Abstract
Prostate cancer (PCa) is typically found as a multifocal disease suggesting the potential for molecular defects within the morphologically normal tissue. The frequency and spatial extent of DNA methylation changes encompassing a potential field defect are unknown. A comparison of non-tumor-associated (NTA) prostate to histologically indistinguishable tumor-associated (TA) prostate tissues detected a distinct profile of DNA methylation alterations (0.2%) using genome-wide DNA arrays based on the Encyclopedia of DNA Elements 18 sequence that tile both gene-rich and poor regions. Hypomethylation (87%) occurred more frequently than hypermethylation (13%). Several of the most significantly altered loci (CAV1, EVX1, MCF2L, and FGF1) were then used as probes to map the extent of these DNA methylation changes in normal tissues from prostates containing cancer. In TA tissues, the extent of methylation was similar both adjacent (2 mm) and at a distance (>1 cm) from tumor foci. These loci were also able to distinguish NTA from TA tissues in a validation set of patient samples. These mapping studies indicate that a spatially widespread epigenetic defect occurs in the peripheral prostate tissues of men who have PCa that may be useful in the detection of this disease.
Introduction
“Field cancerization” and “field defect” were terms first used in head and neck tumors to describe an increased frequency of cancer development found outside the visible boundaries of the primary tumor [1]. In the modern era, this definition has been refined to include genetically or epigenetically compromised cells identified in histologically normal appearing tissues. A field defect has been identified in gastric, colorectal, bladder, and esophageal cancers [2–4]. Epigenetic alterations limited solely to the immediate peritumor environment suggest a response of the surrounding tissue to the primary cancer. Single-gene epigenetic studies have identified these changes in a subset of specimens adjacent to the primary prostate cancers (PCa) [5–7]. Greater controversy exists whether widespread epigenetic alterations arise within the susceptible peripheral zone of the prostate that might underlie the regional multifocality at diagnosis, as well as the increased incidence with aging [8].
PCa development and progression are driven by the interplay of genetic and epigenetic changes [9]. One important epigenetic process is the reversible methylation of cytosine at CpG dinucleotides, a sequence underrepresented in the genome except at CpG islands [10]. DNA hypermethylation at gene promoters leads to gene silencing and has been observed in preneoplastic lesions and cancers [11,12]. A decrease in global methylation is a signature of neoplastic processes and a predisposition to chromosomal instability and cancer [13,14]. Demethylation of LINE-1 elements in normal colonic mucosa in patients with multiple colon cancers has recently suggested a role for demethylation in cancer predisposition associated with a field defect [4].
Herein, we used an immunocapture approach to enrich methylated DNA and combined this with DNA microarrays based on the Encyclopedia of DNA Elements (ENCODE) 18 sequence that query functional elements throughout gene regions and are not focused solely on CpG island promoter regions. We then used these loci to evaluate the extent of the field defect in histologically normal tissues from men with PCa. Our data support a spatially widespread change in DNA methylation throughout the peripheral prostate at multiple loci that has implications for the development of the disease as well as clinical treatment.
Materials and Methods
Tissue Samples and Histopathology
Samples termed non-tumor-associated (NTA; mean, 63; range, 55–81 years) were obtained from organ donation or cystoprostatectomy and had extensive histologic evaluation to rule out associated PCa. Tumor-associated (TA; mean, 61; range, 57–64 years) prostate tissues were obtained from patients who underwent radical prostatectomy for PCa. The clinical-pathologic characteristics for all samples used in these studies are presented (Table W1). A separate validation group consisting of 12 NTA (mean, 60; range, 55–70 years) and 11 TA (mean, 57; range, 51–67 years) samples were evaluated using quantitative pyrosequencing.
To define the relationship of methylation to tumor foci, histologic sections containing both cancer and normal regions were generated from 26 (mean, 58; range, 44–69 years) radical prostatectomy specimens. Microdissection was performed to obtain tumor (T) and normal tissue adjacent (2 mm) to tumor foci (TAA) and at a greater distance (10 mm, TAD) as described [15]. Additional histopathologic criteria for the TA and NTA samples included similar epithelial-to-stromal ratios and lack of inflammation. Prostate specimens were confirmed to have no tumor by both hematoxylin and eosin (H&E) staining in three dimensions using step sectioning and α-methylacyl-CoA expression. For AMACR analysis, RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA), and 300 ng of RNA were reverse transcribed with Ominscript. Quantitative real-time polymerase chain reaction (PCR) for total AMACR was performed using primer sequences, as reported [16]. This study was approved by Institutional Review Boards at the University of Wisconsin-Madison and the University of Pittsburgh.
DNA Methylation Microarrays
Genomic DNA was isolated using the DNeasy Blood and Tissue Kit (Qiagen). Roche NimbleGen ENCODE HG18 DNA methylation arrays were used. These arrays contain 385,000 50mer oligonucleotides (probes) that cover elements such as protein-coding genes, transcription units, protein binding sites, conserved DNA elements, chromatin assembly and modification features, and single-nucleotide polymorphisms. Notably, the probes are placed at 60-bp intervals to include both CpG and non-CpG islands within gene bodies, promoters, as well as intergenic regions (UCSC Genome browser, http://genome.ucsc.edu/ENCODE/encode.hg18.html). All chromosomes are represented on the arrays with the exception of 3 and 17.
