Abstract
Cancer has long been considered a disease that mimics an “unhealed wound,” with oncogene-induced secretory activation signals from epithelial cancer cells facilitating stromal fibroblast, endothelial, and inflammatory cell participation in tumor progression. However, the underlying mechanisms that orchestrate cooperative interaction between malignant epithelium and the stroma remain largely unknown. Here, we identified interleukin-1β (IL-1β) as a stromal-acting chemokine secreted by ovarian cancer cells, which suppresses p53 protein expression in cancer-associated fibroblasts (CAFs). Elevated expression of IL-1β and cognate receptor IL-1R1 in ovarian cancer epithelial cells and CAFs independently predicted reduced overall patient survival, as did repressed nuclear p53 in ovarian CAFs. Knockdown of p53 expression in ovarian fibroblasts significantly enhanced the expression and secretion of chemokines IL-8, growth regulated oncogene-alpha (GRO-α), IL-6, IL-1β, and vascular endothelial growth factor (VEGF), significantly increased in vivo mouse xenograft ovarian cancer tumor growth, and was entirely dependent on interaction with, and transcriptional up-regulation of, nuclear factor-kappaB (NF-κB) p65. Our results have uncovered a previously unrecognized circuit whereby epithelial cancer cells use IL-1β as a communication factor instructing stromal fibroblasts through p53 to generate a protumorigenic inflammatory microenvironment. Attenuation of p53 protein expression in stromal fibroblasts generates critical protumorigenic functionality, reminiscent of the role that oncogenic p53 mutations play in cancer cells. These findings implicate CAFs as an important target for blocking inflammation in the tumor microenvironment and reducing tumor growth.
Introduction
Cancers interact with a richly diverse stromal microenvironment composed of mesenchymal, endothelial, immunoregulatory, and hematopoietic cells to foster tumor growth and progression [1]. Many microenvironment components involved in cancer development are also involved in the wound healing process. Cancer has been long recognized as an “unhealed wound” [2], and the key component of wound healing is inflammatory signal network modulation. Although well regulated in normal wound healing, the role of inflammatory mediators in regulating cancer is largely undefined. As the predominant cellular component of the tumor microenvironment [3], fibroblasts play a critical synergistic role by working with oncogenic cells to facilitate initiation and progression toward clinically recognizable tumor [4,5]. Indeed, paracrine secretory activation of tumor stroma yields a microenvironment replete with inflammatory mediators, growth factors, extracellular matrix (ECM) modifiers, and angiogenic factors [6–8], selectively stimulating aggressive tumorigenesis. Cancer epithelium-secreted stimulatory factors induce transition of normal fibroblasts to a reactive, synthetic, tumor-promoting cancer-associated fibroblast (CAF) phenotype. Yet, a detailed mechanism linking coordinate regulation of these disparate components to promote growth of ovarian cancer is not well understood.
Ovarian cancer is the deadliest gynecologic malignancy in women in the United States [9]. Dismal prognosis is due, in part, to incomplete understanding of the mechanisms controlling development. Integrated genomic analysis has identified mutation of the TP53 tumor suppressor gene, encoding the p53 transcription factor, in 96% of high-grade serous ovarian cancer patients [10]. In addition to the well-known effect of p53 in maintaining genomic integrity despite DNA damage or instability through apoptosis regulation [11], p53 is known to function in regulation of inflammation [12]. Whereas most studies have focused on p53 function in epithelial cancer cells, limited knowledge is available for the role of p53 in the most abundant component of cancer stroma, fibroblasts. While p53 expression in CAFs has been recognized [13,14], its regulation and functional role in tumor pathogenesis is not known.
Inflammation is a long-recognized risk factor for ovarian cancer [15] and a hallmark of almost all cancers [16]. The inflammatory response is involved in almost all stages of tumor development [17]. Inflammation of the peritoneum, classically associated with ovarian cancer, has been tied to elevated ovarian stromal expression of multiple cytokines and chemokines, all of which are downstream targets of the transcriptional factor NF-κB [18,19]. NF-κB is constitutively activated in many tumor cells [20]. NF-κB activation provides a critical link between inflammation and cancer development [21], whereas NF-κB inhibition suppresses tumor growth, leading to the concept of “NF-κB addiction” in cancer cells [22]. We previously demonstrated that overexpression of oncogenic alleles of HRAS and KRAS in immortalized human ovarian surface epithelial cells activated secretion of a group of multiple cytokines and chemokines in an NF-κB-dependent manner [23], identifying one group member, GRO-α, as capable of inducing ovarian stromal fibroblast senescent activation, promoting ovarian tumorigenesis [24]. However, although senescent fibroblasts composed only a small percentage of the tumor microenvironment, the signaling mechanism by which ovarian cancer cells communicated successfully with nonsenescent fibroblasts (the largest component of the tumor microenvironment) remained unknown. Therefore, we hypothesized that additional chemokines secreted by ovarian cancer cells may function as critical communication signals promoting synergistic alteration and reprogramming of adjacent stromal fibroblasts to synchronize with cancer epithelial cells in promoting cancer growth. We present data demonstrating that interleukin-1β (IL-1β) is a critical chemokine involved in the communication between cancer cells and stromal fibroblasts, acting to facilitate the development of ovarian cancer through the generation of a protumorigenic inflammatory phenotype in stromal fibroblasts.
Materials and Methods
Patients, Clinicopathologic Data, and Ovarian Cancer Tissue Microarrays
Five hundred twenty-seven representative tumor samples and relevant clinical data were obtained from women diagnosed with primary ovarian carcinoma that underwent initial surgery at The University of Texas MD Anderson Cancer Center (UTMDACC) between January 1990 and April 2010. Relevant clinical data were collected by retrospective review of the patient pathology reports and used in generating an identity-free patient clinical factor follow-up database. The Institutional Review Board at the UTMDACC approved the use of both archival tissue blocks and chart reviews. The median age of the 527 patients in our study was 60 years (range, 20–92 years). The mean overall survival time was 7.8 years (95% CI, 3.5–4.6 years), and the overall survival rates were 59% (95% CI, 0.55–0.63) at 3 years, 39% (95% CI, 0.34–0.44) at 5 years, and 27% (0.22–0.32) at 10 years. Histopathology diagnoses were based on World Health Organization criteria, tumor grading for nonserous carcinomas was based on the Gynecologic Oncology Group criteria, and disease staging was assigned according to the International Federation of Gynecology and Obstetrics staging system. Serous carcinomas were graded by using a two-tier system (low grade and high grade) according to established criteria. Archived tissue microarray paraffin blocks were constructed from representative patient core samples using morphologically representative areas of blocks, as we have previously described [25].
