that can sialylate recombinant glycoproteins in the absence of fetal bovine serum. This new cell line will be widely useful as an improved host for baculovirus-mediated recombinant glycoprotein production.
Keywords: baculovirus expression system, cell transformation, insect cells, protein glycosylation, sialic acid synthesis
Introduction
A striking physiological difference between insects and higher eukaryotes is the absence of terminal sialic acids on most insect cell–derived N-glycoproteins as a result of fundamental differences in their N-glycan processing pathways (reviewed by Kornfeld and Kornfeld, 1985; Marz et al., 1995; Jarvis, 1997; Marchal et al., 2001). In general, the dominant pathway in insect cells appears to include the processing glycosidases involved in N-glycan trimming but only a small subset of the glycosyltransferases involved in N-glycan elongation. More specifically, the inability of insect cells to routinely produce terminally sialylated, complex N-glycans is related to the fact that they have no detectable sialyltransferase activities (Stollar et al., 1976; Butters et al., 1981; Hooker et al., 1999) or cytidine 5′-monophosphate (CMP)–sialic acids (Hooker et al., 1999; Tomiya et al., 2001). These functions do not appear to be totally absent from insect cells because terminally sialylated, complex N-glycans have been found on insect cell–derived glycoproteins under certain conditions (Davidson et al., 1990; Joshi et al., 2001; Watanabe et al., 2001; Jooster and Shuler, 2003), sialic acids have been detected in insect tissues (Roth et al., 1992; Karacali et al., 1997, 1999) and cell lines (Lawrence et al., 2000), and Drosophila melanogaster encodes at least one enzyme potentially involved in sialic acid metabolism (Kim et al., 2002). However, the major processed N-glycans found on insect cell–derived N-glycoproteins are trimannosyl core structures [Manα6 (Manα3)Manβ4GlcNAcβ2GlcNAc-R] with or without fucose residues linked to the chitobiose core, indicating that the dominant processing pathway in these cells is truncated relative to the mammalian pathway (reviewed by Marz et al., 1995; Jarvis, 1997; Marchal et al., 2001).
The inability of insect cells to routinely produce terminally sialylated N-glycans is important because insect cell systems are widely used to produce recombinant mammalian glycoproteins (reviewed by O’Reilly et al., 1992; Jarvis, 1993, 1997; Pfeifer, 1998). But due to the truncated nature of the major insect N-glycan processing pathway, recombinant glycoproteins produced by insect cells usually have truncated trimannosyl core glycans in place of the complex, terminally sialylated glycans found on the native products. This structural difference can be a serious problem, depending on the nature of the recombinant glycoprotein of interest and its intended application (Jenkins and Curling, 1994; Weigel, 1994; Raju et al., 1996; Grossmann et al., 1997; Chitlaru et al., 1998).
Over the past few years, our group has been addressing this problem by genetically engineering established insect cell lines to create transgenic lines that encode and express mammalian glycosyltransferases (Hollister et al., 1998, 2002; Breitbach and Jarvis, 2001; Hollister and Jarvis, 2001). The addition of these mammalian functions was designed to extend the endogenous insect cell N-glycan processing machinery and enable these cells to produce more authentic recombinant mammalian glycoproteins. Sfβ4GalT/ST6 is one transgenic insect cell line that encodes and constitutively expresses mammalian β1,4-galactosyltransferase and α2,6-sialyltransferase genes. We have shown that these cells can indeed produce terminally sialylated recombinant N-glycoproteins (Hollister and Jarvis, 2001). But we also found that the terminally sialylated N-glycans produced by these cells have artificial monoantennary structures in which only the α1,3 branch is elongated (Hollister et al., 2002). This finding suggested that Sfβ4GalT/ST6 cells have insufficient levels of N-acetylglucosaminyltransferase II activity, which led us to introduce a mammalian N-acetylglucosaminyltransferase II gene, among others, into these cells (Hollister et al., 2002). The resulting transgenic insect cell line, SfSWT-1, produced recombinant glycoproteins with biantennary sialylated N-glycans, confirming that the Sfβ4GalT/ST6 cells had insufficient levels of N-acetylglucosaminyltransferase II. However, the ability of our transgenic cell lines to produce sialylated glycoproteins raised another compelling question: If they have no CMP–sialic acids (Hooker et al., 1999; Tomiya et al., 2001), how do these cell lines sialylate their newly synthesized glycoproteins?
A partial answer to this question was obtained in the accompanying study, in which we discovered that these cell lines can produce terminally sialylated recombinant glycoproteins when cultured in the presence of an exogenous source of sialic acid, particularly the covalently bound sialic acids found in fetal bovine serum and fetuin (Hollister et al., 2003). Another requirement for glycoprotein sialylation by SWT-1 and Sfβ4GalT/ST6 cells is the mammalian α2,6-sialyltransferase gene in these cells because Sfβ4GalT cells, which lack this gene, cannot produce sialylated N-glycans, even in the presence of serum (Hollister and Jarvis, 2001; Seo et al., 2001). The precise mechanism by which extracellular sialoglycoproteins effectively support recombinant glycoprotein sialylation by the sialyltransferase in Sfβ4GalT/ST6 and SfSWT-1 cells remains to be determined. But it seems likely that these cells can somehow salvage sialic acids from extracellular sialoglycoproteins and use them to produce CMP–sialic acid, which is transported into the Golgi apparatus and used for de novo glycoprotein sialylation by the intracellular sialyltransferase.
The purpose of the present study was to extend our previous engineering efforts by transforming SfSWT-1 cells with genes encoding two mammalian enzymes involved in CMP–sialic acid biosynthesis to allow them to produce their own CMP–sialic acid (Munster et al., 1998; Nakata et al., 2000). The resulting cell line both produced CMP–sialic acid and efficiently sialylated a recombinant glycoprotein when cultured in the absence of fetal bovine serum, other exogenous sialoglycoproteins, or free sialic acid. This new transgenic insect cell line will be widely useful as an improved host for baculovirus-mediated glycoprotein production due to its ability to produce sialylated recombinant glycoproteins in serum-free media. Eliminating the serum requirement will greatly facilitate the purification, decrease the cost, and enhance the safety of these end products for various biomedical applications.
Results
Transformation of SfSWT-1 cells with mammalian genes encoding sialic acid synthase and CMP–sialic acid synthetase
SfSWT-1 cells, a transgenic derivative of Sf9 cells, produce monosialylated, biantennary N-glycans, which are the most extensively processed structures produced by any transgenic insect cell line described to date (Hollister et al., 2002). Therefore, SfSWT-1 was the logical cell line to be transformed with mammalian sialic acid synthase (SAS) (Nakata et al., 2000) and CMP–sialic acid synthetase (CMP-SAS) (Munster et al., 1998) genes. Mouse cDNAs encoding these enzymes were inserted into a dual immediate early expression plasmid, and the resulting construct was used to cotransfect SfSWT-1 cells together with a selectable marker, as described in Materials and methods. Antibiotic-resistant clones were isolated; then total RNA was prepared from each clone. Samples were analyzed for expression of the unselected markers by dot-blot hybridization. The results showed that two clones were strongly positive for both SAS and CMP-SAS RNAs (Figure 1; clones 3C3 and 4E2 in lanes 2 and 6, respectively). Additional dot-blot assays established that both clones remained strongly positive for RNA from all five of the glycosyltransferase genes originally incorporated into SfSWT-1 cells (data not shown). Both of these clones were amplified and characterized during the course of this study, and both had similar properties, but the remainder of the data presented in this report were obtained with a single clone (4E2), which was designated SfSWT-3.
