Abstract
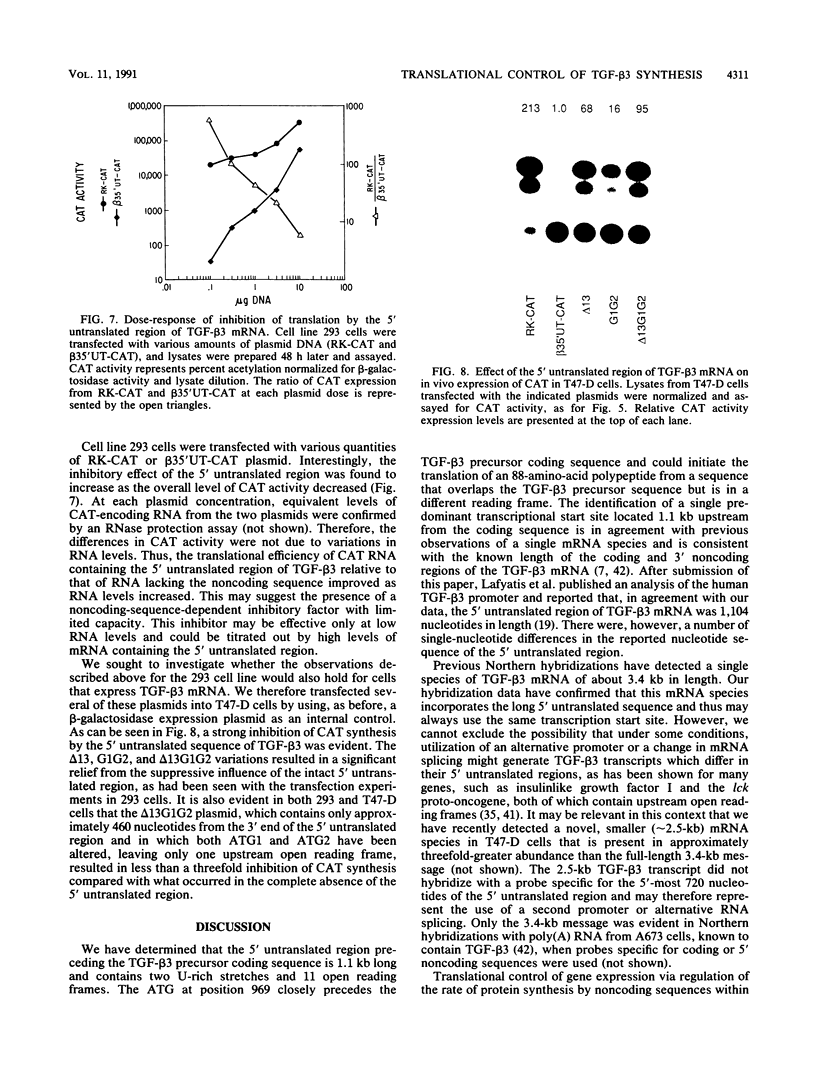
We have cloned and sequenced the 5' untranslated region of the transforming growth factor-beta 3 (TGF-beta 3) mRNA as well as the adjacent genomic sequence. S1 nuclease analysis identified a single transcription start site. We have thus determined that the 5' untranslated region is about 1.1 kb long and contains 11 open reading frames. In vitro translation of the TGF-beta 3 precursor coding sequence was markedly inhibited by the presence of the 5' untranslated region. Similarly, when the 5' untranslated region of TGF-beta 3 was introduced upstream of the coding sequence of chloramphenicol acetyltransferase, in vitro translation was inhibited. Furthermore, upon transfection into 293 cells, chloramphenicol acetyltransferase expression was inhibited by the 5' untranslated region of TGF-beta 3. The degree of translational inhibition was inversely proportional to the amount of transfected DNA. Mutation analysis implicated multiple segments of the 5' untranslated region as contributing to the inhibitory effect. Deletion of much of the 5'-most 640 nucleotides, including 8 of the 11 upstream ATGs, relieved much but not all of the inhibitory influence of the 5' untranslated region of TGF-beta 3 mRNA. The two upstream open reading frames closest to the initiator codon for the TGF-beta 3 coding sequence also decreased translational efficiency, since mutation of either ATG resulted in increased translation. Transfection results with T47-D cells, a cell line which expresses TGF-beta 3 mRNA, were similar to those obtained with the 293 cell line. Thus, TGF-beta 3 mRNA is a recent example of an expanding group of growth-related mRNAs in which the 5' untranslated region contains upstream open reading frames and other sequences which inhibit translation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Korc M., Derynck R. Differential regulation of expression of three transforming growth factor beta species in human breast cancer cell lines by estradiol. Cancer Res. 1990 Jan 15;50(2):299–303. [PubMed] [Google Scholar]
- Bascom C. C., Wolfshohl J. R., Coffey R. J., Jr, Madisen L., Webb N. R., Purchio A. R., Derynck R., Moses H. L. Complex regulation of transforming growth factor beta 1, beta 2, and beta 3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors beta 1 and beta 2. Mol Cell Biol. 1989 Dec;9(12):5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darveau A., Pelletier J., Sonenberg N. Differential efficiencies of in vitro translation of mouse c-myc transcripts differing in the 5' untranslated region. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2315–2319. doi: 10.1073/pnas.82.8.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Lindquist P. B., Lee A., Wen D., Tamm J., Graycar J. L., Rhee L., Mason A. J., Miller D. A., Coffey R. J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988 Dec 1;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick A. B., Flanders K. C., Danielpour D., Yuspa S. H., Sporn M. B. Retinoic acid induces transforming growth factor-beta 2 in cultured keratinocytes and mouse epidermis. Cell Regul. 1989 Nov;1(1):87–97. doi: 10.1091/mbc.1.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graycar J. L., Miller D. A., Arrick B. A., Lyons R. M., Moses H. L., Derynck R. Human transforming growth factor-beta 3: recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and -beta 2. Mol Endocrinol. 1989 Dec;3(12):1977–1986. doi: 10.1210/mend-3-12-1977. [DOI] [PubMed] [Google Scholar]
- Grens A., Scheffler I. E. The 5'- and 3'-untranslated regions of ornithine decarboxylase mRNA affect the translational efficiency. J Biol Chem. 1990 Jul 15;265(20):11810–11816. [PubMed] [Google Scholar]