Immunohistochemical Staining and Statistical Analysis
Ovarian cancer tissue microarray slides were subjected to immunohistochemical (IHC) staining according to the manufacturer's protocol (Biocare Medical, Concord, CA) and following our previously published protocols [25,26]. Briefly, after initial deparaffinization/hydration, epitopes were unmasked using the Universal Decloaker (UD1000) and the Decloaking Chamber pressure cooker (DC2002), endogenous peroxidases were blocked by Peroxidazed 1 (PX968), and nonspecific binding was blocked by Background Sniper (BS966) and Avidin-Biotin blocking reagents (AB972). Slides were then incubated overnight at 4°C with primary antibodies: p53 (1:75; Santa Cruz Biotechnology, Dallas, TX, DO-1 sc-126), phospho-Y496-IL-1R1 (1:500; Abcam, Cambridge, MA, ab59995), or IL-1β (1:1000; Santa Cruz Biotechnology, sc-7884). Primary antibodies were followed by incubation with biotin-labeled Universal Goat Link secondary reagent (GU600) for 15 minutes, Streptavidin-HRP (HP604) for 15 minutes, staining for 5 minutes with 3,3-diaminobenzidine (DB801), counterstaining with hematoxylin, dehydration, and mounting in Permount. Negative controls included replacing the primary antibody with phosphate-buffered saline (PBS). IHC staining was analyzed by two gynecological pathologists (by J.L. and J.Z.). Staining was scored according to tumor stroma nuclear p53, cytoplasmic IL-1β, and IL-1R1 immunoreactivity in ovarian cancer epithelial cells and cancer-proximal stromal fibroblasts using the software and hardware of the Applied Imaging Ariol automated image analysis system (Molecular Devices, Inc, Sunnyvale, CA, SL-50). The degree of staining in tumor microarray cores was quantified using a grading system based on positive percentage: score = 0 for <10% positive fibroblasts, score = 1 for 10% to 20% positive fibroblasts, score = 2 for 20% to 50% positive fibroblasts, score = 3 for 50% to 70% positive fibroblasts, and score = 4 for 70% to 100% positive fibroblasts. For all statistical analyses, scoring was grouped according to low expression and high expression, as indicated in the figures and figure legends. Fisher's exact test was used to evaluate the association of p53, cytoplasmic IL-1β, and IL-1R1 with clinical factors. The Kaplan-Meier method was used to estimate the probability of overall survival, and the Mantel-Cox log-rank test was used to compare the overall survival or disease-free survival between different comparison groups. Multivariate Cox regression models were fitted to determine whether p53, cytoplasmic IL-1β, and IL-1R1 correlation with overall patient survival achieved independent predictor status after adjusting/challenging by the clinical factors of International Federation of Gynecology and Obstetrics (FIGO) stage (I–IV), clinical response (as defined above), and grade (1–3). Results were considered statistically significant at the P ≤ .05 level. SPSS PASW17.0.2 software (IBM, Inc, Armonk, NY) was used for all statistical analyses.
Ovarian Tissue-Derived and Established Ovarian Line Cell Culture
For the purpose of continuous in vitro culturing, we previously established human telomerase reverse transcriptase overexpressing normal ovarian fibroblast cell lines [24]. Multiple lines of these extended replication fibroblasts were used for this study, which achieve consistently higher passage number and display elevated proliferation capacity relative to unaltered primary parent fibroblast lines (Figure W1). CAF cell cultures were collected and isolated according to our protocol approved by the UTMDACC Institutional Review Board and were grown and maintained as we have previously published [24]. T29 cells were derived from the normal, immortalized nontumorigenic ovarian surface epithelial cell line IOSE-29 expressing human telomerase catalytic subunit and SV40 T/t antigens by transfection and confirmed as nontumorigenic as previously described [23]. SKOV3 malignant ovarian cancer cells were maintained and used as we have previously published [23].
Establishment of Stably Expressing hTERT, p53i, and p65i Ovarian Fibroblasts
Retroviruses carrying hTERT cDNA or p53 siRNA, or lenti-viruses carrying p65 shRNA plasmid, were generated and harvested as described previously [23]. All fibroblast lines were generated by two rounds of infection by generated retrovirus or lentivirus, followed by stable selection with hygromycin (100 µg/ml), neomycin (400 µg/ml), or puromycin (1.0 µg/ml) for 10 to 14 days. Retrovirus infection efficiency was determined by follow-up with Western blot for the factor being repressed (as shown in figures), and lentiviral infection efficiency was determined by green fluorescence observed using the integrated internal ribosome entry site fused to the enhanced variant of green fluorescent protein (IRES-EGFP) element in the pGIPZ vector.
Western Blot Analysis
Western blot analysis was performed as we have previously described [27]. Briefly, cell lysates were prepared using a radioimmunoassay-based lysis buffer: 50 mM Tris-HCl (pH 8), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate with a protease inhibitor cocktail added before use (Roche, Indianapolis, IN). Protein concentrations were measured using the Bio-Rad (Hercules, CA) protein assay kit, followed by overnight primary antibody incubation at 4°C at the following dilutions: β-actin at 1:30,000 (Sigma, St Louis, MO, A5691), CXCR2 at 1:500 (R&D Systems, Minneapolis, MN, MAB331), GRO-α at 1:500 (Sigma, G7659), hTERT at 1:1000 (GeneTex, Irvine, CA, GTX30410), IL-1α at 1:500 (R&D Systems, MAB200), IL-1β at 1:500 (Santa Cruz Biotechnology, sc-7884), IL-1R1 at 1:1000 (Santa Cruz Biotechnology, sc-25775), IL-6 at 1:500 (Santa Cruz Biotechnology, sc-7920), IL-8 at 1:200 (R&D Systems, AF-208-NA), p53 at 1:500 (Santa Cruz Biotechnology, DO-1 sc-126), p65 at 1:500 (Abcam, ab7970), phospho-S276-p65 at 1:000 (Cell Signaling Technology, Danvers, MA, #3037), SDF-1α at 1:1000 (Abcam, ab80118), super-activator of chemokine signaling 1 (SOCS1) at 1:500 (Abcam, ab62584), TFIIB at 1:1000 (Santa Cruz Biotechnology, sc-274), tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF-6) at 1:1000 (Abcam, ab13853), γ-tubulin at 1:1000 (Sigma, T5192), and vascular endothelial growth factor (VEGF) at 1:1000 (Santa Cruz Biotechnology, sc-152), and then secondary antibody incubation using sheep anti-mouse HRP (GE Healthcare, Pittsburgh, PA, RPN4201), goat anti-rabbit HRP (GE Healthcare, RPN4301), or donkey anti-goat (Santa Cruz Biotechnology, sc-2020) at 1:3000. Films were developed from blots incubated using the ECLplus kit (Amersham Biosciences, Piscataway, NJ) and exposure times of 30 seconds to 15 minutes.
Analysis of Cell Proliferation
To analyze cell proliferation, 2750 cells or 3500 cells were seeded onto 96-well plates in N = 8 wells, including three control wells with cell growth media only. Cells were incubated at 37°C and 5% CO2. Proliferation was assessed 24, 48, 60, and 72 hours later in triplicate based on the colorimetric MTT cell proliferation assay (ATCC, Manassas, VA, 30-1010K) according to the manufacturer's protocol. Assays were repeated in triplicate and are reported in the Supplementary Materials.