Fig. 1.
Identification of SfSWT-3 clones by RNA dot-blot analysis. SfSWT-1 cells were cotransfected with an expression plasmid encoding mouse SAS and mouse CMP-SAS plus an expression plasmid encoding a zeocin resistance marker. Then zeocin-resistant clones were isolated and assayed for the presence of the SAS (A) and CMP-SAS (B) markers by RNA dot-blot analysis, as described in Materials and methods. SfSWT-1 cell extracts were used as negative controls.
Growth properties of SfSWT-3 cells
Morphologically, SfSWT-3, SfSWT-1, and Sf9 are all small, round cells with a highly refractile appearance under the phase-contrast microscope (data not shown). SfSWT-1 cells are noticeably smaller than the other two cell types, with an average diameter of about 13.5 µm, as compared with about 18.5 and 17.5 µm for SfSWT-3 and Sf9, respectively. A comparison of the growth properties of these three cell lines under identical culture conditions showed that SfSWT-3 and Sf9 cells were very similar, though the former grew to slightly lower final densities (Figure 2). In contrast, SfSWT-1 cells grew to a much higher final density after an initial period of slower growth, relative to the other cell lines. These same general trends were observed in three independent experiments. A serum-free medium supplemented with N-acetylmannosamine was used in these experiments because it includes the requisite CMP–sialic acid precursor and would facilitate purification of recombinant glycoproteins secreted from SWT-3 cells (closed symbols in Figure 2). However, similar growth curves were obtained when the experiments were performed using this same serum-free medium with no added N-acetylmannosamine (open symbols in Figure 2).
Fig. 2.
Growth properties of SfSWT-3 cells. Triplicate samples were removed from shake flask cultures of Sf9 (squares), SfSWT-1 (circles), or SfSWT-3 (triangles) cells at various times after being incubated in a serum-free medium supplemented with nothing (open symbols) or with 10 mM N-acetylmannosamine (closed symbols). Viable cell counts were performed using the trypan blue dye exclusion method, as described in Materials and methods, and average cell densities were plotted against time with standard errors indicated by the error bars (in most cases, the error bars were too small to show up on the plot).
Recombinant glycoprotein production by SfSWT-3 cells
The most common way to use insect cells for recombinant glycoprotein production is to use them as hosts for baculovirus expression vectors (reviewed by Summers and Smith, 1987; O’Reilly et al., 1992; Jarvis, 1997). Typically, the cells are infected with a recombinant baculovirus in which the sequence encoding a glycoprotein of interest is positioned under the control of the strong baculovirus polyhedrin promoter. A virus-encoded transcription complex produced during the very late phase of infection transcribes the foreign gene under the control of this promoter at high levels, and the resulting mRNA is translated and processed by cellular machinery to yield the final end product. Thus we compared baculovirus-mediated recombinant glycoprotein production by SfSWT-3 cells and the parental cell lines. Sf9, SfSWT-1, and SfSWT-3 cells were infected with AcGST-SfManI, a baculovirus expression vector encoding a glutathione-S-transferase (GST)–tagged soluble domain of the Spodoptera frugiperda class I α-mannosidase (Kawar et al., 1997, 2000), which was the model recombinant glycoprotein used in this study. Samples were taken from each culture at various times after infection and assayed by immunoblotting, as described in Materials and methods.
The results showed that these three different cell lines produced similar amounts of GST-SfManI, although SfSWT-3 cells produced slightly less (Figure 3). In Sf9 cells, GST-SfManI expression reached a plateau earlier in infection, and this protein was undetectable at 5 days postinfection. In lanes containing smaller amounts of this recombinant glycoprotein, it clearly migrates as a doublet, but in lanes containing larger amounts, it appears to migrate as a single broader band. Previous studies have shown that the doublet consists of unglycosylated (lower band) and N-glycosylated (upper band) forms of GST-SfManI (Kawar et al., 2000; Kawar and Jarvis, 2001). The presence of these two bands indicates that the efficiency of GST-SfManI glycosylation is less than 100%. Furthermore, the data in Figure 3 show that the efficiency of glycosylation decreases with increasing GST-SfManI production, suggesting that the translocation and/or initial N-glycosylation reactions become saturated. As in the growth curve experiment, we used a serum-free medium supplemented with N-acetylmannosamine in these experiments because it includes the requisite CMP–sialic acid precursor and would facilitate purification of recombinant glycoproteins secreted from SfSWT-3 cells. However, similar results were obtained using the same serum-free medium with no added N-acetylmannosamine (data not shown).
Fig. 3.
Recombinant glycoprotein production by baculovirus-infected SfSWT-3 cells. Sf9 (top row), SfSWT-1 (middle row), and SfSWT-3 (bottom row) cells cultured in serum-free medium supplemented with N-acetylmannosamine were infected with AcGST-SfManI, cell extracts were prepared from culture each at various times after infection, and equivalent aliquots were assayed by immunoblotting, as described in Materials and methods. The position of the 97-kDa protein standard in each blot is indicated by the arrows.
Production of CMP-NeuAc by SfSWT-3 cells
The next set of experiments was designed to determine if SfSWT-3 cells could produce CMP–sialic acid when cultured in the presence of the sialic acid precursor N-acetylmannosamine. Soluble fractions were prepared from SfSWT-3 cells cultured in a serum-free medium supplemented with nothing or N-acetylmannosamine, as described in Materials and methods. Cytosolic fractions were then prepared and fractionated by preparative fast-performance liquid chromatography (FPLC) and high pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD), as described in Materials and methods. A peak that coeluted with standard CMP N-acetylneuraminic acid (Neu5Ac) was observed in the cytosolic fraction of SfSWT-3 cells grown in the presence (Figure 4A) but not in the absence (data not shown) of N-acetylmannosamine. This material was recovered after preparative HPAEC-PAD, split into two equal aliquots, and either held as a control or used for mild acid hydrolysis. A subsequent HPAEC-PAD analysis showed that the untreated material still coeluted with the CMP-Neu5Ac standard (data not shown), whereas the hydrolyzed material yielded two peaks, one of which coeluted with free Neu5Ac and the other of which was free CMP (Figure 4B). These results indicated that SfSWT-3 cells were able to produce CMP–sialic acid when cultured in a serum-free medium supplemented with N-acetylmannosamine.
Fig. 4.
CMP–sialic acid production by SfSWT-3 cells. Cytosolic extracts were prepared from SfSWT-3 cells cultured in a serum-free medium supplemented with N-acetylmannosamine, and a putative peak of CMP–sialic acid was isolated by preparative FPLC and HPAEC-PAD, as described in Materials and methods. This material was split into two equal aliquots and held on ice (A) or subjected to mild acid hydrolysis (B). Then each sample was reanalyzed by HPAEC-PAD, as described in Materials and methods. The elution positions of standard CMP-Neu5Ac and Neu5Ac and the presumptive elution position of CMP are indicated by the arrows.