- Hinnebusch A. G. Involvement of an initiation factor and protein phosphorylation in translational control of GCN4 mRNA. Trends Biochem Sci. 1990 Apr;15(4):148–152. doi: 10.1016/0968-0004(90)90215-w. [DOI] [PubMed] [Google Scholar]
- Kondaiah P., Sands M. J., Smith J. M., Fields A., Roberts A. B., Sporn M. B., Melton D. A. Identification of a novel transforming growth factor-beta (TGF-beta 5) mRNA in Xenopus laevis. J Biol Chem. 1990 Jan 15;265(2):1089–1093. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Lechleider R., Kim S. J., Jakowlew S., Roberts A. B., Sporn M. B. Structural and functional characterization of the transforming growth factor beta 3 promoter. A cAMP-responsive element regulates basal and induced transcription. J Biol Chem. 1990 Nov 5;265(31):19128–19136. [PubMed] [Google Scholar]
- Marth J. D., Overell R. W., Meier K. E., Krebs E. G., Perlmutter R. M. Translational activation of the lck proto-oncogene. Nature. 1988 Mar 10;332(6160):171–173. doi: 10.1038/332171a0. [DOI] [PubMed] [Google Scholar]
- Massagué J. The TGF-beta family of growth and differentiation factors. Cell. 1987 May 22;49(4):437–438. doi: 10.1016/0092-8674(87)90443-0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Lee A., Matsui Y., Chen E. Y., Moses H. L., Derynck R. Complementary DNA cloning of the murine transforming growth factor-beta 3 (TGF beta 3) precursor and the comparative expression of TGF beta 3 and TGF beta 1 messenger RNA in murine embryos and adult tissues. Mol Endocrinol. 1989 Dec;3(12):1926–1934. doi: 10.1210/mend-3-12-1926. [DOI] [PubMed] [Google Scholar]
- Miller D. A., Lee A., Pelton R. W., Chen E. Y., Moses H. L., Derynck R. Murine transforming growth factor-beta 2 cDNA sequence and expression in adult tissues and embryos. Mol Endocrinol. 1989 Jul;3(7):1108–1114. doi: 10.1210/mend-3-7-1108. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin N., Darveau A., Nicholson R., Sonenberg N. cis-acting translational effects of the 5' noncoding region of c-myc mRNA. Mol Cell Biol. 1988 Jul;8(7):2875–2883. doi: 10.1128/mcb.8.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton R. W., Dickinson M. E., Moses H. L., Hogan B. L. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development. 1990 Oct;110(2):609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Pelton R. W., Hogan B. L., Miller D. A., Moses H. L. Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev Biol. 1990 Oct;141(2):456–460. doi: 10.1016/0012-1606(90)90401-4. [DOI] [PubMed] [Google Scholar]
- Rao C. D., Pech M., Robbins K. C., Aaronson S. A. The 5' untranslated sequence of the c-sis/platelet-derived growth factor 2 transcript is a potent translational inhibitor. Mol Cell Biol. 1988 Jan;8(1):284–292. doi: 10.1128/mcb.8.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L. Regulation of expression of the c-sis proto-oncogene. Nucleic Acids Res. 1989 Jun 12;17(11):4101–4115. doi: 10.1093/nar/17.11.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C. T., Jr, Lasky S. R., Lowe W. L., Jr, LeRoith D. Rat IGF-I cDNA's contain multiple 5'-untranslated regions. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1154–1159. doi: 10.1016/0006-291x(87)90768-6. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takadera T., Leung S., Gernone A., Koga Y., Takihara Y., Miyamoto N. G., Mak T. W. Structure of the two promoters of the human lck gene: differential accumulation of two classes of lck transcripts in T cells. Mol Cell Biol. 1989 May;9(5):2173–2180. doi: 10.1128/mcb.9.5.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Hansen P., Iwata K. K., Pieler C., Foulkes J. G. Identification of another member of the transforming growth factor type beta gene family. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4715–4719. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]