Evaluation of Cell Cycle Progression and Apoptotic Status
The cell cycle progression status cell lines was evaluated following trypsinization, two washes in PBS, and resuspension at 1 x 106 to 2 x 106 in 200 µl of PBS. Cells were fixed in cold 75% ethanol for a minimum of 4 hours at 4°C, washed twice with PBS, resuspended in 200 µl of PBS, and incubated with 20 µl of RNase (1 mg/ml, Sigma) at 37°C for 15 to 20 minutes before staining with 200 µl of propidium iodide (PI; 50 µg/ml, Sigma). For apoptosis analysis, cells were harvested by trypsinization at 75% confluence, washed twice with PBS, and stained with fluorescein isothiocyanate-Annexin V and PI (BD Pharmingen, Franklin Lakes, NJ, 556547). Experiments were performed in duplicate and repeated twice. Determination of both cell cycle progression and apoptotic index was performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) running BD CellQuest Pro version 5.1 software in the Flow Cytometry and Cellular Imaging Facility at UTMDACC. The percentage of apoptotic cells was calculated on the basis of the height of the M1 peak along the Annexin V and PI biparametric cytogram, representing the combined early and late apoptotic cell populations among the total cell population analyzed.
Co-immunoprecipitation
Binding complex association between p53 and p65 was evaluated using a co-immunoprecipitation (co-IP) kit (Thermo Scientific, Waltham, MA, Cat. No. 26149), following the manufacturer's suggested protocol. Briefly, 10 µg of p53 (Santa Cruz Biotechnology, sc-126) or p65 (Abcam, ab7970) antibody was conjugated to the AminoLink Plus coupling resin beads and washed extensively. Normal ovarian fibroblast cell lysates were harvested from 80% confluent 60-mm dishes (seeded equivalently) using the IP/lysis buffer by alternating incubation on ice and extensive scrapping (two each), then pre-cleared using the control agarose resin beads for 2 hours at room temperature, and incubated overnight at 4°C on separate p53- and p65-conjugated bead resin columns. After extensive washing, bound proteins were eluted using elution buffer in 50-µl total volume for immediate analysis of total elution by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels, subsequent Western blot analysis.
Dual-Luciferase Reporter Assay
NF-κB promoter activation status was determined using the Dual-Luciferase Reporter Assay System (Promega, Sunnyvale, CA, TM040) following the manufacturer's suggested protocol. Briefly, the above-listed normal ovarian fibroblast cell lines were transiently co-transfected using the FuGene6 commercial protocol with plasmids containing either two wild-type or two mutant truncated engineered NF-κB response minimal promoter elements upstream of the firefly luciferase 3 gene [23], in combination with the pRL-CMV vector (Promega, E2261) that expresses Renilla luciferase driven by the CMV intermediate promoter. Normal ovarian fibroblast cell lysates were harvested 36 hours after co-transfection in 400 µl of cold passive lysis buffer using a plastic scrapper from 60-mm dishes at 80% confluence (seeded equivalently), immediately subjected to two cycles of freeze/thaw, and then centrifuged at 4°C at maximum speed for 15 minutes to pellet cell debris. Twenty microliters of cleared cell lysates was used for each assay reading on a DLR Luminometer (Turner BioSystems, Sunnyvale, CA, P/N 9600-001), setup for a 20-second wavelength pulse read for each luciferase. Data are reported as the ratio of detection for firefly/Renilla luciferases in relative light units standardized according to total protein concentration for each lysate. Experiments were repeated twice with N = 4 samples.
Xenograft in Athymic Nude Mouse and Statistical Analysis
To determine the effect on fibroblast tumor contribution in the absence of p53 or p65 on xenograft tumor formation and growth in vivo, normal ovarian fibroblast 151 cells expressing hTERT, hTERT plus p65 inhibition, hTERT plus p53 inhibition, or hTERT plus double-inhibition constructs targeting p53 and p65 were counted using trypan blue to indicate viable cells and then were combined in a 1:4 ratio (fibroblasts/epithelial cells) with viable-counted T29 or SKOV3 ovarian epithelial cells. T29 cells (4 x 106) were combined with 1 x 106 fibroblasts, and 1 x 106 SKOV3 cells were combined with 2.5 x 105 fibroblasts for each injection type. Cell combinations were immediately washed twice with PBS, resuspended in 150 µl of PBS, and injected subcutaneously into 4- to 6-week-old Swiss nu-nu/Ncr athymic nude mouse hind flank (outbred mouse colony maintained by the Department of Experimental Radiation Oncology at UTMDACC). The mice were housed and cared for in the UTMDACC pathogen-free experimental animal facility and checked every 2 days for tumors. The date on which the first grossly visible tumors appeared was recorded, and tumor size was monitored thereafter every 2 to 3 days. Mice were sacrificed after 13 weeks of follow-up, or according to the occurrence of excessive tumor burden. Tumors were removed, fixed in 10%formalin, and subjected to routine histologic examination. All mouse experiments followed our Institutional Animal Care and Use Committee (IACUC)-approved animal protocol.
Statistically significant differences in the rate of tumor growth between xenografts produced with cancer cells combined with fibroblasts attenuated for p53 or p65 (or both) and their parental control cell injections were calculated using linear, nonparametric regression analysis. Statistically significant differences in tumor growth curves between xenograft implants of ovarian epithelial cells with fibroblasts with attenuated p53 or p65 (or both) and their parental control cells were calculated using the Student's t test or analysis of variance (ANOVA), as indicated. All results were considered statistically significant at the P < .05 confidence level, using SPSS17.02 software (IBM, Inc).
Results
Ovarian Cancer Cell-Derived IL-1β Paracrinely Represses p53 Protein Expression in Ovarian Fibroblasts
We hypothesized that p53 is the key sensor in stromal fibroblasts involved in the regulation of communication between cancer epithelial cells and fibroblasts. Thus, we first analyzed p53 protein levels in human telomerase reverse transcriptase (hT) immortalized ovarian fibroblasts (Figure W1) following treatment with media conditioned by ovarian cancer cells. As shown in Figure 1A, attenuation of p53 levels in ovarian fibroblasts occurred after only 8 hours of exposure to media conditioned by the SKOV3 ovarian cancer cell line, while p53 attenuation occurred 12, 16, and especially 24 hours after exposure to HRAS-transformed ovarian epithelial cell (T29H)-conditioned media (Figure 1B). To identify specific paracrine factors involved in repression of p53, we examined multiple cytokines commonly secreted by cancer epithelial cells and RAS-transformed tumorigenic cells including IL-1β, IL-8, IL-6, and GRO-α [23]. Ovarian fibroblasts treated with exogenous IL-6 or IL-8 did not display p53 attenuation (Figure 2, C and D), as did treatment with GRO-α (data not shown). However, IL-1β treatment in contrast specifically repressed p53 protein levels in ovarian fibroblasts (Figure 1E). To address the mechanisms of IL-1β-mediated p53 suppression, we examined if the repressed level of p53 protein by IL-1β is mediated by p65, as IL-1β is a known potent inducer of the NF-κB signaling pathway. We treated the p65 knockdown fibroblasts and the parental fibroblasts with IL-1β, but we failed to detect major change in the p53 protein level when p65 was knocked down (Figure W2). These results demonstrated that IL-1β-mediated repression of p53 protein may operate through a p65-independent pathway.