Sialylation of a recombinant glycoprotein by SfSWT-3 cells
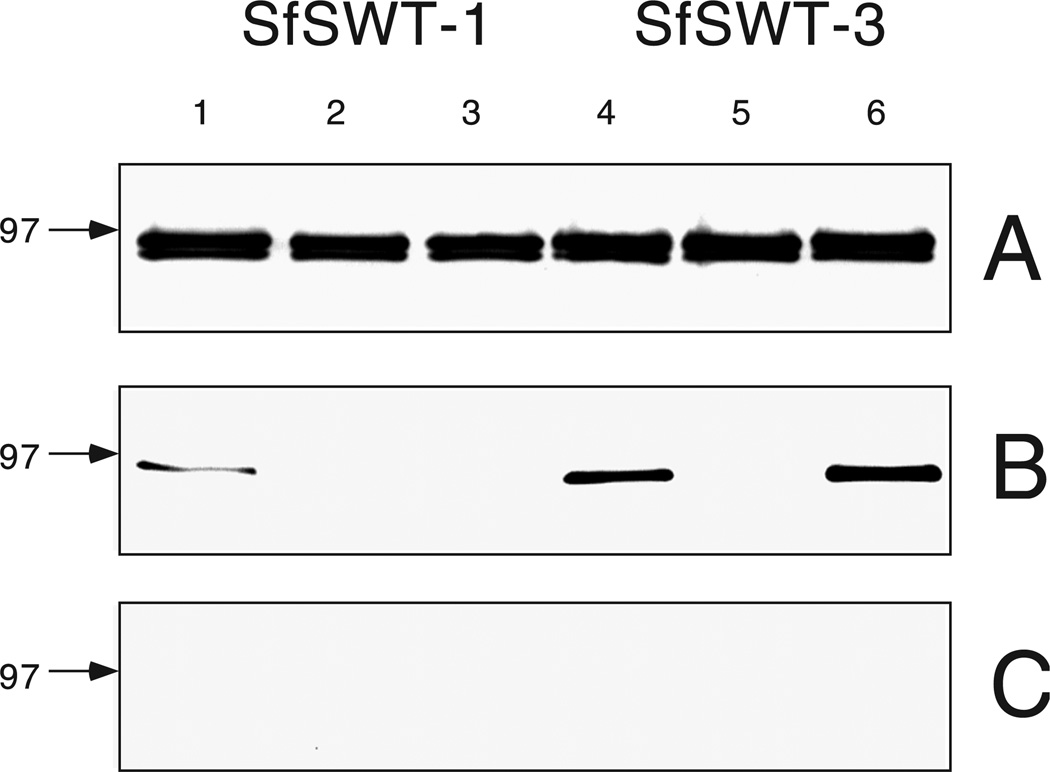
If sialic acid can be salvaged from exogenous sialoglycoproteins and reutilized for de novo glycoprotein sialylation in SfSWT-1 cells, then SfSWT-3 cells, which can produce their own CMP–sialic acid, should not require exogenous sialoglycoproteins for this function. We tested this hypothesis by analyzing the N-glycans isolated from the recombinant GST-SfManI produced by SfSWT-3 and SfWT-1 cells under various culture conditions. Each cell line was infected with AcGST-SfManI, and the recombinant GST-SfManI was affinity purified and assayed by immunoblotting and lectin blotting, as described in Materials and methods. The immunoblotting results showed that approximately equal amounts of GST-SfManI were loaded in each case (Figure 5A). The lectin blotting results showed that Sambucus nigra agglutinin (SNA) bound to the GST-SfManI produced by SfSWT-1 cells cultured in the presence but not in the absence of serum, as expected (Figure 5B, lanes 1–3). In contrast, SNA bound to the GST-SfManI produced by SfSWT-3 cells cultured in the presence or absence of serum if the serum-free medium was supplemented with N-acetylmannosamine (Figure 5B, lanes 4–6). Furthermore, SNA binding was most intense with the GST-SfManI produced by SfSWT-3 cells cultured in serum-free medium supplemented with N-acetylmannosamine, suggesting that this protein contained the highest level of terminal sialic acids.
Fig. 5.
Lectin blotting analysis of GST-SfManI. GST-SfManI was affinity-purified from AcGST-SfManI-infected SfSWT-1 (lanes 1–3) or SfSWT-3 (lanes 4–6) cells cultured in the presence of FCS (lanes 1 and 4), in a serum-free medium (lanes 2 and 5), or in a serum-free medium supplemented with N-acetylmannosamine (lanes 3 and 6), as described in Materials and methods. Normalized samples were resolved by SDS–PAGE and transferred to immobilon filters; then the filters were cut into panels and probed with anti-GST (A), SNA (B), or SNA that was preincubated with a competing sugar (C). The position of the 97-kDa protein standard in each blot is indicated by the arrows.
The specificity of the lectin blotting assays was evidenced by the fact that SNA bound only to the upper band in the GST-SfManI doublet, which is the N-glycosylated form. In addition, SNA failed to bind to any proteins after it had been preincubated with a competing sugar (Figure 5C). Finally, SNA failed to bind to GST-SfManI from SfSWT-3 cells cultured in serum-free medium supplemented with N-acetylmannosamine when it was pretreated with either peptide: N-glycosidase F (PNGase-F) or neuraminidase (Figure 6). It is noteworthy that PNGase-F converted the GST-SfManI doublet into the single, lower Mr species (Figure 6, lane 2), whereas neuraminidase treatment slightly increased the Mr of the upper band (Figure 6, compare lanes 3 and 4), because these results confirmed that the lower species is unglycosylated and the upper contains terminal sialic acids.
Fig. 6.
Specificity of lectin blotting analysis. Normalized samples of GST-SfManI purified from AcGST-SfManI-infected SfSWT-3 cells cultured in serum-free medium supplemented with N-acetylmannosamine were treated with buffer alone (lanes 1 and 4), PNGase-F (lanes 2 and 5), or neuraminidase (lanes 3 and 6), then resolved by SDS–PAGE and transferred to an immobilon filter. The filter was cut into two panels, and one was probed with anti-GST (lanes 1–3) while the other was probed with SNA (lanes 4–6), as described in Materials and methods. The position of the 97-kDa protein standard is indicated by the arrows.
Finally, it is noteworthy that SNA did not detectably bind to the GST-SfManI produced by SfSWT-1 cells cultured in serum-free medium supplemented with N-acetylmannosamine, whereas in the accompanying study, SNA did bind weakly to the GST-SfManI produced by Sfβ4GalT/ST6 cells under these same culture conditions. This difference is most likely explained by the fact that the Sfβ4GalT/ST6 cells were exposed to N-acetylmannosamine for twice as long as the SfSWT-1 cells. However, it also is possible that this simply reflects differences in the properties of these two clonal cell lines.
The results of the lectin blotting assays were extended by direct N-glycan structural analyses. Gel electrophoresis with Coomassie blue staining showed that GST-SfManI was the only detectable protein in affinity-purified preparations from SfSWT-1 and SfSWT-3 cells cultured under various conditions (data not shown). Subsequently, each preparation was treated with PNGase-F alone or PNGase-F plus neuraminidase, and then the N-glycans were recovered and analyzed by HPAEC-PAD, as described in Materials and methods.