Sample preparation for the microarray was performed following the manufacturer's protocol as previously detailed [11]. Briefly, 2 to 6 µg of high-quality genomic DNA was digested with MseI (New England Biolabs, Ipswich, MA) to produce 200 to 1000 bp fragments (keeping CpG islands intact) and then heat denatured to form single strands. Methylated DNA fragments were immunoprecipitated (IP) with 1 µg of antibody against 5-methylcytidine (Abcam, Cambridge, MA) and captured with agarose beads. The DNA/antibody/bead mixture was digested with Proteinase K and purified with phenol-chloroform. Enrichment of the methylated IP DNA was validated with PCR primers for the H19-ICR specific for methylated DNA and FLJ25439 specific for unmethylated regions as described [17]. The labeling of IP and input DNA, microarray hybridization, and scanning were performed by NimbleGen Laboratories (Reykjavik, Iceland) as described [18]. Data were extracted from scanned images by using NimbleScan 2.4 extraction software (NimbleGen Systems, Inc, Madison, WI). The samples were assayed in duplicate.
Quantitative Pyrosequencing
Sodium bisulfite modification of genomic DNA was performed using the EpiTect Bisulfite Kit (Qiagen). Bisulfite-modified DNA was then amplified using PCR in preparation for pyrosequencing, with either the forward or reverse primer biotinylated [19]. All PCR and sequence primers for pyrosequencing were designed using PyroMark Assay Design 2.0 (Qiagen), and primer sequences are listed in Table W2A. The biotinylated PCR products were captured with streptavidin sepharose beads, denatured to single strand, and annealed to the sequencing primer for the pyrosequencing assay. SssI methylase-treated bisulfite-converted DNA from human prostate epithelial cell and PPC1 were used as positive controls, and water substituted for DNA was used as a negative control. Methylation was quantified with the PyroMark MD Pyrosequencing System (Qiagen) within the linear range of the assay. All samples were analyzed by three independent experiments in duplicate.
Analysis of Methylation and Gene Expression
DNA and RNA were obtained from normal human prostate epithelial cells primary cultured from five patients and four human PCa cell lines (PC3, PPC1, Du145, and LNCaP). DNA methylation was evaluated by pyrosequencing as described above. Gene expression was measured by reverse transcription-quantitative PCR (RTQPCR) using a Bio-Rad CFX 96 Real-Time PCR System and SYBR Green PCR Master Mix (Applied Biosystems, Grand Island, NY) as described. Primer sequences are listed in Table W2B. mRNA levels of each gene were normalized to β-actin as ΔCT. Experiments were performed in duplicate in three independent experiments; a t test was used to compare the PCa cells to the normal prostate epithelial cells.
Data Analysis
Scaled log2 ratio gene-finding format (GFF) file and P value GFF files provided by NimbleGen Systems, Inc were used for microarray analysis. The scaled log2 ratio is the ratio of the test sample to the input signal when co-hybridized to the array. Scaling was performed by subtracting the biweight mean for all array features. From the scaled log2 ratio data, a fixed-length window was placed around each consecutive probe and the one-sided Kolmogorov-Smirnov test was applied to determine whether the probes had a significantly more positive distribution of intensity log ratios than those in the rest of array. The resulting score for each probe was the -log10 P value. The probe IDs were first chosen on the basis of a -log10[P] value that ranged from 2 to 10 resulting in around 1000 probes on each chromosome and 18,101 probes in total. A statistical analysis comparing the log2 ratios between the NTA and TA groups for the probes was then determined using a t test (P < .05). We used the more appropriate standard t test or the Satterthwaite approximation t test, depending on testing for equal variances when evaluating these data. Significantly changed probes were clustered by Java MultiExperiment View (MEV 4.6.2) with unsupervised hierarchical clustering [20]. For quantitative pyrosequencing, the methylation at each CpG site was expressed as a percentage. A two-tailed t test was used to test for differences between groups; P < .05 was considered statistically significant.
Results
Distinct Patterns of DNA Methylation Define TA from NTA Prostate Tissues
To identify probes, we used 385,000 locus arrays based on the ENCODE18 sequences that densely tile a series of biologically significant regions in ∼1% of the human genome. These arrays query functional elements throughout gene regions and are not focused solely on CpG island promoter regions. DNA was initially prepared in duplicate from four TA and five NTA prostate specimens, restriction digested, and enriched for methylated DNA by IP with an antibody against 5-methylcytidine, as described [11]. Peripheral zone prostate tissues were used for all of these studies as PCa demonstrates a predilection for this region.