Figure 1.
p53 attenuation in ovarian fibroblasts is mediated by ovarian cancer cell-secreted IL-1β in vitro. Normal ovarian fibroblasts were treated for 24 hours with conditioned media from either malignant ovarian cancer SKOV3 cells (A) or HRAS-transformed ovarian surface epithelial T29H cells (B) and total cell lysate was harvested every 4 hours for Western blot (N = 3). Normal ovarian fibroblasts were treated with increasing concentrations of exogenous, recombinant human IL-6 (C), IL-8 (D), and IL-1β (E) for 48 hours, after which total cell lysate was harvested for Western blot (N = 3).
Figure 2.
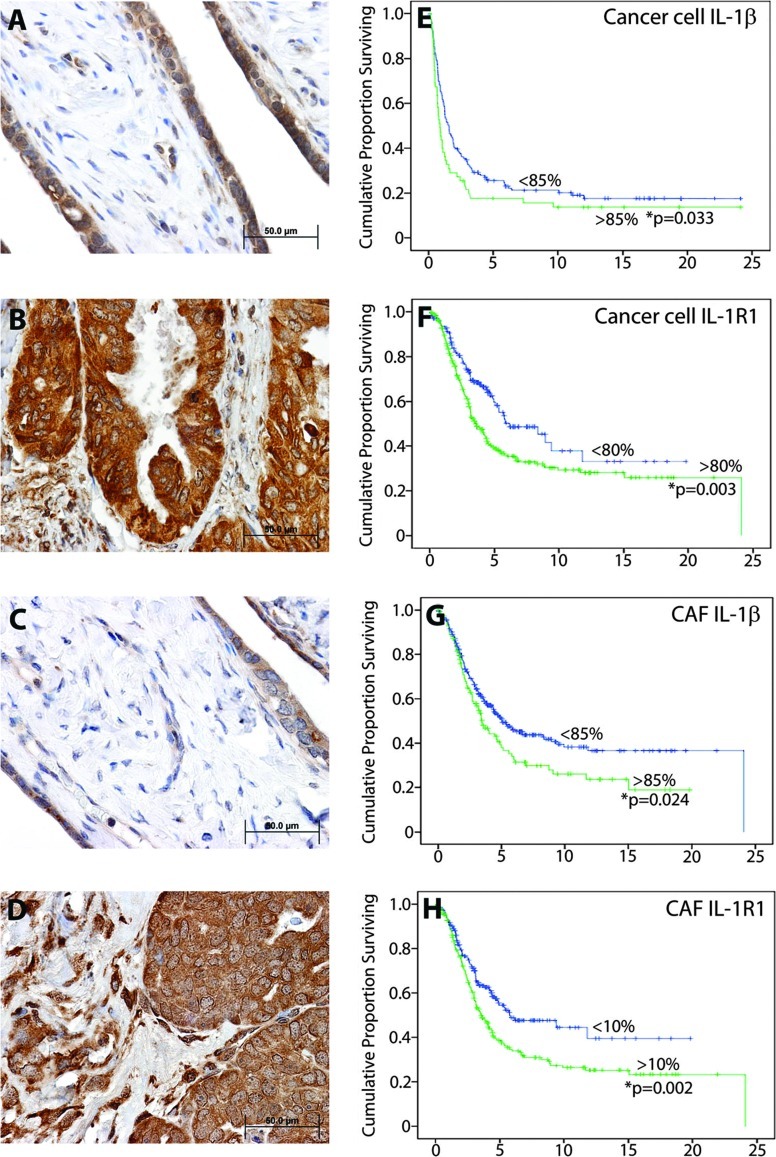
The IL-1β/IL-1R1 signaling axis is highly elevated in ovarian cancer and correlates with poor ovarian cancer patient survival. Background IL-1β IHC detection is observed in both the epithelium and stromal compartment of normal ovarian cyst (A), as opposed to highly elevated IHC staining in both compartments in ovarian cancer tissue (B). IL-1β cognate receptor IL-1R1 displays a similar pattern of background detection in normal ovarian cyst tissue (C), compared to elevated staining in ovarian cancer epithelial cells and CAFs (D). Kaplan-Meier patient overall survival analysis curves (E–H) for ovarian carcinoma patients show reduced survival time as a function of (E) ≥85% IL-1β expression in epithelial cancer cells (P = .033; N = 397), (F) ≥80% IL-1R1 expression in epithelial cancer cells (P = .003; N = 406), (G) ≥85% IL-1β expression in ovarian cancer fibroblasts (P = .024; N = 204), and (H) ≥10% IL-1R1 expression in ovarian cancer fibroblasts (P = .002; N = 231).
Elevated Expression of IL-1β/IL-1R1 or Loss of p53 Expression in Stromal Fibroblasts Predicts Poor Survival in Patients with Ovarian Cancer
Next, we reasoned that IL-1β, IL-1R1, and p53 may be able to impact the clinical outcome of patients if this signaling axis plays a critical role in ovarian tumorigenesis. Toward this end, we examined the prognostic significance of expression of IL-1β and IL-1R1 in human ovarian cancer using an ovarian cancer tissue array of 527 patients linked to detailed pathologic and clinical outcome information. Upregulation of IL-1β expression was observed in the ovarian cancer epithelium (Figure 2B) relative to basal expression in fallopian tubal epithelium (Figure 2A). Similarly, IL-1R1 was elevated in both ovarian cancer epithelial cells and CAFs (Figure 2D) compared to basal expression levels observed in normal fallopian tubal epithelial cells (Figure 2C). Moreover, elevated ovarian cancer epithelial cell cytoplasmic and plasma surface expression of both IL-1β (Figure 2E) and its receptor IL-1R1 (Figure 2F) significantly predicted reduced overall ovarian cancer patient survival. Similarly, elevated immunodetection of both IL-1β (Figure 2G) and its receptor IL-1R1 (Figure 2H) in ovarian CAFs significantly predicted poor ovarian cancer patient overall survival. Complete patient clinicopathologic factors and indicators are shown in Tables W1–W5. Our analysis showed a significant correlation between ovarian cancer histologic type (histotype) and elevated expression of IL-1β (P < .033; Table W1) or IL-1R1 (P < .016; Table W3), as well as a significant cross-correlation between elevated expression of these factors in the oncogenic epithelium and the tumor stroma (P < .0001; Table W5). Therefore, our findings demonstrated that the IL-1β/IL-1R1 chemokine signaling axis is indeed critical to ovarian cancer patient outcome.