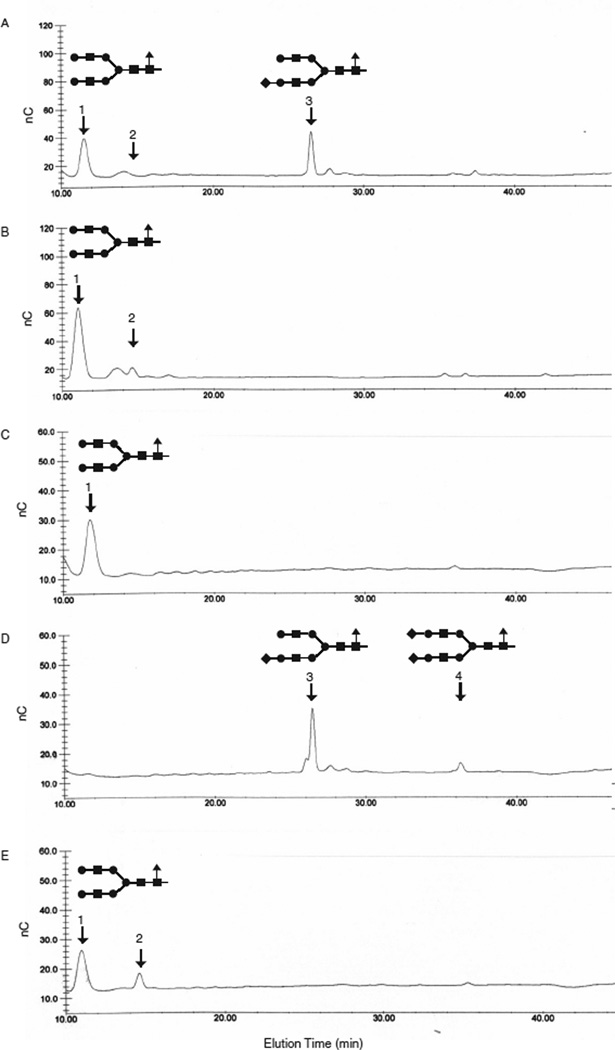
The results demonstrated that the GST-SfManI produced by SWT-1 cells cultured in the presence of serum had two major processed N-glycans (peaks 1 and 3 in Figure 7A). As expected, these glycans coeluted with terminally galactosylated (peak 1) or monosialylated (peak 3) biantennary N-glycan standards, as they had been shown to be structurally identical to these standards in previous tandem mass spectroscopy experiments (Hollister et al., 2002). The presence of sialic acid on the N-glycan in peak 3 was confirmed by neuraminidase treatment, which eliminated peak 3, increased the size of peak 1, and produced a new peak that coeluted with free Neu5Ac (peak 2 in Figure 7B). The GST-SfManI produced by SfSWT-3 cells cultured in serum-free medium with no added N-acetylmannosamine contained only the terminally galactosylated N-glycan (peak 1 in Figure 7C) with no detectable sialylated N-glycans. However, culturing these same cells in serum-free medium supplemented with N-acetylmannosamine allowed them to produce the monosialylated biantennary glycan (peak 3 in Figure 7D). The presence of terminal sialic acid on this N-glycan was confirmed by neuraminidase treatment, which converted it to the terminally galactosylated biantennary N-glycan and free Neu5Ac (peaks 1 and 2, respectively, in Figure 7E). Unexpectedly, the GST-SfManI produced by SfSWT-3 cells cultured under these conditions had no detectable terminally galactosylated N-glycans, which make up at least half of the total N-glycan population found on the GST-SfManI produced by SfSWT-1 cells cultured in serum (Figure 7A). Furthermore, SfSWT-3 cells cultured in serum-free medium supplemented with N-acetylmannosamine produce a minor N-glycan subpopulation that coeluted with a biantennary disialylated N-glycan standard (peak 4 in Figure 7D). This structure, like the monosialylated N-glycan, was eliminated by neuraminidase treatment (Figure 7E).
Fig. 7.
HPAEC-PAD analysis of N-glycans from GST-SfManI. GST-SfManI was affinity-purified from AcGST-SfManI-infected SfSWT-1 (A–B) or SfSWT-3 (C–E) cells cultured in medium containing serum (A–B), serum-free medium (C), or serum-free medium supplemented with 10 mM N-acetylmannosamine (D–E), as described in Materials and methods. Each protein was then digested with PNGase-F alone (A, C, D) or with PNGase-F and neuraminidase (B, E), and the N-glycans were recovered and analyzed by HPAEC-PAD, as described in Materials and methods. The elution positions of standards, including a digalactosylated biantennary N-glycan (1), a monosialylated N-glycan (2), free Neu5Ac (3), and a disialylated biantennary N-glycan (4), are indicated by the arrows.
Discussion
The first reports of baculovirus-mediated recombinant protein production (Smith et al., 1983; Pennock et al., 1984) were followed by a flurry of efforts to improve baculoviral vectors and facilitate their use. Over the following decade, these goals were largely accomplished, and a vast array of tools and methods became available for the isolation and identification of highly efficacious recombinant baculovirus expression vectors (reviewed by O’Reilly et al., 1992; Jarvis, 1997). These technical advances were facilitated by great progress in our basic understanding of baculovirus molecular biology (Miller, 1997), which included completion of the first baculovirus genome project (Ayres et al., 1994). Conversely, relatively little effort was dedicated to understanding or developing the other half of this important recombinant protein production system, the insect cell. This was ironic, considering that the baculovirus-insect cell system was highly regarded as a tool for recombinant glycoprotein production because of its protein processing capabilities. Equally ironically, the widespread use of this system for recombinant glycoprotein production ultimately improved our understanding of insect cell glycobiology.
Studies on enzyme activities and N-glycan structures in mosquito cells provided an early view of the insect protein N-glycosylation pathway (Butters and Hughes, 1981; Butters et al., 1981; Hsieh and Robbins, 1984). In essence, these studies indicated that the insect pathway is a truncated version of the mammalian pathway because it includes enzymes involved in N-glycan trimming but not those involved in elongation. Most structural studies of N-glycans isolated from baculovirus-expressed recombinant glycoproteins, together with the apparent absence of sialyltransferase activity and CMP–sialic acid in lepidopteran insect cells, supported this conclusion (reviewed by Marz et al., 1995; Jarvis, 1997; Marchal et al., 2001). In addition, some data indicated that insects have more extensive N-glycan processing capabilities, which allow them to produce complex, even terminally sialylated N-glycans under some conditions (Davidson et al., 1990; Roth et al., 1992; Karacali et al., 1997, 1999; Joshi et al., 2001; Watanabe et al., 2001; Kim et al., 2002; Jooster and Shuler, 2003). However, these capabilities appear to be relatively limited and do not reflect the major N-glycan processing pathway in insect cell systems (Marchal et al., 2001).
The deduced difference between the major N-glycan processing pathways of lepidopteran insect and mammalian cells posed a serious problem for those interested in using the baculovirus-insect cell system for recombinant glycoprotein production. Thus our group began to use mammalian glycosyltransferase genes to create transgenic insect cell lines with extended N-glycan processing capabilities. The addition of mammalian β1,4-galactosyltransferase and α2,6-sialyltransferase genes yielded Sf9 and Tn-5B1-4 variants that could produce terminally sialylated N-glycans (Breitbach and Jarvis, 2001; Hollister and Jarvis, 2001). But, these were artificial monoantennary structures in which only the α3 branch was elongated, presumably because the cells had inadequate levels of N-acetylglucosaminyltransferase II activity. This presumption was confirmed with the isolation of a new cell line, SfSWT-1, which included a mammalian N-acetylglucosaminyltransferase II gene and produced biantennary, terminally sialylated N-glycans (Hollister et al., 2002). However, the ability of transgenic insect cell lines to produce sialylated N-glycans was somewhat inexplicable because Sf9 cells have no detectable CMP–sialic acid (Hooker et al., 1999; Tomiya et al., 2001) and the new cell lines had only been engineered to produce mammalian glycosyltransferases, not nucleotide sugar precursors.