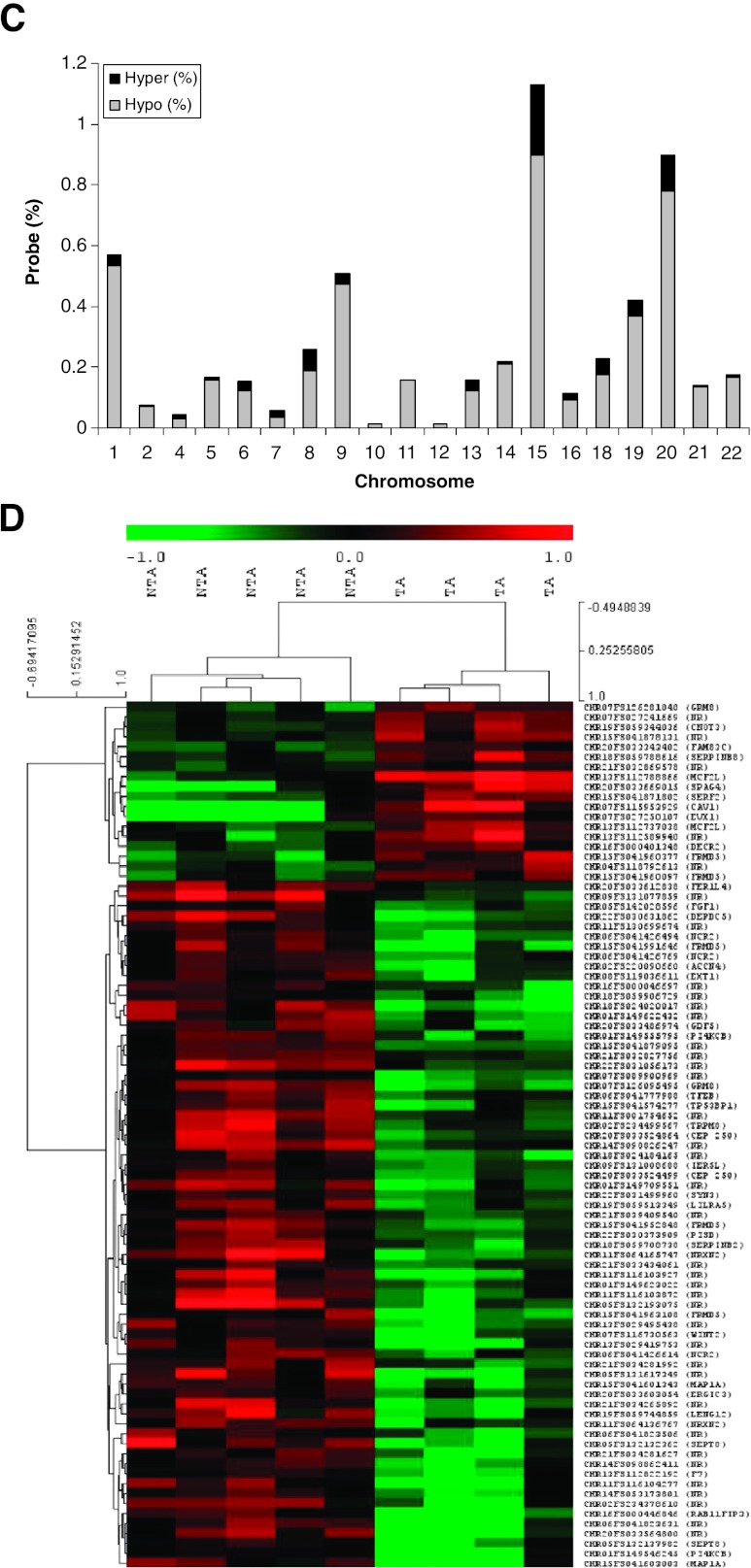
To validate the IP, single-gene PCR was applied to confirm an enrichment of methylation in IP DNA compared to total genomic DNA (Input; Figure 1A). Total IP DNA and Input DNA were labeled with Cy5 and Cy3 dye, respectively, and then co-hybridized to the array (Figure 1B) [17]. There were 18,101 significant loci identified that were then statistically compared between TA and NTA tissue groups [21,22]. This generated 615 probes (0.16% of total) that were differentially methylated in TA tissues, of which 537 (87%) were hypomethylated and 78 (13%) were hypermethylated (P < .05; Figure 1C). Microarray data are available at http://www.ncbi.nlm.nih.gov/geo (Accession No. GSE38982).
Figure 1.
DNA methylation analysis using ENCODE18-based arrays of TA and NTA prostate tissues. (A) Verification of the IP for methylated DNA. Single-gene PCR was applied to both total genomic (Input) DNA and IP DNA before array analysis as described in Materials and Methods section. The H19 ICR served as a positive control for methylation and FLJ 25439 as a demethylation control. The IP shows lower demethylated levels. (B) Schema of methods. Genes aberrantly methylated in TA compared to NTA were identified by using an intensity cutoff (-log10[P] = 2–10), then a t test was used to compare the log2 ratios between groups. For further validation, statistical significance was used to rank the probes. (C) Genome-wide distribution of DNA methylation array differences at 385,000 loci in histologically normal TA prostate tissues compared to NTA tissues. Significant differences in methylation between TA and NTA prostate tissues were generated using a cutoff of probe score of -log10[P] = 2–10 and t test between two groups (P < .05). A total of 615 probes were differentially methylated in TA tissues with 537 demonstrating hypomethylation and 78 hypermethylation. The percentage (axis) is the significantly altered probe number versus the total probe number analyzed for each chromosome. Chromosomes 15 and 20 were differentially methylated to a greater extent than other chromosomes. (D) Heat map of significant DNA methylation array changes using unsupervised hierarchical clustering. Using more stringent criteria (t test, P < .01), 86 probes are shown comparing sets of NTA (left) to TA (right) and hierarchically ordered from top to bottom by relatively hypermethylation to hypomethylation. Green indicates relative hypomethylation, whereas the red shaded areas demonstrate hypermethylation of the TA set.
Chromosome 15 demonstrated the greatest number of significantly differentially methylated probes (1.1%) in TA tissues, followed by chromosome 20 (0.9%). Across genomic regions, specific peaks were identified and defined as regions containing greater than two probes within a 500-bp length (Table W3A). From these results, we concluded that altered methylation between TA and NTA tissues occurs infrequently when using ENCODE-based genome arrays. Furthermore, arrays that query areas beyond promoter CpG islands reveal that hypomethylation was more prevalent than increased methylation in tissues associated with tumors.
Identification of Probes for Analyzing the Field Defect in Prostate Specimens
To limit the number of loci for further study, more stringent statistical parameters (P < .01) were applied identifying 86 probes (Figure 1B). These probes were subjected to unsupervised hierarchical clustering using TMEV software to pictorially generate a heat map of methylation distinguishing TA from NTA prostate tissues (Figure 1D). Among these loci, 68 were hypomethylated and 18 were hypermethylated in TA tissues. Of these, 38 probes were not closely related to genes (Table W3B), and 48 were within 35 genes (Table 1). Loci associated with genes were primarily located within introns (78%) and were distant from transcription start sites.
Table 1.