To examine the effect of p53 attenuation in stromal fibroblasts on clinical outcome in patients with ovarian cancer, we first examined p53 expression levels in two independent normal ovarian fibroblast primary cell lines, NOF150 and NOF151, as well as in independent CAF cell lines, CAF147 and CAF187. Relative to wild-type p53 protein levels in normal ovarian fibroblasts, we observed a significant decrease in CAF p53 expression in two separate lines (Figure W3), demonstrating downregulated p53 protein in CAFs. Next, we examined whether p53 expression is also attenuated in ovarian CAFs in vivo by determining the level of p53 immunoreactivity in the nuclei of CAFs within microarrays containing normal ovary, normal fallopian tube, and ovarian cancer tissue. Relative to a robust population of p53 nuclear-positive fibroblasts (red arrowheads) and epithelial cells (green arrowheads) in normal ovarian or fallopian tube tissue (Figure 3, A and B, respectively), very weak or negligible nuclear p53 staining was observed in ovarian CAFs (red arrowheads) proximal to strongly positive high-grade serous ovarian carcinoma epithelial cells (green arrowheads; Figure 3, B and D). These findings were anticipated due to a high rate of p53 oncogenic mutation in ovarian cancer cells. Moreover, late-stage, high-grade serous ovarian carcinoma patients with low or negative p53 nuclear expression in their CAFs displayed significantly reduced overall survival (P = .018; Figure 3E). Thus, our data demonstrated that the level of p53 expression in ovarian CAFs is predictive of poor ovarian cancer patient outcome. Collectively, these data strongly designate IL-1β and p53 as key signaling molecules in stromal fibroblasts in vivo, with alteration in expression significantly impacting patient clinical outcome.
Figure 3.
p53 attenuation in ovarian CAFs correlates with reduced patient survival. IHC detection indicated healthy, elevated nuclear expression of p53 in fallopian tube (A) or ovarian cyst (B) epithelial (green arrowheads) and stromal compartment fibroblasts (red arrowheads). (C and D) In contrast, ovarian cancer tissue IHC staining indicated highly increased p53 detection in cancer epithelial cell nuclei (green arrowheads), with significantly attenuated p53 nuclear expression in ovarian CAFs (red arrowheads). Formicrographs A to D:magnification, x400; scale bars, 50 µm; p53 antibody dilution of 1:75. (E) Kaplan-Meier analysis indicated that overall survival rates were significantly lower for late-stage, high-grade serous ovarian carcinoma patients with CAF displaying very low nuclear levels of p53 (P = .018; N = 301).
p53 Attenuation in Ovarian Fibroblasts Leads to Elevated Expression and Secretion of Chemokines in an NF-κB p65-Dependent Fashion
To test the direct effect of p53 on phenotypic conversion of normal fibroblasts to a protumorigenic phenotype, p53 was forcibly attenuated in normal ovarian fibroblasts through stable knockdown [28]. Attenuation of the p53 tumor suppressor in normal ovarian fibroblasts specifically increased expression of the immunoregulators IL-8, IL-6, GRO-α, precursor and mature secreted forms of IL-1α and IL-1β (Figure 4A), as well as a panel of other chemokines, receptors, and microenvironment activators (Figures W4 and W5). As these are all downstream target genes of NF-κB transcriptional activation, our data indicated that p53 suppression may involve NF-κB signaling in stromal fibroblasts similar to oncogenic RAS activation in ovarian epithelial cells [23]. To directly test this hypothesis, we examined p65 protein level in both the cytoplasm and nucleus following knockdown of p65 gene expression in the presence or absence of p53 knockdown. As shown in Figure 4B, in hTERT (hT) immortalized fibroblasts, p53 is primarily located in the cytoplasm; the expression of p65 is largely undetectable, while the serine 276 phosphorylation form (S276) of p65 is readily detectable. Knockdown of p53 expression increased the nuclear expression of p65 (hTp53i) specifically phosphorylated at serine 276, as compared with the hT lane control (Figure 4B). These results suggest that p53 repression activates nuclear-localized, S276-phosphorylated p65, and phospho-modification at this residue enhances NF-κB p65 transcriptional activity by facilitating interaction with the transcriptional co-activator p300/CBP [29]. Our results showed that the p53 knockdown in the fibroblast upregulates expression of several inflammatory cytokines (Figure 4A, lanes 1 and 3); however, the p65 knockdown almost completely abolishes these effects (lane 4). On the basis of these data, we believe that the up-regulation of cytokines by p53 knockdown is largely dependent on p65, although we cannot completely rule out the role of p50 and inhibition of nuclear factor kappa-B kinase subunit β (IKKβ) in this process. We also observed a concurrent increase in SOCS1, a downstream p53-binding partner modulating the senescence response [30]. As anticipated, we detected a concurrent increase in the level of expression of IL-1α, IL-1β, IL-6, and IL-8 in p53-attenuated fibroblasts (Figure 4B). Furthermore, we observed a corresponding increase in the level of transcripts following inhibition of p53 alone or double inhibition of p53 and p65 (Figure 4C). Additionally, as shown in Figure 5, A to F, we examined the level of chemokine and growth factor extracellular secretion by ELISA for IL-1β, IL-6, GRO-α, IL-1α, IL-8, and VEGF, respectively. All factors displayed significantly increased detection in p53-attenuated normal ovarian fibroblasts, demonstrating enhanced secretion of these factors to the microenvironment. Collectively, our results establish that p53 repression directly activates phosphorylation of p65 at S276, altering the phenotype of ovarian fibroblasts through increased expression, and elevated extracellular secretion, of potent inflammatory modulators, allowing for paracrine interaction with local cancer cells or other cell types.
Figure 4.
p53 attenuation in normal ovarian fibroblasts elevates expression of critical secreted paracrine communication chemokines and ECM remodeling factors in an NF-κB p65-dependent fashion. (A) Attenuation of p53 in ovarian fibroblasts increases expression of IL-8, IL-6, GRO-α, both immature and mature secreted forms of IL-1α and IL-1β, TRAF-6, and SOCS1, relative to γ-tubulin total cell levels. Double attenuation of p53 and NF-κB p65 either disrupt this stimulation (nearly all chemokines shown and SOCS1) or enhance the stimulation (TRAF-6), indicating that p53 suppression requires p65 signaling to stimulate chemokine secretion. (B) Cytoplasmic (C) and nuclear (N) fractionation before protein lysate harvest indicates that p53 repression activates nuclear localization of p65 phosphorylated at serine 273 and confirms elevated cytoplasmic expression of chemokines IL-1α, IL-1β, IL-6, and IL-8 following p53 attenuation that is primarily dependent on p65 as a driving mechanism, relative to stable cytoplasmic levels of HSP-90 and nuclear protein Lamin A/C. (C) Observations at the translational level are confirmed at the transcriptional level by reverse transcription-polymerase chain reaction amplification of cDNA from total RNA lysates following p53, or p53 and p65, attenuation, relative to stable β-actin RNA levels. Each blotting result indicates representative results from N = 3 experiments.