The accompanying study provided our first clues about how transgenic insect cells with no obvious source of CMP–sialic acid can sialylate newly synthesized glycoproteins. That study defined a set of minimal requirements as (1) the intracellular sialyltransferase activity encoded by a mammalian transgene in these cells and (2) the presence of an exogenous sialoglycoprotein. Our working model is that these cells can salvage terminal sialic acid from extracellular sialoglycoproteins and process it to CMP–sialic acid, which can be utilized by the intracellular sialyltransferase. This model is highly speculative and must be investigated in much greater detail. But the discovery of the exogenous sialoglycoprotein requirement was important because it revealed that our transgenic insect cell lines could not produce recombinant sialoglycoproteins when cultured in serum-free media. This was a problem because the use of a serum-containing growth medium would raise cost, safety, and regulatory issues in the biotechnology industry and complicate efforts to recover and purify any recombinant glycoprotein produced by these cell lines. Another potential problem was that the putative salvaging mechanism might limit the efficiency of recombinant glycoprotein sialylation by SfSWT-1 cells.
In this study, we addressed these problems by engineering SfSWT-1 cells to produce their own CMP–sialic acid. This was accomplished by transforming these cells with mammalian SAS and CMP-SAS genes to produce a new transgenic insect cell line, designated SfSWT-3. Sf9, SfSWT-1, and SfSWT-3 cells are morphologically similar, though SfSWT-1 cells are slightly smaller, and all three cell lines have similar growth properties. SfSWT-3 cells express seven transgenes, including the five glycosyltransferase genes of SfSWT-1 and the two new mouse genes, and can produce both CMP–sialic acid and a sialylated recombinant glycoprotein when grown in serum-free medium supplemented with N-acetylmannosamine. Furthermore, SfSWT-3 cells grown under these conditions sialylated recombinant GST-SfManI more efficiently and extensively than SfSWT-1 cells grown in serum. Thus SfSWT-3 cells have the most extensive N-glycan processing pathway of any transgenic insect cell line described to date and should be widely useful as an improved host for baculovirus-mediated recombinant glycoprotein production.
In addition to the practical utility of SfSWT-3 cells, this study provided some additional information about lepidopteran insect cell glycobiology. The ability of SfSWT-3 cells to produce sialylated N-glycans in the absence of an exogenous sialoglycoprotein supports the idea that exogenous sialoglycoproteins can provide a source of sialic acid for de novo glycoprotein sialylation by SfSWT-1 cells. This finding justifies further efforts to elucidate this putative insect cell salvaging mechanism. In addition, the ability of SfSWT-3 cells to produce CMP–sialic acid and a sialoglycoprotein when cultured in serum-free media supplemented with N-acetylmannosamine supports two previous conclusions about sialic acid metabolism in these insect cells and their Sf9 cell progenitors. The ability to produce CMP–sialic acid supports the idea that these cells have a functional N-acetylmannosamine kinase activity because the murine SAS gene used in this study actually encodes a Neu5Ac 9-phosphate synthase, not a Neu5Ac synthase, which utilizes N-acetylmannosamine-6-phosphate, not N-acetylmannosamine, as a substrate (Nakata et al., 2000). This conclusion is consistent with the finding that D. melanogaster (Kim et al., 2002) and human (Lawrence et al., 2000) SAS genes, which have the same substrate specificity as murine SAS, were able to produce intracellular Neu5Ac when expressed in Sf9 cells cultured in the presence of N-acetylmannosamine. Furthermore, it has been demonstrated that uninfected Sf9 cell lysates have N-acetylmannosamine kinase activity (Effertz et al., 1999), and two groups (Angata and Varki, 2000; Kim et al., 2002) have identified a Drosophila gene with similarity to the kinase domain of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (Stasche et al., 1997). The ability to produce CMP–sialic acid also suggests that SfSWT-3 cells and their progenitors have a phosphatase that can convert N-acetylneuraminate-9-phosphate, the product of murine SAS, to N-acetylneuraminic acid, a substrate for murine CMP-SAS. Finally, the ability of SfSWT-3 cells to produce a sialoglycoprotein supports the idea that these cells and their progenitors have a nucleotide sugar transporter that can transport CMP–sialic acid into the Golgi apparatus.
Although this conclusion was previously suggested by the ability of other transgenic insect cell lines to produce sialylated glycoproteins, the actual sialylation mechanism in these cells is entirely open to speculation. In contrast, because SfSWT-3 cells produce sialylated glycoproteins only under conditions that allow them to produce CMP–sialic acid, the sialylation mechanism in these cells most likely requires transport of CMP–sialic acid into the Golgi apparatus, where it is used as the conventional donor substrate for the mammalian α2,6-sialyltransferase. Two putative Drosophila CMP–sialic acid transporter genes have been identified (Ferraz et al., 1999; Kim et al., 2002), but one has been experimentally shown to be a UDP-galactose/UDP-N-acetylgalactosamine transporter (Aumiller and Jarvis, 2002; Segawa et al., 2002) and the other has not yet been tested. We are currently using molecular genetic approaches to extend these observations as part of a continuing effort to unequivocally define the nature of insect N-glycan processing pathways.
Materials and methods
Construction of an expression plasmid encoding SAS and CMP-SAS
pBS-DIE1SAS/CMP-SAS is a dual immediate early expression plasmid that encodes mouse SAS (Nakata et al., 2000) and CMP-SAS (Munster et al., 1998) cDNAs under the control of back-to-back baculovirus ie1 promoters and the baculovirus hr5 enhancer element. The ie1 promoter and hr5 enhancer provide constitutive foreign gene expression in uninfected insect cells (Jarvis et al., 1990, 1996). Therefore, pBS-DIE1SAS/CMP-SAS can be used to stably transform insect cell lines.
The SAS and CMP-SAS cDNAs used in this study were I.M.A.G.E. Consortium (Lennon et al., 1996) clones 3154822 (GenBank accession number BC003307) and 3465964 (GenBank accession number BE689556), each purchased from Invitrogen (Carlsbad, CA). The full-length SAS cDNA sequence had already been determined. Thus we simply excised a 1.3-kb StuI–XhoI fragment containing the SAS open reading frame from the commercial cDNA clone, blunted the XhoI end with T4 DNA polymerase, and subcloned the gel-purified fragment into the SmaI site of pAc64KDIETV1 (Jarvis et al., 2001) to create an intermediate plasmid called pAc64KDIE1SAS.
In contrast, the commercial CMP-SAS clone was a 5′ expressed sequence tag, and only 413 bp of reliable sequence data were available. Therefore, we sequenced the cDNA insert in this clone and found that it includes a full-length open reading frame identical to the published mouse DNA sequence (Munster et al., 1998), except for two nucleotide differences (data not shown). One of these differences induced no change in the deduced amino acid sequence, whereas the other changed the ninth amino acid from valine to alanine. The 1.7-kb XhoI–NotI fragment containing this full-length CMP-SAS open reading frame was subsequently excised from this clone, both ends were blunted with T4 DNA polymerase, and the gel-purified fragment was subcloned into the SpeI (repaired) site of pAc64KDIE1SAS to create a second intermediate plasmid called pAc64KDIE1SAS/ CMP-SAS. Finally, the dual ie1-driven SAS/CMP-SAS cassette was excised from pAc64KDIE1SAS/CMP-SAS, and the gel-purified cassette was subcloned into pBS-KS (Stratagene, La Jolla, CA) to obtain pBS-DIE1SAS/ CMP-SAS.