Gene-related Loci Ranked by Significance.
| Gene | Gene Name | Accession No. | Location | Hypermethylation or Hypomethylation | Distance to TSS | CpG Island | Gene Body | Exon/Intron |
| MCF2L* | Cell line derived transforming sequence-like | NM_024979 | Chr13: 112,704,068–112,792,498 | Hyper | +117331 | Y | Y | Intron |
| RAB11FIP3* | RAB11 family interacting protein 3 | NM_014700 | Chr16: 415,668–512,482 | Hypo | +31178 | Y | Y | Intron |
| CNOT3 | CCR4-NOT transcription complex 3 | BC016474 | Chr19: 59,333,294–59,351,230 | Hyper | +10789 | Y | Y | Exon |
| NCR2* | Natural cytotoxicity triggering receptor 2 | AJ010100 | Chr6: 41,411,566–41,426,579 | Hypo | +14989 | Y | Y | Exon |
| IER5L | Immediate early response 5-like | NM_203434 | Chr9: 130,978,873–131,012,683 | Hypo | -28327 | N | N | - |
| SERF2 | Small EDRK-rich factor 2 | BC015491 | Chr15: 41,871,834–41,873,536 | Hyper | +15217 | Y | Y | Exon |
| FRMD5 | FERM domain containing 5 | NM_032892 | Chr15: 41,952,764–42,274,683 | Hypo | +311563 | N | Y | Intron |
| MCF2L | Cell line derived transforming sequence-like | NM_024979 | Chr13: 112,704,068–112,792,498 | Hyper | +65503 | N | Y | Intron |
| FAM83C | Family with sequence similarity 83 C | NM_178468 | Chr20: 33,336,947–33,343,639 | Hyper | +189 | Y | Y | Exon |
| FRMD5 | FERM domain containing 5 | NM_032892 | Chr15: 41,952,764–42,274,683 | Hypo | +321825 | N | N | - |
| DEPDC5 | DEP domain containing 5 | AJ698951 | Chr22: 30,480,852–30,632,599 | Hypo | +151926 | N | Y | Intron |
| EVX1* | Even-skipped homeobox 1 | NM_001989 | Chr7: 27,248,945–27,252,717 | Hyper | +1419 | Y | Y | Intron |
| MAP1A | Microtubule-associated protein 1A | NM_002373 | Chr15: 41,597,132–41,611,110 | Hypo | +4246 | Y | Y | Exon |
| FGF1* | Fibroblast growth factor 1 | NM_000800 | Chr5: 141,953,305–142,045,812 | Hypo | +29175 | Y | Y | Intron |
| SYN3 | Synapsin III | NM_003490 | Chr22: 31,238,539–31,732,683 | Hypo | +284369 | N | Y | Intron |
| GRM8 | Glutamate receptor, metabotropic 8 | NM_000845 | Chr7: 125,865,894–126,670,548 | Hypo | +584116 | N | Y | Intron |
| SERPINB8 | Serpin peptidase inhibitor B8 | BC034528 | Chr18: 59,788,332–59,804,866 | Hyper | +374 | Y | Y | Intron |
| CAV1* | Caveolin 1 | AF 172085 | Chr7: 115,953,642–115,986,904 | Hyper | +1855 | Y | Y | Exon |
| WNT2* | Wingless-type MMTV integration site | BC 078170 | Chr7: 116,704,514–116750565 | Hypo | +19961 | Y | Y | Intron |
| CEP250* | Centrosomal protein | AF022655 | Chr20: 33,511,231–33,563,123 | Hypo | +17863 | N | Y | Intron |
| PI4KCB* | Phosphatidylinositol 4-kinase | NM_002651 | Chr1: 149,531,036–149,565,348 | Hypo | +11021 | N | Y | Intron |
| PISD | Phosphatetidylserine decarboxylase | CR456540 | Chr22: 30,345,379–30,388,195 | Hypo | 13468 | N | N | - |
| GRM8 | Glutamate receptor, metabotropic 8 | NM_000845 | Chr7: 125,865,894–126,670,548 | Hyper | +398576 | N | Y | Intron |
| SPAG4 | Sperm associated antigen 4 | NM_003116 | Chr20: 33,667,222–33,672,379 | Hyper | +1793 | Y | Y | Intron |
| NCR2 | Natural cytotoxicity triggering receptor 2 | AJ010100 | Chr6: 41,411,566–41,426,579 | Hypo | +15264 | Y | N | - |
| FRMD5 | FERM domain containing 5 | NM_032892 | Chr15: 41,952,764–42,274,683 | Hypo | +293027 | Y | Y | Intron |
| NRXN2 | Neurexin 2 | NM_138734 | Chr11: 64,130,221–64,167,363 | Hypo | +81441 | N | Y | Intron |
| ACCN4 | Amiloride-sensitive cation channel 4 | NM_182847 | Chr2: 220,087,295–220,111.738 | Hypo | +3525 | Y | Y | Intron |
| SERPINB2 | Serpin peptidase inhibitor, B2 | NM_002575 | Chr18: 59,705,921–59,722,100 | Hypo | +2820 | N | Y | Intron |
| CEP250 | Centrosomal protein | AF022655 | Chr20: 33,511,231–33,563,123 | Hypo | +18228 | N | Y | Intron |
| FRMD5 | FERM domain containing 5 | NM_032892 | Chr15: 41,952,764–42,274,683 | Hyper | +314296 | N | Y | Intron |
| FER1L4 | Fer-1-like 4 | AL833764 | Chr20: 33,609,920–33,651,008 | Hypo | +46003 | N | Y | Intron |
| ERGIC3 | ERGIC and golgi 3 | AF116614 | Chr20: 33,593,290–33,608,818 | Hypo | +9863 | Y | Y | Intron |
| TRPM8 | Transient receptor potential cation channel | NM_024080 | Chr2: 234,490,781–234,592,905 | Hypo | +8786 | N | Y | Intron |
| TFEB | Transcription factor EB | NM_007162 | Chr6: 41,759,693–41,810,766 | Hypo | +33939 | Y | Y | Intron |
| SEPT8 | Septin | AF440762 | Chr5: 132,114,415–132,140,966 | Hypo | +9050 | Y | Y | Intron |
| EXT1 | Exostoses 1 | BC001174 | Chr8: 118,880,782–119,192,632 | Hypo | +156580 | N | Y | Intron |
| GDF5 | Growth differentiation factor 5 | NM_000557 | Chr20: 33,484,562–33,489,441 | Hypo | +2409 | N | Y | Intron |
| SEPT8 | Septin | AF440762 | Chr5: 132,114,415–132,140,966 | Hypo | +3430 | Y | Y | Intron |