Figure 5.
p53 inhibition in ovarian fibroblasts promotes extracellular secretion of inflammatory modulators of ovarian cancer in a p65-dependent manner. ELISA, following harvest of growth media conditioned for 48 hours by normal ovarian fibroblasts expressing hTERT (hT), p65 attenuation (hTp65i), p53 attenuation (hTp53i), or p53 and p65 attenuation (hTp53ip65i) viral constructs, was used to measure extracellular secretion of IL-1β (A), IL-6 (B), GRO-α (C), IL-1α (D), IL-8 (E), and VEGF (F). All ELISA data were standardized according to detection of known concentrations of recombinant proteins respective to each molecule listed and normalized to total, viable cell number at the time of conditioned medium collection.
p53 Attenuation Facilitates Protein Complex Formation between p53 and NF-κB p65 and Transcription Activation of NF-κB in Ovarian Fibroblasts
To further characterize the mechanism by which p53 regulates NF-κB-mediated transcription, we tested whether p53 forms a protein complex with NF-κB p65 in vitro by co-IP. A high level of p53 protein was detected in wild-type normal ovarian fibroblasts regardless of the presence or absence of p65 knockdown. However, p65 (both total and phospho-forms) associated directly with the isolated p53 protein complex only when p53 levels were repressed (Figure 6A, left panel). In contrast, when p65 was the moiety being immunoprecipitated, p53 was undetectable regardless of the status of knockdown targeting either p53 or p65, except for a faint p53–p65 interaction in hTERT-immortalized normal ovarian fibroblasts (Figure 6B, right panel). These data revealed a unidirectional signal from p53 to p65, evidenced by the observation that loss of p53 increased the level of total and S276-phosphorylated p65 in ovarian fibroblasts, without a reciprocal activation of p53 in the absence of p65. Thus, our results signify that p65 not only interacts within a stable complex with p53 but is also itself a downstream target of this interaction. To directly test whether p53 repression alters transcriptional activation of NF-κB, we used an NF-κB-responsive reporter construct. Parental normal ovarian fibroblasts (hT), those with p65 knockdown, p53 knockdown, or both, were transiently co-transfected with plasmids containing promoter elements engineered with either two wild-type or two mutant sites for NF-κB binding for dual-luciferase reporter detection assay. Our data show that p53 attenuation in ovarian fibroblasts strongly activated transcription of the wild-type NF-κB-responsive promoter, in comparison to nearly undetectable activity in p53-inhibited normal ovarian fibroblasts expressing the mutant NF-κB promoter or to p53–p65 double-knockdown normal ovarian fibroblasts expressing wild-type NF-κB promoter (Figure 6B). Collectively, these findings demonstrate that p53 normally suppresses activation of NF-κB in ovarian fibroblasts. Moreover, repression of p53 protein levels facilitates protein complex formation with NF-κB p65, phosphorylation of p65 at S276, and thus downstream activation of multiple inflammatory cytokine and chemokine targets.
Figure 6.
Interaction between p53 and NF-κB p65 governs the activated secretion profile in ovarian fibroblasts. (A) Co-IP from precleared lysates of the same cell lines listed above using either p53-linked or p65-linked agarose beads, followed by Western immunoblot (IB) analysis for p65, phospho-p65 at serine 276, or p53 (N = 2). (B) Normal ovarian fibroblasts expressing hTERT, p65 attenuation, p53 attenuation, or p53 and p65 attenuation viral constructs were transiently co-transfected with dual-luciferase reporter plasmids bearing either wild-type (wt) or mutated (mut) NF-κB promoter driving firefly luciferase [48] and control plasmid bearing CMV intermediate promoter controlling Renilla luciferase (pRL-CMV). The ratio of detection of firefly/Renilla luciferases in relative light units was standardized according to total protein concentration for each lysate (N = 4).
p53 Attenuation in Fibroblasts Facilitates Ovarian Tumor Growth In Vivo in an NF-κB p65-Dependent Manner
We next investigated the significance of secreted interaction between p53-attenutated ovarian fibroblasts and ovarian cancer cells during tumor growth in vivo [23]. p53-attenuated ovarian fibroblasts (green curve) significantly increased the tumorigenic growth from immortalized, nontumorigenic ovarian epithelial cells (T29) (P < .0001), compared to parental T29 cells (blue curve; P < .005; Figure 7A). Notably, double attenuation of both p53 and p65 (purple curve), or only p65 knockdown (red curve), in hTERT-expressing normal ovarian fibroblasts was sufficient to completely abrogate the growth-stimulatory effect of p53-attenuated normal ovarian fibroblasts exerted on T29 cells. The tumors were significantly infiltrated by neutrophils (arrow points, Figure W6). These results indicated that the inflammatory cells were recruited to the tumors and may contribute to the tumor growth. Similarly, p53-attenuated ovarian fibroblasts (green curve) significantly increased the tumorigenic growth from the SKOV3 ovarian cancer cell line, relative to all control co-injections (Figure 7B; P < .005). Attenuation of both p53 and p65 in ovarian fibroblasts (purple curve) completely abrogated the effect of p53-attenuated fibroblastic stimulation on SKOV3 tumor growth. These results demonstrate that p53-reduced normal ovarian fibroblasts activate NF-κB-mediated chemokine and growth factor secretion, indeed exerting a robust promotion of tumor growth in vivo.
Figure 7.
p53 attenuation in ovarian fibroblasts promotes in vivo tumorigenesis through an NF-κB p65-dependent mechanism. (A) p53-attenuated ovarian fibroblasts (green curve) significantly increased the tumorigenic potential of preneoplastic ovarian surface epithelial T29 cells, compared to hTERT-immortalized ovarian fibroblast stimulation of T29 cells (blue curve), relative to all listed control co-implantations. Double attenuation of p53 and p65 (purple curve) or only p65 knockdown (red curve) completely ameliorated the effect of p53 attenuation. (B) p53-attenuated ovarian fibroblasts (green curve) significantly decreased the tumorigenic lag phase of malignant ovarian cancer SKOV3 cells, relative to all control coinjections. p53 and p65 co-attenuation (purple curve) completely abrogated the effect of p53 attenuation; * indicates P < .005 and ** indicates P < .0001 by calculating ANOVA comparing p53-attenuated fibroblast co-injections with either hTERT-only, p65 knockdown, or SKOV3-only controls (N = 8 injections per experimental group or N = 3 injections per control group). (C) Model based on the hypothesis and results demonstrating that IL-1β secreted from ovarian cancer epithelial cells effectively attenuates adjacent ovarian fibroblast p53 tumor suppressor protein levels, which transcriptionally activates NF-κB promoter leading to the transcriptional activation and release of key chemokines and activator molecules that synergistically act in paracrine on the initiator ovarian epithelial cells to further alter the phenotype to that of invasive and metastatic.
Collectively, our data suggest a novel model for ovarian epithelial cancer cells working in synergy with stromal fibroblasts through an inflammatory chemokine (Figure 7C): Following oncogenic activation mediated by mutated RAS or loss of a tumor suppressor gene like TP53, epithelial cancer cells express a high level of IL-1β, which stimulates IL-1R1 on adjacent stromal fibroblasts leading to attenuation of p53 tumor suppressor protein levels. Repression of p53 leads to transactivation of NF-κB, which in turn activates the transcription and release of multiple key immunomodulatory chemokines, including IL-6, IL-8, GRO-α, IL-1β, as well as VEGF. These proinflammatory and proangiogenic factors synergistically interact with each other to generate a tumor-prone microenvironment, jointly fostering a paracrine synergy with cancer cells to support malignant tumor progression mimicking an unhealed wound.