Each plasmid was isolated from large-scale bacterial cultures by standard alkaline lysis and CsCl–ethidium bromide density gradient ultracentrifugation methods, as described previously (Sambrook et al., 1989), and their genetic structures were confirmed by restriction mapping prior to being used for subcloning or cotransfection experiments.
Cells and viruses
Sf9 (Summers and Smith, 1987), SfSWT-1 (Hollister et al., 2002), and SfSWT-3 (this study) cells were routinely maintained as shake-flask cultures at densities of about 0.3–3.0 × 106 cells/ml in serum-free media or complete TNM-FH medium. The latter medium consisted of Trichoplusia ni medium–Fred Hink (TNM-FH) prepared from powdered chemicals and supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT) and 1% (w/v) pluronic F68, as described previously (Summers and Smith, 1987; Murhammer and Goochee, 1988). The serum-free media used in this study included HyQ Sfx-Insect (Hyclone) and Sf 900-II SFM (Invitrogen). AcGST-SfManI was a recombinant baculovirus used to express a soluble GST-SfManI fusion protein under the control of the polyhedrin promoter, as described previously (Kawar et al., 2000).
Isolation of SfSWT-3 cells
SfSWT-3, a stably transformed insect cell line ultimately derived from Sf9 cells, was produced using a modification of an established procedure for genetic transformation of lepidopteran insect cells (Jarvis et al., 1990; Jarvis and Guarino, 1995). Briefly, a modified calcium phosphate method was used to cotransfect SfSWT-1 cells with a mixture of pBS-DIE1SAS/CMP-SAS and pIZ/V5-HIS (Invitrogen), the latter of which encodes a zeocin resistance marker under the control of a baculovirus ie2 promoter. The transfected cells were washed, fed with complete TNM-FH, and allowed to recover for 2 days; then the growth medium was replaced with complete TNM-FH medium containing sequentially increasing concentrations of zeocin, ranging from 0.3–1.0 mg/ml. After a 2-week selection period, antibiotic-resistant clones were isolated by limiting dilution in 96-well plates. Multiple clones were amplified in stepwise fashion into larger cultures. Then total RNA was prepared from each clone using the Tri-Reagent (Molecular Research Center, Cincinnati, OH), and samples were assayed for SAS and CMP-SAS expression by dot-blot hybridization, as described previously (Jarvis et al., 1996). Additional dot blot assays were performed to check each clone for expression of N-acetylglucosaminyltransferase I, N-acetylglucosaminyltransferase II, β1,4-galactosyltransferase, α2,6-sialyltransferase, and α2,3-sialyltransferase RNAs, which were the transgenes expressed by SfSWT-1 cells. Several clones expressed all seven mammalian transgenes, and clone 4E2, which was used for the experiments described in this study, was designated SfSWT-3.
Cellular growth curves
Sf9, SfSWT-1, or SfSWT-3 cells from Sf 900-II SFM maintenance cultures were seeded at a density of 0.6 × 106 cells/ml into 125-ml shake flasks containing a total of 50 ml of the same growth medium supplemented with 10 mM N-acetylmannosamine. The flasks were transferred to a Forma Model 4580 console shaker-incubator adjusted to 125 rpm and 28°C. Then triplicate samples were removed from each flask at various times after seeding, and viable cell counts were performed using the trypan blue dye exclusion method, as described previously (Summers and Smith, 1987).
Immunoblotting analyses
Sf9, SfSWT-1, or SfSWT-3 cells were maintained for one passage in Sf 900-II SFM medium supplemented with 10 mM N-acetylmannosamine. The cells were then freshly seeded in the same medium, allowed to grow for 48 h, and then incubated together with 5 plaque forming units/cell of AcGST-SfManI on a rocking platform for 1 h at 28°C. The cells were then repelleted, washed twice with Sf900-II SFM, resuspended in Sf 900-II SFM medium supplemented with 10 mM N-acetylmannosamine, and dispensed into six-well tissue culture plates (Corning, Corning, NY) at a density of 1.0 × 106 cells/well in a total of 1.5 ml.
At various times after infection, the medium was harvested from individual wells containing each virus-infected cell type and clarified in a microcentrifuge. Equivalent samples of the cell-free supernatants were then resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), the proteins were electrophoretically transferred to immobilon membranes (Millipore, Bedford, MA), and the membranes were blocked and used for immunoblotting analysis, as described previously (Laemmli, 1970; Towbin et al., 1979; Jarvis et al., 1996). The primary antibody used in this study was a commercial polyclonal rabbit anti-GST (Sigma, St. Louis, MO), the secondary antibody was a commercial goat anti-rabbit IgG conjugated to alkaline phosphatase (Sigma), and immune complexes were detected using a standard alkaline phosphatase–based color reaction (Blake et al., 1984).
CMP–sialic acid determinations
SfSWT-3 cells from shake flask cultures maintained in Sf 900-II SFM were seeded at a density of 0.5 × 106 cells/ml in fresh medium supplemented with nothing or 10 mM N-acetylmannosamine. The cells were grown for 24 h, to a density of 1 million cells/ml, harvested by centrifugation, and washed twice with phosphate buffered saline (10 mM sodium phosphate, 140 mM NaCl, pH 7.4). The washed cells were resuspended in 40 mM ammonium bicarbonate, pH 8.0, and sonicated for 0.5 min on ice using a Branson Model 450 Sonifier (Danbury, CT) adjusted to 50% P and 40% duty. The sonicate was clarified by centrifugation at 4°C for 1 h at 100,000 × g in a SW28 rotor in an Optima Model XL-100K ultracentrifuge (Beckman-Coulter, Palo Alto, CA). The cytosolic supernatants were harvested, ethanol was added to a final concentration of 60%, and then, after 10 min on ice, the resulting precipitate was removed by ultracentrifugation, as described. The supernatant was harvested, evaporated, redissolved in water, and injected into an FPLC system (Pharmacia Biotech, Piscataway, NJ) equipped with a Mono-Q column. The column was washed with water from 0–10.1 min, washed with 6% buffer B (2.0 M ammonium acetate) from 10.1–12.0 min, and eluted with a linear gradient of 6–15% buffer B from 12.0–30.0 min. Finally, the column was stripped with 100% buffer B for 10 min and reequilibrated with water for 10 min. Absorption was monitored at 280 nm, and the material eluting at about 14.5 min during the gradient, which corresponded to the elution time of standard CMP-Neu5Ac (Sigma), was collected. This material was concentrated by evaporation, resuspended in water, and further resolved by preparative HPEAC-PAD on a PA1 column. The HPAEC-PAD conditions included a 10-min wash with 0.2 N NaOH, followed by elution with a linear gradient of 0–500 mM sodium acetate in 0.2N NaOH developed over 30 min. The material eluting in fractions corresponding to standard CMP-Neu5Ac was collected, concentrated, and split into equal aliquots. One aliquot was held on ice while the other was hydrolyzed with 2 N formic acid for 1 h at 80°C. Finally, the control and hydrolyzed samples were reanalyzed by HPAEC-PAD as described.