| NRXN2 | Neurexin 2 | NM_138734 | Chr11: 64,130,221–64,167,363 | Hypo | +110421 | Y | Y | Intron |
| TP53BP1 | Tumor protein p53 binding protein 1 | NM_005657 | Chr15: 41,486,698–41,590,028 | Hypo | +15672 | N | Y | Intron |
| F7 | Coagulation factor VII | NM_019616 | Chr13: 112,808,105–112,822,346 | Hypo | +14087 | Y | Y | Exon |
| LILRA5 | Leukocyte immunoglobulin-like receptor A5 | NM_181985 | Chr19: 59,510,164–59,516,221 | Hypo | +2824 | N | Y | Intron |
| DECR2 | 2,4-dienoyl CoA reductase 2 | AK128012 | Chr16: 391,893–402,371 | Hyper | +9490 | Y | Y | Intron |
| FRMD5 | FERM domain containing 5 | NM_032892 | Chr15: 41,952,764–42,274,683 | Hyper | +314576 | N | Y | Intron |
| PI4KCB | Phosphatidylinositol 4-kinase | NM_002651 | Chr1: 149,531,036–149,565,348 | Hypo | +20522 | N | Y | Intron |
| NCR2 | Natural cytotoxicity triggering receptor 2 | AJ010100 | Chr6: 41,411,566–41,426,579 | Hypo | +15109 | Y | N | - |
| MAP1A | Microtubule-associated protein 1A | NM_002373 | Chr15: 41,597,132–41,611,110 | Hypo | +5906 | Y | Y | Exon |
Probes subjected to validation using quantitative pyrosequencing. Of the initial 20 loci listed, 11 were not able to be assessed due to technical limitations regarding probe design and performance.
Of the 48 gene-related probes (representing 35 genes), we evaluated 20 loci that demonstrated the most significant methylation changes on our arrays (Table 1). This included both hypermethylated and hypomethylated probes. In a separate set of 11 TA and 12 NTA prostate specimens, quantitative pyrosequencing was employed to validate these DNA methylation differences [19]. Because of technical reasons (mainly high CG density and mispriming; both limitations of pyrosequencing), we were unable to generate functional primers that covered 11 loci. NCR2, WNT2, and three others were not initially validated, although NCR2 and WNT2 were significantly altered in a high-grade disease subset (see below). We validated CAV1, EVX1, and MCF2L (hypermethylated) and FGF1 (hypomethylated) in this tissue set (Figure 2A). The location of these four loci relative to gene structure is demonstrated in Figure 2B. These regions were downstream from the transcription start site and included both exonic and intronic regions making them less likely to alter gene expression. To evaluate this issue, we performed a correlation analysis for these four loci examining methylation and gene expression in a series of normal prostate epithelial cells (five) and PCa cell lines (four). Changes in methylation did not correlate with altered transcript expression from these four associated genes (Figure W2). The above data validated these four primer sets as tools marking an epigenetic field defect present in men with associated PCa that were then used in subsequent mapping experiments.
Figure 2.
Validation of loci identified by DNA methylation array in additional prostate tissues using quantitative pyrosequencing. (A) Analysis of methylation was performed using quantitative pyrosequencing as described in Materials and Methods section. An additional validation set of NTA tissues (12) was compared to TA tissues (11). All loci shownwere significantly altered in TAwhen compared toNTAsamples (P < .05). (B) Location of selected loci within genes that were analyzed by quantitative pyrosequencing. Exon and intron boundaries are shown, as well as the transcription start site. The genome-wide analysis resulted in loci that were not typically located in promoter CpG islands. Tick marks represent CG sites analyzed.
Identification of a Widespread Methylation Field Defect in the Peripheral Prostate
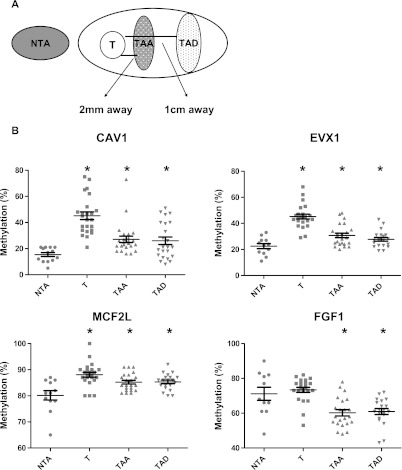
We then used these probes to test whether tissues adjacent to PCa tumor foci are preferentially altered, suggesting only an immediate peritumor response, or whether aberrant methylation occurs at a distance from the tumor indicating a more widespread field defect. The extent of the field defect was assessed using four differentially methylated markers in 26 additional histologically normal tissues from men with cancer. Clinical and pathologic information is presented (Table W1). We dissected normal tissues 2 mm adjacent (TAA) and compared these to tissue more than 10 mm distant (TAD) from the main tumor focus for each of these 26 specimens (Figure 3A). H&E staining of step sections was used to exclude contamination by tumor cells, significant inflammation, and the presence of high-grade prostatic intraepithelial neoplasia (PIN), a putative cancer precursor [23]. AMACR expression, a PCa marker, was determined by QPCR, and samples contaminated by overt cancer were removed (Figure W1).