Discussion
Twenty-five years ago, Dvorak first described that human cancer is similar to an “unhealed” wound [31]. Cancer epithelial cells are highly effective in their mutation-selected ability at co-opting the stromal microenvironment, by inappropriately amplifying the normal inflammatory and cellular processes of wound healing to facilitate cancer progression, directly initiating cancer cell proliferation, angiogenesis, and invasion [32–34]. More specifically, this perturbation to a state of chronic repair relies on reciprocal paracrine interaction between epithelial tumor cells and neighboring stromal fibroblasts [35]. Although this is a universal, heterotypic signaling paradigm across tumor types that leads to a stromal inflammatory recruitment [29], details pertaining to the underlying mechanism for this well-described synergy between tumor and stroma remain largely uncharted. Here, we demonstrate that IL-1β mediates this paracrine communication between ovarian epithelial cancer cells and neighboring stroma. In addition, we show that the IL-1β/IR-1R1 signaling axis is highly elevated in both the cancer epithelium and cancer stroma of ovarian cancer patient tissue samples in vivo and that increased IHC detection of both ligand and receptor significantly predicts reduced overall patient survival. Further, we show that due to ovarian cancer cell-elevated expression of IL-1β, activation of IL-1R1 in stromal fibroblasts leads to repression of p53 and a concurrent activation of NF-κB transcriptional targets like IL-8, GRO-α, IL-6, and IL-1β. Therefore, IL-1β may act in synchrony with other cytokines to synergize its effect on the tumor promotion. It is interesting to note that fibroblasts with p53 knockdown are quite significant in promoting tumor growth from T29 cells while the effect on SKOV3 cells was minimal, as the T29 cell line was immortalized with SV40 T/t antigens and the human catalytic subunit of telomerase and is not tumorigenic. However, the human epithelial ovarian cancer cell line SKOV3 is highly tumorigenic and aggressive. The effects of p53 knockdown fibroblasts are quite significant on the tumor promotion from T29 cells, suggesting that the proinflammatory fibroblast may play important roles in oncogenic transformation rather than in the promotion of advanced tumors. Moreover, our findings revealed that targeted and sustained knockdown of p53 protein in normal ovarian fibroblasts in vitro fosters a highly activated stromal phenotype characterized by a significant increase in proliferation and progression through the cell cycle, with concurrent inhibition of apoptosis. Therefore, our report demonstrates the critical function of IL-1β as a secreted mediator used by cancer epithelial cells to synergistically activate adjacent stroma to a proinflammatory and protumorigenic phenotype.
Almost all high-grade ovarian serous carcinoma cells acquire TP53 mutations [10], and multiple reports have indicated that altered p53 expression in cancer fibroblasts, perhaps not mutational events, act to promote stromal cell involvement in stimulating tumorigenesis [36]. Indeed, evidence suggests that human prostate and breast cancer fibroblasts express a nonmutated, but functionally deficient, form of p53 [37,38], expanding on mouse and cell-based studies pointing to a selective mechanism exerted by tumorigenic epithelial cells for synergistic growth and development with p53-attenuated fibroblasts [13,39–42]. Offering novel evidence of this mechanism in the context of ovarian cancer, we show that specifically IL-1β secreted by ovarian cancer epithelial cells acts as a critical signal instructing normal ovarian fibroblasts to repress p53, indicating that p53 in stromal fibroblasts is a key sensor for perceiving and interpreting this signal. Furthermore, attenuated expression of p53 in fibroblasts significantly predicts reduced survival among serous ovarian carcinoma patients. Therefore, these findings suggest that repression of p53 in ovarian fibroblasts may be as crucial to ovarian cancer progression as TP53 mutational events in epithelial tumor cells.
The NF-κB pathway is well known for the central role it plays in human cancer, in which hyperactivation is a common occurrence due primarily to the promotion this exerts on cell cycle progression, antiapoptosis, angiogenesis, and inflammatory activation [20]. NF-κB has been shown to be functionally antagonistic with p53 in multiple tumor types [43]. Moreover, it has been observed across disparate tumor types that constitutive activation of the NF-κB pathway leads in part to repression of wild-type p53 tumor suppressor function [44]. Interestingly, this mutual repression balancing act between p53 and p65 involves competition for critical nuclear interaction partners [45], including the ARF/BRCA1/ATR/Chk1 tumor suppressor and proliferation inhibitory complex [46] and the stress activation-responsive nuclear protein proenkephalin controlling apoptosis [47]. Our study reveals a novel mechanism of interaction between p53 and NF-κB in activation of stromal fibroblasts. In contrast to the previously described mutual activation between p53 and NF-κB in cancer cells, p53 repression in ovarian fibroblasts increased the total and S276-phosphorylated p65; the latter is nuclear localized and this may facilitate the interaction with nuclear p53, thereby facilitated formation of a p53/phospho-p65 protein complex, leading to a p65-dependent transcriptional induction at the NF-κB promoter, phenotypically switching ovarian fibroblasts to potent stimulators of ovarian epithelial cell transformation and tumor growth. Therefore, p53-mediated repression of p65 transcriptional activation is a critical sensor in ovarian fibroblasts that capacitates the cancer cell-secreted signal IL-1β to induce a tumor-prone phenotype and generate a microenvironment selectively conducive to malignant progression.
Our results provide a long-sought mechanism characterizing how epithelial cancer cells, fibroblasts, and inflammation are coordinately regulated as an “unhealed wound” to generate a malignant phenotype. Elucidating additional details regarding the mechanism of regulation and interaction among IL-1R1 signaling, p53 function, and NF-κB p65 transactivation in ovarian fibroblasts is essential. New findings along these lines would increase our understanding of the role that the stroma plays in ovarian tumorigenesis and aid in the development of novel therapeutic approaches for ovarian cancer as well as other solid epithelial tumors.
Supplementary Material
Acknowledgments
The authors thank all the members of the Liu laboratory for their helpful scientific discussion and assistance. X.G. is a Visiting Professor at the University Texas MD Anderson Cancer Center from the Department of Obstetrics and Gynecology, Shihezi University School of Medicine, Shihezi, Xinjiang, China.
Footnotes
This work was supported by the National Institutes of Health National Cancer Institute (5R01 CA131183) and the Ovarian Cancer Research Fund (to J.L.). We also acknowledge the support of the Ovarian Cancer Specialized Program of Research Excellence (5P50 CA83639), as well as shared resources funded in part by the Cancer Center Support Grant (5P30 CA016672) at The University of Texas MD Anderson Cancer Center.
This article refers to supplementary materials, which are designated by Tables W1 to W5 and Figures W1 to W6 and are available online at www.neoplasia.com.