N-glycan structural analyses
SfSWT-1 or SfSWT-3 cells were maintained for one passage in serum-free media supplemented with 10% fetal bovine serum, nothing, or 10 mM N-acetylmannosamine. The cells were then freshly seeded in the same media, allowed to grow for 48 h, and then infected with AcGST-SfManI at a multiplicity of 5 plaque forming units/cell as described (see Immunoblotting analyses). After being washed twice, the infected cells were resuspended in the same media and returned to the incubator shaker until 72 h postinfection; then cells and debris were removed by low-speed centrifugation. The GST-SfManI was purified from the supernatants by glutathione affinity chromatography, as described previously (Hollister et al., 2002). Equal amounts of each purified GST-SfManI preparation were then analyzed by immunoblotting and lectin blotting analyses, as described previously (Hollister and Jarvis, 2001). Prior to some lectin blotting analyses, the GST-SfManI was pretreated with buffer alone, with 17,500 U/ml of PNGase-F (New England Biolabs, Beverly, MA), or with 100 mU/ml of Arthrobacter urefaciens neuraminidase (Calbiochem, La Jolla, CA) overnight at 37°C. For the more direct structural analyses, larger amounts of purified GST-SfManI were purified at 96 h postinfection from SfSWT-1 and SfSWT-3 cells, deglycosylated with PNGase-F alone or PNGase-F plus neuraminidase, and the N-glycans were recovered and analyzed by HPAEC-PAD, as described previously (Hollister and Jarvis, 2001; Hollister et al., 2002). Prior to these latter analyses, the purity of the samples was verified by SDS–PAGE with Coomassie blue staining and immunoblotting. The N-glycan standards used for these HPAEC-PAD analyses included terminally digalactosylated (NA2F, eluted at 11.5 min), monosialylated (A1F, eluted at 26.5 min), and disialylated (A2F, eluted at 36.3 min) biantennary structures, purchased from Glyko (Novato, CA), and Neu5Ac (eluted at 14.9 min), purchased from Sigma.
Acknowledgments
This work was supported by NIH Grant number GM49734 and NSF Grant BES-9818001.
Abbreviations
- CMP
cytidine 5′-monophosphate
- FPLC
fast-performance liquid chromatography
- GST
glutathione-S-transferase
- HPAEC-PAD
high pH anion-exchange chromatography with pulsed amperometric detection
- PNGase-F
peptide: N-glycosidase F
- SAS
sialic acid synthase or N-acetylneuraminic acid 9-phosphate synthase
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SNA
Sambucus nigra agglutinin
- TNM-FH
Trichoplusia ni medium–Fred Hink
References
- Angata T, Varki A. Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 2000;275:22127–22135. doi: 10.1074/jbc.M002775200. [DOI] [PubMed] [Google Scholar]
- Aumiller JJ, Jarvis DL. Expression and functional characterization of a nucleotide sugar transporter from Drosophila melanogaster: relevance to protein glycosylation in insect cell expression systems. Prot. Exp. Purif. 2002;26:438–448. doi: 10.1016/s1046-5928(02)00550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres MD, Howard SC, Kuzio J, Lopez-Ferber M, Possee RD. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase conjugated anti-antibody on western blot. Anal. Biochem. 1984;36:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Breitbach K, Jarvis DL. Improved glycosylation of a foreign protein by Tn-5B1-4 cells engineered to express mammalian glycosyltransferases. Biotech. Bioengr. 2001;74:230–239. doi: 10.1002/bit.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters TD, Hughes RC. Isolation and characterization of mosquito cell membrane glycoproteins. Biochim. Biophys. Acta. 1981;640:655–671. doi: 10.1016/0005-2736(81)90096-1. [DOI] [PubMed] [Google Scholar]
- Butters TD, Hughes RC, Vischer P. Steps in the biosynthesis of mosquito cell membrane glycoproteins and the effects of tunicamycin. Biochim. Biophys. Acta. 1981;640:672–686. doi: 10.1016/0005-2736(81)90097-3. [DOI] [PubMed] [Google Scholar]
- Chitlaru T, Kronman C, Zeevi M, Kam M, Harel A, Ordentlich A, Velan B, Shafferman A. Modulation of circulatory residence of recombinant acetylcholinesterase through biochemical or genetic manipulation of sialylation levels. Biochem. J. 1998;336:647–658. doi: 10.1042/bj3360647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson DJ, Fraser MJ, Castellino FJ. Oligosaccharide processing in the expression of human plasminogen cDNA by lepidopteran insect (Spodoptera frugiperda) cells. Biochemistry. 1990;29:5584–5590. doi: 10.1021/bi00475a024. [DOI] [PubMed] [Google Scholar]
- Effertz K, Hinderlich S, Reutter W. Selective loss of either the epimerase or kinase activity of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase due to site- directed mutagenesis based on sequence alignments. J. Biol. Chem. 1999;274:28771–28778. doi: 10.1074/jbc.274.40.28771. [DOI] [PubMed] [Google Scholar]
- Ferraz C, Vidal S, Brun C, Bucheton A, Demaille JG. Sequencing the distal × chromosome of Drosophila melanogaster. 1999 GenBank Acc. No. AL023874. [Google Scholar]
- Grossmann M, Wong R, Teh NG, Tropea JE, East-Palmer J, Weintraub BD, Szkudlinski MW. Expression of biologically active human thyrotropin (hTSH) in a baculovirus system: effect of insect cell glycosylation on hTSH activity in vitro and in vivo. Endocrinology. 1997;138:92–100. doi: 10.1210/endo.138.1.4897. [DOI] [PubMed] [Google Scholar]
- Hollister J, Jarvis DL. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian β1,4-galactosyltransferase and alpha 2,6-sialyltransferase genes. Glycobiology. 2001;11:1–9. doi: 10.1093/glycob/11.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Hollister JR, Shaper JH, Jarvis DL. Stable expression of mammalian beta 1,4-galactosyltransferase extends the N-glycosylation pathway in insect cells. Glycobiology. 1998;8:473–480. doi: 10.1093/glycob/8.5.473. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Grabenhorst E, Nimtz M, Conradt HO, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister J, Conradt H, Jarvis DL. Evidence for a sialic acid salvaging pathway in lepidopteran insect cells. Glycobiology. 2003;13:487–495. doi: 10.1093/glycob/cwg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker AD, Green NH, Baines AJ, Bull AT, Jenkins N, Strange PG, James DC. Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotech. Bioengr. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hsieh P, Robbins PW. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J. Biol. Chem. 1984;259:2375–2382. [PubMed] [Google Scholar]
- Jarvis DL. Continuous foreign gene expression in stably-transformed insect cells. In: Goosen MFA, Daugulis A, Faulkner P, editors. Insect cell culture engineering. New York: Marcel Dekker; 1993. pp. 193–217. [Google Scholar]
- Jarvis DL. Baculovirus expression vectors. In: Miller LK, editor. The baculoviruses. New York: Plenum Press; 1997. pp. 389–431. [Google Scholar]