Figure 3.
Analysis of extent of field defect in prostatectomy samples using identified probes. (A) Schematic showing prostate tumor (T) and adjacent (TAA) and distant (TAD) normal tissue dissection as well as NTA specimens. Radical prostatectomy samples containing tumor foci were sectioned and samples dissected for subsequent analyses. Adjacent and distant TA tissues did not contain histologic cancer. Samples were assessed three-dimensionally using step sectioning. (B) Analysis of methylation at differentially methylated loci in above dissected prostate tissues and NTA prostate tissues using quantitative pyrosequencing. Methylation analyses for CAV1, EVX1, MCF2L, and FGF1 were significantly different when comparing T, TAA, or TAD to NTA (*P < .05). No significant erosion in methylation with increasing distances from the primary tumor was observed when TAA was compared to TAD. Technical replicates obtained from independent PCR reactions showed average SDs of 2.4% for CAV1, 1.5% for EVX1, 1.6% for MCF2L, and 2.1% for FGF1, respectively.
When compared to NTA tissues, hypermethylation of probes associated with CAV1, EVX1, and MCF2L and hypomethylation of FGF1 demonstrated significant changes in both adjacent (TAA), as well as distant tissues (Figure 3B). Results for each CpG tested are listed in Table W4. We noted no significant difference in the extent of methylation seen at different distances from the tumor using these unbiased probes. We also evaluated methylation in the associated tumor (T) for each sample. The methylation change representing the field defect was also present in the tumor samples at three loci. At FGF1, the tumor did not demonstrate hypomethylation similar to the TA tissue, suggesting that these defects are not always enriched for in the associated tumors. In summary, similar methylation extent in both adjacent and distant tissues indicates that the epigenetic field defect in the prostate is spatially widespread and not localized solely to the immediate peritumor environment.
We also performed a subset analysis on TA tissues looking at these changes in intermediate (Gleason 5–7) or high-grade (Gleason 8–10) cancers. Two loci, NCR2 and WNT2, that were not significantly altered when all tissue grades were examined were found to be significantly altered in the high-grade but not intermediate-grade subset (Figure 4A). The location of NCR2 and WNT2 relative to gene structure is demonstrated in Figure 4B. Other loci did not distinguish between the field defect associated with high- or low-grade disease. These data suggest that distinct field methylation alterations may be solely associated with the presence of high-grade cancer.
Figure 4.
Analysis demonstrates a relationship between grade and methylation for several tested loci. (A) Quantitative pyrosequencing was performed on nine loci (Table 1). Prostate tumor (T), adjacent (TAA), and distant (TAD) tissues were divided into high- (Gleason score of 8–10) and intermediate- (Gleason score of 5–7) grade tissues. Tissues without associated cancer were also evaluated (NTA). NCR2 and WNT2 were found to only be significantly hypomethylated in TA tissues associated with the presence of high-grade cancer when compared to NTA (P < .05). (B) Location of NCR2 and WNT2 region where the probes are located.
Discussion
Research has led to a proposal that a field defect may underlie the development of multifocal cancer [1]. Initial efforts in characterizing this process focused on genetic alterations [24,25]. More recently, epigenetic changes have been proposed as an etiology [26,27]. In the current study, we used high-resolution genome-wide arrays that cover a range of biologically significant regions (not limited to promoter regions) to identify significant changes in DNA methylation that occur within histologically normal tissues associated with PCa. We validated several of these loci and used them as probes to demonstrate for the first time that methylation changes are spatially widespread and not restricted to the immediate peritumor environment as previously thought [2,4,15]. Finally, these methylation alterations permit a clear distinction between TA and NTA prostate tissues.
To date, epigenetic profiling of TA histologically normal tissues has not been performed in the prostate. Our genome-wide assessment of specific loci demonstrates that hypomethylation was seen more commonly than hypermethylation in TA prostate tissues. This surprising finding may be a result of using arrays that do not solely examine promoter CpG islands but densely assess gene-enriched regions, including intergenic regions, of the genome. The use of these arrays also explains the relatively low frequency of methylation changes (0.2%) found compared to studies in other cancers using CpG promoter arrays [28]. Hypomethylation was recently found at LINE-1 sequences in normal colonic mucosa of patients with multiple colon cancers, suggesting that demethylation may underlie the field defect found in these patients [4]. These findings were also increased with aging, and the authors proposed a “wear and tear” model to explain the propensity of colon cancer with aging [4]. In the prostate, DNA hypomethylation may be found in PIN [29], a cancer precursor. Our data that widespread hypomethylation occurs in the prostate field defect extend these findings and lead to speculation that epigenetic alterations in TA tissues may possibly precede the histologic changes of PIN and cancer.