References
- 1.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–3474. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1(1):46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 4.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11(1):54–59. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 5.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 7.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth—bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 10.Network CGAR, author. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aylon Y, Oren M. New plays in the p53 theater. Curr Opin Genet Dev. 2011;21(1):86–92. doi: 10.1016/j.gde.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25(5):400–410. 413. [PubMed] [Google Scholar]
- 13.Bar J, Feniger-Barish R, Lukashchuk N, Shaham H, Moskovits N, Goldfinger N, Simansky D, Perlman M, Papa M, Yosepovich A, et al. Cancer cells suppress p53 in adjacent fibroblasts. Oncogene. 2009;28(6):933–936. doi: 10.1038/onc.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivier M, Petitjean A, Marcel V, Pétré A, Mounawar M, Plymoth A, de Fromentel CC, Hainaut P. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 2009;16(1):1–12. doi: 10.1038/cgt.2008.69. [DOI] [PubMed] [Google Scholar]
- 15.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman RS, Deavers M, Liu J, Wang E. Peritoneal inflammation—a microenvironment for epithelial ovarian cancer (EOC) J Transl Med. 2004;2(1):23. doi: 10.1186/1479-5876-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Wang E, Kavanagh JJ, Freedman RS. Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med. 2005;3:25. doi: 10.1186/1479-5876-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 21.Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1(5):a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30(14):1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Yang G, Thompson-Lanza JA, Glassman A, Hayes K, Patterson A, Marquez RT, Auersperg N, Yu Y, Hahn WC, et al. A genetically defined model for human ovarian cancer. Cancer Res. 2004;64(5):1655–1663. doi: 10.1158/0008-5472.can-03-3380. [DOI] [PubMed] [Google Scholar]
- 24.Yang G, Rosen DG, Zhang Z, Bast RC, Jr, Mills GB, Colacino JA, Mercado-Uribe I, Liu J. The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA. 2006;103(44):16472–16477. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen DG, Yang G, Deavers MT, Malpica A, Kavanagh JJ, Mills GB, Liu J. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer. 2006;106(9):1925–1932. doi: 10.1002/cncr.21767. [DOI] [PubMed] [Google Scholar]
- 26.Chang B, Liu G, Xue F, Rosen DG, Xiao L, Wang X, iu J. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22(6):817–823. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang G, Thompson JA, Fang B, Liu J. Silencing of H-ras gene expression by retrovirus-mediated siRNA decreases transformation efficiency and tumor growth in a model of human ovarian cancer. Oncogene. 2003;22(36):5694–5701. doi: 10.1038/sj.onc.1206858. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, Rosen DG, Mercado-Uribe I, Colacino JA, Mills GB, Bast RC, Jr, Zhou C, Liu J. Knockdown of p53 combined with expression of the catalytic subunit of telomerase is sufficient to immortalize primary human ovarian surface epithelial cells. Carcinogenesis. 2007;28(1):174–182. doi: 10.1093/carcin/bgl115. [DOI] [PubMed] [Google Scholar]
- 29.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22(6):1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calabrese V, Mallette FA, Deschenes-Simard X, Ramanathan S, Gagnon J, Moores A, Ilangumaran S, Ferbeyre G. SOCS1 links cytokine signaling to p53 and senescence. Mol Cell. 2009;36(5):754–767. doi: 10.1016/j.molcel.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 31.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 32.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 33.Schauer IG, Rowley DR. The functional role of reactive stroma in benign prostatic hyperplasia. Differentiation. 2011;82(4–5):200–210. doi: 10.1016/j.diff.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schauer IG, Sood AK, Mok S, Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia. 2011;13(5):393–405. doi: 10.1593/neo.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22(42):6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 36.Campbell IG, Qiu W, Polyak K, Haviv I. Breast-cancer stromal cells with TP53 mutations. N Engl J Med. 2008;358(15):1634–1635. doi: 10.1056/NEJMc086024. author reply 1636. [DOI] [PubMed] [Google Scholar]
- 37.Dudley AC, Shih SC, Cliffe AR, Hida K, Klagsbrun M. Attenuated p53 activation in tumour-associated stromal cells accompanies decreased sensitivity to etoposide and vincristine. Br J Cancer. 2008;99(1):118–125. doi: 10.1038/sj.bjc.6604465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawsawi NM, Ghebeh H, Hendrayani SF, Tulbah A, Al-Eid M, Al-Tweigeri T, Ajarim D, Alaiya A, Dermime S, Aboussekhra A. Breast carcinoma-associated fibroblasts and their counterparts display neoplastic-specific changes. Cancer Res. 2008;68(8):2717–2725. doi: 10.1158/0008-5472.CAN-08-0192. [DOI] [PubMed] [Google Scholar]
- 39.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123(6):1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 40.Kiaris H, Chatzistamou I, Trimis G, Frangou-Plemmenou M, Pafiti-Kondi A, Kalofoutis A. Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res. 2005;65(5):1627–1630. doi: 10.1158/0008-5472.CAN-04-3791. [DOI] [PubMed] [Google Scholar]
- 41.Komarova EA, Diatchenko L, Rokhlin OW, Hill JE, Wang ZJ, Krivokrysenko VI, Feinstein E, Gudkov AV. Stress-induced secretion of growth inhibitors: a novel tumor suppressor function of p53. Oncogene. 1998;17(9):1089–1096. doi: 10.1038/sj.onc.1202303. [DOI] [PubMed] [Google Scholar]
- 42.Addadi Y, Moskovits N, Granot D, Lozano G, Carmi Y, Apte RN, Neeman M, Oren M. p53 Status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner. Cancer Res. 2010;70(23):9650–9658. doi: 10.1158/0008-5472.CAN-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey A, Tergaonkar V, Lane DP. Double-edged swords as cancer therapeutics: simultaneously targeting p53 and NF-κB pathways. Nat Rev Drug Discov. 2008;7(12):1031–1040. doi: 10.1038/nrd2759. [DOI] [PubMed] [Google Scholar]
- 44.Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, Tararova ND, et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-κB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci USA. 2005;102(48):17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadgaonkar R, Phelps KM, Haque Z, Williams AJ, Silverman ES, Collins T. CREB-binding protein is a nuclear integrator of nuclear factor-κB and p53 signaling. J Biol Chem. 1999;274(4):1879–1882. doi: 10.1074/jbc.274.4.1879. [DOI] [PubMed] [Google Scholar]
- 46.Rocha S, Garrett MD, Campbell KJ, Schumm K, Perkins ND. Regulation of NF-κB and p53 through activation of ATR and Chk1 by the ARF tumour suppressor. EMBO J. 2005;24(6):1157–1169. doi: 10.1038/sj.emboj.7600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McTavish N, Copeland LA, Saville MK, Perkins ND, Spruce BA. Proenkephalin assists stress-activated apoptosis through transcriptional repression of NF-κB- and p53-regulated gene targets. Cell Death Differ. 2007;14(9):1700–1710. doi: 10.1038/sj.cdd.4402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niu J, Chang Z, Peng B, Xia Q, Lu W, Huang P, Tsao MS, Chiao PJ. Keratinocyte growth factor/fibroblast growth factor-7-regulated cell migration and invasion through activation of NF-κB transcription factors. J Biol Chem. 2007;282(9):6001–6011. doi: 10.1074/jbc.M606878200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.