- Jarvis DL, Guarino LA. Continuous foreign gene expression in transformed lepidopteran insect cells. In: Richardson CD, editor. Baculovirus expression protocols. vol. 39. Clifton NJ: Humana Press; 1995. pp. 187–202. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Fleming JA, Kovacs GR, Summers MD, Guarino LA. Use of early baculovirus promoters for continuous expression and efficient processing of foreign gene products in stably transformed lepidopteran cells. Bio/Technology. 1990;8:950–955. doi: 10.1038/nbt1090-950. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Weinkauf C, Guarino LA. Immediate early baculovirus vectors for foreign gene expression in transformed or infected insect cells. Prot. Expr. Purif. 1996;8:191–203. doi: 10.1006/prep.1996.0092. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Howe D, Aumiller JJ. Novel baculovirus expression vectors that provide sialylation of recombinant glycoproteins in lepidopteran insect cells. J. Virol. 2001;75:6223–6227. doi: 10.1128/JVI.75.13.6223-6227.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins N, Curling EMA. Glycosylation of recombinant proteins: problems and prospects. Enz. Micr. Technol. 1994;16:354–364. doi: 10.1016/0141-0229(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Shuler ML. Production of a sialylated N-linked glycoprotein in insect cells: Role of glycosidases and effect of harvest time on glycosylation. Biotech. Progr. 2003;19:193–201. doi: 10.1021/bp025695h. [DOI] [PubMed] [Google Scholar]
- Joshi L, Shuler ML, Wood HA. Production of a sialylated N-linked glycoprotein in insect cells. Biotech. Progr. 2001;17:822–827. doi: 10.1021/bp010071h. [DOI] [PubMed] [Google Scholar]
- Karacali S, Kirmizigul S, Deveci R, Deveci O, Onat T, Gurcu B. Presence of sialic acid in prothoracic glands of Galleria mellonella (Lepidoptera) Tiss. Cell. 1997;29:315–321. doi: 10.1016/s0040-8166(97)80007-9. [DOI] [PubMed] [Google Scholar]
- Karacali S, Kirmizigul S, Deveci R. Sialic acids in developing testis of Galleria mellonella (Lepidoptera) Invert. Repr. Dev. 1999;35:225–229. [Google Scholar]
- Kawar ZS, Jarvis DL. Biosynthesis and intracellular localization of a lepidopteran insect alpha 1,2-mannosidase. Insect Biochem. Mol. Biol. 2001;31:289–297. doi: 10.1016/s0965-1748(00)00121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawar Z, Herscovics A, Jarvis DL. Isolation and characterization of an alpha 1,2-mannosidase cDNA from the lepidopteran insect cell line Sf9. Glycobiology. 1997;7:433–443. doi: 10.1093/glycob/7.3.433. [DOI] [PubMed] [Google Scholar]
- Kawar Z, Romero PA, Herscovics A, Jarvis DL. N-glycan processing by a lepidopteran insect alpha 1,2-mannosidase. Glycobiology. 2000;10:347–355. doi: 10.1093/glycob/10.4.347. [DOI] [PubMed] [Google Scholar]
- Kim K, Lawrence SM, Park J, Pitts L, Vann WF, Betenbaugh MJ, Palter KB. Expression of a functional Drosophila melanogaster N-acetylneuraminic acid (Neu5Ac) phosphate synthase gene: evidence for endogenous sialic acid biosynthetic ability in insects. Glycobiology. 2002;12:73–83. doi: 10.1093/glycob/12.2.73. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Ann. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence SM, Huddleston KA, Pitts LR, Nguyen N, Lee YC, Vann WF, Coleman TA, Betenbaugh MJ. Cloning and expression of the human N-acetylneuraminic acid phosphate synthase gene with 2-keto-3-deoxy-D-glycero-D-galacto-nononic acid biosynthetic ability. J. Biol. Chem. 2000;275:17869–17877. doi: 10.1074/jbc.M000217200. [DOI] [PubMed] [Google Scholar]
- Lennon G, Auffray C, Polymeropoulos M, Soares MB. The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996;33:151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: sialylated or not? Biol. Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins. vol. 29a. Amsterdam: Elsevier; 1995. pp. 543–563. [Google Scholar]
- Miller LK. The baculoviruses. In: Fraenkel-Conrat H, Wagner RR, editors. The viruses. New York: Plenum Press; 1997. [Google Scholar]
- Munster AK, Eckhardt M, Potvin B, Muhlenhoff M, Stanley P, Gerardy-Schahn R. Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: a nuclear protein with evolutionarily conserved structural motifs. Proc. Natl Acad. Sci. USA. 1998;95:9140–9145. doi: 10.1073/pnas.95.16.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhammer DW, Goochee CF. Scaleup of insect cell cultures: protective effects of pluronic F-68. Bio/Technology. 1988;6:1411–1418. [Google Scholar]
- Nakata D, Close BE, Colley KJ, Matsuda T, Kitajima K. Molecular cloning and expression of the mouse N-acetylneuraminic acid 9-phosphate synthase which does not have deaminoneuraminic acid (KDN) 9-phosphate synthase activity. Biochem. Biophys. Res. Commun. 2000;273:642–648. doi: 10.1006/bbrc.2000.2983. [DOI] [PubMed] [Google Scholar]
- O’Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors. New York: W. H. Freeman; 1992. [Google Scholar]
- Pennock GD, Shoemaker C, Miller LK. Strong and regulated expression of Escherichia coli beta-galactosidase in insect cells with a baculovirus vector. Mol. Cell. Biol. 1984;4:399–406. doi: 10.1128/mcb.4.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer TA. Expression of heterologous proteins in stable insect cell culture. Curr. Opin. Biotechnol. 1998;9:518–521. doi: 10.1016/s0958-1669(98)80039-6. [DOI] [PubMed] [Google Scholar]
- Raju TS, Lerner L, O’Connor JV. Glycopinion: biological significance and methods for the analysis of complex carbohydrates of recombinant glycoproteins. Biotechnol. Appl. Biochem. 1996;24:191–194. [PubMed] [Google Scholar]
- Roth J, Kempf A, Reuter G, Schauer R, Gehring W. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. New York: Cold Spring Harbor Press, Cold Spring Harbor; 1989. [Google Scholar]
- Segawa H, Kawakita M, Ishida N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem. 2002;269:128–138. doi: 10.1046/j.0014-2956.2001.02632.x. [DOI] [PubMed] [Google Scholar]
- Seo NS, Hollister JR, Jarvis DL. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Prot. Expr. Purif. 2001;22:234–241. doi: 10.1006/prep.2001.1432. [DOI] [PubMed] [Google Scholar]
- Smith GE, Summers MD, Fraser MJ. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983;3:2156–2165. doi: 10.1128/mcb.3.12.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasche R, Hinderlich S, Weise C, Effertz K, Lucka L, Moormann P, Reutter W. A bifunctional enzyme catalyzes the first two steps in N-acetylneuraminic acid biosynthesis of rat liver. Molecular cloning and functional expression of UDP-N-acetyl-glucosamine 2-epimerase/N-acetylmannosamine kinase. J. Biol. Chem. 1997;272:24319–24324. doi: 10.1074/jbc.272.39.24319. [DOI] [PubMed] [Google Scholar]
- Stollar V, Stollar BD, Koo R, Harrap KA, Schlesinger RW. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976;69:104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Summers MD, Smith GE. A manual of methods for baculovirus vectors and insect cell culture procedures. Tx. Ag. Expt. Stn. Bull. 1987 No. 1555. [Google Scholar]
- Tomiya N, Ailor E, Lawrence SM, Betenbaugh MJ, Lee YC. Determination of nucleotides and sugar nucleotides involved in protein glycosylation by high-performance anion-exchange chromatography: sugar nucleotide contents in cultured insect cells and mammalian cells. Anal. Biochem. 2001;293:129–137. doi: 10.1006/abio.2001.5091. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kokuho T, Takahashi H, Takahashi M, Kubota T, Inumaru S. Sialylation of N-glycans on the recombinant proteins expressed by a baculovirus-insect cell system under β-N-acetylglucosaminidase inhibition. J. Biol. Chem. 2001;277:5090–5093. doi: 10.1074/jbc.M110548200. [DOI] [PubMed] [Google Scholar]
- Weigel PH. Galactosyl and N-acetylgalactosaminyl homeostasis: a function for mammalian asialoglycoprotein receptors. Bioessays. 1994;16:519–524. doi: 10.1002/bies.950160713. [DOI] [PubMed] [Google Scholar]