A debate exists regarding whether the peripheral prostate may undergo widespread methylation changes similar to bladder cancer, a disease also characterized by multifocality and recurrence [27]. In bladder, there was no dependence on distance from the primary tumor. Previously, methylation studies using single genes GSTP1, RASSF1A, APC, and RARβ2 altered in PCa identified methylation changes in a subset of specimens only adjacent to the primary tumor [5–7]. This suggested that methylation alterations were limited solely to the immediate peritumor environment in the prostate. In contrast, in the present epigenomic profiling study, we find that methylation alterations consistently extended even to regions distant (>1 cm) from tumor foci. A similar widespread field defect was demonstrated during evaluation of insulin-like growth factor 2 loss of imprinting in peripheral prostate tissues [15]. Insulin-like growth factor 2 loss of imprinting is an agerelated event in the human prostate that is associated with cancer formation [30]. This epigenetic association has also been seen in the colon and predisposes to cancer formation [31]. These data suggest a more widespread methylation change in the histologically normal peripheral prostate of men with cancer that we postulate predisposes the gland to multiple tumor formation with aging. Further research will be needed to address this hypothesis and how a widespread methylation field defect might arise, whether due to diet or environmental exposures [32,33].
Our primary goal was to use methylation arrays to define an unbiased panel of probes with which to evaluate whether a widespread field defect occurs in PCa. Thus, we used a more limited number of samples initially and further validated a panel of genes with quantitative pyrosequencing. We found six of nine loci to be validated in our other tissue sets, indicating a false discovery rate of 33%. To select loci for validation, we used approaches commonly employed in gene array studies (e.g., statistical value) [11]. The majority of probes identified fell within CpG islands [34], but interestingly none fell into defined gene promoter regions (Table 1). Hypermethylation within promoters has been linked to decreased gene expression [35,36], but the function of CpG islands outside these regions remains uncertain. No correlation with expression and methylation was noted at the majority of these loci when normal and cancer cell lines were examined (Figure W2). At NCR2 and WNT2, a correlation was seen between increased methylation and expression. Methylation within the gene bodies has been associated with elevated gene expression in genome-wide studies [37]. These changes may reflect alternate promoter usage that could impact expression in a sense or antisense manner. Alternatively, given the potential for long-range epigenetic silencing through chromatin looping, we postulate that the methylation changes identified may herald alterations in nuclear structure that influence gene expression at distant sites [38].
The current findings suggest a methylation field defect that may encompass peripheral prostate tissue, the susceptible region of the gland, even at a distance from the tumor. These findings have several additional implications. Several of the loci identified in this study were recently used as a test to successfully identify the presence of PCa elsewhere in the prostate solely from normal biopsy tissue in a group of clinical patients with an excellent area under the curve of 0.79 [39]. An additional finding of several regions that were associated only with high-grade cancer might suggest an early marker for more aggressive PCa. Finally, the assessment of alterations that occur in PCa have typically compared tumor to “normal” tissues within the same prostate gland. The current study indicates that histologically normal tissue from men who have PCa may already contain methylation abnormalities leading to an underestimation of epigenetic changes within the tumor.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health (No. 2R01CA097131; http://grants.nih.gov/grants/) and the John Livesey endowment. The authors have declared that no competing interests exist.
This article refers to supplementary materials, which are designated by Tables W1 to W4 and Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 2.Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi T, Habuchi T, Kakehi Y, Mitsumori K, Akao T, Terachi T, Yoshida O. Clonal and chronological genetic analysis of multifocal cancers of the bladder and upper urinary tract. Cancer Res. 1998;58:5835–5841. [PubMed] [Google Scholar]
- 4.Kamiyama H, Suzuki K, Maeda T, Koizumi K, Miyaki Y, Okada S, Kawamura YJ, Samuelsson JK, Alonso S, Konishi F, et al. DNA demethylation in normal colon tissue predicts predisposition to multiple cancers. Oncogene. 2012;31:5029–5037. doi: 10.1038/onc.2011.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aitchison A, Warren A, Neal D, Rabbitts P. RASSF1A promoter methylation is frequently detected in both pre-malignant and non-malignant microdissected prostatic epithelial tissues. Prostate. 2007;67:638–644. doi: 10.1002/pros.20475. [DOI] [PubMed] [Google Scholar]
- 6.Hanson JA, Gillespie JW, Grover A, Tangrea MA, Chuaqui RF, Emmert-Buck MR, Tangrea JA, Libutti SK, Linehan WM, Woodson KG. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst. 2006;98:255–261. doi: 10.1093/jnci/djj051. [DOI] [PubMed] [Google Scholar]
- 7.Henrique R, Jeronimo C, Teixeira MR, Hoque MO, Carvalho AL, Pais I, Ribeiro FR, Oliveira J, Lopes C, Sidransky D. Epigenetic heterogeneity of high-grade prostatic intraepithelial neoplasia: clues for clonal progression in prostate carcinogenesis. Mol Cancer Res. 2006;4:1–8. doi: 10.1158/1541-7786.MCR-05-0113. [DOI] [PubMed] [Google Scholar]
- 8.Miller GJ, Cygan JM. Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol. 1994;152:1709–1713. doi: 10.1016/s0022-5347(17)32368-6. [DOI] [PubMed] [Google Scholar]
- 9.Schulz WA, Hoffmann MJ. Epigenetic mechanisms in the biology of prostate cancer. Semin Cancer Biol. 2009;19:172–180. doi: 10.1016/j.semcancer.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 11.Morris MR, Ricketts CJ, Gentle D, McRonald F, Carli N, Khalili H, Brown M, Kishida T, Yao M, Banks RE, et al. Genome-wide methylation analysis identifies epigenetically inactivated candidate tumour suppressor genes in renal cell carcinoma. Oncogene. 2011;30:1390–1401. doi: 10.1038/onc.2010.525. [DOI] [PubMed] [Google Scholar]
- 12.Wu YH, Tsai Chang JH, Cheng YW, Wu TC, Chen CY, Lee H. Xeroderma pigmentosum group C gene expression is predominantly regulated by promoter hypermethylation and contributes to p53 mutation in lung cancers. Oncogene. 2007;26:4761–4773. doi: 10.1038/sj.onc.1210284. [DOI] [PubMed] [Google Scholar]
- 13.Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300:455. doi: 10.1126/science.1083557. [DOI] [PubMed] [Google Scholar]
- 14.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 15.Bhusari S, Yang B, Kueck J, Huang W, Jarrard DF. Insulin-like growth factor-2 (IGF2) loss of imprinting marks a field defect within human prostates containing cancer. Prostate. 2011;71:1621–1630. doi: 10.1002/pros.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang B, Leung YK, Wang V, Chung E, Levin L, Bracken B, Cheng L, Ho SM. α-Methylacyl-CoA racemase spliced variants and their expression in normal and malignant prostate tissues. Urology. 2011;77:249.e1–249.e7. doi: 10.1016/j.urology.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schubeler D. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet. 2005;37:853–862. doi: 10.1038/ng1598. [DOI] [PubMed] [Google Scholar]
- 18.Roche.NimbleGen, author. Arrays User's Guide DNA Methylation Arrays Version 7.2. 2010. [Google Scholar]
- 19.Tost J, El abdalaoui H, Gut IG. Serial pyrosequencing for quantitative DNA methylation. Biotechniques. 2006;40:721–722. 724, 726. doi: 10.2144/000112190. [DOI] [PubMed] [Google Scholar]
- 20.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- 21.Ruike Y, Imanaka Y, Sato F, Shimizu K, Tsujimoto G. Genomewide analysis of aberrant methylation in human breast cancer cells using methyl-DNA immunoprecipitation combined with high-throughput sequencing. BMC Genomics. 2010;11:137. doi: 10.1186/1471-2164-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson IM, Davies JJ, Weber M, Brown CJ, Alvarez CE, MacAulay C, Schübeler D, Lam WL. Epigenomics:mapping themethylome. Cell Cycle. 2006;5:155–158. doi: 10.4161/cc.5.2.2367. [DOI] [PubMed] [Google Scholar]
- 23.Ayala AG, Ro JY. Prostatic intraepithelial neoplasia: recent advances. Arch Pathol Lab Med. 2007;131:1257–1266. doi: 10.5858/2007-131-1257-PINRA. [DOI] [PubMed] [Google Scholar]
- 24.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of slaughter's concept of field cancerization. Cancer Res. 2003;63:1727–1730. [PubMed] [Google Scholar]
- 25.Garcia SB, Park HS, Novelli M, Wright NA. Field cancerization, clonality, and epithelial stem cells: the spread of mutated clones in epithelial sheets. J Pathol. 1999;187:61–81. doi: 10.1002/(SICI)1096-9896(199901)187:1<61::AID-PATH247>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37:899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 27.Wolff EM, Chihara Y, Pan F, Weisenberger DJ, Siegmund KD, Sugano K, Kawashima K, Laird PW, Jones PA, Liang G. Unique DNA methylation patterns distinguish noninvasive and invasive urothelial cancers and establish an epigenetic field defect in premalignant tissue. Cancer Res. 2010;70:8169–8178. doi: 10.1158/0008-5472.CAN-10-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamaru H, Yamamoto E, Suzuki H, Nojima M, Maruyama R, Yamano HO, Yoshikawa K, Kimura T, Harada T, Ashida M, et al. Aberrant methylation of RASGRF1 is associated with an epigenetic field defect and increased risk of gastric cancer. Cancer Prev Res (Phila) 2012;5:1203–1212. doi: 10.1158/1940-6207.CAPR-12-0056. [DOI] [PubMed] [Google Scholar]
- 29.Yang B, Sun H, Lin W, Hou W, Li H, Zhang L, Li F, Gu Y, Song Y, Li Q, et al. Evaluation of global DNA hypomethylation in human prostate cancer and prostatic intraepithelial neoplasm tissues by immunohistochemistry. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.05.009. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Fu VX, Dobosy JR, Desotelle JA, Almassi N, Ewald JA, Srinivasan R, Berres M, Svaren J, Weindruch R, Jarrard DF. Aging and cancer-related loss of insulin-like growth factor 2 imprinting in the mouse and human prostate. Cancer Res. 2008;68:6797–6802. doi: 10.1158/0008-5472.CAN-08-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakatani T, Kaneda A, Iacobuzio-Donahue CA, Carter MG, de Boom Witzel S, Okano H, Ko MS, Ohlsson R, Longo DL, Feinberg AP. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 32.Mathers JC, Strathdee G, Relton CL. Induction of epigenetic alterations by dietary and other environmental factors. Adv Genet. 2010;71:3–39. doi: 10.1016/B978-0-12-380864-6.00001-8. [DOI] [PubMed] [Google Scholar]
- 33.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci USA. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JY. Promoter hypermethylation in prostate cancer. Cancer Control. 2010;17:245–255. doi: 10.1177/107327481001700405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper CS, Foster CS. Concepts of epigenetics in prostate cancer development. Br J Cancer. 2009;100:240–245. doi: 10.1038/sj.bjc.6604771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ball MP, Li JB, Gao Y, Lee JH, LeProust EM, Park IH, Xie B, Daley GQ, Church GM. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol. 2009;27:361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark SJ. Action at a distance: epigenetic silencing of large chromosomal regions in carcinogenesis. Hum Mol Genet. 2007;16:R88–R95. doi: 10.1093/hmg/ddm051. [DOI] [PubMed] [Google Scholar]
- 39.Truong M, Yang B, Livermore A, Wagner J, Weeratunga P, Huang W, Dhir R, Nelson J, Lin DW, Jarrard DF. Employing the epigenetic field defect to detect prostate cancer in biopsy, negative patients. J Urol. 2012 doi: 10.1016/j.juro.2012.11.074. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.